Abstract
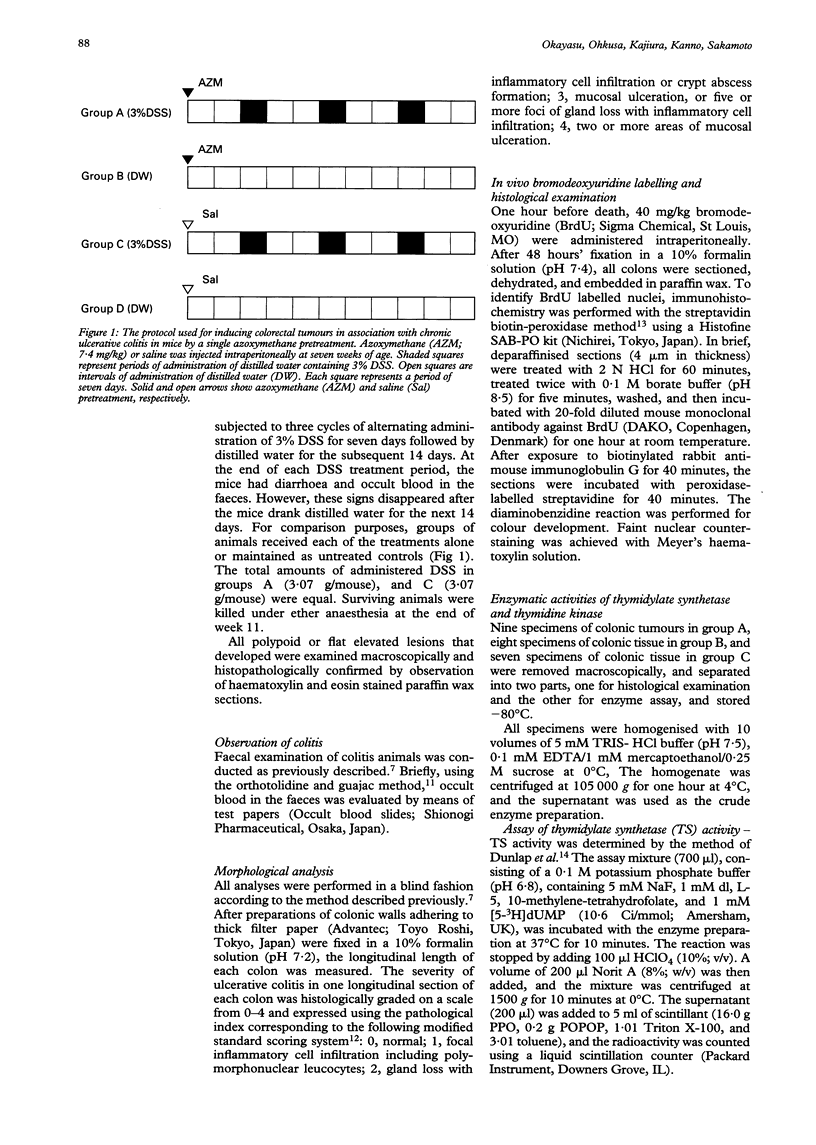
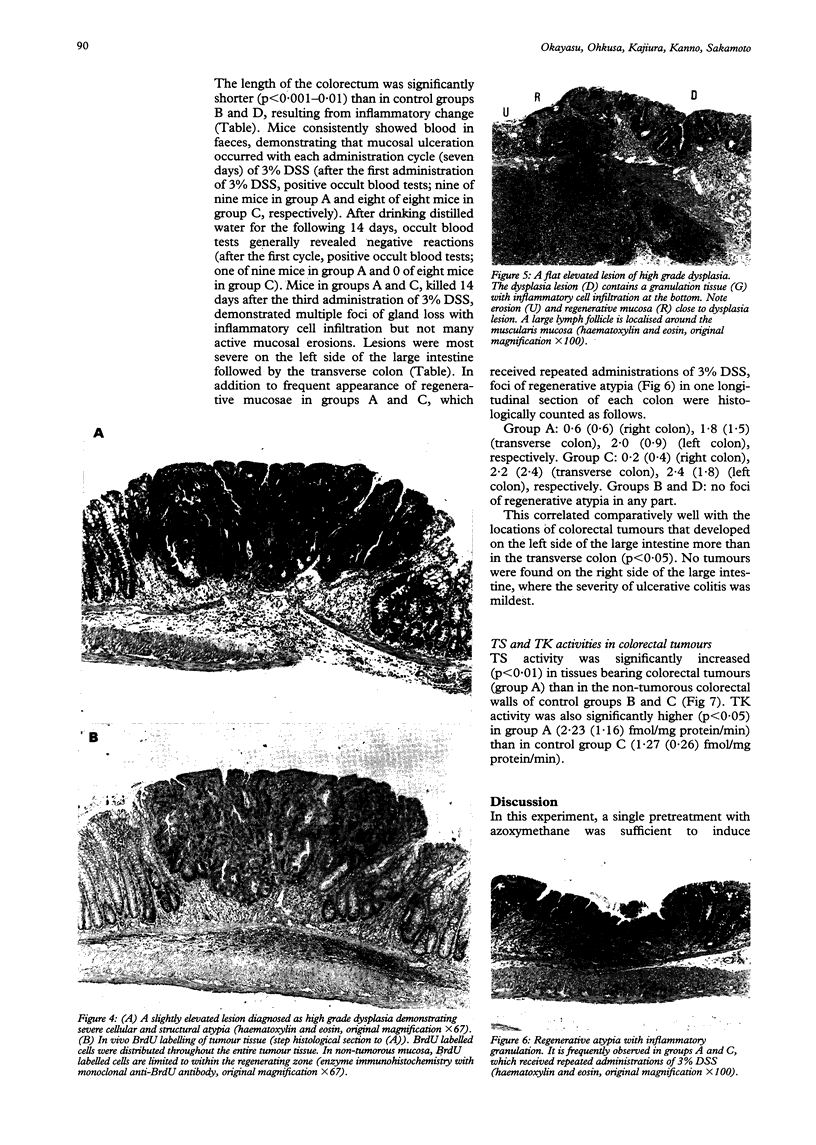
BACKGROUND: The mechanisms underlying the frequent development of colorectal carcinomas in patients with ulcerative colitis are still unknown. AIMS: To evaluate whether mucosal necrosis and regeneration act as enhancing or promoting factors in colorectal tumorigenesis, development of multiple colorectal tumours was studied in a murine model of ulcerative colitis with azoxymethane pretreatment. METHODS: Periods of chronic ulcerative colitis in mice were induced by three repeated administrations of 3% dextran sulphate sodium subsequent to a single azoxymethane pretreatment, to give conditions similar to the clinically observed active and remission phases. RESULTS: In the chronic colitis group with carcinogen exposure, multiple mucosal tumours (10.5/mouse) developed in the colorectum. This occurred primarily on the left side of the large intestine or transverse colon, the sites of the most severe colitic injury. The observed lesions were high grade dysplasias and invasive adenocarcinomas. Increased cell proliferation was evidenced by high uptake of bromodeoxyuridine, and increased activities of thymidylate synthetase and thymidine kinase. No tumours were induced in the control groups with azoxymethane pretreatment or chronic colitis alone. CONCLUSIONS: Repeated mucosal erosion with necrosis and regeneration is critical for the development of colorectal tumours in this experimental colitis system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTLER V. B., COLLER F. A. The prognostic significance of direct extension of carcinoma of the colon and rectum. Ann Surg. 1954 Jun;139(6):846–852. doi: 10.1097/00000658-195406000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashi K. W., Inagaki T., Fujimoto Y., Fukuda Y. Induction by degraded carrageenan of colorectal tumors in rats. Cancer Lett. 1978 Mar;4(3):171–176. doi: 10.1016/s0304-3835(78)94237-4. [DOI] [PubMed] [Google Scholar]
- Boyland E. The correlation of experimental carcinogenesis and cancer in man. Prog Exp Tumor Res. 1969;11:222–234. doi: 10.1159/000391396. [DOI] [PubMed] [Google Scholar]
