Abstract
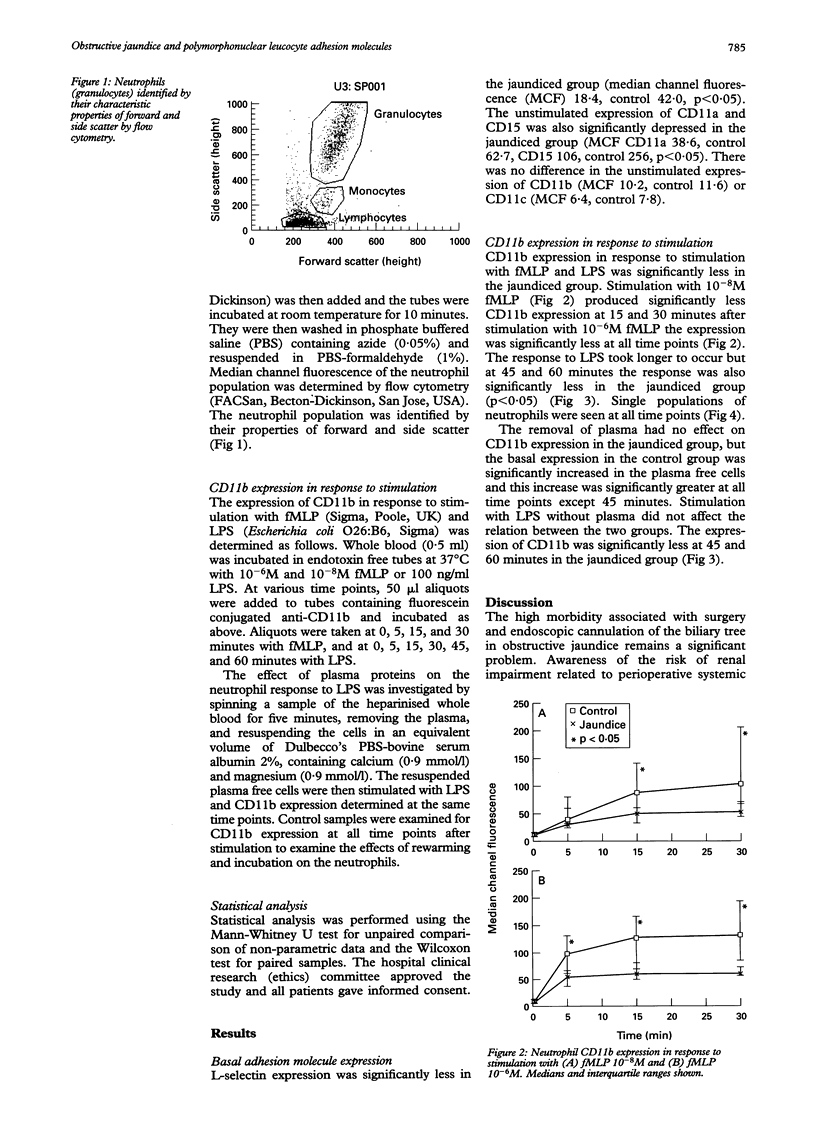
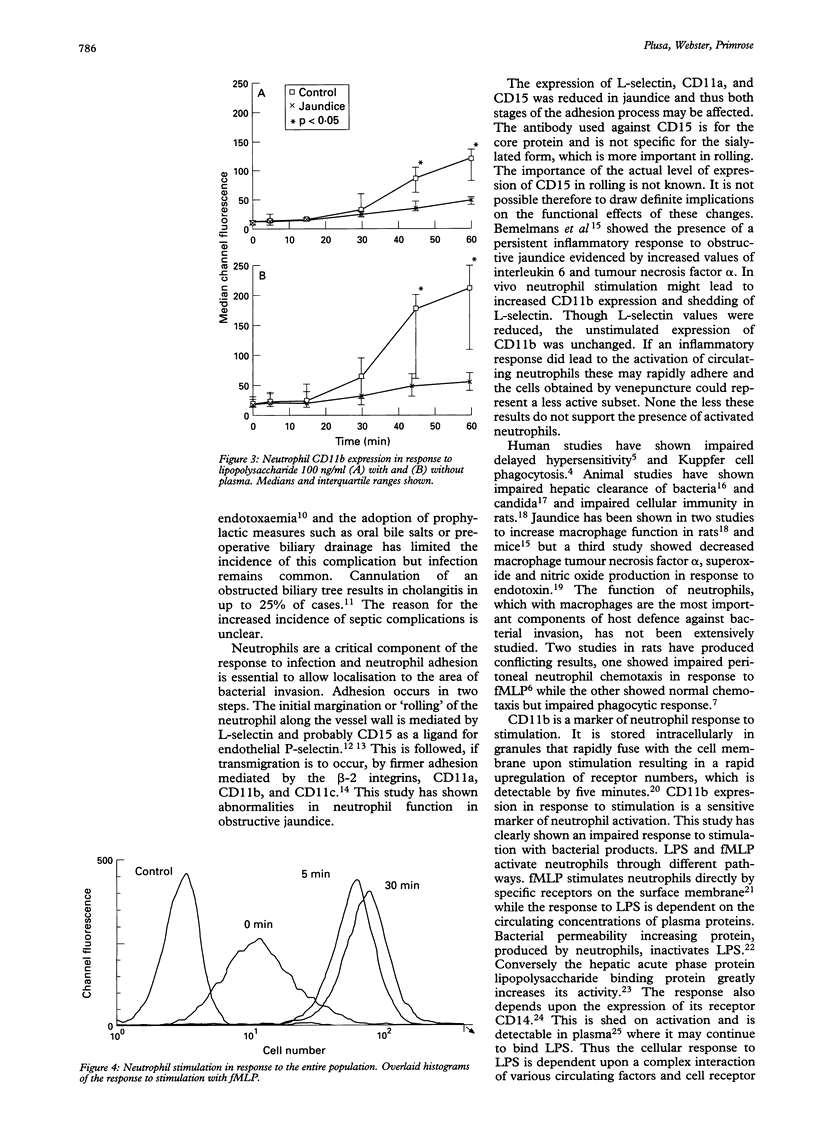
BACKGROUND--Obstructive jaundice is associated with an increased incidence of infection and endotoxaemia, which may result from impaired host immunity. Neutrophil adhesion to vascular endothelium is a key part of the inflammatory response. AIMS--To investigate neutrophil adhesion molecule expression and activation in obstructive jaundice. PATIENTS--Nine adult patients with obstructive jaundice and 11 control subjects. METHODS--The expression of the neutrophil adhesion receptors L-selectin, CD11a, CD11b, CD11c, and CD15 was determined using flow cytometry. CD11b expression in response to stimulation with fMLP and endotoxin was measured. RESULTS--The basal expression of L-selectin, CD11a, and CD15 was significantly decreased in jaundiced patients (p < 0.05) and the expression of CD11b in response to stimulation with fMLP and endotoxin was significantly impaired in the jaundiced group. Endotoxin stimulation without plasma did not reverse the impaired response showing that it is not caused by endotoxin inactivation by plasma proteins. CONCLUSIONS--Neutrophils from patients with obstructive jaundice show decreased adhesion receptor expression and an impaired response to stimulation with bacterial products. This cellular dysfunction may be responsible for the high incidence of septic complications in these patients.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andy O. J., Jr, Grogan J. B., Griswold J. A., Scott-Conner C. E. Peritoneal neutrophil chemotaxis is impaired in biliary obstruction. Am Surg. 1992 Jan;58(1):28–31. [PubMed] [Google Scholar]
- Bemelmans M. H., Gouma D. J., Greve J. W., Buurman W. A. Cytokines tumor necrosis factor and interleukin-6 in experimental biliary obstruction in mice. Hepatology. 1992 Jun;15(6):1132–1136. doi: 10.1002/hep.1840150626. [DOI] [PubMed] [Google Scholar]
- Burkey T. H., Webster R. O. Adenosine inhibits fMLP-stimulated adherence and superoxide anion generation by human neutrophils at an early step in signal transduction. Biochim Biophys Acta. 1993 Feb 17;1175(3):312–318. doi: 10.1016/0167-4889(93)90223-c. [DOI] [PubMed] [Google Scholar]
