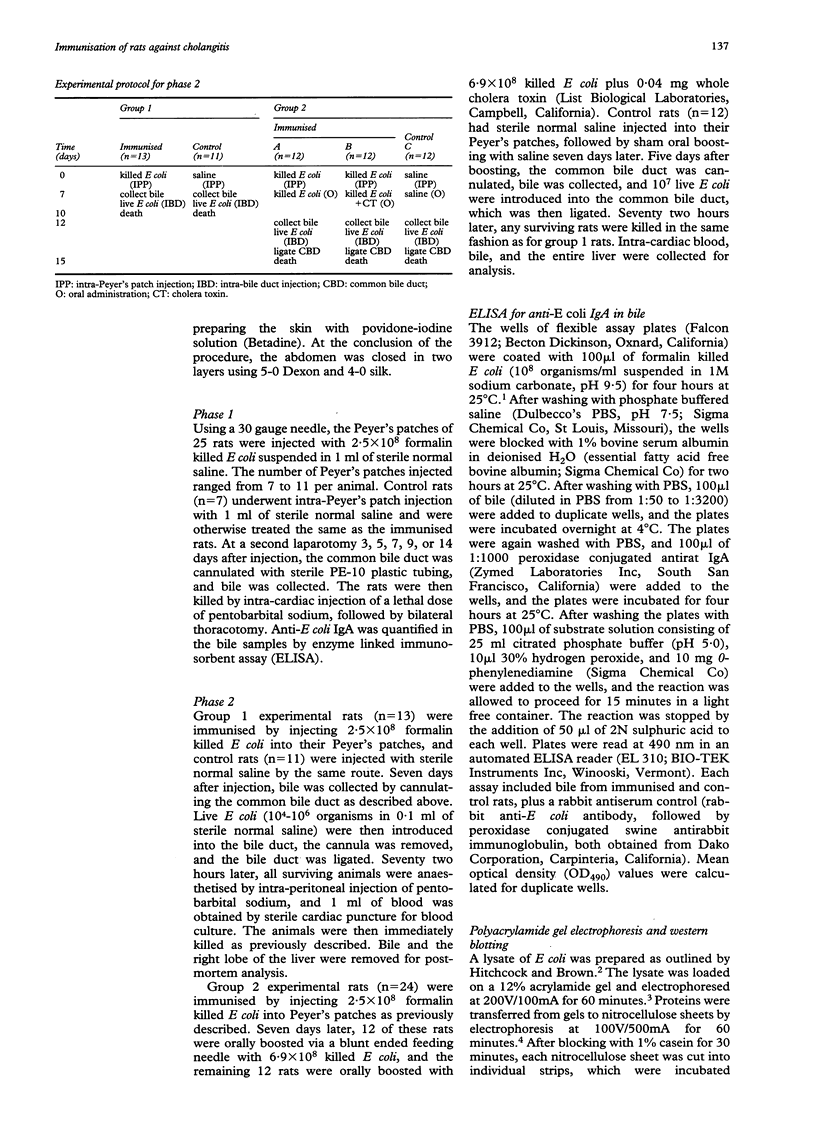
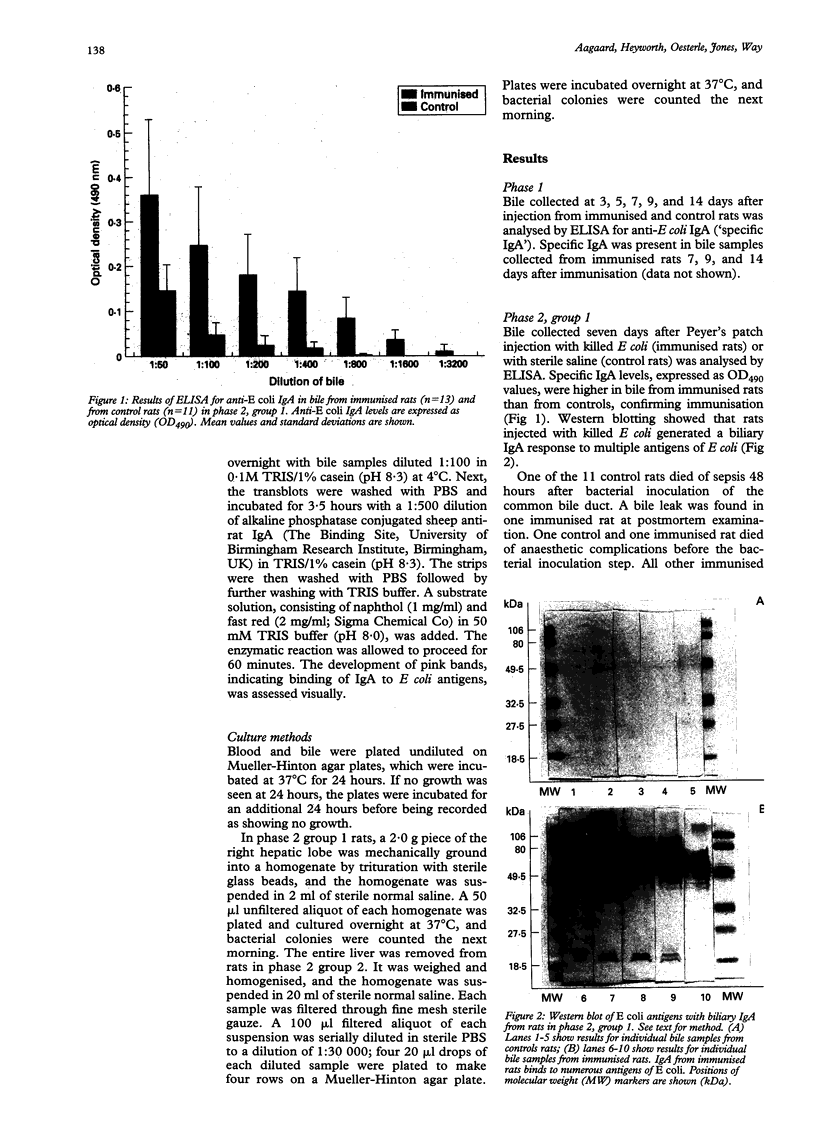
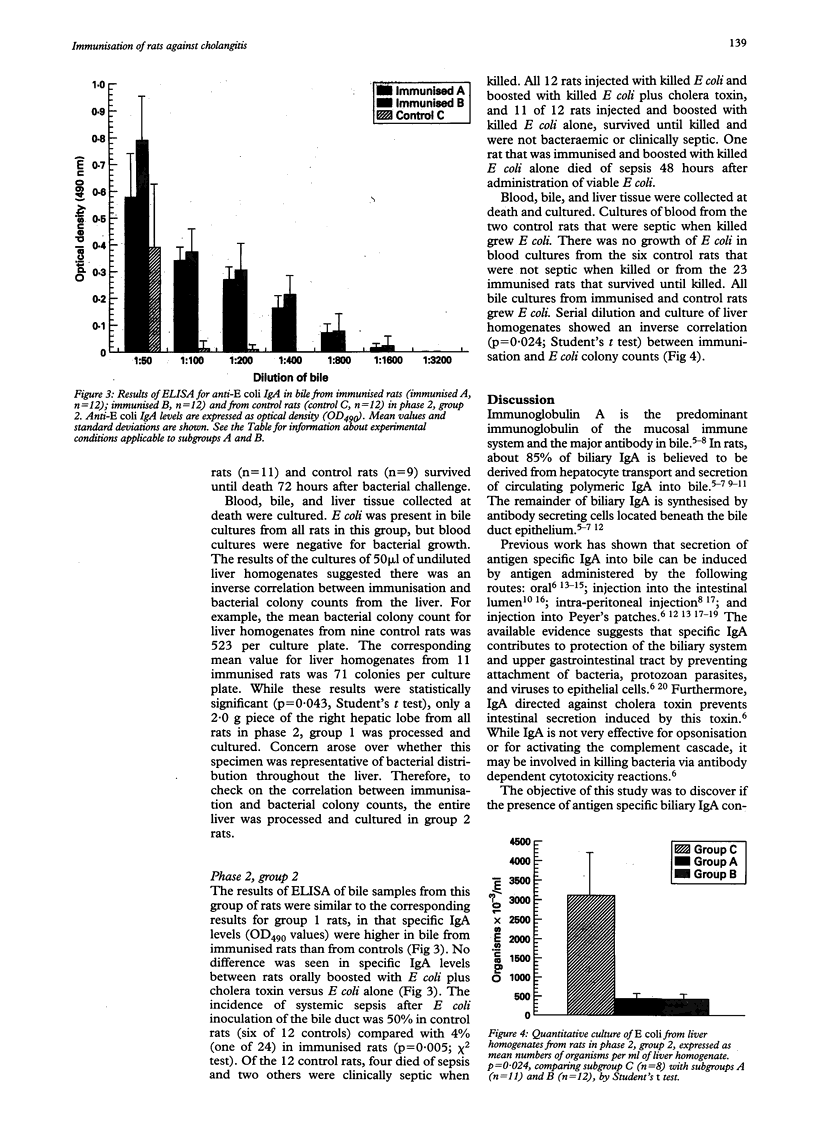
Abstract
BACKGROUND: Cholangitis, an infection of the biliary tract, is most commonly caused by Gram negative bacteria, particularly Escherichia coli. Factors governing the severity of cholangitis, including the role of biliary IgA, are poorly understood. AIMS: The aim of this work was to find out if biliary IgA directed against E coli protects rats against hepatobiliary infection with E coli. SUBJECTS: Male Sprague-Dawley rats weighing 270-350 grams were used in all of the experiments. METHODS: At laparotomy, rats were immunised by injecting killed E coli or normal saline (controls) into Peyer's patches. With or without subsequent antigenic boosting (by oral administration of killed E coli), bile was collected at a second laparotomy, and rats were infected by introducing viable E coli into the bile duct. Production of IgA anti-E coli antibody was measured by enzyme linked immunosorbent assay of bile, and the presence of hepatobiliary infection was determined by quantitative culture of liver homogenates. RESULTS: Systemic infection was present in six of 12 control rats and in one of 24 immunised rats (p = 0.005) after death. There was an inverse correlation between immunisation and E coli colony counts in cultured liver homogenates (p = 0.024). CONCLUSION: The findings suggest that biliary IgA directed against E coli protected rats against hepatobiliary E coli infection and systemic sepsis.
