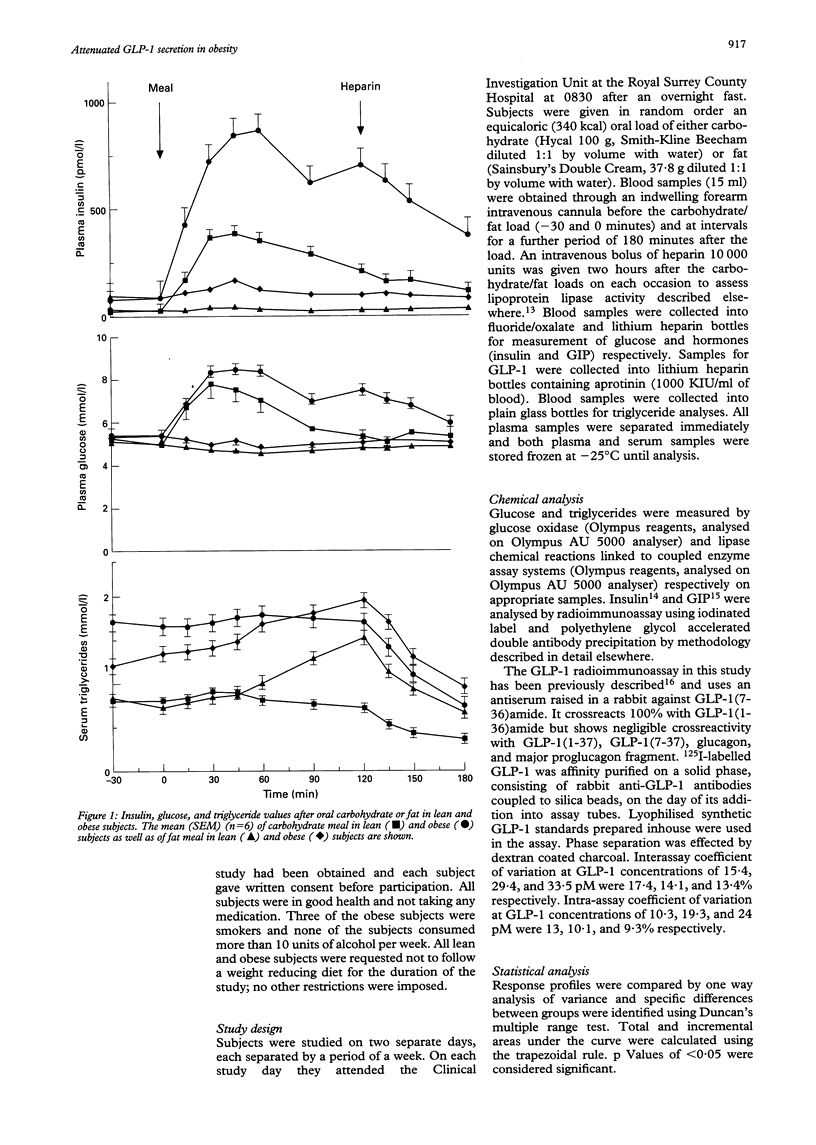
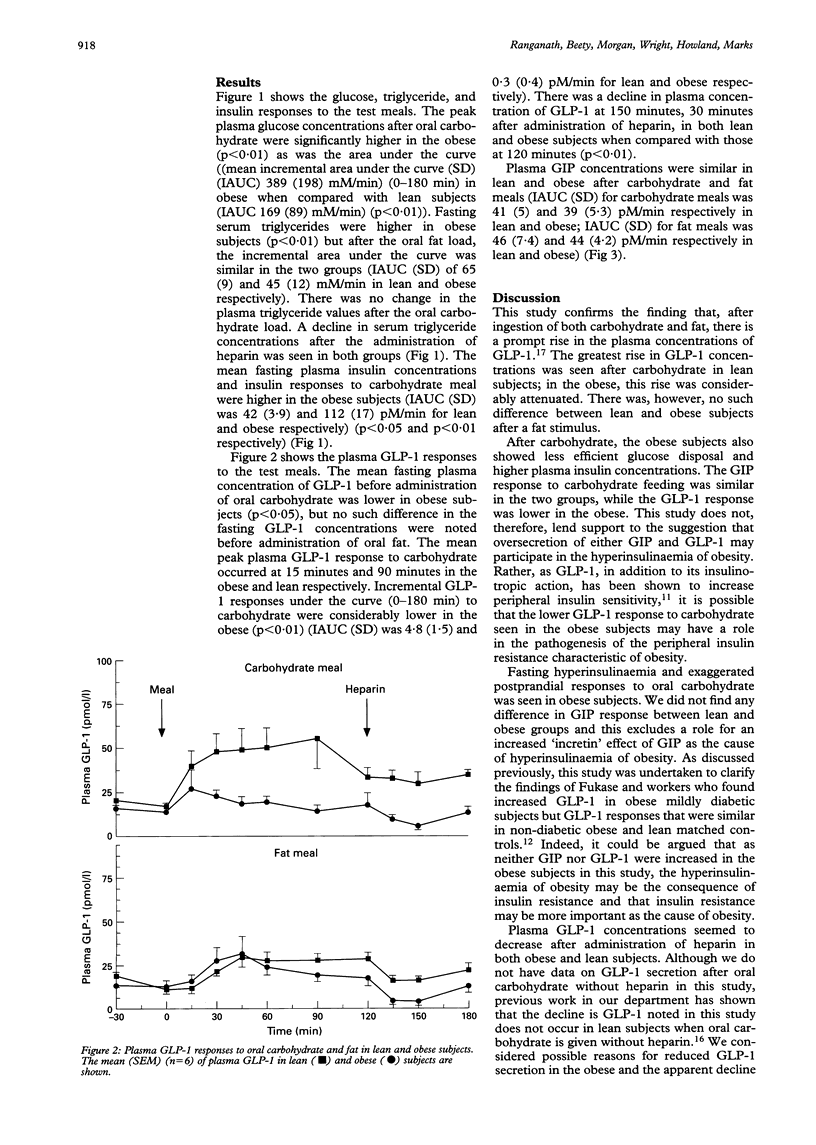
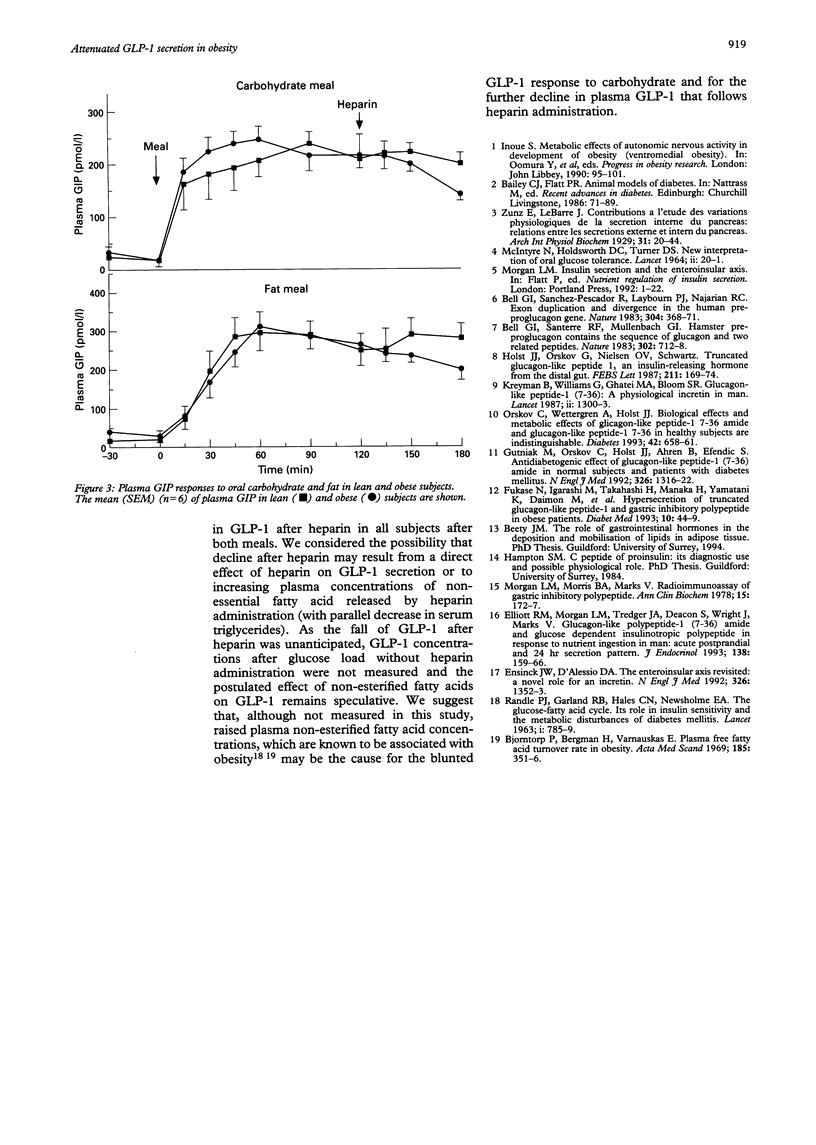
Abstract
BACKGROUND: Hypersecretion of insulinotropic factors such as glucose dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1(7-36)amide (GLP-1) have been postulated to account for the hyperinsulinaemia of obesity. AIMS: To examine the role of GLP-1 and GIP in obese women and matched controls. SUBJECTS: Six lean and six obese women subjects matched for age. METHODS: The gut hormone, plasma glucose, and serum triglyceride responses were studied over 180 minutes after oral carbohydrate and fat meals. Heparin (10,000 units) was given intravenously at 120 minutes. RESULTS: There was pronounced attenuation of plasma GLP-1 secretion to oral carbohydrate in the obese compared with lean subjects but no such difference in response to oral fat load. There were no differences in the plasma GIP responses to carbohydrate or fat feeding. There was an apparent fall in plasma GLP-1 values in all subjects after administration of heparin. CONCLUSION: Postprandial GLP-1 secretion in response to oral carbohydrate is considerably attenuated in obese subjects. The cause of this attenuation of GLP-1 secretion is not known although we suggest that both this fall and the overall reduction in GLP-1 values in obese subjects may be related to an increase in plasma non-esterified fatty acids.