Abstract
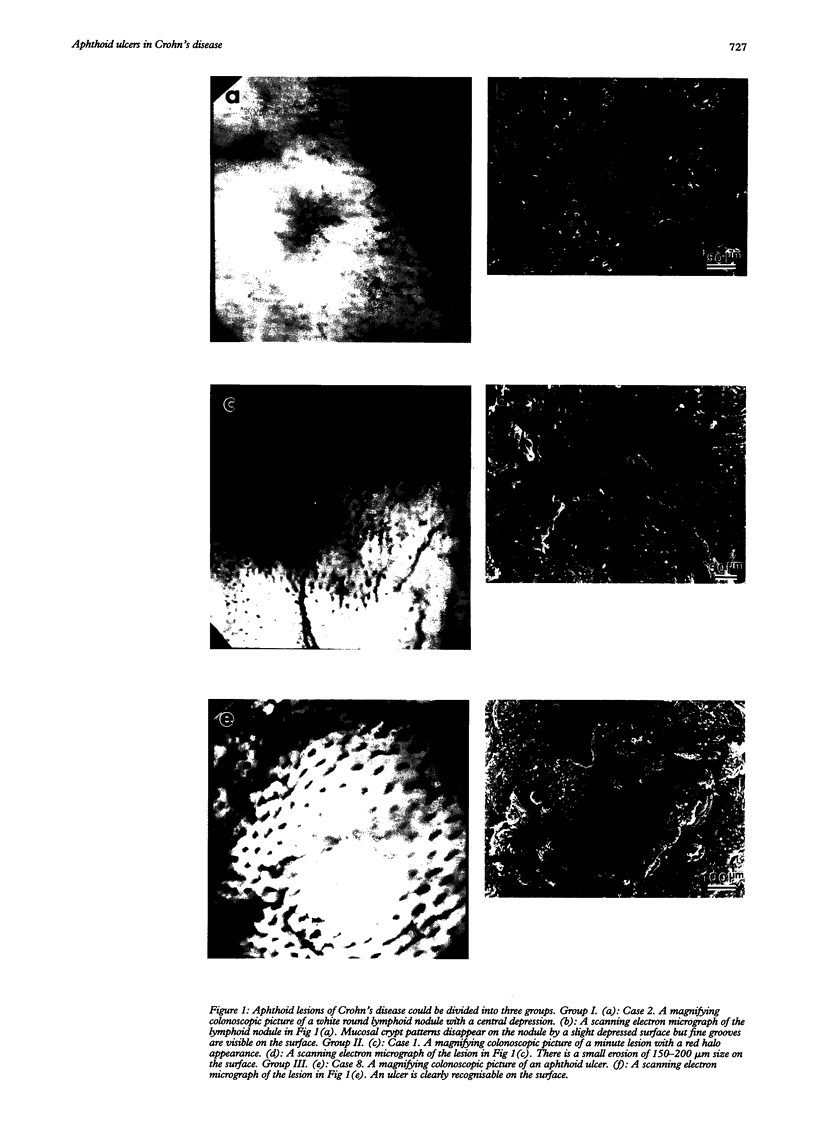
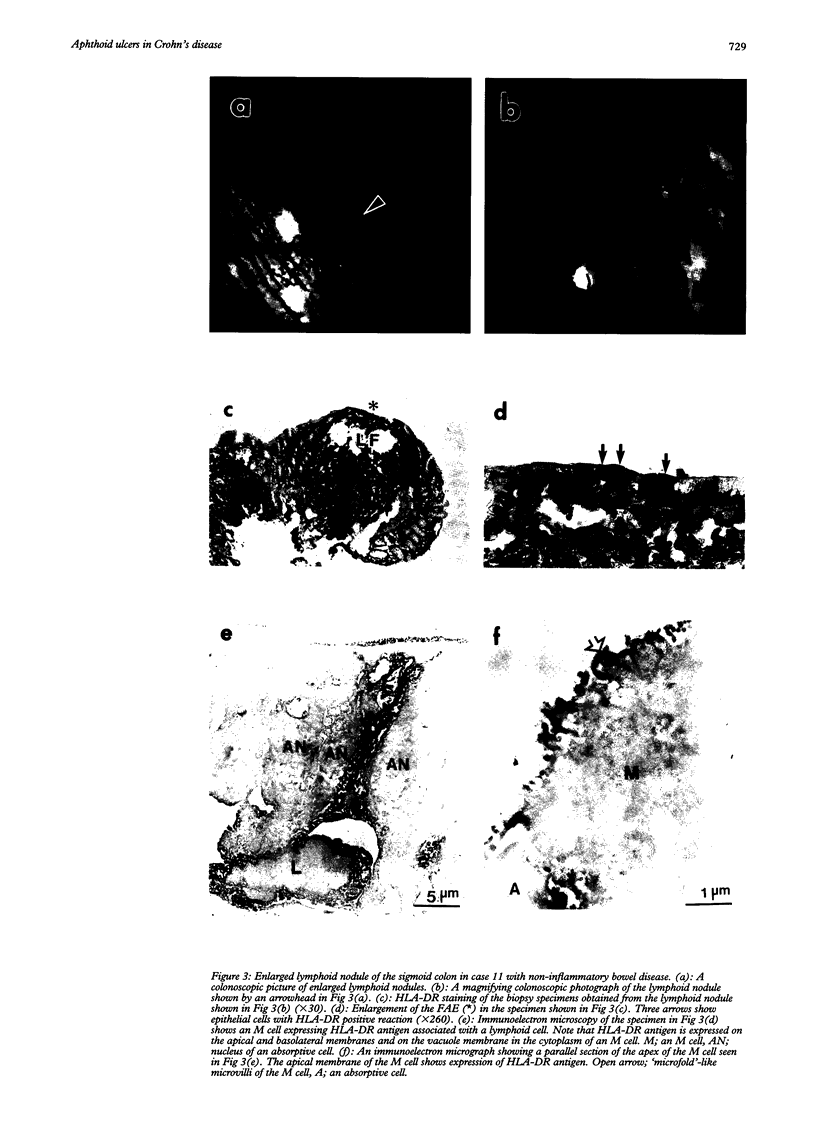
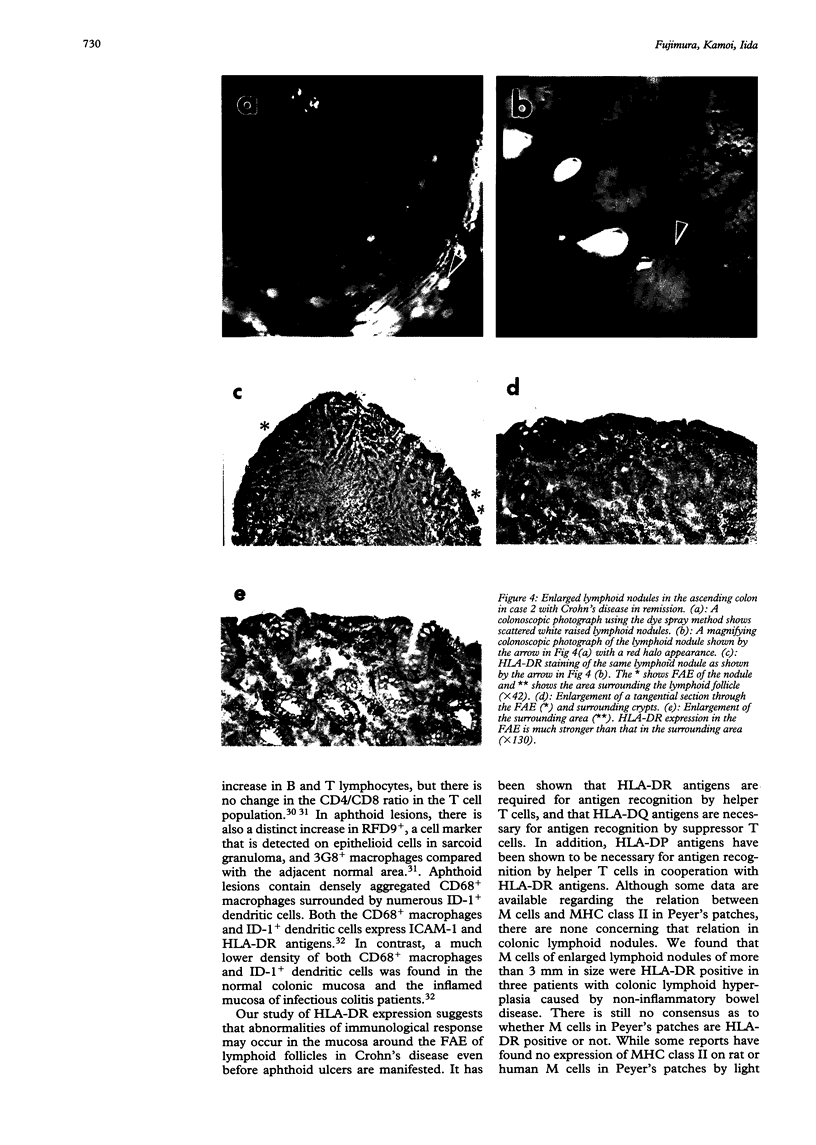
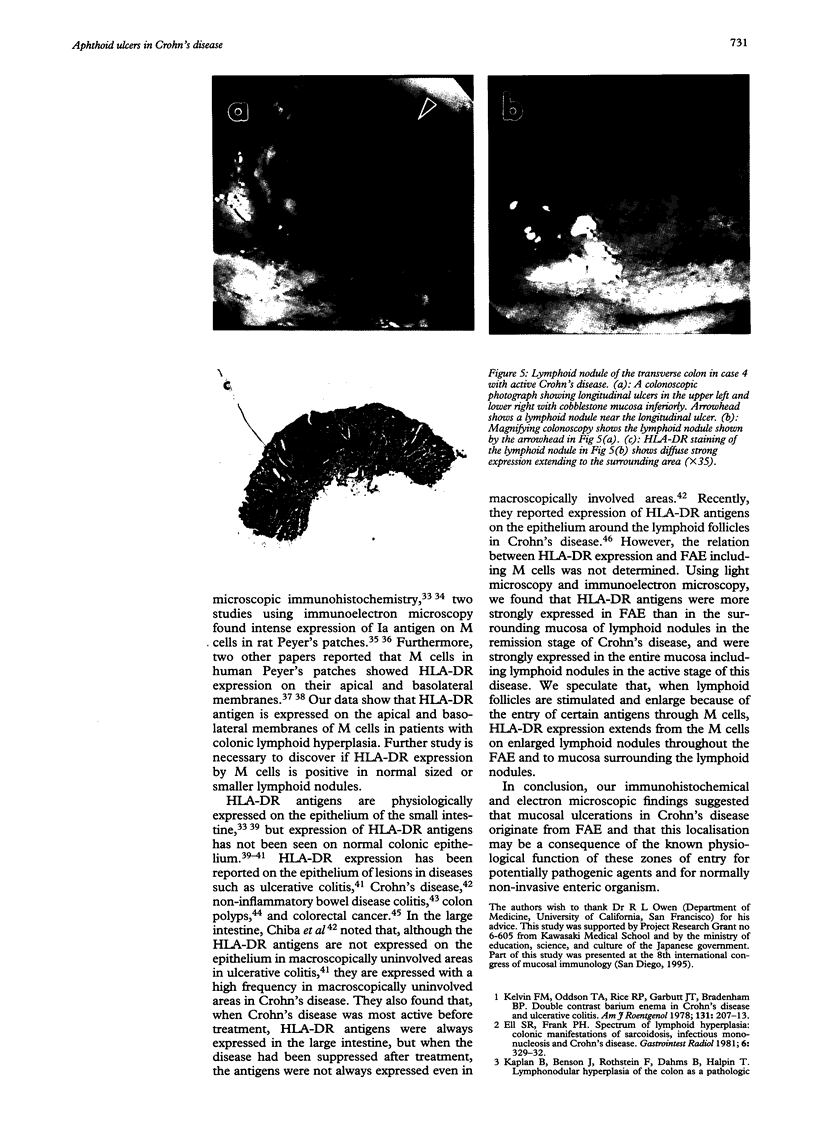
BACKGROUND--The mechanism of ulceration in Crohn's disease remains unknown. AIMS--To clarify the role of the follicle associated epithelium (FAE) of colonic lymphoid nodules in the formation of ulcers in Crohn's disease. METHODS--After identification of colonic lymphoid nodules and aphthoid lesions by magnifying colonoscopy, 76 biopsy specimens were obtained from 10 patients with Crohn's disease and three patients with colonic lymphoid hyperplasia. This study correlated magnifying colonoscopic, electron microscopic, and immunohistochemical findings of biopsy specimens. RESULTS--In Crohn's disease, scanning electron microscopy of lymphoid nodules surrounded by a red halo without visible erosions by magnifying colonoscopy, showed surface erosions 150-200 microns in size. These lymphoid nodules with red halos had small erosions either light microscopically or electron microscopically in 18 of 21 specimens (86%). Correlation of scanning and transmission electron microscopy showed residues of FAE including M cells at the edges of the erosions. In immunohistochemical studies, HLA-DR antigen was limited in M cells of FAE in the patients with lymphoid hyperplasia without inflammatory bowel disease. In Crohn's disease patients in remission, however, HLA-DR antigen was strongly expressed over the entire FAE of lymphoid nodules with a red halo endoscopically, while the expression was weak and irregular in the mucosa surrounding the lymphoid nodules. HLA-DR was strongly expressed in the entire inflamed colonic mucosa in the active stage. CONCLUSION--The red halo appearance surrounding lymphoid follicles seems to precede visible aphthoid ulcers and suggests that ulcerations in Crohn's disease originate from FAE, possibly related to its physiological role as a portal of entry for potentially pathogenic agents.