Abstract
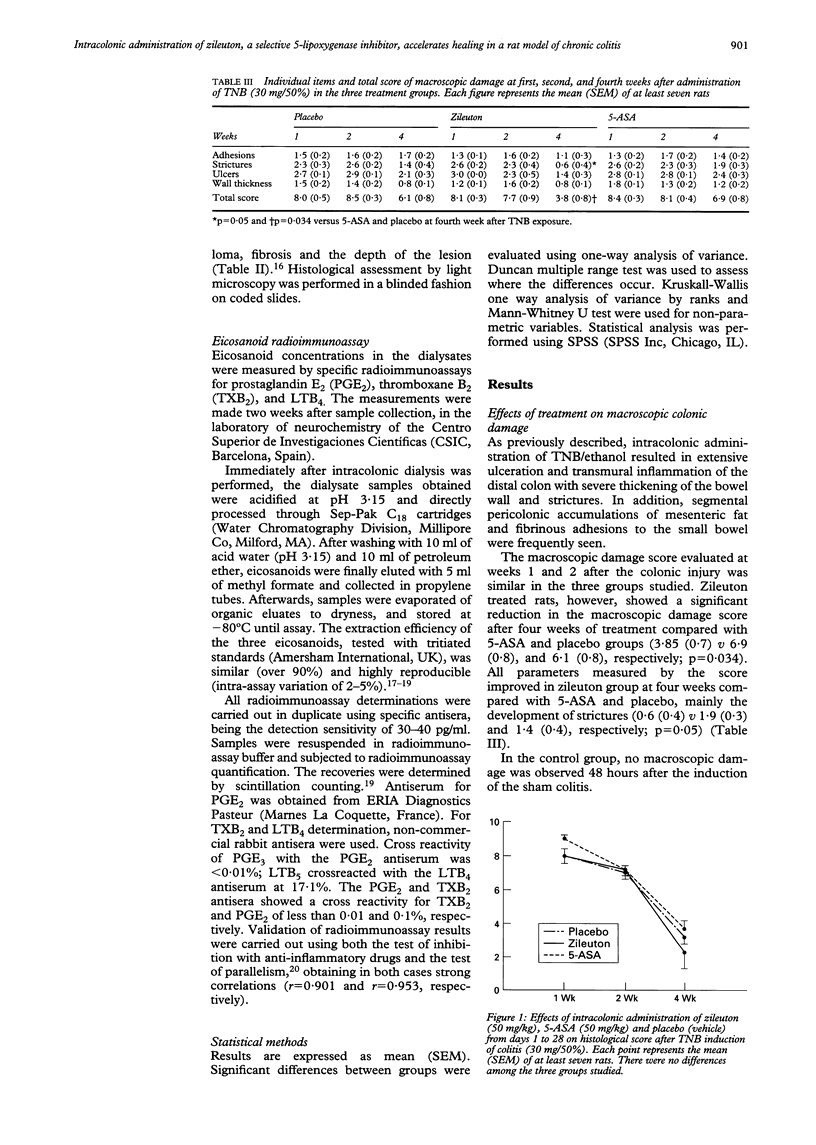
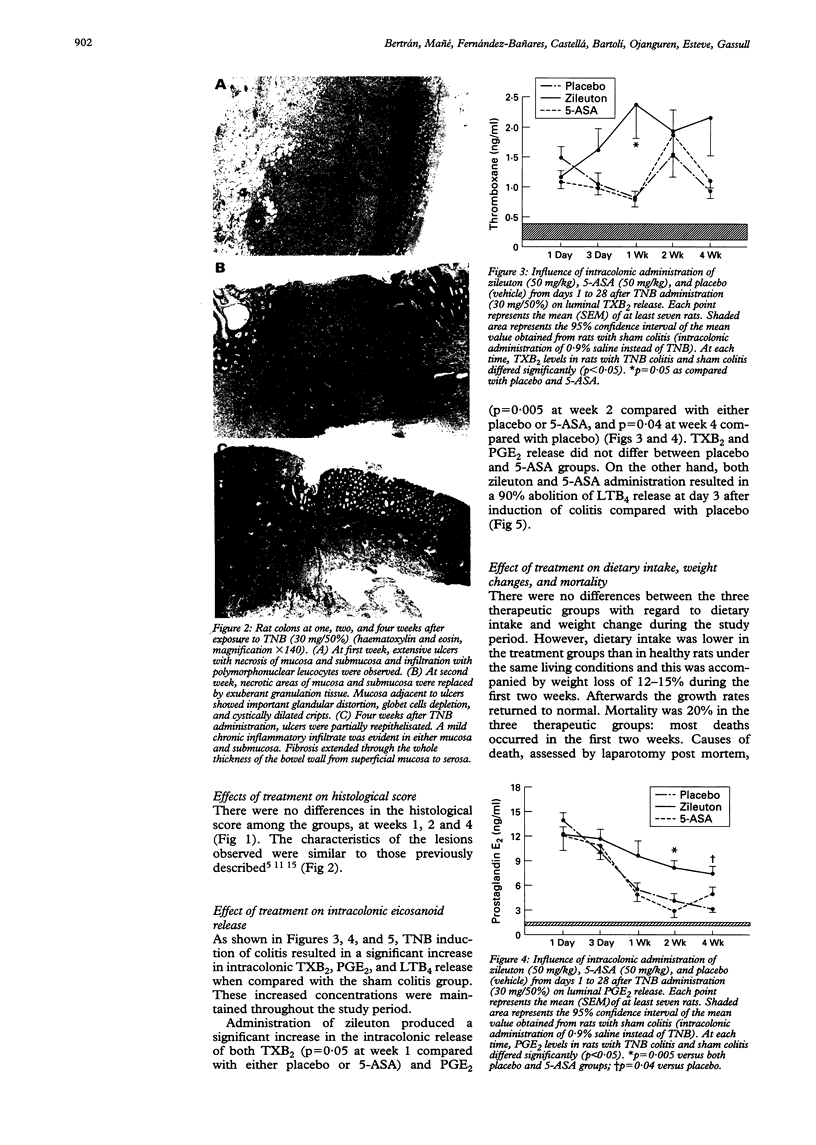
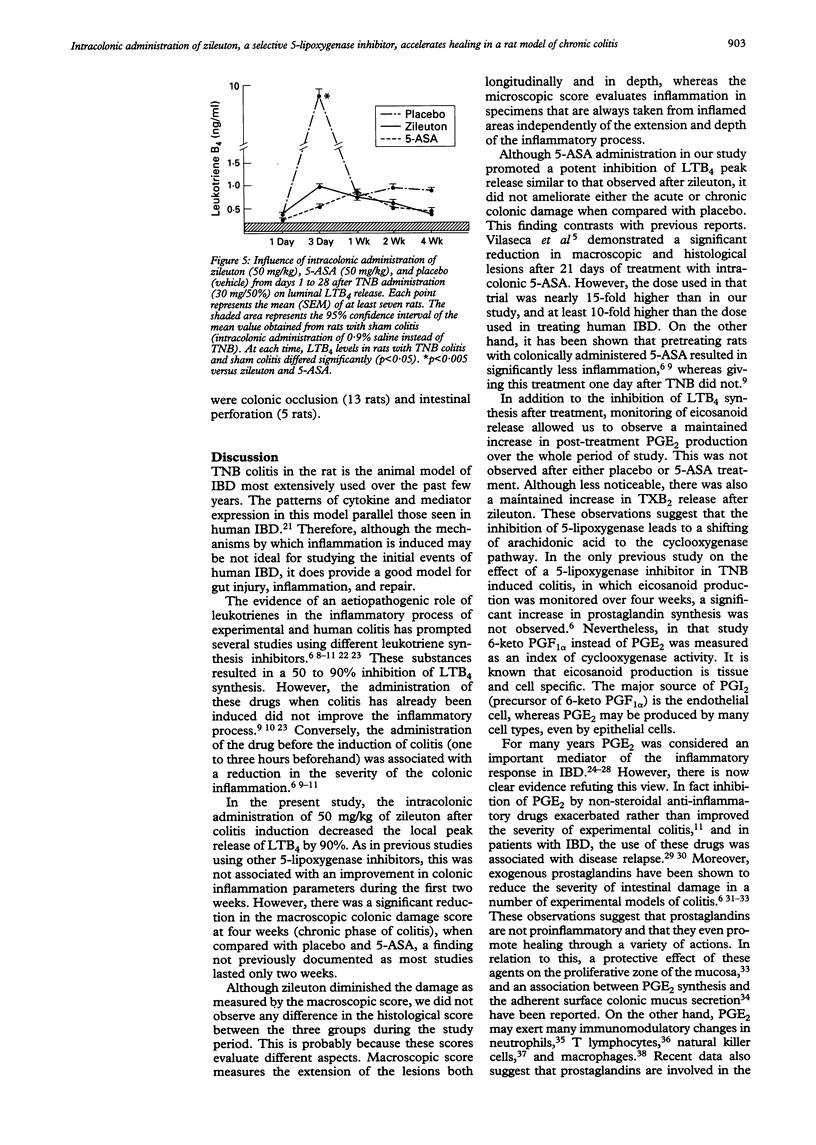
BACKGROUND: 5-Lipoxygenase products play a part in inflammatory response. AIMS: The effect of intracolonic administration of zileuton (a 5-lipoxygenase inhibitor) on colonic damage and eicosanoid local release was assessed in a rat model of colitis. METHODS: Ninety rats with trinitrobenzenesulphonic acid induced colitis were randomised to receive placebo, 5-aminosalicylic acid (50 mg/kg), or zileuton (50 mg/kg) intracolonically for four weeks. Local eicosanoid release was monitored by intracolonic dialysis throughout the study. The colon was removed for macroscopic and histological assessment at weeks 1, 2, and 4 after colitis induction in 10 rats of each group. RESULTS: Zileuton significantly reduced macroscopic damage score after four weeks of treatment in comparison with the other two groups (p = 0.034). In addition, zileuton administration significantly increased the intracolonic release of both thromboxane B2 at week 1 (p = 0.05) and prostaglandin E2 at weeks 2 and 4 (p < 0.05). Zileuton and 5-aminosalicylic acid decreased leukotriene B4 release by 90% at day 3. CONCLUSIONS: Intracolonic zileuton, compared with 5-aminosalicylic acid and placebo, seems to improve the course of the disease in a model of chronic colitis. This effect may be related to an increased and maintained production of prostaglandin E2 together with inhibition of leukotriene B4 synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allgayer H., Deschryver K., Stenson W. F. Treatment with 16,16'-dimethyl prostaglandin E2 before and after induction of colitis with trinitrobenzenesulfonic acid in rats decreases inflammation. Gastroenterology. 1989 May;96(5 Pt 1):1290–1300. doi: 10.1016/s0016-5085(89)80016-2. [DOI] [PubMed] [Google Scholar]
- Beno D. W., Espinal R., Edelstein B. M., Davis B. H. Administration of prostaglandin E1 analog reduces rat hepatic and Ito cell collagen gene expression and collagen accumulation after bile duct ligation injury. Hepatology. 1993 Apr;17(4):707–714. doi: 10.1002/hep.1840170427. [DOI] [PubMed] [Google Scholar]