- Chester J. F., Gaissert H. A., Ross J. S., Malt R. A., Weitzman S. A. Colonic cancer induced by 1,2-dimethylhydrazine: promotion by experimental colitis. Br J Cancer. 1989 May;59(5):704–705. doi: 10.1038/bjc.1989.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieleman L. A., Ridwan B. U., Tennyson G. S., Beagley K. W., Bucy R. P., Elson C. O. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994 Dec;107(6):1643–1652. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- Dobbins W. O., 3rd Dysplasia and malignancy in inflammatory bowel disease. Annu Rev Med. 1984;35:33–48. doi: 10.1146/annurev.me.35.020184.000341. [DOI] [PubMed] [Google Scholar]
- Dunlap R. B., Harding N. G., Huennekens F. M. Thymidylate synthetase from amethopterin-resistant Lactobacillus casei. Biochemistry. 1971 Jan 5;10(1):88–97. doi: 10.1021/bi00777a014. [DOI] [PubMed] [Google Scholar]
- Fath R. B., Jr, Deschner E. E., Winawer S. J., Dworkin B. M. Degraded carrageenan-induced colitis in CF1 mice. A clinical, histopathological and kinetic analysis. Digestion. 1984;29(4):197–203. doi: 10.1159/000199033. [DOI] [PubMed] [Google Scholar]
- Fiala E. Investigations into the metabolism and mode of action of the colon carcinogen 1, 2-dimethylhydrazine. Cancer. 1975 Dec;36(6 Suppl):2407–2412. doi: 10.1002/1097-0142(197512)36:6<2407::aid-cncr2820360620>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Herzfeld A., Legg M. A., Greengard O. Human colon tumors: enzymic and histological characteristics. Cancer. 1978 Sep;42(3):1280–1283. doi: 10.1002/1097-0142(197809)42:3<1280::aid-cncr2820420337>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Hinton J. M. Risk of malignant change in ulcerative colitis. Gut. 1966 Oct;7(5):427–432. doi: 10.1136/gut.7.5.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lennard-Jones J. E., Melville D. M., Morson B. C., Ritchie J. K., Williams C. B. Precancer and cancer in extensive ulcerative colitis: findings among 401 patients over 22 years. Gut. 1990 Jul;31(7):800–806. doi: 10.1136/gut.31.7.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkusa T., Okayasu I., Tokoi S., Araki A., Ozaki Y. Changes in bacterial phagocytosis of macrophages in experimental ulcerative colitis. Digestion. 1995;56(2):159–164. doi: 10.1159/000201236. [DOI] [PubMed] [Google Scholar]
