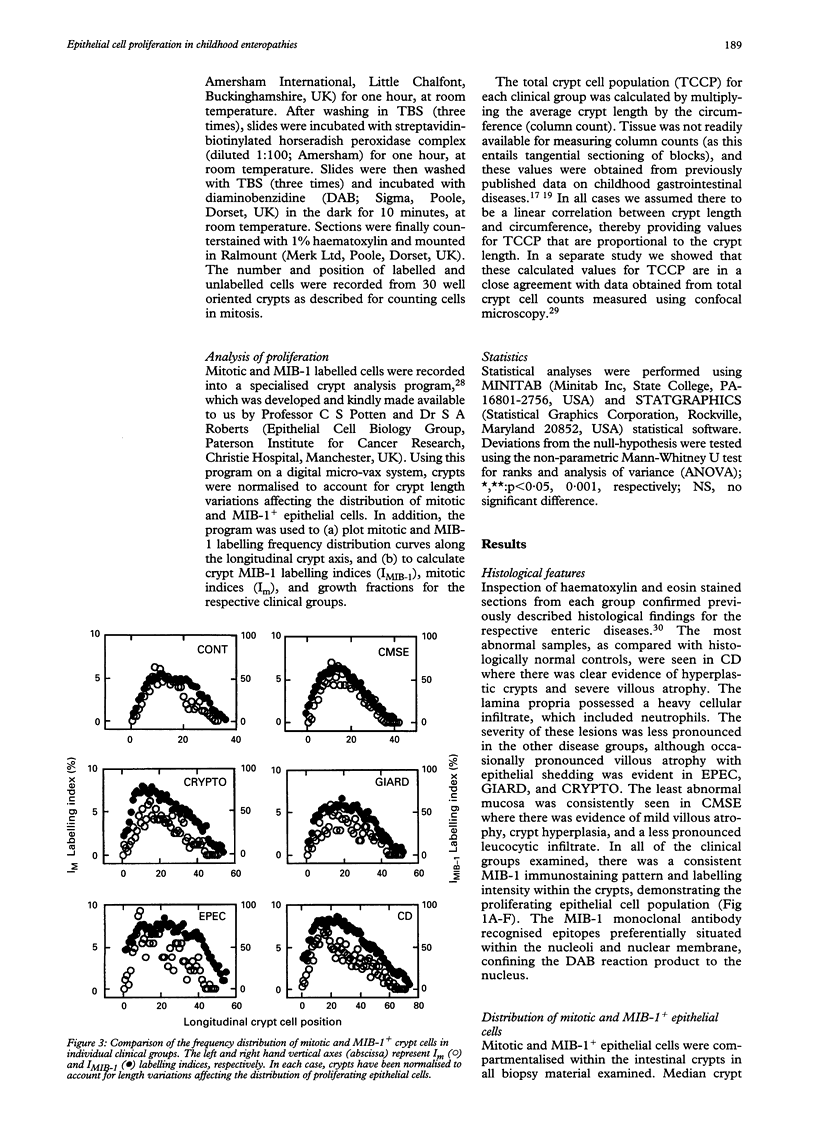
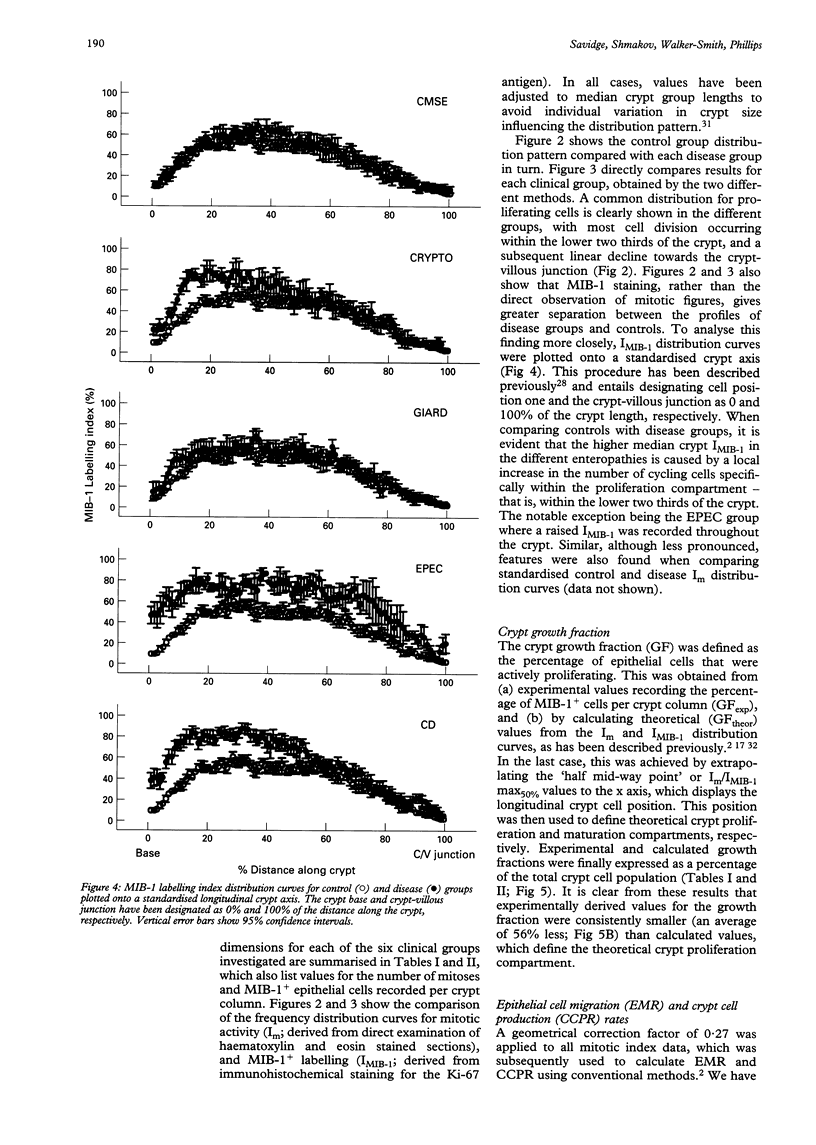
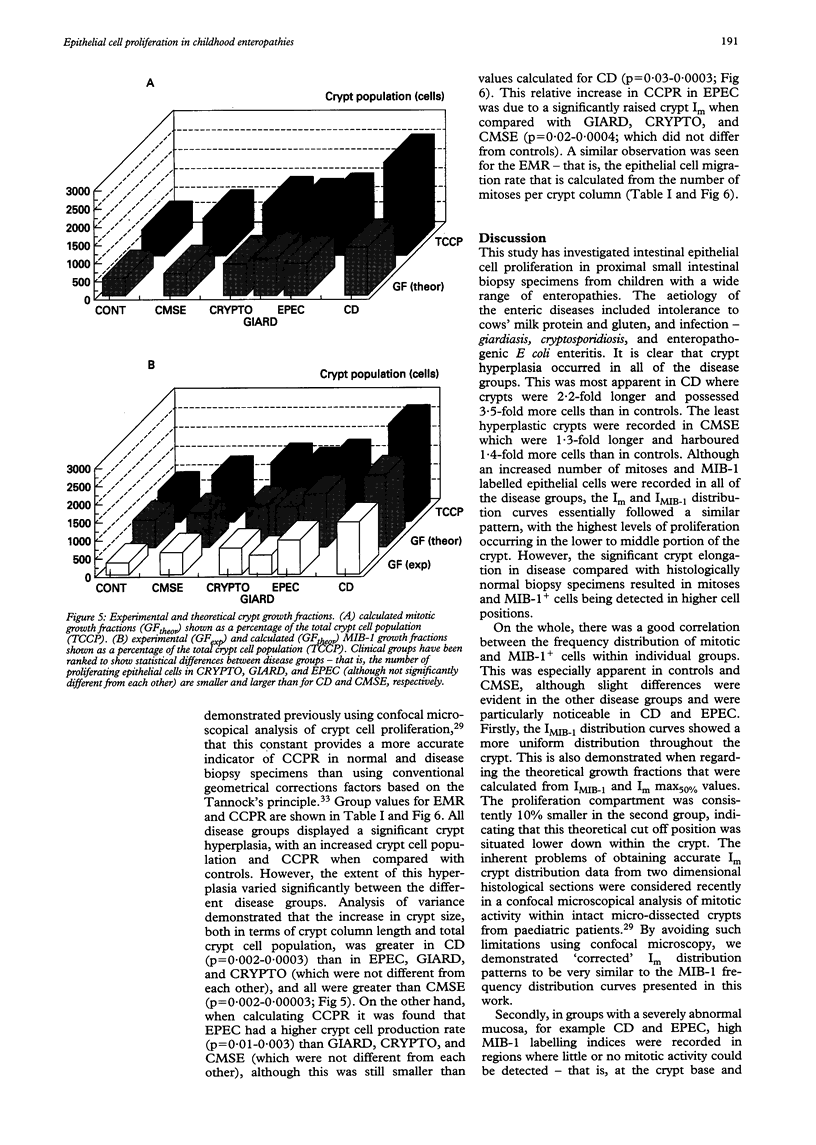
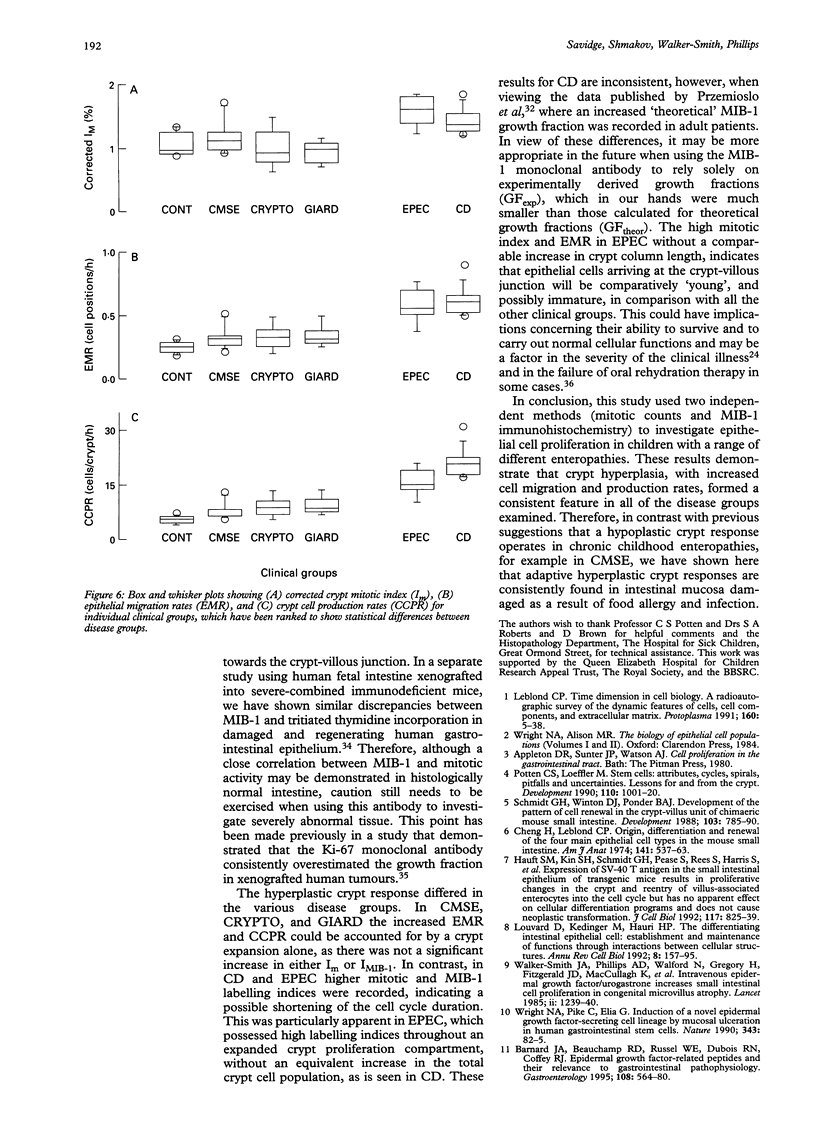
Abstract
BACKGROUND/AIM: The aim of this study was to investigate epithelial cell turnover in childhood enteropathy to establish whether common disease related mechanisms operate. Levels of epithelial cell proliferation were measured in children with food intolerance (cows' milk protein intolerance and coeliac disease), and after infection with Giardia lamblia, Cryptosporidium, and enteropathogenic Escherichia coli. METHODS: Comparative measures of epithelial cell proliferation were performed by recording mitotic activity and MIB-1 immunoreactivity in proximal small intestinal biopsy specimens. RESULTS/CONCLUSIONS: A hyperplastic crypt response was evident in all of the disease states examined and was particularly pronounced in coeliac disease and in infection with enteropathogenic E coli, where mitotic and MIB-1 labelling indices were significantly raised above control values. In contrast with coeliac disease, increased crypt cell production rates in enteropathogenic E coli infection were also due to an expansion