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan C. H., Mendrick D. L., Trier J. S. Rat intestinal M cells contain acidic endosomal-lysosomal compartments and express class II major histocompatibility complex determinants. Gastroenterology. 1993 Mar;104(3):698–708. doi: 10.1016/0016-5085(93)91004-2. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Bjerke K. Human Peyer's patches: lympho-epithelial relationships and characteristics of immunoglobulin-producing cells. Immunol Invest. 1989 Jan-May;18(1-4):29–45. doi: 10.3109/08820138909112225. [DOI] [PubMed] [Google Scholar]
- Burbige E. J., Sobky R. Z. Endoscopic appearance of colonic lymphoid nodules: a normal variant. Gastroenterology. 1977 Mar;72(3):524–526. [PubMed] [Google Scholar]
- Chiba M., Iizuka M., Horie Y., Igarashi K., Masamune O. HLA-DR antigen expression in macroscopically uninvolved areas of intestinal epithelia in Crohn's disease. Gastroenterol Jpn. 1989 Aug;24(4):365–372. doi: 10.1007/BF02774341. [DOI] [PubMed] [Google Scholar]
- Chiba M., Iizuka M., Horie Y., Ishii N., Masamune O. Expression of HLA-DR antigens on colonic epithelium around lymph follicles. An anomalous expression in Crohn's disease. Dig Dis Sci. 1994 Jan;39(1):83–90. doi: 10.1007/BF02090065. [DOI] [PubMed] [Google Scholar]
- Chiba M., Iizuka M., Ohtaka M., Kuwabara T., Masamune O., Mukoujima T., Ito M. Four cases of the red ring sign. Gastrointest Endosc. 1992 Nov-Dec;38(6):728–730. doi: 10.1016/s0016-5107(92)70583-5. [DOI] [PubMed] [Google Scholar]
- De Smet A. A., Tubergen D. G., Martel W. Nodular lymphoid hyperplasia of the colon associated with dysgammaglobulinemia. AJR Am J Roentgenol. 1976 Sep;127(3):515–517. doi: 10.2214/ajr.127.3.515. [DOI] [PubMed] [Google Scholar]
- Ell S. R., Frank P. H. Spectrum of lymphoid hyperplasia: colonic manifestations of sarcoidosis, infectious mononucleosis, and Crohn's disease. Gastrointest Radiol. 1981;6(4):329–332. doi: 10.1007/BF01890279. [DOI] [PubMed] [Google Scholar]