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Sanchez-Pescador R., Laybourn P. J., Najarian R. C. Exon duplication and divergence in the human preproglucagon gene. 1983 Jul 28-Aug 3Nature. 304(5924):368–371. doi: 10.1038/304368a0. [DOI] [PubMed] [Google Scholar]
- Björntorp P., Bergman H., Varnauskas E. Plasma free fatty acid turnover rate in obesity. Acta Med Scand. 1969 Apr;185(4):351–356. doi: 10.1111/j.0954-6820.1969.tb07347.x. [DOI] [PubMed] [Google Scholar]
- Elliott R. M., Morgan L. M., Tredger J. A., Deacon S., Wright J., Marks V. Glucagon-like peptide-1 (7-36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol. 1993 Jul;138(1):159–166. doi: 10.1677/joe.0.1380159. [DOI] [PubMed] [Google Scholar]
- Ensinck J. W., D'Alessio D. A. The enteroinsular axis revisited. A novel role for an incretin. N Engl J Med. 1992 May 14;326(20):1352–1353. doi: 10.1056/NEJM199205143262009. [DOI] [PubMed] [Google Scholar]
- Fukase N., Igarashi M., Takahashi H., Manaka H., Yamatani K., Daimon M., Tominaga M., Sasaki H. Hypersecretion of truncated glucagon-like peptide-1 and gastric inhibitory polypeptide in obese patients. Diabet Med. 1993 Jan-Feb;10(1):44–49. doi: 10.1111/j.1464-5491.1993.tb01995.x. [DOI] [PubMed] [Google Scholar]