of the crypt proliferation compartment, without a comparable increase in crypt cell numbers. Crypt hyperplasia is therefore a common tissue response to mucosal damage in food allergy and infection, although disease specific mechanisms are evident.
Full text
PDF
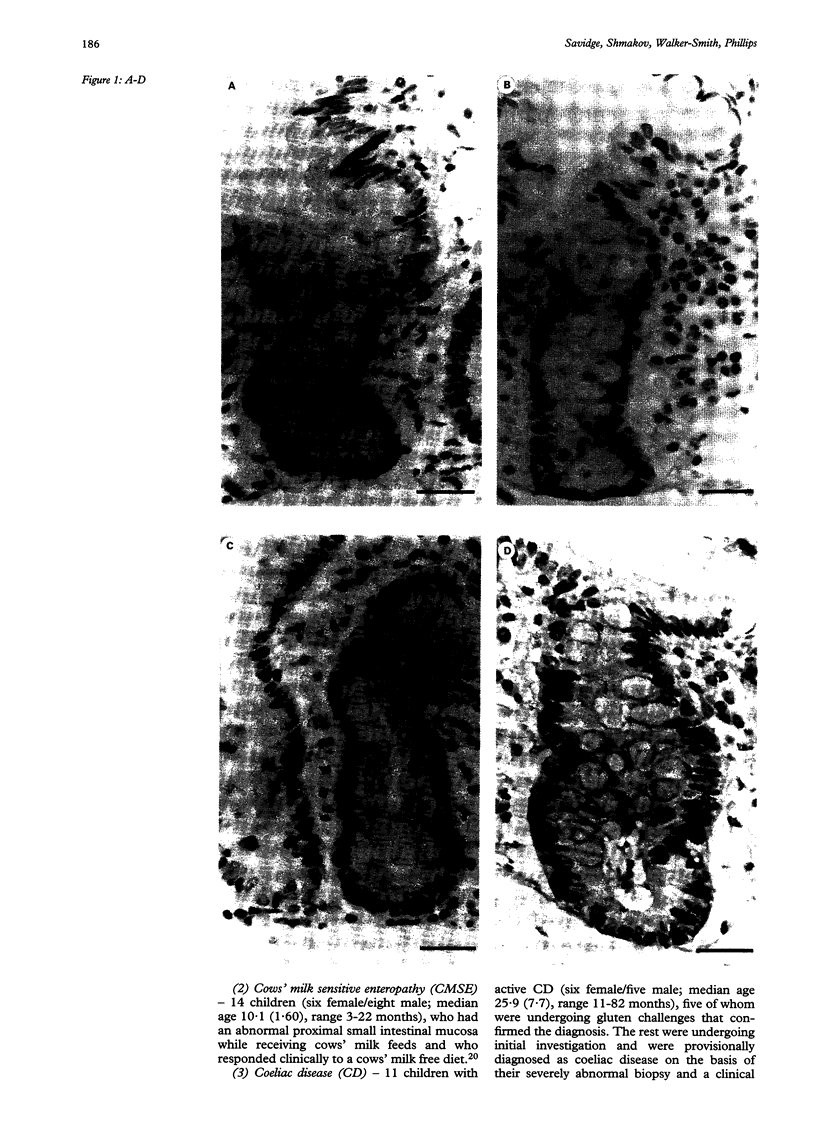
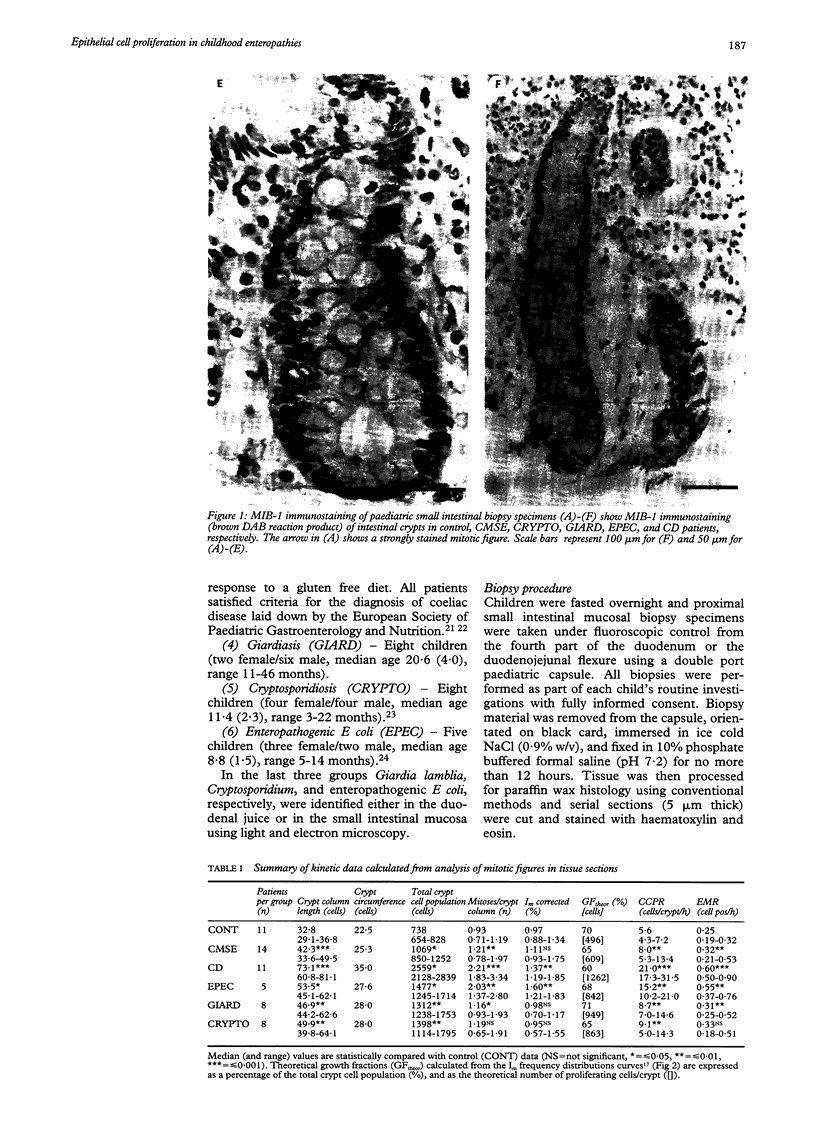
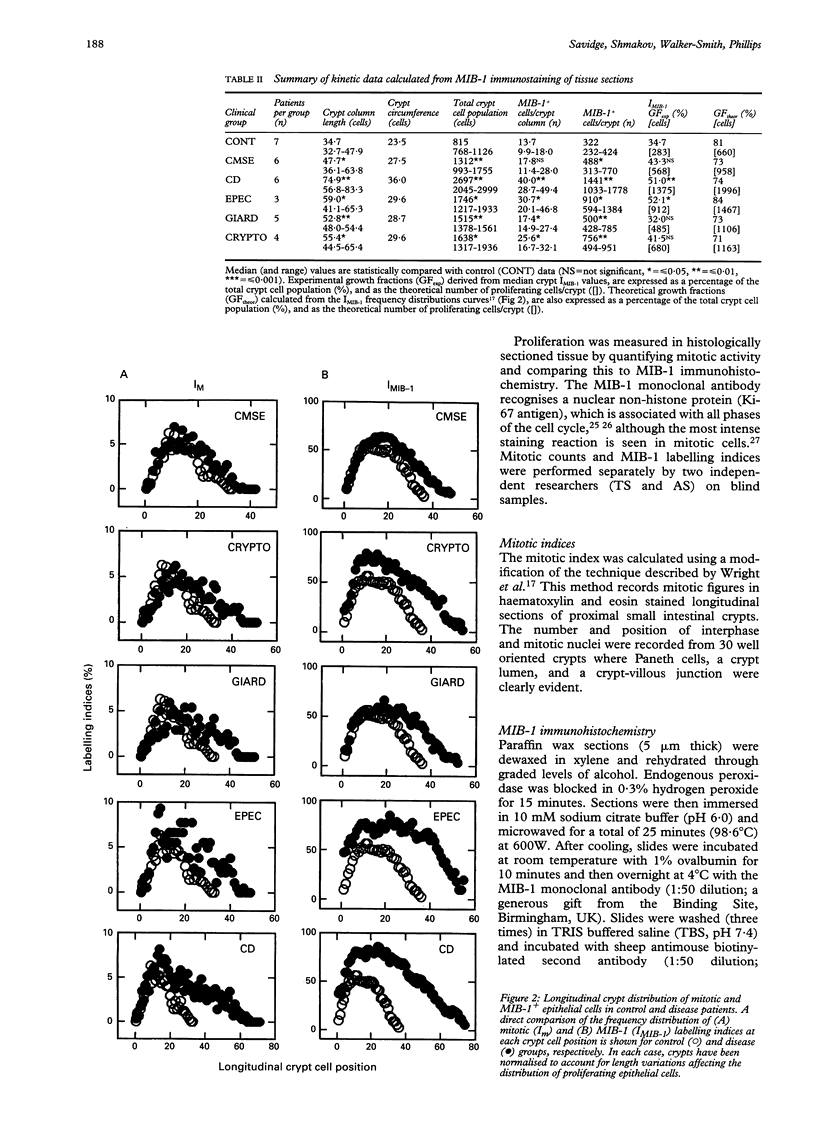

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnard J. A., Beauchamp R. D., Russell W. E., Dubois R. N., Coffey R. J. Epidermal growth factor-related peptides and their relevance to gastrointestinal pathophysiology. Gastroenterology. 1995 Feb;108(2):564–580. doi: 10.1016/0016-5085(95)90087-x. [DOI] [PubMed] [Google Scholar]
- Cheng H., Leblond C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974 Dec;141(4):537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- Cutz E., Rhoads J. M., Drumm B., Sherman P. M., Durie P. R., Forstner G. G. Microvillus inclusion disease: an inherited defect of brush-border assembly and differentiation. N Engl J Med. 1989 Mar 9;320(10):646–651. doi: 10.1056/NEJM198903093201006. [DOI] [PubMed] [Google Scholar]