- Fujimura Y., Hosobe M., Kihara T. Ultrastructural study of M cells from colonic lymphoid nodules obtained by colonoscopic biopsy. Dig Dis Sci. 1992 Jul;37(7):1089–1098. doi: 10.1007/BF01300292. [DOI] [PubMed] [Google Scholar]
- Gionchetti P., Mahida Y. R., Patel S., Jewell D. P. Macrophage and lymphocyte subpopulations in magnifying endoscopic lesions of Crohn's disease. Clin Exp Immunol. 1988 Jun;72(3):373–376. [PMC free article] [PubMed] [Google Scholar]
- Hirata I., Austin L. L., Blackwell W. H., Weber J. R., Dobbins W. O., 3rd Immunoelectron microscopic localization of HLA-DR antigen in control small intestine and colon and in inflammatory bowel disease. Dig Dis Sci. 1986 Dec;31(12):1317–1330. doi: 10.1007/BF01299810. [DOI] [PubMed] [Google Scholar]
- Hizawa K., Iida M., Kohrogi N., Kuroki F., Yao T., Sakamoto K., Fujishima M. Crohn disease: early recognition and progress of aphthous lesions. Radiology. 1994 Feb;190(2):451–454. doi: 10.1148/radiology.190.2.8284398. [DOI] [PubMed] [Google Scholar]
- Horie Y., Chiba M., Iizuka M., Igarashi K., Masamune O. Colonic lymphoid cell subsets and epithelial HLA-DR antigens in familial polyposis coli. Gastroenterol Jpn. 1989 Dec;24(6):632–639. doi: 10.1007/BF02774161. [DOI] [PubMed] [Google Scholar]
- Horie Y., Chiba M., Iizuka M., Masamune O. Class II (HLA-DR, -DP, and -DO) antigens on intestinal epithelia in ulcerative colitis, Crohn's disease, colorectal cancer and normal small intestine. Gastroenterol Jpn. 1990 Oct;25(5):575–584. doi: 10.1007/BF02779357. [DOI] [PubMed] [Google Scholar]
- Iida M., Kobayashi H., Matsumoto T., Okada M., Fuchigami T., Niizeki H., Yao T., Fujishima M. Intestinal Behçet disease: serial changes at radiography. Radiology. 1993 Jul;188(1):65–69. doi: 10.1148/radiology.188.1.8511319. [DOI] [PubMed] [Google Scholar]