- Okayasu I., Hatakeyama S., Yamada M., Ohkusa T., Inagaki Y., Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990 Mar;98(3):694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Bartlett J. G. Bacteriological studies of experimental ulcerative colitis. Am J Clin Nutr. 1979 Jan;32(1):258–265. doi: 10.1093/ajcn/32.1.258. [DOI] [PubMed] [Google Scholar]
- Oohashi Y., Ishioka T., Wakabayashi K., Kuwabara N. A study on carcinogenesis induced by degraded carrageenan arising from squamous metaplasia of the rat colorectum. Cancer Lett. 1981 Dec;14(3):267–272. doi: 10.1016/0304-3835(81)90153-1. [DOI] [PubMed] [Google Scholar]
- Riddell R. H., Goldman H., Ransohoff D. F., Appelman H. D., Fenoglio C. M., Haggitt R. C., Ahren C., Correa P., Hamilton S. R., Morson B. C. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983 Nov;14(11):931–968. doi: 10.1016/s0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- Sakamoto S., Iwama T., Tsukada K., Utsunomiya J., Kawasaki T., Okamoto R. Increased activity of thymidine kinase isozyme in human colon tumor. Carcinogenesis. 1984 Feb;5(2):183–185. doi: 10.1093/carcin/5.2.183. [DOI] [PubMed] [Google Scholar]
- Sakamoto S., Kuwa K., Tsukada K., Sagara T., Kasahara N., Okamoto R. Relative activities of thymidylate synthetase and thymidine kinase in 1,2-dimethylhydrazine-induced colon carcinomas in rats. Carcinogenesis. 1987 Mar;8(3):405–408. doi: 10.1093/carcin/8.3.405. [DOI] [PubMed] [Google Scholar]
- Shi Z. R., Itzkowitz S. H., Kim Y. S. A comparison of three immunoperoxidase techniques for antigen detection in colorectal carcinoma tissues. J Histochem Cytochem. 1988 Mar;36(3):317–322. doi: 10.1177/36.3.3278057. [DOI] [PubMed] [Google Scholar]
- Taylor A. T., Stafford M. A., Jones O. W. Properties of thymidine kinase partially purified from human fetal and adult tissue. J Biol Chem. 1972 Mar 25;247(6):1930–1935. [PubMed] [Google Scholar]
- Tokoi S., Ohkusa T., Okayasu I., Nakamura K. Population changes in immunoglobulin-containing mononuclear cells in dextran sulfate sodium-induced coltitis. J Gastroenterol. 1996 Apr;31(2):182–188. doi: 10.1007/BF02389516. [DOI] [PubMed] [Google Scholar]
- Yamada M., Ohkusa T., Okayasu I. Occurrence of dysplasia and adenocarcinoma after experimental chronic ulcerative colitis in hamsters induced by dextran sulphate sodium. Gut. 1992 Nov;33(11):1521–1527. doi: 10.1136/gut.33.11.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]