- Davidson G. P., Cutz E., Hamilton J. R., Gall D. G. Familial enteropathy: a syndrome of protracted diarrhea from birth, failure to thrive, and hypoplastic villus atrophy. Gastroenterology. 1978 Nov;75(5):783–790. [PubMed] [Google Scholar]
- Falini B., Flenghi L., Fagioli M., Stein H., Schwarting R., Riccardi C., Manocchio I., Pileri S., Pelicci P. G., Lanfrancone L. Evolutionary conservation in various mammalian species of the human proliferation-associated epitope recognized by the Ki-67 monoclonal antibody. J Histochem Cytochem. 1989 Oct;37(10):1471–1478. doi: 10.1177/37.10.2476477. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Schwab U., Lemke H., Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983 Jan 15;31(1):13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Hauft S. M., Kim S. H., Schmidt G. H., Pease S., Rees S., Harris S., Roth K. A., Hansbrough J. R., Cohn S. M., Ahnen D. J. Expression of SV-40 T antigen in the small intestinal epithelium of transgenic mice results in proliferative changes in the crypt and reentry of villus-associated enterocytes into the cell cycle but has no apparent effect on cellular differentiation programs and does not cause neoplastic transformation. J Cell Biol. 1992 May;117(4):825–839. doi: 10.1083/jcb.117.