- Betz M., Fox B. S. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol. 1991 Jan 1;146(1):108–113. [PubMed] [Google Scholar]
- Donowitz M. Arachidonic acid metabolites and their role in inflammatory bowel disease. An update requiring addition of a pathway. Gastroenterology. 1985 Feb;88(2):580–587. doi: 10.1016/0016-5085(85)90525-6. [DOI] [PubMed] [Google Scholar]
- Gelpí E., Ramis I., Hotter G., Bioque G., Bulbena O., Roselló J. Modern high-performance liquid chromatographic-radioimmunoassay strategies for the study of eicosanoids in biological samples. J Chromatogr. 1989 Aug 11;492:223–250. doi: 10.1016/s0378-4347(00)84470-9. [DOI] [PubMed] [Google Scholar]
- Gibson G. R., Whitacre E. B., Ricotti C. A. Colitis induced by nonsteroidal anti-inflammatory drugs. Report of four cases and review of the literature. Arch Intern Med. 1992 Mar;152(3):625–632. [PubMed] [Google Scholar]
- Goto T., Herberman R. B., Maluish A., Strong D. M. Cyclic AMP as a mediator of prostaglandin E-induced suppression of human natural killer cell activity. J Immunol. 1983 Mar;130(3):1350–1355. [PubMed] [Google Scholar]
- Gould S. R. Assay of prostaglandin-like substances in faeces and their measurement in ulcerative colitis. Prostaglandins. 1976 Mar;11(3):489–497. doi: 10.1016/0090-6980(76)90095-2. [DOI] [PubMed] [Google Scholar]
- Gould S. R., Brash A. R., Conolly M. E., Lennard-Jones J. E. Studies of prostaglandins and sulphasalazine in ulcerative colitis. Prostaglandins Med. 1981 Feb;6(2):165–182. doi: 10.1016/0161-4630(81)90088-4. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Szczeklik A., Wandzilak M. The effect of six prostaglandins, prostacyclin and iloprost on generation of superoxide anions by human polymorphonuclear leukocytes stimulated by zymosan or formyl-methionyl-leucyl-phenylalanine. Biochem Pharmacol. 1987 Dec 15;36(24):4209–4213. doi: 10.1016/0006-2952(87)90660-5. [DOI] [PubMed] [Google Scholar]
- Kaufmann H. J., Taubin H. L. Nonsteroidal anti-inflammatory drugs activate quiescent inflammatory bowel disease. Ann Intern Med. 1987 Oct;107(4):513–516. doi: 10.7326/0003-4819-107-4-513. [DOI] [PubMed] [Google Scholar]
