Abstract
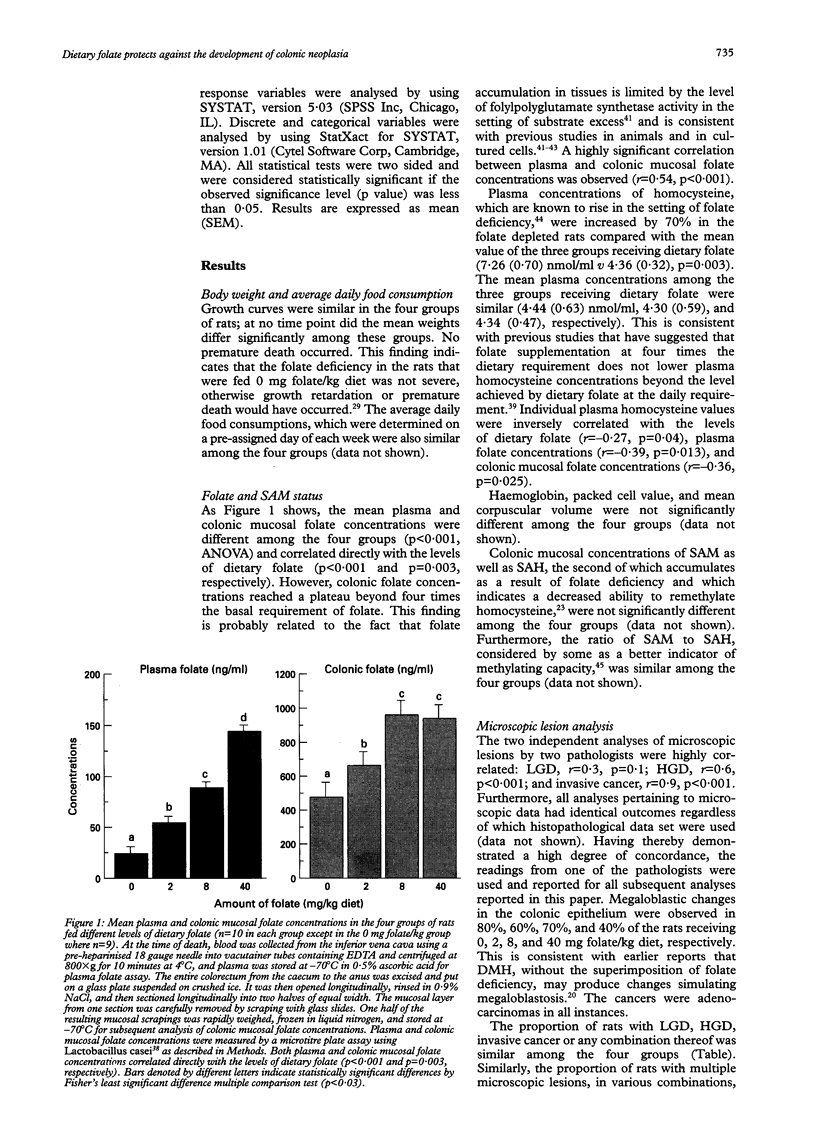
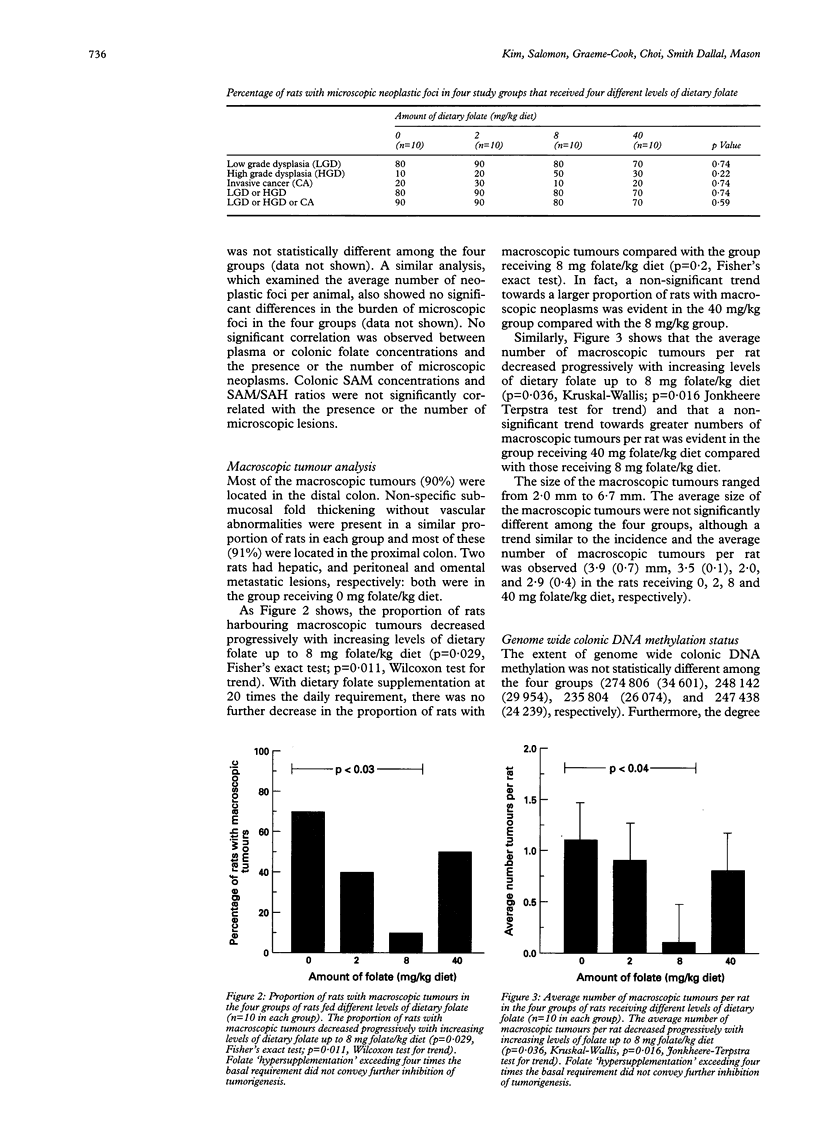
BACKGROUND AND AIMS: Diminished folate status is associated with enhanced colorectal carcinogenesis. This study investigated the potential chemopreventive role of dietary folate in the dimethylhydrazine colorectal cancer model. SUBJECTS AND METHODS: Sprague-Dawley rats were fed diets containing either 0, 2 (daily dietary requirement), 8 or 40 mg folate/kg diet for 20 weeks. After five weeks of diet, rats were injected with dimethyl-hydrazine (44 mg/kg) weekly for 15 weeks. Fifteen weeks after the first injection of dimethylhydrazine, all rats were killed. Folate status was determined, and the entire colorectum from each rat was analysed for macroscopic and microscopic neoplasms. RESULTS: Plasma and colonic folate concentrations correlated directly with dietary folate levels (p < 0.005). The incidence of microscopic neoplasms was similar among the four groups. However, the incidence and the average number of macroscopic tumours per rat decreased progressively with increasing dietary folate levels up to 8 mg/kg diet (p < 0.05). In the strongly procarcinogenic milieu used in this study, folate supplementation at 20 times the basal requirement was associated with rates of macroscopic tumour development that were intermediate, and not statistically distinct, from rates observed at either 0 or 8 mg/kg diet. CONCLUSIONS: These data indicate that in this rat model, (a) increasing dietary folate up to four times the basal requirement leads to a progressive reduction in the evolution of macroscopic neoplasms from microscopic foci; and (b) folate supplementation beyond four times the requirement does not convey further benefit.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balaghi M., Wagner C. DNA methylation in folate deficiency: use of CpG methylase. Biochem Biophys Res Commun. 1993 Jun 30;193(3):1184–1190. doi: 10.1006/bbrc.1993.1750. [DOI] [PubMed] [Google Scholar]
- Barkla D. H., Tutton P. J. Surface changes in the descending colon of rats treated with dimethylhydrazine. Cancer Res. 1977 Jan;37(1):262–271. [PubMed] [Google Scholar]
- Benito E., Cabeza E., Moreno V., Obrador A., Bosch F. X. Diet and colorectal adenomas: a case-control study in Majorca. Int J Cancer. 1993 Sep 9;55(2):213–219. doi: 10.1002/ijc.2910550208. [DOI] [PubMed] [Google Scholar]
- Benito E., Stiggelbout A., Bosch F. X., Obrador A., Kaldor J., Mulet M., Muñoz N. Nutritional factors in colorectal cancer risk: a case-control study in Majorca. Int J Cancer. 1991 Sep 9;49(2):161–167. doi: 10.1002/ijc.2910490202. [DOI] [PubMed] [Google Scholar]