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S. M., Phillips A. D., Walker-Smith J. A. Enteropathogenic Escherichia coli and life threatening chronic diarrhoea. Gut. 1991 Feb;32(2):154–158. doi: 10.1136/gut.32.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellett M., Potten C. S., Rew D. A. A comparison of in vivo cell proliferation measurements in the intestine of mouse and man. Epithelial Cell Biol. 1992 Oct;1(4):147–155. [PubMed] [Google Scholar]
- Kosnai I., Kuitunen P., Savilahti E., Rapola J., Köhegyi J. Cell kinetics in the jejunal crypt epithelium in malabsorption syndrome with cow's milk protein intolerance and in coeliac disease of childhood. Gut. 1980 Dec;21(12):1041–1046. doi: 10.1136/gut.21.12.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvard D., Kedinger M., Hauri H. P. The differentiating intestinal epithelial cell: establishment and maintenance of functions through interactions between cellular structures. Annu Rev Cell Biol. 1992;8:157–195. doi: 10.1146/annurev.cb.08.110192.001105. [DOI] [PubMed] [Google Scholar]
- Maluenda C., Phillips A. D., Briddon A., Walker-Smith J. A. Quantitative analysis of small intestinal mucosa in cow's milk-sensitive enteropathy. J Pediatr Gastroenterol Nutr. 1984 Jun;3(3):349–356. doi: 10.1097/00005176-198406000-00008. [DOI] [PubMed] [Google Scholar]
- Marin L., Aperia A., Zetterström R., Günóz H., Sökücü S., Saner G., Neyzi O. Unsuccessful oral rehydration therapy in an infant with enteropathogenic E. coli diarrhoea. Studies of fluid and electrolyte homeostasis. Acta Paediatr Scand. 1985 May;74(3):477–479. doi: 10.1111/j.1651-2227.1985.tb11012.x. [DOI] [PubMed] [Google Scholar]
- Phillips A. D., Thomas A. G., Walker-Smith J. A. Cryptosporidium, chronic diarrhoea and the proximal small intestinal mucosa. Gut. 1992 Aug;33(8):1057–1061. doi: 10.1136/gut.33.8.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten C. S., Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990 Dec;110(4):1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- Potten C. S. The significance of spontaneous and induced apoptosis in the gastrointestinal tract of mice. Cancer Metastasis Rev. 1992 Sep;11(2):179–195. doi: 10.1007/BF00048063. [DOI] [PubMed] [Google Scholar]
