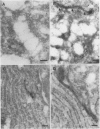
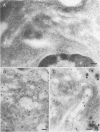
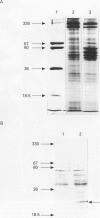
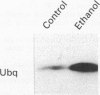
Abstract
BACKGROUND: Cytoskeletal changes after longterm exposure to ethanol have been described in a number of cell types in adult rat and humans. These changes can play a key part in the impairment of nutrient assimilation and postnatal growth retardation after prenatal damage of the intestinal epithelium produced by ethanol intake. AIMS: To determine, in the newborn rat, which cytoskeletal proteins are affected by longterm ethanol exposure in utero and to what extent. ANIMALS: The offspring of two experimental groups of female Wistar rats: ethanol treated group receiving up to 25% (w/v) of ethanol in the drinking fluid and control group receiving water as drinking fluid. METHODS: Single and double electron microscopy immunolocalisation and label density estimation of cytoskeletal proteins on sections of proximal small intestine incubated with monoclonal antibodies against actin, alpha-tubulin, cytokeratin (polypeptides 1, 5, 6, 7, 8, 10, 11, and 18), and with a polyclonal antibody anti-beta 1,4-galactosyl transferase as trans golgi (TG) or trans golgi network (TGN) marker, or both. SDS-PAGE technique was also performed on cytoskeletal enriched fractions from small intestine. Western blotting analysis was carried out by incubation with the same antibodies used for immunolocalisation. RESULTS: Intestinal epithelium of newborn rats from the ethanol treated group showed an overexpression of cytoskeletal polypeptides ranging from 39 to 54 kDa, affecting actin and some cytokeratins, but not tubulin. Furthermore, a cytokeratin related polypeptide of 28-29 kDa was identified together with an increase in free ubiquitin in the same group. It was noteworthy that actin and cytokeratin were abnormally located in the TG or the TGN, or both. CONCLUSIONS: Longterm exposure to ethanol in utero causes severe dysfunction in the cytoskeleton of the developing intestinal epithelium. Actin and cytokeratins, which are involved in cytoskeleton anchoring to plasma membrane and cell adhesion, are particularly affected, showing overexpression, impaired proteolysis, and mislocalisation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adickes E. D., Mollner T. J. Ethanol-induced cytoskeletal dysgenesis with dietary protein manipulations. Alcohol Alcohol. 1986;21(4):347–355. [PubMed] [Google Scholar]
- Akamatsu M., Hori S., Tsutsumi Y., Osamura R. Y., Ohkido M. Ubiquitinated cytokeratin inclusions in lichen amyloidosus: an immunohistochemical analysis. Pathol Int. 1995 Feb;45(2):116–122. doi: 10.1111/j.1440-1827.1995.tb03431.x. [DOI] [PubMed] [Google Scholar]
- Barbatis C., Morton J., Woods J. C., Burns J., Bradley J., McGee J. O. Disorganisation of intermediate filament structure in alcoholic and other liver diseases. Gut. 1986 Jul;27(7):765–770. doi: 10.1136/gut.27.7.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs J. M. Lipid intermolecular hydrogen bonding: influence on structural organization and membrane function. Biochim Biophys Acta. 1987 Oct 5;906(3):353–404. doi: 10.1016/0304-4157(87)90017-7. [DOI] [PubMed] [Google Scholar]
- Buts J. P., Sokal E. M., Van Hoof F. Prenatal exposure to ethanol in rats: effects on postnatal maturation of the small intestine and liver. Pediatr Res. 1992 Nov;32(5):574–579. doi: 10.1203/00006450-199211000-00018. [DOI] [PubMed] [Google Scholar]
- Charness M. E., Safran R. M., Perides G. Ethanol inhibits neural cell-cell adhesion. J Biol Chem. 1994 Mar 25;269(12):9304–9309. [PubMed] [Google Scholar]
- Corsi D., Galluzzi L., Crinelli R., Magnani M. Ubiquitin is conjugated to the cytoskeletal protein alpha-spectrin in mature erythrocytes. J Biol Chem. 1995 Apr 14;270(15):8928–8935. doi: 10.1074/jbc.270.15.8928. [DOI] [PubMed] [Google Scholar]