- Bianchi A., Chipman D. W., Dreskin A., Rosensweig N. S. Nutritional folic acid deficiency with megaloblastic changes in the small-bowel epithelium. N Engl J Med. 1970 Apr 9;282(15):859–861. doi: 10.1056/NEJM197004092821510. [DOI] [PubMed] [Google Scholar]
- Borek C., Ong A., Morgan W. F., Cleaver J. E. Morphological transformation of 10T1/2 mouse embryo cells can be initiated by DNA double-strand breaks alone. Mol Carcinog. 1991;4(3):243–247. doi: 10.1002/mc.2940040311. [DOI] [PubMed] [Google Scholar]
- Branda R. F., Blickensderfer D. B. Folate deficiency increases genetic damage caused by alkylating agents and gamma-irradiation in Chinese hamster ovary cells. Cancer Res. 1993 Nov 15;53(22):5401–5408. [PubMed] [Google Scholar]
- Bruce W. R., Eyssen G. M., Ciampi A., Dion P. W., Boyd N. Strategies for dietary intervention studies in colon cancer. Cancer. 1981 Mar 1;47(5 Suppl):1121–1125. doi: 10.1002/1097-0142(19810301)47:5+<1121::aid-cncr2820471310>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Clifford A. J., Heid M. K., Müller H. G., Bills N. D. Tissue distribution and prediction of total body folate of rats. J Nutr. 1990 Dec;120(12):1633–1639. doi: 10.1093/jn/120.12.1633. [DOI] [PubMed] [Google Scholar]
- Clifford A. J., Wilson D. S., Bills N. D. Repletion of folate-depleted rats with an amino acid-based diet supplemented with folic acid. J Nutr. 1989 Dec;119(12):1956–1961. doi: 10.1093/jn/119.12.1956. [DOI] [PubMed] [Google Scholar]
- Cox R. DNA methylase inhibition in vitro by N-methyl-N'-nitro-N-nitrosoguanidine. Cancer Res. 1980 Jan;40(1):61–63. [PubMed] [Google Scholar]
- Cravo M. L., Mason J. B., Dayal Y., Hutchinson M., Smith D., Selhub J., Rosenberg I. H. Folate deficiency enhances the development of colonic neoplasia in dimethylhydrazine-treated rats. Cancer Res. 1992 Sep 15;52(18):5002–5006. [PubMed] [Google Scholar]
- Cravo M., Fidalgo P., Pereira A. D., Gouveia-Oliveira A., Chaves P., Selhub J., Mason J. B., Mira F. C., Leitao C. N. DNA methylation as an intermediate biomarker in colorectal cancer: modulation by folic acid supplementation. Eur J Cancer Prev. 1994 Nov;3(6):473–479. doi: 10.1097/00008469-199411000-00004. [DOI] [PubMed] [Google Scholar]
- Decaens C., Gautier R., Daher N., Bara J., Burtin P. Induction of rat intestinal carcinogenesis with single doses, low and high repeated doses of 1,2-dimethylhydrazine. Carcinogenesis. 1989 Jan;10(1):69–72. doi: 10.1093/carcin/10.1.69. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Jones P. A. Progressing toward a molecular description of colorectal cancer development. FASEB J. 1992 Jul;6(10):2783–2790. doi: 10.1096/fasebj.6.10.1321771. [DOI] [PubMed] [Google Scholar]
