Abstract
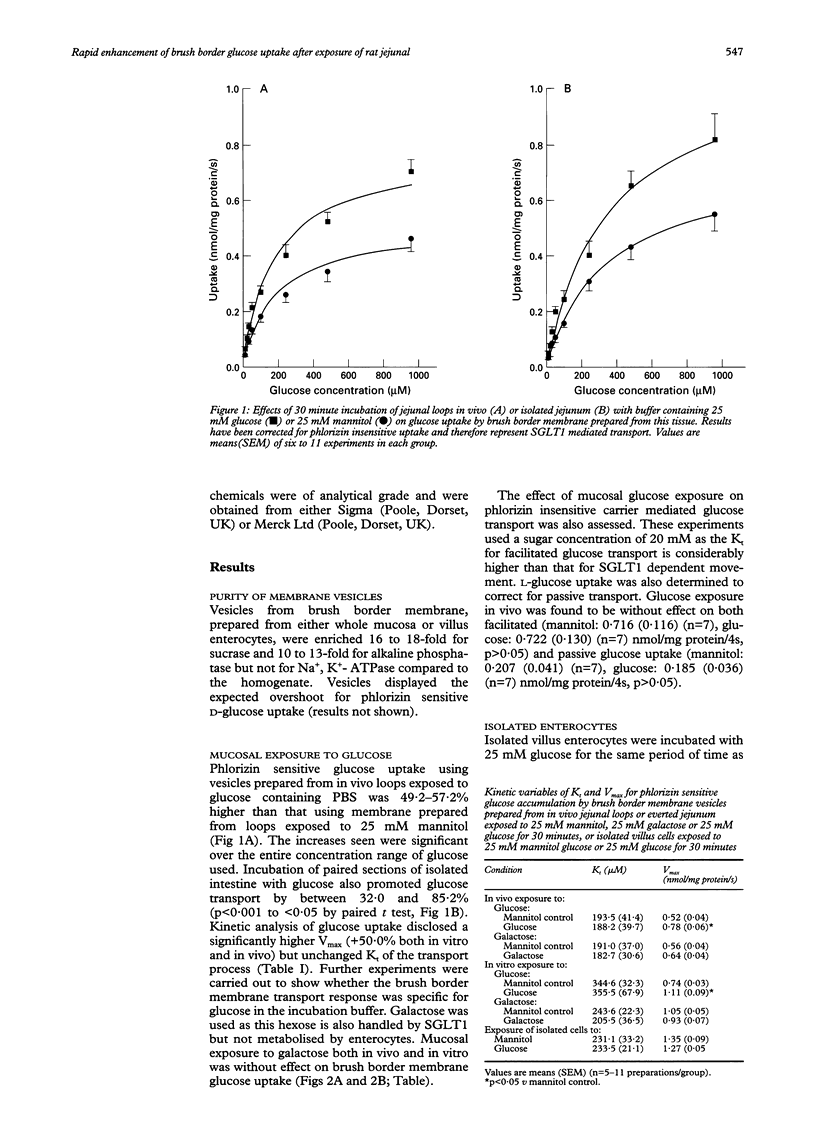
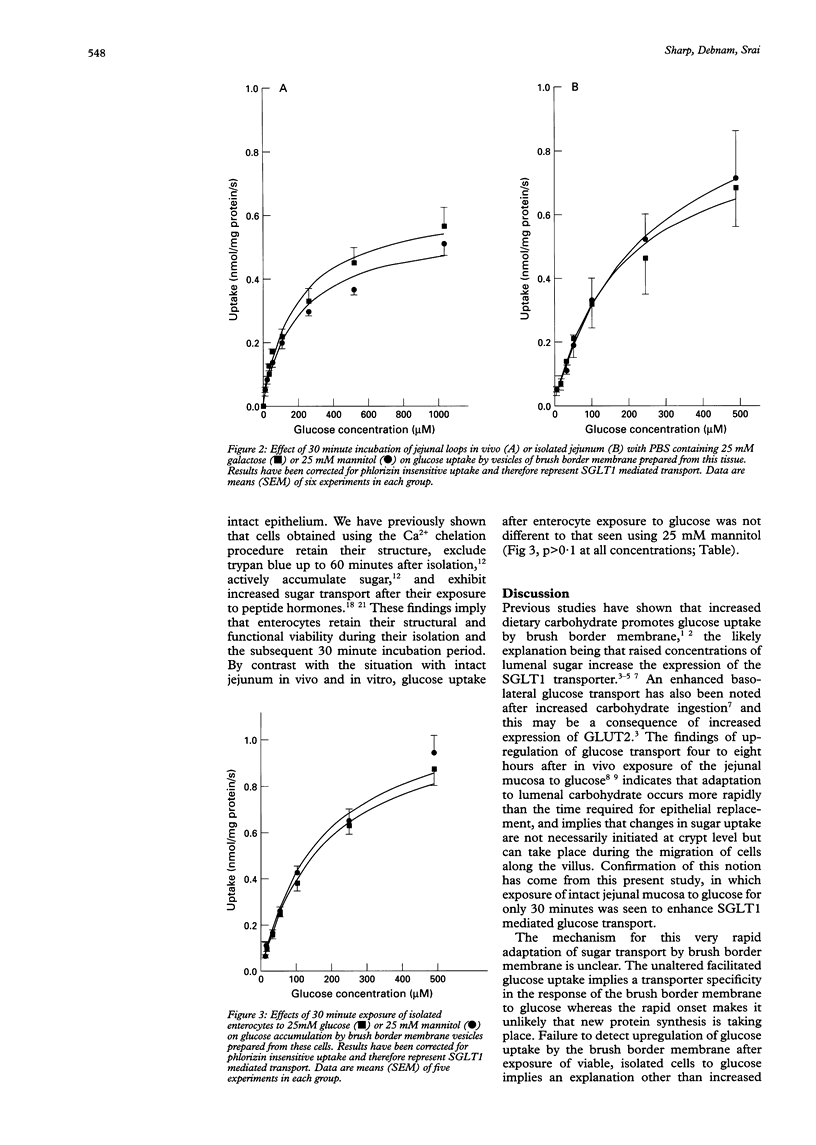
BACKGROUND: Increased jejunal glucose transport after ingestion of carbohydrate rich diets may reflect higher concentrations of lumenal glucose. Normal processing of carbohydrate causes wide fluctuations in glucose concentration in the jejunal lumen and this raises the question of whether the high lumenal concentrations seen at peak digestion affect glucose uptake. AIMS: To study the effects of 30 minute exposure of rat jejunal mucosa to glucose on sodium-glucose transporter (SGLT1) mediated glucose transport across the brush border membrane. METHODS: Jejunal mucosa was exposed in vitro or in vivo to 25 mM glucose or 25 mM mannitol for 30 minutes. In addition, isolated villus enterocytes were incubated with mannitol or glucose for the same time. Brush border membrane vesicles were isolated from these preparations and phlorizin sensitive 3H-D-glucose accumulation was measured. RESULTS: Lumenal glucose in vivo significantly enhanced SGLT1 mediated glucose uptake by 49.2-57.2%. For jejunal loops in vitro, the increase was 32.0-85.2%. Kinetic analysis disclosed a 50% greater Vmax for glucose uptake in each preparation. The facilitated and passive components of uptake were, however, unaffected by prior exposure to glucose. Incubation of villus enterocytes with 25 mM glucose did not influence glucose uptake by brush border membranes. Finally, exposure of intact mucosa to 20 mM galactose, a nonmetabolised sugar also transported by SGLT1, did not alter glucose transport. CONCLUSIONS: Lumenal glucose promotes glucose transport by brush border membrane within 30 minutes. An intact mucosa is necessary for upregulation and evidence suggests that the response is mediated by locally acting mechanisms.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRY R. J., DIKSTEIN S., MATTHEWS J., SMYTH D. H., WRIGHT E. M. ELECTRICAL POTENTIALS ASSOCIATED WITH INTESTINAL SUGAR TRANSFER. J Physiol. 1964 Jun;171:316–338. doi: 10.1113/jphysiol.1964.sp007379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman C. I., Harley B. Adaptation of glucose transport across rat enterocyte basolateral membrane in response to altered dietary carbohydrate intake. J Physiol. 