- Doyle K. M., Bird D. A., al-Salihi S., Hallaq Y., Cluette-Brown J. E., Goss K. A., Laposata M. Fatty acid ethyl esters are present in human serum after ethanol ingestion. J Lipid Res. 1994 Mar;35(3):428–437. [PubMed] [Google Scholar]
- Gordon B. H., Baraona E., Miyakawa H., Finkelman F., Lieber C. S. Exaggerated acetaldehyde response after ethanol administration during pregnancy and lactation in rats. Alcohol Clin Exp Res. 1985 Jan-Feb;9(1):17–22. doi: 10.1111/j.1530-0277.1985.tb05041.x. [DOI] [PubMed] [Google Scholar]
- Gottlieb T. A., Ivanov I. E., Adesnik M., Sabatini D. D. Actin microfilaments play a critical role in endocytosis at the apical but not the basolateral surface of polarized epithelial cells. J Cell Biol. 1993 Feb;120(3):695–710. doi: 10.1083/jcb.120.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregori L., Marriott D., West C. M., Chau V. Specific recognition of calmodulin from Dictyostelium discoideum by the ATP, ubiquitin-dependent degradative pathway. J Biol Chem. 1985 May 10;260(9):5232–5235. [PubMed] [Google Scholar]
- Griffiths G., Hoppeler H. Quantitation in immunocytochemistry: correlation of immunogold labeling to absolute number of membrane antigens. J Histochem Cytochem. 1986 Nov;34(11):1389–1398. doi: 10.1177/34.11.3534077. [DOI] [PubMed] [Google Scholar]
- Guasch R., Renau-Piqueras J., Guerri C. Chronic ethanol consumption induces accumulation of proteins in the liver Golgi apparatus and decreases galactosyltransferase activity. Alcohol Clin Exp Res. 1992 Oct;16(5):942–948. doi: 10.1111/j.1530-0277.1992.tb01897.x. [DOI] [PubMed] [Google Scholar]
- Guerri C., Sanchis R. Acetaldehyde and alcohol levels in pregnant rats and their fetuses. Alcohol. 1985 Mar-Apr;2(2):267–270. doi: 10.1016/0741-8329(85)90057-6. [DOI] [PubMed] [Google Scholar]
- Hoek J. B., Thomas A. P., Rooney T. A., Higashi K., Rubin E. Ethanol and signal transduction in the liver. FASEB J. 1992 Apr;6(7):2386–2396. [PubMed] [Google Scholar]
- Hu G., Querimit L. A., Downing L. A., Charness M. E. Ethanol differentially increases alpha 2-adrenergic and muscarinic acetylcholine receptor gene expression in NG108-15 cells. J Biol Chem. 1993 Nov 5;268(31):23441–23447. [PubMed] [Google Scholar]
- Kesäniemi Y. A., Sippel H. W. Placental and foetal metabolism of acetaldehyde in rat. I. Contents of ethanol and acetaldehyde in placenta and foets of the pregnant rat during ethanol oxidation. Acta Pharmacol Toxicol (Copenh) 1975 Jul;37(1):43–48. doi: 10.1111/j.1600-0773.1975.tb00820.x. [DOI] [PubMed] [Google Scholar]
- Lieber C. S. Biochemical mechanisms of alcohol-induced hepatic injury. Alcohol Alcohol Suppl. 1991;1:283–290. [PubMed] [Google Scholar]
- López-Tejero D., Arilla E., Colás B., Llobera M., Herrera E. Low intestinal lactase activity in offspring from ethanol-treated mothers. Biol Neonate. 1989;55(4-5):204–213. doi: 10.1159/000242918. [DOI] [PubMed] [Google Scholar]
- Mays R. W., Beck K. A., Nelson W. J. Organization and function of the cytoskeleton in polarized epithelial cells: a component of the protein sorting machinery. Curr Opin Cell Biol. 1994 Feb;6(1):16–24. doi: 10.1016/0955-0674(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Miles M. F., Diaz J. E., DeGuzman V. Ethanol-responsive gene expression in neural cell cultures. Biochim Biophys Acta. 1992 Apr 14;1138(4):268–274. doi: 10.1016/0925-4439(92)90003-6. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D., Chang F. H., Cheever L., Kim M., Diamond I., Gordon A. S. Chronic ethanol causes heterologous desensitization of receptors by reducing alpha s messenger RNA. Nature. 1988 Jun 30;333(6176):848–850. doi: 10.1038/333848a0. [DOI] [PubMed] [Google Scholar]
