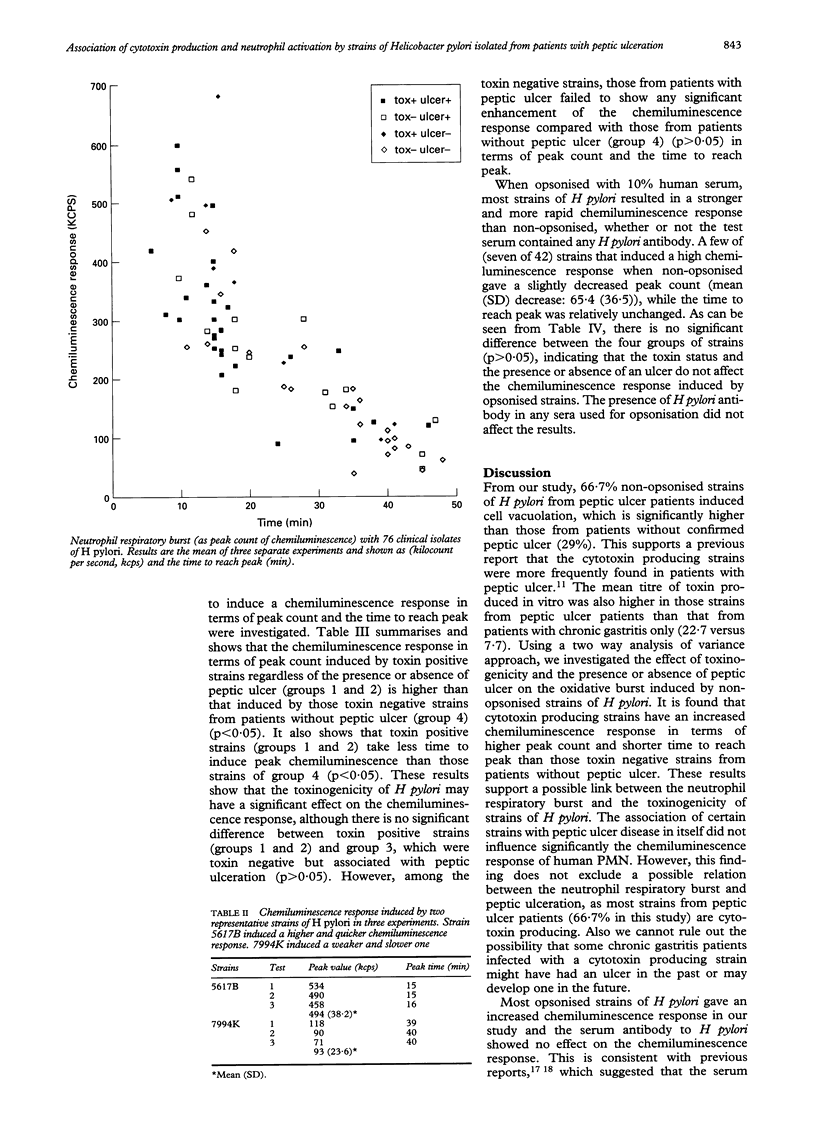
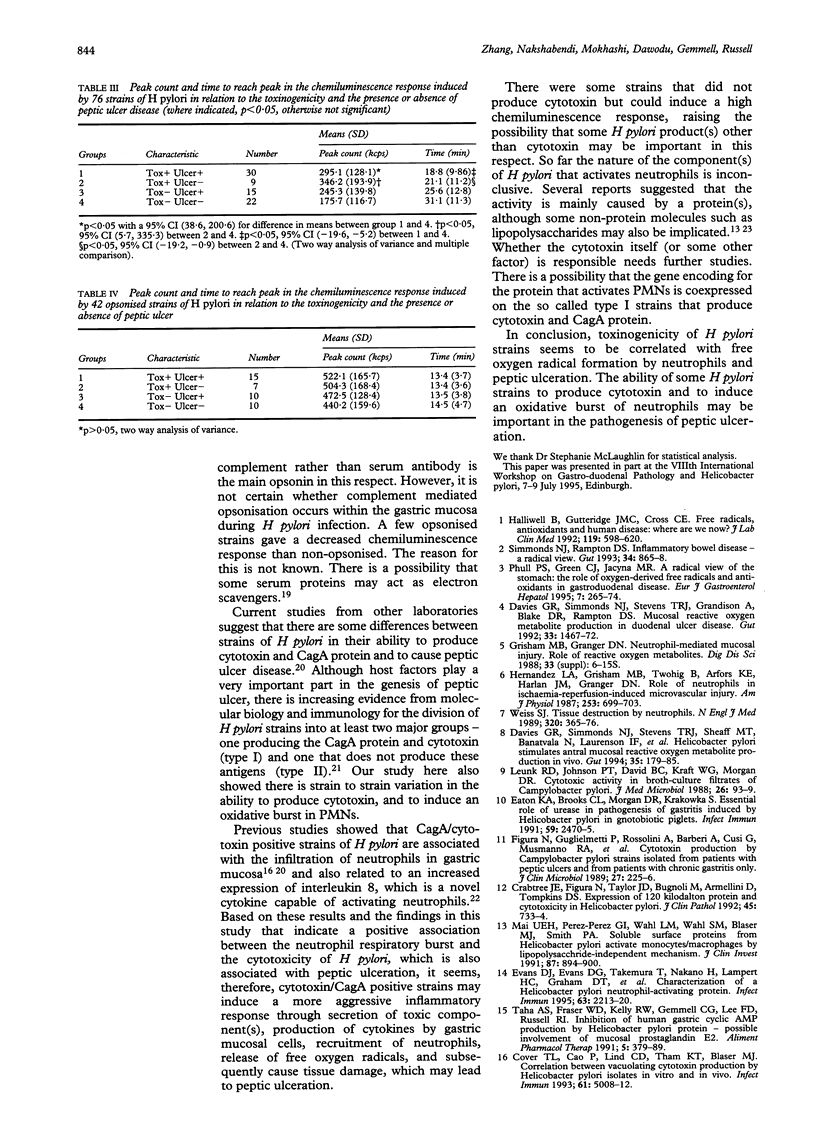
Abstract
BACKGROUND: Helicobacter pylori is associated with neutrophil infiltration within the gastroduodenal mucosa. Neutrophil activation provides a major source of oxygen free radicals, which have been implicated in the pathogenesis of peptic ulceration. AIM: To investigate if cytotoxin producing strains of H pylori are associated with the generation of oxidative burst in polymorphonuclear neutrophils (PMNs). PATIENTS: 76 patients undergoing endoscopy of whom 45 had peptic ulcer and 31 chronic gastritis only were studied. METHODS: Strains of H pylori were cultured in Brucella broth. After 48 hours, bacteria were harvested by centrifugation and a bacterial suspension prepared as a stimulus for PMN oxidative burst using chemiluminescence. PMNs were prepared from health blood donors. To test the ability of strains to produce cytotoxin, culture supernatants of each were concentrated by polyethylene glycol and tested on cultured Vero cells for intracellular vacuolation. RESULTS: 30 of 45 (66.7%) peptic ulcer patients induced cell vacuolation versus nine of 31 (29%) strains from patients with chronic gastritis only (p < 0.01). Cytotoxin positive strains of H pylori regardless of the presence or absence of peptic ulcer displayed an increased induction of respiratory burst in PMNs compared with toxin negative strains from patients with chronic gastritis only (p < 0.05). Among the toxin negative strains, those from patients with peptic ulcer did not show a significant increase of the oxidative burst than those from patients without peptic ulcer (NS). CONCLUSION: Toxinogenicity of strains of H pylori seems to be correlated with neutrophil respiratory burst and peptic ulceration. The ability of some strains of H pylori to produce cytotoxin and to induce the oxidative burst in neutrophils may be important in the pathogenesis of peptic ulcer disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briheim G., Stendahl O., Dahlgren C. Intra- and extracellular events in luminol-dependent chemiluminescence of polymorphonuclear leukocytes. Infect Immun. 1984 Jul;45(1):1–5. doi: 10.1128/iai.45.1.1-5.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T. L., Cao P., Lind C. D., Tham K. T., Blaser M. J. Correlation between vacuolating cytotoxin production by Helicobacter pylori isolates in vitro and in vivo. Infect Immun. 