- Iizuka M., Chiba M., Horie Y., Masamune O., Ohta H. Lymphoid cell subsets in colonic mucosa and HLA-DR antigens on colonic epithelia in colitis excluding ulcerative colitis and Crohn's disease. Gastroenterol Jpn. 1990 Dec;25(6):700–707. doi: 10.1007/BF02779183. [DOI] [PubMed] [Google Scholar]
- Iizuka M., Chiba M., Ohta H., Kodama K., Igarashi K., Arakawa H., Masamune O. Expression of HLA-DR antigens on colonic epithelium in ulcerative colitis. Gastroenterol Jpn. 1987 Oct;22(5):571–577. doi: 10.1007/BF02776716. [DOI] [PubMed] [Google Scholar]
- Jarry A., Robaszkiewicz M., Brousse N., Potet F. Immune cells associated with M cells in the follicle-associated epithelium of Peyer's patches in the rat. An electron- and immuno-electron-microscopic study. Cell Tissue Res. 1989 Feb;255(2):293–298. doi: 10.1007/BF00224111. [DOI] [PubMed] [Google Scholar]
- Kaplan B., Benson J., Rothstein F., Dahms B., Halpin T. Lymphonodular hyperplasia of the colon as a pathologic finding in children with lower gastrointestinal bleeding. J Pediatr Gastroenterol Nutr. 1984 Nov;3(5):704–708. doi: 10.1097/00005176-198411000-00012. [DOI] [PubMed] [Google Scholar]
- Kelvin F. M., Oddson T. A., Rice R. P., Garbutt J. T., Bradenham B. P. Double contrast barium enema in Crohn's disease and ulcerative colitis. AJR Am J Roentgenol. 1978 Aug;131(2):207–213. doi: 10.2214/ajr.131.2.207. [DOI] [PubMed] [Google Scholar]
- LOCKHART-MUMMERY H. E., MORSON B. C. Crohn's disease (regional enteritis) of the large intestine and its distinction from ulcerative colitis. Gut. 1960 Jun;1:87–105. doi: 10.1136/gut.1.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makiyama K., Bennett M. K., Jewell D. P. Endoscopic appearances of the rectal mucosa of patients with Crohn's disease visualised with a magnifying colonoscope. Gut. 1984 Apr;25(4):337–340. doi: 10.1136/gut.25.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T., Iida M., Tada S., Fuchigami T., Iwashita A., Sakamoto K., Fujishima M. The value of double-contrast barium enema in amebic colitis. Gastrointest Radiol. 1989 Winter;14(1):73–78. doi: 10.1007/BF01889160. [DOI] [PubMed] [Google Scholar]
