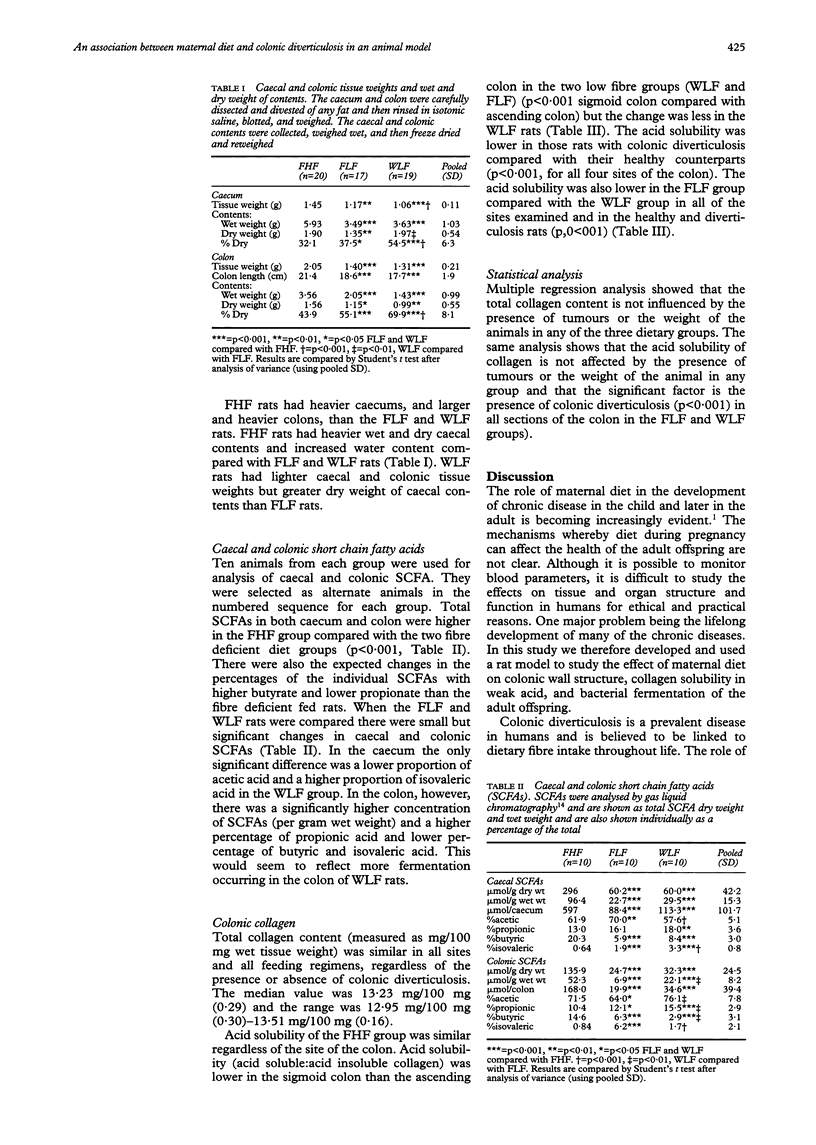
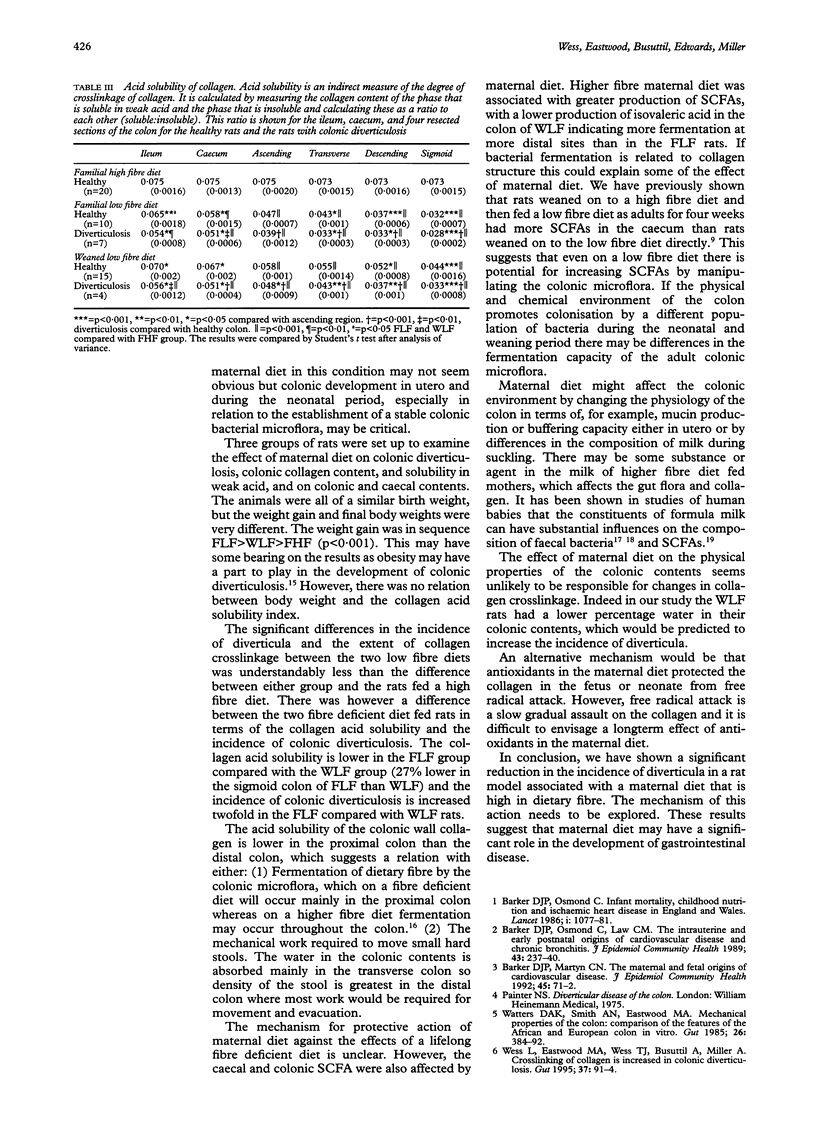
Abstract
BACKGROUND/AIMS: Maternal diet may have an effect on the health of the offspring in middle and later life. This study used the laboratory rat as an animal model to examine whether the fibre content of the maternal diet during pregnancy affected subsequent development of colonic diverticula in the offspring fed lifelong fibre deficient or higher fibre diets. METHODS: The parents of experimental animals were fed either a diet that was known to predispose to colonic diverticulosis or a control diet for one month prior to mating. The offspring were fed one of these diets for 18 months. The incidence of colonic diverticulosis, submucosal collagen content, collagen solubility in weak acid, and the composition of intestinal contents were then measured. RESULTS: Offspring of rats fed a higher fibre diet from higher fibre diet fed parents had 0% incidence of colonic diverticulosis. When offspring (regardless of parental diet) were fed a low fibre diet for life the acid solubility was lowered compared with rats fed lifelong higher fibre diet mean (SD) (0.044 (0.0007) v 0.073 (0.0015) sigmoid colon (ratio of soluble:insoluble collagen)); 21.1% had diverticulosis and there was reduced fibre fermentation. However, when the diet of the parents of the fibre deficient diet fed rats was considered, the animals whose mothers had a fibre deficient diet had lower acid solubility (0.032 (0.0007)) and an increased incidence of colonic diverticulosis (42.1%) than the animals fed a fibre deficient diet from higher fibre diet fed parents (p < 0.01 in all instances). CONCLUSION: Maternal diet and the subsequent nutrition of the progeny seem to be of importance in the development of colonic diverticulosis in the rat.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong E. F., Eastwood M. A., Edwards C. A., Brydon W. G., Macintyre C. C. The effect of weaning diet on the subsequent colonic metabolism of dietary fibre in the adult rat. Br J Nutr. 1992 Nov;68(3):741–751. doi: 10.1079/bjn19920130. [DOI] [PubMed] [Google Scholar]