1991 Jun;437:563–575. doi: 10.1113/jphysiol.1991.sp018611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- Dakka T., Cuber J. C., Chayvialle J. A. Functional coupling between the active transport of glucose and the secretion of intestinal neurotensin in rats. J Physiol. 1993 Sep;469:753–765. doi: 10.1113/jphysiol.1993.sp019841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnam E. S. Adaptation of hexose uptake by the rat jejunum induced by the perfusion of sugars into the distal ileum. Digestion. 1985;31(1):25–30. doi: 10.1159/000199173. [DOI] [PubMed] [Google Scholar]
- Debnam E. S., Chowrimootoo G. Streptozotocin diabetes and sugar transport by rat ileal enterocytes: evidence for adaptation caused by an increased luminal nutrient load. Biochim Biophys Acta. 1992 Jun 11;1107(1):86–92. doi: 10.1016/0005-2736(92)90332-g. [DOI] [PubMed] [Google Scholar]
- Debnam E. S., Ebrahim H. Y. Diabetes mellitus and the sodium electrochemical gradient across the brush border membrane of rat intestinal enterocytes. J Endocrinol. 1989 Dec;123(3):453–459. doi: 10.1677/joe.0.1230453. [DOI] [PubMed] [Google Scholar]
- Debnam E. S., Ebrahim H. Y., Swaine D. J. Diabetes mellitus and sugar transport across the brush-border and basolateral membranes of rat jejunal enterocytes. J Physiol. 1990 May;424:13–25. doi: 10.1113/jphysiol.1990.sp018052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnam E. S., Levin R. J. Influence of specific dietary sugars on the jejunal mechanisms for glucose, galactose, and alpha-methyl glucoside absorption: evidence for multiple sugar carriers. Gut. 1976 Feb;17(2):92–99. doi: 10.1136/gut.17.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnam E. S., Sharp P. A. Acute and chronic effects of pancreatic glucagon on sugar transport across the brush-border and basolateral membranes of rat jejunal enterocytes. Exp Physiol. 1993 Mar;78(2):197–207. doi: 10.1113/expphysiol.1993.sp003680. [DOI] [PubMed] [Google Scholar]
- Debnam E. S., Smith M. W., Sharp P. A., Srai S. K., Turvey A., Keable S. J. The effects of streptozotocin diabetes on sodium-glucose transporter (SGLT1) expression and function in rat jejunal and ileal villus-attached enterocytes. Pflugers Arch. 1995 Jun;430(2):151–159. doi: 10.1007/BF00374645. [DOI] [PubMed] [Google Scholar]
