Abstract
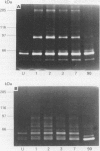
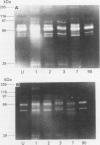
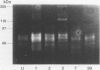
BACKGROUND: Uncontrolled and increased extracellular matrix degradation during early anastomotic repair in the intestine may reduce wound strength increasing the risk of anastomotic dehiscence. AIMS: To characterise the metalloproteinases present in intact and anastomosed ileum and colon to study their role in matrix degradation after surgery. SUBJECTS: Tissue extracts of uninjured, and of anastomosed rat ileum and colon at postoperative days 1, 2, 3, 7, and 90, were used. METHODS: Metalloproteinases were identified by gelatin and casein zymography. Aminophenylmercuric acetate (APMA) treatment was used to activate latent metalloproteinases. RESULTS: Both uninjured ileum and colon contained a 60 and 67 kDa activity, but a 54 and 72 kDa gelatinase was present in ileum only, and a 51 kDa activity in colon only. APMA treatment converted the 60 kDa protease to 54 and 51 kDa forms and the 72 kDa protease to the 67 kDa form. These gelatinases may correspond to latent and active forms of MMP 1 and MMP 2, respectively. Additional metalloproteinases were observed after anastomotic construction. Both ileum and colon contained 95 and 230 kDa gelatinases, which were converted to 83 and 76 kDa forms by APMA. They may be the latent and active forms of MMP 9, respectively. Gelatinolytic activities of 25 and 28 kDa were only found in anastomosed ileum. Caseinolytic activities were only found in ileum extracts and those were most prominent at day 1, 2, and 3 after surgery. CONCLUSIONS: The metalloproteinase pattern in ileum and colon differ considerably suggesting that matrix degradation after anastomotic construction may also vary.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agren M. S. Gelatinase activity during wound healing. Br J Dermatol. 1994 Nov;131(5):634–640. doi: 10.1111/j.1365-2133.1994.tb04974.x. [DOI] [PubMed] [Google Scholar]
- Agren M. S., Taplin C. J., Woessner J. F., Jr, Eaglstein W. H., Mertz P. M. Collagenase in wound healing: effect of wound age and type. J Invest Dermatol. 1992 Dec;99(6):709–714. doi: 10.1111/1523-1747.ep12614202. [DOI] [PubMed] [Google Scholar]
- Bendeck M. P., Zempo N., Clowes A. W., Galardy R. E., Reidy M. A. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res. 1994 Sep;75(3):539–545. doi: 10.1161/01.res.75.3.539. [DOI] [PubMed] [Google Scholar]
- Chen W. Y., Rogers A. A., Lydon M. J. Characterization of biologic properties of wound fluid collected during early stages of wound healing. J Invest Dermatol. 1992 Nov;99(5):559–564. doi: 10.1111/1523-1747.ep12667378. [DOI] [PubMed] [Google Scholar]
- Chowcat N. L., Savage F. J., Hembry R. M., Boulos P. B. Role of collagenase in colonic anastomoses: a reappraisal. Br J Surg. 1988 Apr;75(4):330–334. doi: 10.1002/bjs.1800750412. [DOI] [PubMed] [Google Scholar]
- Fielding L. P., Stewart-Brown S., Blesovsky L., Kearney G. Anastomotic integrity after operations for large-bowel cancer: a multicentre study. Br Med J. 1980 Aug 9;281(6237):411–414. doi: 10.1136/bmj.281.6237.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg G. I., Strongin A., Collier I. E., Genrich L. T., Marmer B. L. Interaction of 92-kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin. J Biol Chem. 1992 Mar 5;267(7):4583–4591. [PubMed] [Google Scholar]
- Grant G. A., Eisen A. Z., Marmer B. L., Roswit W. T., Goldberg G. I. The activation of human skin fibroblast procollagenase. Sequence identification of the major conversion products. J Biol Chem. 1987 Apr 25;262(12):5886–5889. [PubMed] [Google Scholar]
- Hendriks T., Mastboom W. J. Healing of experimental intestinal anastomoses. Parameters for repair. Dis Colon Rectum. 1990 Oct;33(10):891–901. doi: 10.1007/BF02051930. [DOI] [PubMed] [Google Scholar]
- Hendriks T., Vereecken T. H., Hesp W. L., Schillings P. H., de Boer H. H. Loss of collagen from experimental intestinal anastomoses: early events. Exp Mol Pathol. 1985 Jun;42(3):411–418. doi: 10.1016/0014-4800(85)90090-5. [DOI] [PubMed] [Google Scholar]
- Herron G. S., Banda M. J., Clark E. J., Gavrilovic J., Werb Z. Secretion of metalloproteinases by stimulated capillary endothelial cells. II. Expression of collagenase and stromelysin activities is regulated by endogenous inhibitors. J Biol Chem. 1986 Feb 25;261(6):2814–2818. [PubMed] [Google Scholar]
- Hesp W. L., Lubbers E. J., de Boer H. H., Hendriks T. Anastomotic insufficiency in small bowel surgery--incidence and treatment. Langenbecks Arch Chir. 1986;368(2):105–111. doi: 10.1007/BF01273849. [DOI] [PubMed] [Google Scholar]
- Hibbs M. S., Hasty K. A., Seyer J. M., Kang A. H., Mainardi C. L. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J Biol Chem. 1985 Feb 25;260(4):2493–2500. [PubMed] [Google Scholar]