- Ferraroni M., La Vecchia C., D'Avanzo B., Negri E., Franceschi S., Decarli A. Selected micronutrient intake and the risk of colorectal cancer. Br J Cancer. 1994 Dec;70(6):1150–1155. doi: 10.1038/bjc.1994.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipe M. I. Mucous secretion in rat colonic mucosa during carcinogenesis induced by dimethylhydrazine. A morphological and histochemical study. Br J Cancer. 1975 Jul;32(1):60–77. doi: 10.1038/bjc.1975.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenheim J. L., Graham S., Marshall J. R., Haughey B. P., Cholewinski S., Wilkinson G. Folate intake and carcinogenesis of the colon and rectum. Int J Epidemiol. 1991 Jun;20(2):368–374. doi: 10.1093/ije/20.2.368. [DOI] [PubMed] [Google Scholar]
- Giovannucci E., Stampfer M. J., Colditz G. A., Rimm E. B., Trichopoulos D., Rosner B. A., Speizer F. E., Willett W. C. Folate, methionine, and alcohol intake and risk of colorectal adenoma. J Natl Cancer Inst. 1993 Jun 2;85(11):875–884. doi: 10.1093/jnci/85.11.875. [DOI] [PubMed] [Google Scholar]
- Goldin B. R., Gorbach S. L. Effect of antibiotics on incidence of rat intestinal tumors induced by 1,2-dimethylhydrazine dihydrochloride. J Natl Cancer Inst. 1981 Oct;67(4):877–880. [PubMed] [Google Scholar]
- Halline A. G., Dudeja P. K., Brasitus T. A. 1,2-Dimethylhydrazine-induced premalignant alterations in the S-adenosylmethionine/S-adenosylhomocysteine ratio and membrane lipid lateral diffusion of the rat distal colon. Biochim Biophys Acta. 1988 Sep 15;944(1):101–107. doi: 10.1016/0005-2736(88)90322-7. [DOI] [PubMed] [Google Scholar]
- Hepburn P. A., Margison G. P., Tisdale M. J. Enzymatic methylation of cytosine in DNA is prevented by adjacent O6-methylguanine residues. J Biol Chem. 1991 May 5;266(13):7985–7987. [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Houghton J. A., Williams L. G., de Graaf S. S., Cheshire P. J., Rodman J. H., Maneval D. C., Wainer I. W., Jadaud P., Houghton P. J. Relationship between dose rate of [6RS]Leucovorin administration, plasma concentrations of reduced folates, and pools of 5,10-methylenetetrahydrofolates and tetrahydrofolates in human colon adenocarcinoma xenografts. Cancer Res. 1990 Jun 15;50(12):3493–3502. [PubMed] [Google Scholar]
- James S. J., Cross D. R., Miller B. J. Alterations in nucleotide pools in rats fed diets deficient in choline, methionine and/or folic acid. Carcinogenesis. 1992 Dec;13(12):2471–2474. doi: 10.1093/carcin/13.12.2471. [DOI] [PubMed] [Google Scholar]