- Kunkel S. L., Chensue S. W., Phan S. H. Prostaglandins as endogenous mediators of interleukin 1 production. J Immunol. 1986 Jan;136(1):186–192. [PubMed] [Google Scholar]
- Lauritsen K., Hansen J., Bytzer P., Bukhave K., Rask-Madsen J. Effects of sulphasalazine and disodium azodisalicylate on colonic PGE2 concentrations determined by equilibrium in vivo dialysis of faeces in patients with ulcerative colitis and healthy controls. Gut. 1984 Nov;25(11):1271–1278. doi: 10.1136/gut.25.11.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritsen K., Laursen L. S., Bukhave K., Rask-Madsen J. Effects of topical 5-aminosalicylic acid and prednisolone on prostaglandin E2 and leukotriene B4 levels determined by equilibrium in vivo dialysis of rectum in relapsing ulcerative colitis. Gastroenterology. 1986 Oct;91(4):837–844. doi: 10.1016/0016-5085(86)90684-0. [DOI] [PubMed] [Google Scholar]
- Laursen L. S., Naesdal J., Bukhave K., Lauritsen K., Rask-Madsen J. Selective 5-lipoxygenase inhibition in ulcerative colitis. Lancet. 1990 Mar 24;335(8691):683–685. doi: 10.1016/0140-6736(90)90803-d. [DOI] [PubMed] [Google Scholar]
- LeDuc L. E., Su K. C., Guth E., Reedy T., Guth P. H. Effects of cyclooxygenase and lipoxygenase inhibition on eicosanoids and healing of acetic acid colitis in rats. Dig Dis Sci. 1993 Feb;38(2):289–294. doi: 10.1007/BF01307546. [DOI] [PubMed] [Google Scholar]
- Morris G. P., Beck P. L., Herridge M. S., Depew W. T., Szewczuk M. R., Wallace J. L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989 Mar;96(3):795–803. [PubMed] [Google Scholar]
- Peskar B. M., Dreyling K. W., Peskar B. A., May B., Goebell H. Enhanced formation of sulfidopeptide-leukotrienes in ulcerative colitis and Crohn's disease: inhibition by sulfasalazine and 5-aminosalicylic acid. Agents Actions. 1986 Jun;18(3-4):381–383. doi: 10.1007/BF01965001. [DOI] [PubMed] [Google Scholar]