- Cainzos M., Alcalde J. A., Potel J., Puente J. L. Hyperbilirubinemia, jaundice and anergy. Hepatogastroenterology. 1992 Aug;39(4):330–332. [PubMed] [Google Scholar]
- Dentener M. A., Von Asmuth E. J., Francot G. J., Marra M. N., Buurman W. A. Antagonistic effects of lipopolysaccharide binding protein and bactericidal/permeability-increasing protein on lipopolysaccharide-induced cytokine release by mononuclear phagocytes. Competition for binding to lipopolysaccharide. J Immunol. 1993 Oct 15;151(8):4258–4265. [PubMed] [Google Scholar]
- Drivas G., James O., Wardle N. Study of reticuloendothelial phagocytic capacity in patients with cholestasis. Br Med J. 1976 Jun 26;1(6025):1568–1569. doi: 10.1136/bmj.1.6025.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H. J., Torrealba V., Hudd C., Knight M. The effect of preoperative bile salt administration on postoperative renal function in patients with obstructive jaundice. Br J Surg. 1982 Dec;69(12):706–708. doi: 10.1002/bjs.1800691207. [DOI] [PubMed] [Google Scholar]
- Furie M. B., Tancinco M. C., Smith C. W. Monoclonal antibodies to leukocyte integrins CD11a/CD18 and CD11b/CD18 or intercellular adhesion molecule-1 inhibit chemoattractant-stimulated neutrophil transendothelial migration in vitro. Blood. 1991 Oct 15;78(8):2089–2097. [PubMed] [Google Scholar]
- Greve J. W., Gouma D. J., Soeters P. B., Buurman W. A. Suppression of cellular immunity in obstructive jaundice is caused by endotoxins: a study with germ-free rats. Gastroenterology. 1990 Feb;98(2):478–485. doi: 10.1016/0016-5085(90)90841-n. [DOI] [PubMed] [Google Scholar]
- Jackaman F. R., Hilson G. R., Smith L. Bile bacteria in patients with benign bile duct stricture. Br J Surg. 1980 May;67(5):329–332. doi: 10.1002/bjs.1800670509. [DOI] [PubMed] [Google Scholar]
- Katz S., Grosfeld J. L., Gross K., Plager D. A., Ross D., Rosenthal R. S., Hull M., Weber T. R. Impaired bacterial clearance and trapping in obstructive jaundice. Ann Surg. 1984 Jan;199(1):14–20. doi: 10.1097/00000658-198401000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S., Merkel G. J., Folkening W. J., Rosenthal R. S., Grosfeld J. L. Impaired clearance and organ localization of Candida albicans in obstructive jaundice. J Pediatr Surg. 1991 Aug;26(8):904–907. doi: 10.1016/0022-3468(91)90834-g. [DOI] [PubMed] [Google Scholar]