- Mayrhofer G., Pugh C. W., Barclay A. N. The distribution, ontogeny and origin in the rat of Ia-positive cells with dendritic morphology and of Ia antigen in epithelia, with special reference to the intestine. Eur J Immunol. 1983 Feb;13(2):112–122. doi: 10.1002/eji.1830130206. [DOI] [PubMed] [Google Scholar]
- Morise K., Yamaguchi T., Kuroiwa A., Kanayama K., Matsuura T., Shinoda M., Yamamoto H., Horiuchi Y., Furusawa A., Iwase H. Expression of adhesion molecules and HLA-DR by macrophages and dendritic cells in aphthoid lesions of Crohn's disease: an immunocytochemical study. J Gastroenterol. 1994 Jun;29(3):257–264. doi: 10.1007/BF02358363. [DOI] [PubMed] [Google Scholar]
- Morson B. C. The early histological lesion of Crohn's disease. Proc R Soc Med. 1972 Jan;65(1):71–72. doi: 10.1177/003591577206500129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagura H., Ohtani H., Masuda T., Kimura M., Nakamura S. HLA-DR expression on M cells overlying Peyer's patches is a common feature of human small intestine. Acta Pathol Jpn. 1991 Nov;41(11):818–823. doi: 10.1111/j.1440-1827.1991.tb01624.x. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Iida M., Tominaga M., Yao T., Hirata N., Fujishima M. Salmonella colitis: assessment with double-contrast barium enema examination in seven patients. Radiology. 1992 Aug;184(2):537–540. doi: 10.1148/radiology.184.2.1620861. [DOI] [PubMed] [Google Scholar]
- Owen R. L., Piazza A. J., Ermak T. H. Ultrastructural and cytoarchitectural features of lymphoreticular organs in the colon and rectum of adult BALB/c mice. Am J Anat. 1991 Jan;190(1):10–18. doi: 10.1002/aja.1001900103. [DOI] [PubMed] [Google Scholar]