- Przemioslo R., Wright N. A., Elia G., Ciclitira P. J. Analysis of crypt cell proliferation in coeliac disease using MI-B1 antibody shows an increase in growth fraction. Gut. 1995 Jan;36(1):22–27. doi: 10.1136/gut.36.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revised criteria for diagnosis of coeliac disease. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Arch Dis Child. 1990 Aug;65(8):909–911. doi: 10.1136/adc.65.8.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savidge T. C., Walker-Smith J. A., Phillips A. D. Novel insights into human intestinal epithelial cell proliferation in health and disease using confocal microscopy. Gut. 1995 Mar;36(3):369–374. doi: 10.1136/gut.36.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter C., Duchrow M., Wohlenberg C., Becker M. H., Key G., Flad H. D., Gerdes J. The cell proliferation-associated antigen of antibody Ki-67: a very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J Cell Biol. 1993 Nov;123(3):513–522. doi: 10.1083/jcb.123.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. H., Winton D. J., Ponder B. A. Development of the pattern of cell renewal in the crypt-villus unit of chimaeric mouse small intestine. Development. 1988 Aug;103(4):785–790. doi: 10.1242/dev.103.4.785. [DOI] [PubMed] [Google Scholar]
- Scott R. J., Hall P. A., Haldane J. S., van Noorden S., Price Y., Lane D. P., Wright N. A. A comparison of immunohistochemical markers of cell proliferation with experimentally determined growth fraction. J Pathol. 1991 Oct;165(2):173–178. doi: 10.1002/path.1711650213. [DOI] [PubMed] [Google Scholar]
- Shmakov A. N., Morey A. L., Ferguson D. J., Fleming K. A., O'Brien J. A., Savidge T. C. Conventional patterns of human intestinal proliferation in a severe-combined immunodeficient xenograft model. Differentiation. 1995 Dec;59(5):321–330. doi: 10.1046/j.1432-0436.1996.5950321.x. [DOI] [PubMed] [Google Scholar]
- Totafurno J., Bjerknes M., Cheng H. Variation in crypt size and its influence on the analysis of epithelial cell proliferation in the intestinal crypt. Biophys J. 1988 Nov;54(5):845–858. doi: 10.1016/S0006-3495(88)83021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trier J. S., Browning T. H. Epithelial-cell renewal in cultured duodenal biopsies in celiac sprue. N Engl J Med. 1970 Dec 3;283(23):1245–1250. doi: 10.1056/NEJM197012032832302. [DOI] [PubMed] [Google Scholar]