- Krüger C., Schütt C., Obertacke U., Joka T., Müller F. E., Knöller J., Köller M., König W., Schönfeld W. Serum CD14 levels in polytraumatized and severely burned patients. Clin Exp Immunol. 1991 Aug;85(2):297–301. doi: 10.1111/j.1365-2249.1991.tb05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers T. W., Tool A. T., van der Schoot C. E., Ginsel L. A., Onderwater J. J., Roos D., Verhoeven A. J. Membrane surface antigen expression on neutrophils: a reappraisal of the use of surface markers for neutrophil activation. Blood. 1991 Aug 15;78(4):1105–1111. [PubMed] [Google Scholar]
- Lai E. C., Mok F. P., Fan S. T., Lo C. M., Chu K. M., Liu C. L., Wong J. Preoperative endoscopic drainage for malignant obstructive jaundice. Br J Surg. 1994 Aug;81(8):1195–1198. doi: 10.1002/bjs.1800810839. [DOI] [PubMed] [Google Scholar]
- Lund-Johansen F., Olweus J., Horejsi V., Skubitz K. M., Thompson J. S., Vilella R., Symington F. W. Activation of human phagocytes through carbohydrate antigens (CD15, sialyl-CD15, CDw17, and CDw65). J Immunol. 1992 May 15;148(10):3221–3229. [PubMed] [Google Scholar]
- Marra M. N., Wilde C. G., Collins M. S., Snable J. L., Thornton M. B., Scott R. W. The role of bactericidal/permeability-increasing protein as a natural inhibitor of bacterial endotoxin. J Immunol. 1992 Jan 15;148(2):532–537. [PubMed] [Google Scholar]
- Mathison J. C., Virca G. D., Wolfson E., Tobias P. S., Glaser K., Ulevitch R. J. Adaptation to bacterial lipopolysaccharide controls lipopolysaccharide-induced tumor necrosis factor production in rabbit macrophages. J Clin Invest. 1990 Apr;85(4):1108–1118. doi: 10.1172/JCI114542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavonen T., Renkonen R. Selective expression of sialyl-Lewis x and Lewis a epitopes, putative ligands for L-selectin, on peripheral lymph-node high endothelial venules. Am J Pathol. 1992 Dec;141(6):1259–1264. [PMC free article] [PubMed] [Google Scholar]
- Pain J. A., Cahill C. J., Bailey M. E. Perioperative complications in obstructive jaundice: therapeutic considerations. Br J Surg. 1985 Dec;72(12):942–945. doi: 10.1002/bjs.1800721203. [DOI] [PubMed] [Google Scholar]