- Kim Y. I., Christman J. K., Fleet J. C., Cravo M. L., Salomon R. N., Smith D., Ordovas J., Selhub J., Mason J. B. Moderate folate deficiency does not cause global hypomethylation of hepatic and colonic DNA or c-myc-specific hypomethylation of colonic DNA in rats. Am J Clin Nutr. 1995 May;61(5):1083–1090. doi: 10.1093/ajcn/61.4.1083. [DOI] [PubMed] [Google Scholar]
- Kim Y. I., Giuliano A., Hatch K. D., Schneider A., Nour M. A., Dallal G. E., Selhub J., Mason J. B. Global DNA hypomethylation increases progressively in cervical dysplasia and carcinoma. Cancer. 1994 Aug 1;74(3):893–899. doi: 10.1002/1097-0142(19940801)74:3<893::aid-cncr2820740316>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Laird P. W., Jaenisch R. DNA methylation and cancer. Hum Mol Genet. 1994;3(Spec No):1487–1495. doi: 10.1093/hmg/3.suppl_1.1487. [DOI] [PubMed] [Google Scholar]
- Lashner B. A., Heidenreich P. A., Su G. L., Kane S. V., Hanauer S. B. Effect of folate supplementation on the incidence of dysplasia and cancer in chronic ulcerative colitis. A case-control study. Gastroenterology. 1989 Aug;97(2):255–259. doi: 10.1016/0016-5085(89)90058-9. [DOI] [PubMed] [Google Scholar]
- Lashner B. A. Red blood cell folate is associated with the development of dysplasia and cancer in ulcerative colitis. J Cancer Res Clin Oncol. 1993;119(9):549–554. doi: 10.1007/BF01686465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin B. Nutrition and colorectal cancer. Cancer. 1992 Sep 15;70(6 Suppl):1723–1726. doi: 10.1002/1097-0142(19920915)70:4+<1723::aid-cncr2820701612>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Lewis A. S., Murphy L., McCalla C., Fleary M., Purcell S. Inhibition of mammalian xanthine oxidase by folate compounds and amethopterin. J Biol Chem. 1984 Jan 10;259(1):12–15. [PubMed] [Google Scholar]
- Lowe K. E., Osborne C. B., Lin B. F., Kim J. S., Hsu J. C., Shane B. Regulation of folate and one-carbon metabolism in mammalian cells. II. Effect of folylpoly-gamma-glutamate synthetase substrate specificity and level on folate metabolism and folylpoly-gamma-glutamate specificity of metabolic cycles of one-carbon metabolism. J Biol Chem. 1993 Oct 15;268(29):21665–21673. [PubMed] [Google Scholar]
- Maskens A. P., Dujardin-Loits R. M. Experimental adenomas and carcinomas of the large intestine behave as distinct entities: most carcinomas arise de novo in flat mucosa. Cancer. 1981 Jan 1;47(1):81–89. doi: 10.1002/1097-0142(19810101)47:1<81::aid-cncr2820470115>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Meenan J., O'Hallinan E., Lynch S., Molloy A., McPartlan J., Scott J., Weir D. G. Folate status of gastrointestinal epithelial cells is not predicted by serum and red cell folate values in replete subjects. Gut. 1996 Mar;38(3):410–413. doi: 10.1136/gut.38.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer F., White E. Alcohol and nutrients in relation to colon cancer in middle-aged adults. Am J Epidemiol. 1993 Aug 15;138(4):225–236. doi: 10.1093/oxfordjournals.aje.a116851. [DOI] [PubMed] [Google Scholar]