Full text
PDF

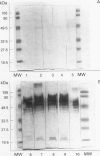
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altorfer J., Hardesty S. J., Scott J. H., Jones A. L. Specific antibody synthesis and biliary secretion by the rat liver after intestinal immunization with cholera toxin. Gastroenterology. 1987 Sep;93(3):539–549. doi: 10.1016/0016-5085(87)90917-6. [DOI] [PubMed] [Google Scholar]
- Brown W. R., Kloppel T. M. The liver and IgA: immunological, cell biological and clinical implications. Hepatology. 1989 May;9(5):763–784. doi: 10.1002/hep.1840090518. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Dahlgren U. I., Svanvik J., Svanborg Edén C. Antibodies to Escherichia coli and anti-adhesive activity in paired serum, hepatic and gall bladder bile samples. Scand J Immunol. 1986 Sep;24(3):251–260. doi: 10.1111/j.1365-3083.1986.tb02092.x. [DOI] [PubMed] [Google Scholar]
- Dahlgren U. I., Wold A. E., Hanson L. A., Midtvedt T. Expression of a dietary protein in E. coli renders it strongly antigenic to gut lymphoid tissue. Immunology. 1991 Aug;73(4):394–397. [PMC free article] [PubMed] [Google Scholar]
- Dahlgren U. I., Wold A. E., Hanson L. A., Midtvedt T. The secretory antibody response in milk and bile against fimbriae and LPS in rats monocolonized or immunized in the Peyer's patches with Escherichia coli. Immunology. 1990 Oct;71(2):295–300. [PMC free article] [PubMed] [Google Scholar]
- Dunkley M. L., Husband A. J. Distribution and functional characteristics of antigen-specific helper T cells arising after Peyer's patch immunization. Immunology. 1987 Aug;61(4):475–482. [PMC free article] [PubMed] [Google Scholar]
- Dunkley M. L., Husband A. J. The induction and migration of antigen-specific helper cells for IgA responses in the intestine. Immunology. 1986 Mar;57(3):379–385. [PMC free article] [PubMed] [Google Scholar]
- Elson C. O., Ealding W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol. 1984 Jun;132(6):2736–2741. [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson G. D., Hansen P. G., Underdown B. J. Further evidence that hepatic sources confer biliary antibody in the rat. Immunology. 1992 Jul;76(3):397–401. [PMC free article] [PubMed] [Google Scholar]
- Jones A. L., Hradek G. T., Schmucker D. L., Underdown B. J. The fate of polymeric and secretory immunoglobulin A after retrograde infusion into the common bile duct in rats. Hepatology. 1984 Nov-Dec;4(6):1173–1183. doi: 10.1002/hep.1840040613. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manning R. J., Walker P. G., Carter L., Barrington P. J., Jackson G. D. Studies on the origins of biliary immunoglobulins in rats. Gastroenterology. 1984 Jul;87(1):173–179. [PubMed] [Google Scholar]
- McKenzie S. J., Halsey J. F. Cholera toxin B subunit as a carrier protein to stimulate a mucosal immune response. J Immunol. 1984 Oct;133(4):1818–1824. [PubMed] [Google Scholar]
- McQueen C. E., Boedeker E. C., Le M., Hamada Y., Brown W. R. Mucosal immune response to RDEC-1 infection: study of lamina propria antibody-producing cells and biliary antibody. Infect Immun. 1992 Jan;60(1):206–212. doi: 10.1128/iai.60.1.206-212.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson K., Dahlgren U. I., Hanson L. A. Different origins of IgA antibodies with various antigen specificities appearing in rat bile. Scand J Immunol. 1988 Nov;28(5):547–551. doi: 10.1111/j.1365-3083.1988.tb01486.x. [DOI] [PubMed] [Google Scholar]
- Reuben A. Biliary proteins. Hepatology. 1984 Sep-Oct;4(5 Suppl):46S–50S. doi: 10.1002/hep.1840040808. [DOI] [PubMed] [Google Scholar]
- Wold A. E., Mestecky J., Tomana M., Kobata A., Ohbayashi H., Endo T., Edén C. S. Secretory immunoglobulin A carries oligosaccharide receptors for Escherichia coli type 1 fimbrial lectin. Infect Immun. 1990 Sep;58(9):3073–3077. doi: 10.1128/iai.58.9.3073-3077.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. Y., Russell M. W. Antibody-secreting cell responses in the mouse liver. Immunology. 1992 Nov;77(3):443–448. [PMC free article] [PubMed] [Google Scholar]