Abstract
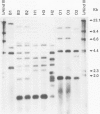
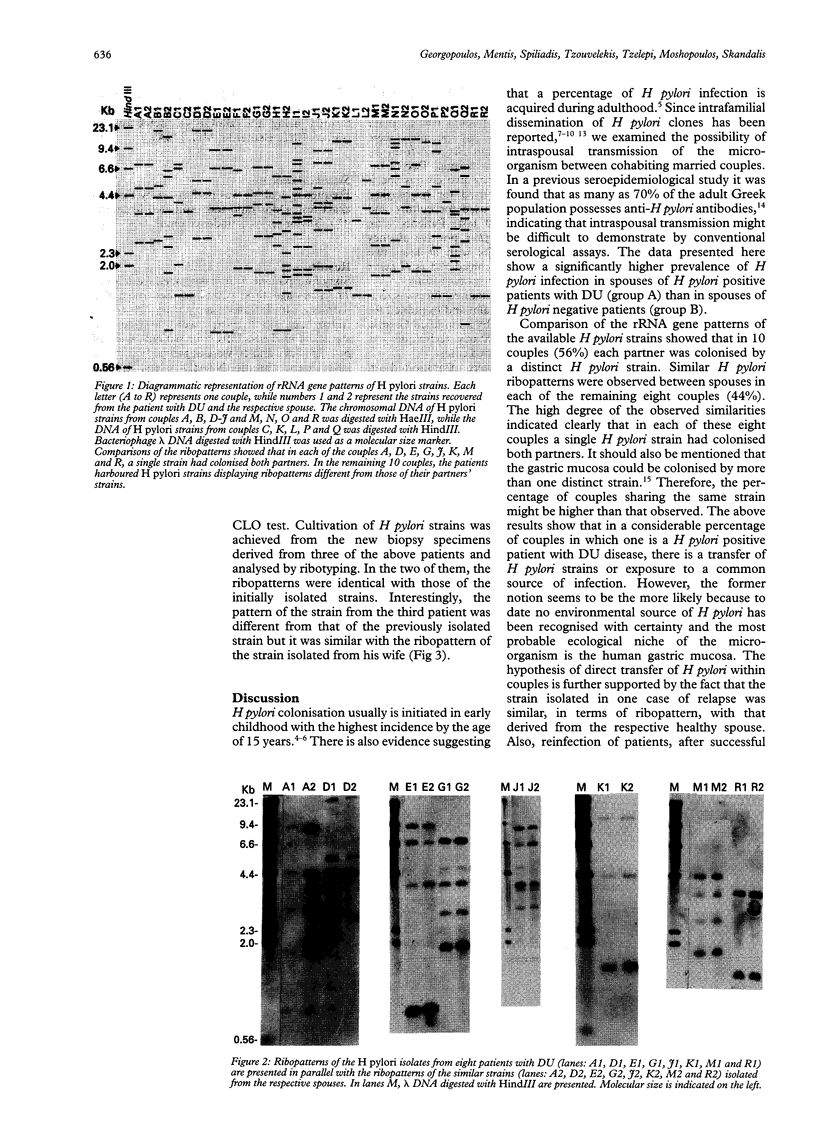
BACKGROUND: In recent studies, familial coinfection with the same Helicobacter pylori strains has been indicated, but more data are necessary to confirm intra-familial spread of the micro-organism. AIMS: The aim of this study was (a) to assess the frequency of H pylori infection in spouses of patients with duodenal ulcers and (b) to investigate the possibility of intraspousal typing of the respective strains. PATIENTS: Sixty four patients with duodenal ulcer and their spouses were included in the study. METHODS: The H pylori infection was confirmed after endoscopy by culture and histological examination of biopsy specimens, and CLO test. The isolates were compared on the basis of their rRNA gene patterns (ribopatterns) after digestion of chromosomal DNA by the restriction endonucleases HaeIII or HindIII. RESULTS: Of the patients, 54 were found to be H pylori positive. Of the respective spouses, 42 (78%) were also H pylori positive. In contrast, only two out of 10 (20%) partners of H pylori negative patients were infected. Ribopatterns of H pylori strains derived from 18 patients and their spouses showed that in each of eight couples a single strain had colonised both partners, while in the remaining 10 couples each partner was colonised by a distinct H pylori strain. CONCLUSIONS: These data suggest person to person transmission within couples or exposure to a common source of infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamford K. B., Bickley J., Collins J. S., Johnston B. T., Potts S., Boston V., Owen R. J., Sloan J. M. Helicobacter pylori: comparison of DNA fingerprints provides evidence for intrafamilial infection. Gut. 1993 Oct;34(10):1348–1350. doi: 10.1136/gut.34.10.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banatvala N., Mayo K., Megraud F., Jennings R., Deeks J. J., Feldman R. A. The cohort effect and Helicobacter pylori. J Infect Dis. 1993 Jul;168(1):219–221. doi: 10.1093/infdis/168.1.219. [DOI] [PubMed] [Google Scholar]
- Foxall P. A., Hu L. T., Mobley H. L. Use of polymerase chain reaction-amplified Helicobacter pylori urease structural genes for differentiation of isolates. J Clin Microbiol. 1992 Mar;30(3):739–741. doi: 10.1128/jcm.30.3.739-741.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan R. P. Helicobacter pylori and gastric cancer. Lancet. 1994 Oct 15;344(8929):1078–1079. doi: 10.1016/s0140-6736(94)91729-9. [DOI] [PubMed] [Google Scholar]
- Mitchell H. M., Li Y. Y., Hu P. J., Liu Q., Chen M., Du G. G., Wang Z. J., Lee A., Hazell S. L. Epidemiology of Helicobacter pylori in southern China: identification of early childhood as the critical period for acquisition. J Infect Dis. 1992 Jul;166(1):149–153. doi: 10.1093/infdis/166.1.149. [DOI] [PubMed] [Google Scholar]