- Balmer S. E., Scott P. H., Wharton B. A. Diet and faecal flora in the newborn: casein and whey proteins. Arch Dis Child. 1989 Dec;64(12):1678–1684. doi: 10.1136/adc.64.12.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer S. E., Wharton B. A. Diet and faecal flora in the newborn: iron. Arch Dis Child. 1991 Dec;66(12):1390–1394. doi: 10.1136/adc.66.12.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. J., Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986 May 10;1(8489):1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- Barker D. J., Osmond C., Law C. M. The intrauterine and early postnatal origins of cardiovascular disease and chronic bronchitis. J Epidemiol Community Health. 1989 Sep;43(3):237–240. doi: 10.1136/jech.43.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood M. A., Brydon W. G., Baird J. D., Elton R. A., Helliwell S., Smith J. H., Pritchard J. L. Fecal weight and composition, serum lipids, and diet among subjects aged 18 to 80 years not seeking health care. Am J Clin Nutr. 1984 Sep;40(3):628–634. doi: 10.1093/ajcn/40.3.628. [DOI] [PubMed] [Google Scholar]
- Edwards C. A., Eastwood M. A. Comparison of the effects of ispaghula and wheat bran on rat caecal and colonic fermentation. Gut. 1992 Sep;33(9):1229–1233. doi: 10.1136/gut.33.9.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C. A., Parrett A. M., Balmer S. E., Wharton B. A. Faecal short chain fatty acids in breast-fed and formula-fed babies. Acta Paediatr. 1994 May;83(5):459–462. doi: 10.1111/j.1651-2227.1994.tb13059.x. [DOI] [PubMed] [Google Scholar]
- Englyst H., Wiggins H. S., Cummings J. H. Determination of the non-starch polysaccharides in plant foods by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst. 1982 Mar;107(1272):307–318. doi: 10.1039/an9820700307. [DOI] [PubMed] [Google Scholar]