- Gutniak M., Orskov C., Holst J. J., Ahrén B., Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7-36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med. 1992 May 14;326(20):1316–1322. doi: 10.1056/NEJM199205143262003. [DOI] [PubMed] [Google Scholar]
- Holst J. J., Orskov C., Nielsen O. V., Schwartz T. W. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett. 1987 Jan 26;211(2):169–174. doi: 10.1016/0014-5793(87)81430-8. [DOI] [PubMed] [Google Scholar]
- Kreymann B., Williams G., Ghatei M. A., Bloom S. R. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987 Dec 5;2(8571):1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- MCINTYRE N., HOLDSWORTH C. D., TURNER D. S. NEW INTERPRETATION OF ORAL GLUCOSE TOLERANCE. Lancet. 1964 Jul 4;2(7349):20–21. doi: 10.1016/s0140-6736(64)90011-x. [DOI] [PubMed] [Google Scholar]
- Morgan L. M., Morris B. A., Marks V. Radioimmunoassay of gastric inhibitory polypeptide. Ann Clin Biochem. 1978 May;15(3):172–177. doi: 10.1177/000456327801500138. [DOI] [PubMed] [Google Scholar]
- Orskov C., Wettergren A., Holst J. J. Biological effects and metabolic rates of glucagonlike peptide-1 7-36 amide and glucagonlike peptide-1 7-37 in healthy subjects are indistinguishable. Diabetes. 1993 May;42(5):658–661. doi: 10.2337/diab.42.5.658. [DOI] [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]