1993 Dec;61(12):5008–5012. doi: 10.1128/iai.61.12.5008-5012.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Farmery S. M., Lindley I. J., Figura N., Peichl P., Tompkins D. S. CagA/cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines. J Clin Pathol. 1994 Oct;47(10):945–950. doi: 10.1136/jcp.47.10.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Figura N., Taylor J. D., Bugnoli M., Armellini D., Tompkins D. S. Expression of 120 kilodalton protein and cytotoxicity in Helicobacter pylori. J Clin Pathol. 1992 Aug;45(8):733–734. doi: 10.1136/jcp.45.8.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Taylor J. D., Wyatt J. I., Heatley R. V., Shallcross T. M., Tompkins D. S., Rathbone B. J. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991 Aug 10;338(8763):332–335. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- Das S. S., Karim Q. N., Easmon C. S. Opsonophagocytosis of Campylobacter pylori. J Med Microbiol. 1988 Oct;27(2):125–130. doi: 10.1099/00222615-27-2-125. [DOI] [PubMed] [Google Scholar]
- Davies G. R., Simmonds N. J., Stevens T. R., Grandison A., Blake D. R., Rampton D. S. Mucosal reactive oxygen metabolite production in duodenal ulcer disease. Gut. 1992 Nov;33(11):1467–1472. doi: 10.1136/gut.33.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G. R., Simmonds N. J., Stevens T. R., Sheaff M. T., Banatvala N., Laurenson I. F., Blake D. R., Rampton D. S. Helicobacter pylori stimulates antral mucosal reactive oxygen metabolite production in vivo. Gut. 1994 Feb;35(2):179–185. doi: 10.1136/gut.35.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton K. A., Brooks C. L., Morgan D. R., Krakowka S. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect Immun. 1991 Jul;59(7):2470–2475. doi: 10.1128/iai.59.7.2470-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Takemura T., Nakano H., Lampert H. C., Graham D. Y., Granger D. N., Kvietys P. R. Characterization of a Helicobacter pylori neutrophil-activating protein. Infect Immun. 1995 Jun;63(6):2213–2220. doi: 10.1128/iai.63.6.2213-2220.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figura N., Guglielmetti P., Rossolini A., Barberi A., Cusi G., Musmanno R. A., Russi M., Quaranta S. Cytotoxin production by Campylobacter pylori strains isolated from patients with peptic ulcers and from patients with chronic gastritis only. J Clin Microbiol. 