- Miller J. W., Nadeau M. R., Smith J., Smith D., Selhub J. Folate-deficiency-induced homocysteinaemia in rats: disruption of S-adenosylmethionine's co-ordinate regulation of homocysteine metabolism. Biochem J. 1994 Mar 1;298(Pt 2):415–419. doi: 10.1042/bj2980415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauss K. M., Locniskar M., Newberne P. M. Effect of alterations in the quality and quantity of dietary fat on 1,2-dimethylhydrazine-induced colon tumorigenesis in rats. Cancer Res. 1983 Sep;43(9):4083–4090. [PubMed] [Google Scholar]
- Nauss K. M., Locniskar M., Pavlina T., Newberne P. M. Morphology and distribution of 1,2-dimethylhydrazine dihydrochloride-induced colon tumors and their relationship to gut-associated lymphoid tissue in the rat. J Natl Cancer Inst. 1984 Oct;73(4):915–924. [PubMed] [Google Scholar]
- Paspatis G. A., Kalafatis E., Oros L., Xourgias V., Koutsioumpa P., Karamanolis D. G. Folate status and adenomatous colonic polyps. A colonoscopically controlled study. Dis Colon Rectum. 1995 Jan;38(1):64–68. doi: 10.1007/BF02053860. [DOI] [PubMed] [Google Scholar]
- Reddy B. S., Watanabe K., Weisburger J. H. Effect of high-fat diet on colon carcinogenesis in F344 rats treated with 1,2-dimethylhydrazine, methylazoxymethanol acetate, or methylnitrosourea. Cancer Res. 1977 Nov;37(11):4156–4159. [PubMed] [Google Scholar]
- Reeves P. G., Nielsen F. H., Fahey G. C., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993 Nov;123(11):1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Riddell R. H., Goldman H., Ransohoff D. F., Appelman H. D., Fenoglio C. M., Haggitt R. C., Ahren C., Correa P., Hamilton S. R., Morson B. C. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983 Nov;14(11):931–968. doi: 10.1016/s0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- Rogers A. E., Herndon B. J., Newberne P. M. Induction by dimethylhydrazine of intestinal carcinoma in normal rats and rats fed high or low levels of vitamin A. Cancer Res. 1973 May;33(5):1003–1009. [PubMed] [Google Scholar]
- Rogers A. E., Nauss K. M. Rodent models for carcinoma of the colon. Dig Dis Sci. 1985 Dec;30(12 Suppl):87S–102S. doi: 10.1007/BF01296986. [DOI] [PubMed] [Google Scholar]