- Niemelä O., Juvonen T., Parkkila S. Immunohistochemical demonstration of acetaldehyde-modified epitopes in human liver after alcohol consumption. J Clin Invest. 1991 Apr;87(4):1367–1374. doi: 10.1172/JCI115141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omodeo-Salé F., Lindi C., Palestini P., Masserini M. Role of phosphatidylethanol in membranes. Effects on membrane fluidity, tolerance to ethanol, and activity of membrane-bound enzymes. Biochemistry. 1991 Mar 5;30(9):2477–2482. doi: 10.1021/bi00223a026. [DOI] [PubMed] [Google Scholar]
- Peterson M. D., Bement W. M., Mooseker M. S. An in vitro model for the analysis of intestinal brush border assembly. II. Changes in expression and localization of brush border proteins during cell contact-induced brush border assembly in Caco-2BBe cells. J Cell Sci. 1993 Jun;105(Pt 2):461–472. doi: 10.1242/jcs.105.2.461. [DOI] [PubMed] [Google Scholar]
- Pinazo-Duran M. D., Renau-Piqueras J., Guerri C. Developmental changes in the optic nerve related to ethanol consumption in pregnant rats: analysis of the ethanol-exposed optic nerve. Teratology. 1993 Oct;48(4):305–322. doi: 10.1002/tera.1420480404. [DOI] [PubMed] [Google Scholar]
- Pösö A. R., Hirsimäki P. Inhibition of proteolysis in the liver by chronic ethanol feeding. Biochem J. 1991 Jan 1;273(Pt 1):149–152. doi: 10.1042/bj2730149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaroni A., Calnek D., Quaroni E., Chandler J. S. Keratin expression in rat intestinal crypt and villus cells. Analysis with a panel of monoclonal antibodies. J Biol Chem. 1991 Jun 25;266(18):11923–11931. [PubMed] [Google Scholar]
- Rechsteiner M. Ubiquitin-mediated pathways for intracellular proteolysis. Annu Rev Cell Biol. 1987;3:1–30. doi: 10.1146/annurev.cb.03.110187.000245. [DOI] [PubMed] [Google Scholar]
- Reddy Y. S., Beesley R. C. Effects of acute and chronic ethanol on cardiac contractile protein ATPase activity of Syrian hamsters. Biochem Med Metab Biol. 1990 Dec;44(3):259–265. doi: 10.1016/0885-4505(90)90070-h. [DOI] [PubMed] [Google Scholar]
- Sanchis R., Guerri C. Alcohol-metabolizing enzymes in placenta and fetal liver: effect of chronic ethanol intake. Alcohol Clin Exp Res. 1986 Jan-Feb;10(1):39–44. doi: 10.1111/j.1530-0277.1986.tb05611.x. [DOI] [PubMed] [Google Scholar]
- Sáez R., Burgal M., Renau-Piqueras J., Marqués A., Guerri C. Evolution of several cytoskeletal proteins of astrocytes in primary culture: effect of prenatal alcohol exposure. Neurochem Res. 1991 Jul;16(7):737–747. doi: 10.1007/BF00965682. [DOI] [PubMed] [Google Scholar]
- Terada N., Nakai T., Yamaguchi M., Hatta A., Arizono K., Ariyoshi T. Effects of maternal ethanol intake during pregnancy on fetal and maternal liver enzyme systems in Wistar rats. J Pharmacobiodyn. 1982 Jan;5(1):49–54. doi: 10.1248/bpb1978.5.49. [DOI] [PubMed] [Google Scholar]
- Testar X., López D., Llobera M., Herrera E. Ethanol administration in the drinking fluid to pregnant rats as a model for the fetal alcohol syndrome. Pharmacol Biochem Behav. 1986 Mar;24(3):625–630. doi: 10.1016/0091-3057(86)90568-x. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. Immunochemistry on ultrathin frozen sections. Histochem J. 1980 Jul;12(4):381–403. doi: 10.1007/BF01011956. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D. S., Jennett R. B., Smith S. L., Sorrell M. F., Tuma D. J. Covalent interactions of acetaldehyde with the actin/microfilament system. Alcohol Alcohol. 1989;24(4):281–289. doi: 10.1093/oxfordjournals.alcalc.a044914. [DOI] [PubMed] [Google Scholar]
- Zern M. A., Chakraborty P. R., Ruiz-Opazo N., Yap S. H., Shafritz D. A. Development and use of a rat albumin cDNA clone to evaluate the effect of chronic ethanol administration on hepatic protein synthesis. Hepatology. 1983 May-Jun;3(3):317–322. doi: 10.1002/hep.1840030307. [DOI] [PubMed] [Google Scholar]
- Zorzano A., Herrera E. Disposition of ethanol and acetaldehyde in late pregnant rats and their fetuses. Pediatr Res. 1989 Jan;25(1):102–106. doi: 10.1203/00006450-198901000-00022. [DOI] [PubMed] [Google Scholar]