- Del Castillo J. R., Robinson J. W. The simultaneous preparation of basolateral and brush-border membrane vesicles from guinea-pig intestinal epithelium, and the determination of the orientation of the basolateral vesicles. Biochim Biophys Acta. 1982 May 21;688(1):45–56. doi: 10.1016/0005-2736(82)90577-6. [DOI] [PubMed] [Google Scholar]
- Dempster J. A., Kellett G. L. A submucosal mechanism of action for prostaglandin E2 on hexose absorption and metabolism in mouse intestine. J Physiol. 1992;453:449–459. doi: 10.1113/jphysiol.1992.sp019238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M., Karasov W. H., Cary C., Enders D., Yung R. Effect of dietary carbohydrate on monosaccharide uptake by mouse small intestine in vitro. J Physiol. 1984 Apr;349:419–440. doi: 10.1113/jphysiol.1984.sp015165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraris R. P., Diamond J. Crypt-villus site of glucose transporter induction by dietary carbohydrate in mouse intestine. Am J Physiol. 1992 Jun;262(6 Pt 1):G1069–G1073. doi: 10.1152/ajpgi.1992.262.6.G1069. [DOI] [PubMed] [Google Scholar]
- Ferraris R. P., Yasharpour S., Lloyd K. C., Mirzayan R., Diamond J. M. Luminal glucose concentrations in the gut under normal conditions. Am J Physiol. 1990 Nov;259(5 Pt 1):G822–G837. doi: 10.1152/ajpgi.1990.259.5.G822. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A., Carter-Su C., Randles J. Energetics of Na+-dependent sugar transport by isolated intestinal cells: evidence for a major role for membrane potentials. Am J Physiol. 1977 Nov;233(5):E357–E362. doi: 10.1152/ajpendo.1977.233.5.E357. [DOI] [PubMed] [Google Scholar]
- Lang F., Paulmichl M., Pfeilschifter J., Friedrich F., Wöll E., Waldegger S., Ritter M., Tschernko E. Cellular mechanisms of bradykinin-induced hyperpolarization in renal epitheloid MDCK-cells. Biochim Biophys Acta. 1991 Apr 9;1073(3):600–608. doi: 10.1016/0304-4165(91)90236-a. [DOI] [PubMed] [Google Scholar]