1989 Jan;27(1):225–226. doi: 10.1128/jcm.27.1.225-226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham M. B., Granger D. N. Neutrophil-mediated mucosal injury. Role of reactive oxygen metabolites. Dig Dis Sci. 1988 Mar;33(3 Suppl):6S–15S. doi: 10.1007/BF01538126. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M., Cross C. E. Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med. 1992 Jun;119(6):598–620. [PubMed] [Google Scholar]
- Leunk R. D., Johnson P. T., David B. C., Kraft W. G., Morgan D. R. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988 Jun;26(2):93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- Mai U. E., Perez-Perez G. I., Wahl L. M., Wahl S. M., Blaser M. J., Smith P. D. Soluble surface proteins from Helicobacter pylori activate monocytes/macrophages by lipopolysaccharide-independent mechanism. J Clin Invest. 1991 Mar;87(3):894–900. doi: 10.1172/JCI115095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinlay A. W., Young A., Russell R. I., Gemmell C. G. Opsonic requirements of Helicobacter pylori. J Med Microbiol. 1993 Mar;38(3):209–215. doi: 10.1099/00222615-38-3-209. [DOI] [PubMed] [Google Scholar]
- Nielsen H., Andersen L. P. Activation of human phagocyte oxidative metabolism by Helicobacter pylori. Gastroenterology. 1992 Dec;103(6):1747–1753. doi: 10.1016/0016-5085(92)91430-c. [DOI] [PubMed] [Google Scholar]
- Phull P. S., Green C. J., Jacyna M. R. A radical view of the stomach: the role of oxygen-derived free radicals and anti-oxidants in gastroduodenal disease. Eur J Gastroenterol Hepatol. 1995 Mar;7(3):265–274. [PubMed] [Google Scholar]
- Simmonds N. J., Rampton D. S. Inflammatory bowel disease--a radical view. Gut. 1993 Jul;34(7):865–868. doi: 10.1136/gut.34.7.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha A. S., Fraser W. D., Kelly R. W., Gemmell C. G., Lee F. D., Russell R. I. Inhibition of human gastric cyclic AMP production by Helicobacter pylori protein--possible involvement of mucosal prostaglandin E2. Aliment Pharmacol Ther. 1991 Aug;5(4):379–389. doi: 10.1111/j.1365-2036.1991.tb00041.x. [DOI] [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Xiang Z., Censini S., Bayeli P. F., Telford J. L., Figura N., Rappuoli R., Covacci A. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect Immun. 1995 Jan;63(1):94–98. doi: 10.1128/iai.63.1.94-98.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]