- Sankey E. A., Dhillon A. P., Anthony A., Wakefield A. J., Sim R., More L., Hudson M., Sawyerr A. M., Pounder R. E. Early mucosal changes in Crohn's disease. Gut. 1993 Mar;34(3):375–381. doi: 10.1136/gut.34.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Bofill M., Jewell D. P. Intestinal lymphocyte subpopulations in inflammatory bowel disease: an analysis by immunohistological and cell isolation techniques. Gut. 1984 Jan;25(1):32–40. doi: 10.1136/gut.25.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Mason D. Y., Jewell D. P. Expression of HLA-DR antigens by colonic epithelium in inflammatory bowel disease. Clin Exp Immunol. 1983 Sep;53(3):614–618. [PMC free article] [PubMed] [Google Scholar]
- Spencer J., Finn T., Isaacson P. G. Expression of HLA-DR antigens on epithelium associated with lymphoid tissue in the human gastrointestinal tract. Gut. 1986 Feb;27(2):153–157. doi: 10.1136/gut.27.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tielbeek A. V., Rosenbusch G., Muytjens H. L., Yap S. H., Strijk S. P., Boetes C. Roentgenologic changes of the colon in Campylobacter infection. Gastrointest Radiol. 1985;10(4):358–361. doi: 10.1007/BF01893132. [DOI] [PubMed] [Google Scholar]
- Vantrappen G., Ponette E., Geboes K., Bertrand P. Yersinia enteritis and enterocolitis: gastroenterological aspects. Gastroenterology. 1977 Feb;72(2):220–227. [PubMed] [Google Scholar]
- Wakefield A. J., Pittilo R. M., Sim R., Cosby S. L., Stephenson J. R., Dhillon A. P., Pounder R. E. Evidence of persistent measles virus infection in Crohn's disease. J Med Virol. 1993 Apr;39(4):345–353. doi: 10.1002/jmv.1890390415. [DOI] [PubMed] [Google Scholar]
- Wakefield A. J., Sankey E. A., Dhillon A. P., Sawyerr A. M., More L., Sim R., Pittilo R. M., Rowles P. M., Hudson M., Lewis A. A. Granulomatous vasculitis in Crohn's disease. Gastroenterology. 1991 May;100(5 Pt 1):1279–1287. [PubMed] [Google Scholar]
- Wakefield A. J., Sawyerr A. M., Dhillon A. P., Pittilo R. M., Rowles P. M., Lewis A. A., Pounder R. E. Pathogenesis of Crohn's disease: multifocal gastrointestinal infarction. Lancet. 1989 Nov 4;2(8671):1057–1062. doi: 10.1016/s0140-6736(89)91078-7. [DOI] [PubMed] [Google Scholar]