- Powell W. S. Rapid extraction of oxygenated metabolites of arachidonic acid from biological samples using octadecylsilyl silica. Prostaglandins. 1980 Nov;20(5):947–957. doi: 10.1016/0090-6980(80)90144-6. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D., Simon P. L., Schwartz L. W., Griswold D. E., Fondacaro J. D., Wasserman M. A. Inflammatory mediators of experimental colitis in rats. Gastroenterology. 1989 Aug;97(2):326–337. doi: 10.1016/0016-5085(89)90068-1. [DOI] [PubMed] [Google Scholar]
- Ramis I., Roselló-Catafau J., Artigot M., Bulbena O., Picado C., Gelpí E. Simultaneous reversed-phase extraction of lipoxygenase and cyclooxygenase metabolites of arachidonic acid in nasal secretions: methodological aspects. J Chromatogr. 1990 Nov 16;532(2):217–225. doi: 10.1016/s0378-4347(00)83773-1. [DOI] [PubMed] [Google Scholar]
- Ramis I., Roselló-Catafau J., Gómez G., Zabay J. M., Fernández Cruz E., Gelpí E. Cyclooxygenase and lipoxygenase arachidonic acid metabolism by monocytes from human immune deficiency virus-infected drug users. J Chromatogr. 1991 Sep 20;557(1-2):507–513. doi: 10.1016/s0021-9673(01)87159-4. [DOI] [PubMed] [Google Scholar]
- Sharon P., Ligumsky M., Rachmilewitz D., Zor U. Role of prostaglandins in ulcerative colitis. Enhanced production during active disease and inhibition by sulfasalazine. Gastroenterology. 1978 Oct;75(4):638–640. [PubMed] [Google Scholar]
- Sharon P., Stenson W. F. Enhanced synthesis of leukotriene B4 by colonic mucosa in inflammatory bowel disease. Gastroenterology. 1984 Mar;86(3):453–460. [PubMed] [Google Scholar]
- Sharon P., Stenson W. F. Metabolism of arachidonic acid in acetic acid colitis in rats. Similarity to human inflammatory bowel disease. Gastroenterology. 1985 Jan;88(1 Pt 1):55–63. doi: 10.1016/s0016-5085(85)80132-3. [DOI] [PubMed] [Google Scholar]
- Surawicz C. M., Belic L. Rectal biopsy helps to distinguish acute self-limited colitis from idiopathic inflammatory bowel disease. Gastroenterology. 1984 Jan;86(1):104–113. [PubMed] [Google Scholar]
- Vilaseca J., Salas A., Guarner F., Rodriguez R., Malagelada J. R. Participation of thromboxane and other eicosanoid synthesis in the course of experimental inflammatory colitis. Gastroenterology. 1990 Feb;98(2):269–277. doi: 10.1016/0016-5085(90)90814-h. [DOI] [PubMed] [Google Scholar]