- Högström H., Haglund U., Zederfeldt B. Suture technique and early breaking strength of intestinal anastomoses and laparotomy wounds. Acta Chir Scand. 1985;151(5):441–443. [PubMed] [Google Scholar]
- Jönsson K., Jiborn H., Zederfeldt B. Breaking strength of small intestinal anastomoses. Am J Surg. 1983 Jun;145(6):800–803. doi: 10.1016/0002-9610(83)90144-7. [DOI] [PubMed] [Google Scholar]
- Kleiner D. E., Stetler-Stevenson W. G. Quantitative zymography: detection of picogram quantities of gelatinases. Anal Biochem. 1994 May 1;218(2):325–329. doi: 10.1006/abio.1994.1186. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lyons J. G., Birkedal-Hansen B., Moore W. G., O'Grady R. L., Birkedal-Hansen H. Characteristics of a 95-kDa matrix metalloproteinase produced by mammary carcinoma cells. Biochemistry. 1991 Feb 12;30(6):1449–1456. doi: 10.1021/bi00220a001. [DOI] [PubMed] [Google Scholar]
- Manicourt D. H., Lefebvre V. An assay for matrix metalloproteinases and other proteases acting on proteoglycans, casein, or gelatin. Anal Biochem. 1993 Dec;215(2):171–179. doi: 10.1006/abio.1993.1572. [DOI] [PubMed] [Google Scholar]
- Martens M. F., Hendriks T. Postoperative changes in collagen synthesis in intestinal anastomoses of the rat: differences between small and large bowel. Gut. 1991 Dec;32(12):1482–1487. doi: 10.1136/gut.32.12.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M. The matrix-degrading metalloproteinases. Bioessays. 1992 Jul;14(7):455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- Quantin B., Murphy G., Breathnach R. Pump-1 cDNA codes for a protein with characteristics similar to those of classical collagenase family members. Biochemistry. 1989 Jun 27;28(13):5327–5334. doi: 10.1021/bi00439a004. [DOI] [PubMed] [Google Scholar]
- Sakata K., Hoshino K., Nakagawa H. Purification and characterization of exudate gelatinases in the chronic-phase of carrageenin-induced inflammation in rats. J Biochem. 1989 Mar;105(3):384–389. doi: 10.1093/oxfordjournals.jbchem.a122673. [DOI] [PubMed] [Google Scholar]
- Seifert W. F., Wobbes T., Hoogenhout J., de Man B. M., Huyben K. M., Hendriks T. Intraoperative irradiation delays anastomotic repair in rat colon. Am J Surg. 1995 Sep;170(3):256–261. doi: 10.1016/s0002-9610(05)80010-8. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Talhouk R. S., Chin J. R., Unemori E. N., Werb Z., Bissell M. J. Proteinases of the mammary gland: developmental regulation in vivo and vectorial secretion in culture. Development. 1991 Jun;112(2):439–449. doi: 10.1242/dev.112.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamakoshi K., Kikkawa F., Nawa A., Maeda O., Kawai M., Sugamuma N., Yamagata S., Tomoda Y. Different pattern of zymography between human gynecologic normal and malignant tissues. Am J Obstet Gynecol. 1994 Aug;171(2):478–484. doi: 10.1016/0002-9378(94)90286-0. [DOI] [PubMed] [Google Scholar]
- Turunen M. J., Peltokallio P. Surgical results in 657 patients with colorectal cancer. Dis Colon Rectum. 1983 Sep;26(9):606–612. doi: 10.1007/BF02552974. [DOI] [PubMed] [Google Scholar]
- Tyagi S. C., Matsubara L., Weber K. T. Direct extraction and estimation of collagenase(s) activity by zymography in microquantities of rat myocardium and uterus. Clin Biochem. 1993 Jun;26(3):191–198. doi: 10.1016/0009-9120(93)90025-2. [DOI] [PubMed] [Google Scholar]
- Vartio T., Hovi T. Polypeptide composition of human macrophage gelatinase. Acta Chem Scand B. 1987 Nov;41(10):754–756. doi: 10.3891/acta.chem.scand.41b-0754. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]
- Woessner J. F., Jr, Taplin C. J. Purification and properties of a small latent matrix metalloproteinase of the rat uterus. J Biol Chem. 1988 Nov 15;263(32):16918–16925. [PubMed] [Google Scholar]
- Wysocki A. B., Staiano-Coico L., Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol. 1993 Jul;101(1):64–68. doi: 10.1111/1523-1747.ep12359590. [DOI] [PubMed] [Google Scholar]
- Zucker S., Mancuso P., DiMassimo B., Lysik R. M., Conner C., Wu C. L. Comparison of techniques for measurement of gelatinases/type IV collagenases: enzyme-linked immunoassays versus substrate degradation assays. Clin Exp Metastasis. 1994 Jan;12(1):13–23. doi: 10.1007/BF01784329. [DOI] [PubMed] [Google Scholar]
- van der Stappen J. W., Hendriks T., de Boer H. H. Collagenolytic activity extracted from intestinal anastomoses of the rat. Matrix. 1989 Jun;9(3):238–243. doi: 10.1016/s0934-8832(89)80056-3. [DOI] [PubMed] [Google Scholar]
- van der Stappen J. W., Hendriks T., de Boer H. H., de Man B. M., de Pont J. J. Collagenolytic activity in experimental intestinal anastomoses. Differences between small and large bowel and evidence for the presence of collagenase. Int J Colorectal Dis. 1992 Jun;7(2):95–101. doi: 10.1007/BF00341294. [DOI] [PubMed] [Google Scholar]