- Moore R. A., Kureishi A., Wong S., Bryan L. E. Categorization of clinical isolates of Helicobacter pylori on the basis of restriction digest analyses of polymerase chain reaction-amplified ureC genes. J Clin Microbiol. 1993 May;31(5):1334–1335. doi: 10.1128/jcm.31.5.1334-1335.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwokolo C. U., Bickley J., Attard A. R., Owen R. J., Costas M., Fraser I. A. Evidence of clonal variants of Helicobacter pylori in three generations of a duodenal ulcer disease family. Gut. 1992 Oct;33(10):1323–1327. doi: 10.1136/gut.33.10.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. J., Hunton C., Bickley J., Moreno M., Linton D. Ribosomal RNA gene restriction patterns of Helicobacter pylori: analysis and appraisal of Hae III digests as a molecular typing system. Epidemiol Infect. 1992 Aug;109(1):35–47. [PMC free article] [PubMed] [Google Scholar]
- Pateraki E., Mentis A., Spiliadis C., Sophianos D., Stergiatou I., Skandalis N., Weir D. M. Seroepidemiology of Helicobacter pylori infection in Greece. FEMS Microbiol Immunol. 1990 Oct;2(3):129–136. doi: 10.1111/j.1574-6968.1990.tb03512.x. [DOI] [PubMed] [Google Scholar]
- Prewett E. J., Bickley J., Owen R. J., Pounder R. E. DNA patterns of Helicobacter pylori isolated from gastric antrum, body, and duodenum. Gastroenterology. 1992 Mar;102(3):829–833. doi: 10.1016/0016-5085(92)90165-u. [DOI] [PubMed] [Google Scholar]
- Riccardi V. M., Rotter J. I. Familial Helicobacter pylori infection. Societal factors, human genetics, and bacterial genetics. Ann Intern Med. 1994 Jun 15;120(12):1043–1045. doi: 10.7326/0003-4819-120-12-199406150-00012. [DOI] [PubMed] [Google Scholar]
- Schütze K., Hentschel E., Dragosics B., Hirschl A. M. Helicobacter pylori reinfection with identical organisms: transmission by the patients' spouses. Gut. 1995 Jun;36(6):831–833. doi: 10.1136/gut.36.6.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltesz V., Zeeberg B., Wadström T. Optimal survival of Helicobacter pylori under various transport conditions. J Clin Microbiol. 1992 Jun;30(6):1453–1456. doi: 10.1128/jcm.30.6.1453-1456.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983 Jun 4;1(8336):1273–1275. [PubMed] [Google Scholar]
- Vincent P., Gottrand F., Pernes P., Husson M. O., Lecomte-Houcke M., Turck D., Leclerc H. High prevalence of Helicobacter pylori infection in cohabiting children. Epidemiology of a cluster, with special emphasis on molecular typing. Gut. 1994 Mar;35(3):313–316. doi: 10.1136/gut.35.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. T., Sheu J. C., Lin J. T., Wang T. H., Wu M. S. Direct DNA amplification and restriction pattern analysis of Helicobacter pylori in patients with duodenal ulcer and their families. J Infect Dis. 1993 Dec;168(6):1544–1548. doi: 10.1093/infdis/168.6.1544. [DOI] [PubMed] [Google Scholar]
- Yoshimura H. H., Evans D. G., Graham D. Y. DNA-DNA hybridization demonstrates apparent genetic differences between Helicobacter pylori from patients with duodenal ulcer and asymptomatic gastritis. Dig Dis Sci. 1993 Jun;38(6):1128–1131. doi: 10.1007/BF01295731. [DOI] [PubMed] [Google Scholar]