- Walker-Smith J. A., Digeon B., Phillips A. D. Evaluation of a casein and a whey hydrolysate for treatment of cow's-milk-sensitive enteropathy. Eur J Pediatr. 1989 Oct;149(1):68–71. doi: 10.1007/BF02024340. [DOI] [PubMed] [Google Scholar]
- Walker-Smith J. A., Phillips A. D., Walford N., Gregory H., Fitzgerald J. D., MacCullagh K., Wright N. A. Intravenous epidermal growth factor/urogastrone increases small-intestinal cell proliferation in congenital microvillous atrophy. Lancet. 1985 Nov 30;2(8466):1239–1240. doi: 10.1016/s0140-6736(85)90762-7. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Pike C., Elia G. Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature. 1990 Jan 4;343(6253):82–85. doi: 10.1038/343082a0. [DOI] [PubMed] [Google Scholar]
- Wright N., Watson A., Morley A., Appleton D., Marks J. Cell kinetics in flat (avillous) mucosa of the human small intestine. Gut. 1973 Sep;14(9):701–710. doi: 10.1136/gut.14.9.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright N., Watson A., Morley A., Appleton D., Marks J., Douglas A. The cell cycle time in the flat (avillous) mucosa of the human small intestine. Gut. 1973 Aug;14(8):603–606. doi: 10.1136/gut.14.8.603. [DOI] [PMC free article] [PubMed] [Google Scholar]