- Fisher N., Berry C. S., Fearn T., Gregory J. A., Hardy J. Cereal dietary fiber consumption and diverticular disease: a lifespan study in rats. Am J Clin Nutr. 1985 Nov;42(5):788–804. doi: 10.1093/ajcn/42.5.788. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Laitinen O., Prockop D. J. Modifications of a specific assay for hydroxyproline in urine. Anal Biochem. 1967 May;19(2):249–255. doi: 10.1016/0003-2697(67)90160-1. [DOI] [PubMed] [Google Scholar]
- Monnier V. M., Kohn R. R., Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc Natl Acad Sci U S A. 1984 Jan;81(2):583–587. doi: 10.1073/pnas.81.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins S. P., Shimokomaki M., Bailey A. J. The chemistry of the collagen cross-links. Age-related changes in the reducible components of intact bovine collagen fibres. Biochem J. 1973 Apr;131(4):771–780. doi: 10.1042/bj1310771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller G. A., Chernoff M. C., Hill R. A., Gates J. E., Nassar J. J., Shipley E. A. Effect of purified cellulose, pectin, and a low-residue diet on fecal volatile fatty acids, transit time, and fecal weight in humans. Am J Clin Nutr. 1980 Apr;33(4):754–759. doi: 10.1093/ajcn/33.4.754. [DOI] [PubMed] [Google Scholar]
- Watters D. A., Smith A. N., Eastwood M. A., Anderson K. C., Elton R. A., Mugerwa J. W. Mechanical properties of the colon: comparison of the features of the African and European colon in vitro. Gut. 1985 Apr;26(4):384–392. doi: 10.1136/gut.26.4.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess L., Eastwood M. A., Edwards C. A., Busuttil A., Miller A. Collagen alteration in an animal model of colonic diverticulosis. Gut. 1996 May;38(5):701–706. doi: 10.1136/gut.38.5.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess L., Eastwood M. A., Wess T. J., Busuttil A., Miller A. Cross linking of collagen is increased in colonic diverticulosis. Gut. 1995 Jul;37(1):91–94. doi: 10.1136/gut.37.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]