- Maenz D. D., Cheeseman C. I. Effect of hyperglycemia on D-glucose transport across the brush-border and basolateral membrane of rat small intestine. Biochim Biophys Acta. 1986 Aug 21;860(2):277–285. doi: 10.1016/0005-2736(86)90524-9. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Hase K., Takagi T., Fujii T., Taketani Y., Minami H., Oka T., Nakabou Y. Differential responses of intestinal glucose transporter mRNA transcripts to levels of dietary sugars. Biochem J. 1993 Oct 1;295(Pt 1):211–215. doi: 10.1042/bj2950211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See N. A., Bass P. Glucose-induced ion secretion in rat jejunum: a mucosal reflex that requires integration by the myenteric plexus. J Auton Nerv Syst. 1993 Jan;42(1):33–40. doi: 10.1016/0165-1838(93)90339-v. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Debnam E. S. Rapid stimulatory effect of bradykinin on glucose transport across the brush-border and basolateral membranes of rat jejunal enterocytes. Exp Physiol. 1992 Nov;77(6):913–916. doi: 10.1113/expphysiol.1992.sp003658. [DOI] [PubMed] [Google Scholar]
- Shirazi-Beechey S. P., Hirayama B. A., Wang Y., Scott D., Smith M. W., Wright E. M. Ontogenic development of lamb intestinal sodium-glucose co-transporter is regulated by diet. J Physiol. 1991 Jun;437:699–708. doi: 10.1113/jphysiol.1991.sp018620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg D. H., Diamond J. M. Comparison of different dietary sugars as inducers of intestinal sugar transporters. Am J Physiol. 1987 Apr;252(4 Pt 1):G574–G584. doi: 10.1152/ajpgi.1987.252.4.G574. [DOI] [PubMed] [Google Scholar]
- Steidl M., Ritter M., Lang F. Regulation of potassium conductance by prostaglandins in cultured renal epitheloid (Madin-Darby canine kidney) cells. Pflugers Arch. 1991 Jun;418(5):431–436. doi: 10.1007/BF00497769. [DOI] [PubMed] [Google Scholar]
- Tsang R., Ao Z., Cheeseman C. Influence of vascular and luminal hexoses on rat intestinal basolateral glucose transport. Can J Physiol Pharmacol. 1994 Apr;72(4):317–326. doi: 10.1139/y94-048. [DOI] [PubMed] [Google Scholar]
- Veyhl M., Spangenberg J., Püschel B., Poppe R., Dekel C., Fritzsch G., Haase W., Koepsell H. Cloning of a membrane-associated protein which modifies activity and properties of the Na(+)-D-glucose cotransporter. J Biol Chem. 1993 Nov 25;268(33):25041–25053. [PubMed] [Google Scholar]