- Vilaseca J., Salas A., Guarner F., Rodríguez R., Martínez M., Malagelada J. R. Dietary fish oil reduces progression of chronic inflammatory lesions in a rat model of granulomatous colitis. Gut. 1990 May;31(5):539–544. doi: 10.1136/gut.31.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. L., Keenan C. M. An orally active inhibitor of leukotriene synthesis accelerates healing in a rat model of colitis. Am J Physiol. 1990 Apr;258(4 Pt 1):G527–G534. doi: 10.1152/ajpgi.1990.258.4.G527. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Keenan C. M., Gale D., Shoupe T. S. Exacerbation of experimental colitis by nonsteroidal anti-inflammatory drugs is not related to elevated leukotriene B4 synthesis. Gastroenterology. 1992 Jan;102(1):18–27. doi: 10.1016/0016-5085(92)91779-4. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., MacNaughton W. K., Morris G. P., Beck P. L. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology. 1989 Jan;96(1):29–36. doi: 10.1016/0016-5085(89)90760-9. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Whittle B. J., Boughton-Smith N. K. Prostaglandin protection of rat colonic mucosa from damage induced by ethanol. Dig Dis Sci. 1985 Sep;30(9):866–876. doi: 10.1007/BF01309518. [DOI] [PubMed] [Google Scholar]
- Yamada T., Specian R. D., Granger D. N., Gaginella T. S., Grisham M. B. Misoprostol attenuates acetic acid-induced increases in mucosal permeability and inflammation: role of blood flow. Am J Physiol. 1991 Aug;261(2 Pt 1):G332–G339. doi: 10.1152/ajpgi.1991.261.2.G332. [DOI] [PubMed] [Google Scholar]
- Zahner G., Disser M., Thaiss F., Wolf G., Schoeppe W., Stahl R. A. The effect of prostaglandin E2 on mRNA expression and secretion of collagens I, III, and IV and fibronectin in cultured rat mesangial cells. J Am Soc Nephrol. 1994 Apr;4(10):1778–1785. doi: 10.1681/ASN.V4101778. [DOI] [PubMed] [Google Scholar]
- Zijlstra F. J., Srivastava E. D., Rhodes M., van Dijk A. P., Fogg F., Samson H. J., Copeman M., Russell M. A., Feyerabend C., Williams G. T. Effect of nicotine on rectal mucus and mucosal eicosanoids. Gut. 1994 Feb;35(2):247–251. doi: 10.1136/gut.35.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]