- Reynolds J. V., Murchan P., Redmond H. P., Watson R. W., Leonard N., Hill A., Clarke P., Marks P., Keane F. B., Tanner W. A. Failure of macrophage activation in experimental obstructive jaundice: association with bacterial translocation. Br J Surg. 1995 Apr;82(4):534–538. doi: 10.1002/bjs.1800820432. [DOI] [PubMed] [Google Scholar]
- Roughneen P. T., Drath D. B., Kulkarni A. D., Kumar S. C., Andrassy R. J., Rowlands B. J. Inflammatory cell function in young rodents with experimental cholestasis: investigations of functional deficits, their etiology, and their reversibility. J Pediatr Surg. 1989 Jul;24(7):668–673. doi: 10.1016/s0022-3468(89)80716-x. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Von Andrian U. H., Hansell P., Chambers J. D., Berger E. M., Torres Filho I., Butcher E. C., Arfors K. E. L-selectin function is required for beta 2-integrin-mediated neutrophil adhesion at physiological shear rates in vivo. Am J Physiol. 1992 Oct;263(4 Pt 2):H1034–H1044. doi: 10.1152/ajpheart.1992.263.4.H1034. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. P., Moodie H., Stamatakis J. D., Kakkar V. V., Williams R. Endotoxaemia and renal failure in cirrhosis and obstructive jaundice. Br Med J. 1976 Dec 11;2(6049):1415–1418. doi: 10.1136/bmj.2.6049.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthen G. S., Avdi N., Vukajlovich S., Tobias P. S. Neutrophil adherence induced by lipopolysaccharide in vitro. Role of plasma component interaction with lipopolysaccharide. J Clin Invest. 1992 Dec;90(6):2526–2535. doi: 10.1172/JCI116146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Hermanowski-Vosatka A., Rockwell P., Detmers P. A. Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J Exp Med. 1991 May 1;173(5):1281–1286. doi: 10.1084/jem.173.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong K. L., Rowles P. M., Patterson K. G., Linch D. C. Granulocyte-macrophage colony-stimulating factor induces neutrophil adhesion to pulmonary vascular endothelium in vivo: role of beta 2 integrins. Blood. 1992 Sep 15;80(6):1565–1575. [PubMed] [Google Scholar]