- Sedwick W. D., Kutler M., Brown O. E. Antifolate-induced misincorporation of deoxyuridine monophosphate into DNA: inhibition of high molecular weight DNA synthesis in human lymphoblastoid cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):917–921. doi: 10.1073/pnas.78.2.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selhub J., Miller J. W. The pathogenesis of homocysteinemia: interruption of the coordinate regulation by S-adenosylmethionine of the remethylation and transsulfuration of homocysteine. Am J Clin Nutr. 1992 Jan;55(1):131–138. doi: 10.1093/ajcn/55.1.131. [DOI] [PubMed] [Google Scholar]
- Stabler S. P., Marcell P. D., Podell E. R., Allen R. H., Savage D. G., Lindenbaum J. Elevation of total homocysteine in the serum of patients with cobalamin or folate deficiency detected by capillary gas chromatography-mass spectrometry. J Clin Invest. 1988 Feb;81(2):466–474. doi: 10.1172/JCI113343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueland P. M., Helland S., Broch O. J., Schanche J. S. Homocysteine in tissues of the mouse and rat. J Biol Chem. 1984 Feb 25;259(4):2360–2364. [PubMed] [Google Scholar]
- Varela-Moreiras G., Selhub J. Long-term folate deficiency alters folate content and distribution differentially in rat tissues. J Nutr. 1992 Apr;122(4):986–991. doi: 10.1093/jn/122.4.986. [DOI] [PubMed] [Google Scholar]
- Vargas P. A., Alberts D. S. Primary prevention of colorectal cancer through dietary modification. Cancer. 1992 Sep 1;70(5 Suppl):1229–1235. doi: 10.1002/1097-0142(19920901)70:3+<1229::aid-cncr2820701507>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Vester B., Rasmussen K. High performance liquid chromatography method for rapid and accurate determination of homocysteine in plasma and serum. Eur J Clin Chem Clin Biochem. 1991 Sep;29(9):549–554. doi: 10.1515/cclm.1991.29.9.549. [DOI] [PubMed] [Google Scholar]
- Walzem R. L., Clifford A. J. Folate deficiency in rats fed diets containing free amino acids or intact proteins. J Nutr. 1988 Sep;118(9):1089–1096. doi: 10.1093/jn/118.9.1089. [DOI] [PubMed] [Google Scholar]
- Wargovich M. J., Baer A. R., Hu P. J., Sumiyoshi H. Dietary factors and colorectal cancer. Gastroenterol Clin North Am. 1988 Dec;17(4):727–745. [PubMed] [Google Scholar]
- Weitzman S. A., Turk P. W., Milkowski D. H., Kozlowski K. Free radical adducts induce alterations in DNA cytosine methylation. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1261–1264. doi: 10.1073/pnas.91.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. D., Horne D. W. Use of glycerol-cryoprotected Lactobacillus casei for microbiological assay of folic acid. Clin Chem. 1982 May;28(5):1198–1200. [PubMed] [Google Scholar]


