Abstract
Clinical and preclinical data suggest that fluctuations in ovarian steroid hormones across the menstrual/estrous cycle influence spontaneous feeding behavior in females. The effects of gender, menstrual cycle phase, and ovarian hormone fluctuations on food-maintained responding under a progressive-ratio schedule were investigated in four female and three male cynomolgus monkeys. Females were studied across 21 menstrual cycles, and ovulatory cycles were defined by analysis of ovarian steroid hormone levels. Data were analyzed for the early and mid-follicular phase and the mid- and late-luteal phase of the menstrual cycle. Progressive-ratio break points for food were significantly higher in males than in females (p < 0.01). However, progressive-ratio break points did not vary consistently as a function of menstrual cycle phase during ovulatory cycles. There were no systematic patterns of progressive ratio break points in anovulatory menstrual cycles. Only one female monkey reached significantly higher break points during the mid- and late luteal phases in comparison to the mid-follicular phase of the menstrual cycle (p < 0.05). There was also a significant positive correlation between progressive-ratio break points and progesterone levels and a significant negative correlation with estradiol in that monkey. Although fluctuations in ovarian steroid hormones may influence food consumption under some conditions, consistent patterns of food-maintained responding were not detected during ovulatory menstrual cycles in cynomolgus monkeys.
Keywords: Menstrual cycle, ovarian steroid hormone, food-maintained responding, Progressive-ratio schedule, gender effects
Introduction
Fluctuations in ovarian steroid hormones across the menstrual cycle influence a variety of behaviors, including eating behavior, in women. Clinical studies report that appetite and eating behavior (Buffenstein et al., 1995; Dye and Blundell, 1997), energy intake (Dalvit, 1981; Lissner et al., 1988; Martini et al., 1994) and energy expenditure vary across the menstrual cycle (Solomon et al., 1982). Food or energy intake (kcal consumed per day) and energy expenditure (kcal per kg of body weight expended per day) usually are higher during the luteal phase, when estradiol levels are moderate and progesterone levels are high, than during the follicular phase, when both estradiol and progesterone levels are low (Dalvit, 1981; Lissner et al., 1988; Li et al., 1999; Pelkman et al., 2001). Eating disorders such as bulimia nervosa may also fluctuate in severity across the menstrual cycle. Bulimia nervosa is characterized by recurrent episodes of binge eating and subsequent purging that can include self-induced vomiting, misuse of laxatives, diuretics and/or enemas or other medications, fasting and/or excessive exercising (DSM-IV, 1997). In one study, binge eating was exacerbated during the late luteal phase of the menstrual cycle (Gladis and Walsh, 1987). Other bulimic women reported an increased frequency of binge eating during both the mid-luteal and premenstrual phases of the menstrual cycle compared to the follicular and ovulatory phases (Lester et al., 2003). Additional evidence that ovarian steroid hormones may influence food intake comes from studies in menopausal women. The onset of menopause induces changes in food intake, weight, fat distribution and metabolism (Rubinoff et al., 1995). Although the exact mechanism(s) underlying these changes are unknown, it is likely that the decrease in levels of ovarian steroid hormones, especially estradiol, may play an important role (Rubinoff et al., 1995; Ainslie et al., 2001).
Preclinical studies also report that spontaneous feeding behavior varies across phases of the menstrual cycle in nonhuman primates and the estrous cycle in rodents. For example, food intake was significantly higher in female rhesus monkeys during the luteal phase when progesterone levels were high, than during the early follicular phase when progesterone levels were low (Czaja, 1978; Rosenblatt et al., 1980). Food rejection occurred more frequently during the periovulatory phase of the menstrual cycle than during the early follicular or the mid-luteal phase (Czaja, 1978; Rosenblatt et al., 1980). In rodents, the estrus phase of the estrous cycle is characterized by declining levels of estradiol from peak levels in proestrus (Freeman, 1994). Food intake was lower during the estrus phase than at other phases of the estrous cycle (Tarttelin and Gorski, 1971; Eckel et al., 2000). Water intake also decreased significantly during estrus compared to other phases of the estrous cycle (Tarttelin and Gorski, 1971).
These several lines of evidence suggest that the ovarian steroid hormones estradiol and progesterone may influence spontaneous feeding behavior across the menstrual and estrous cycles in several species. Consistent with this hypothesis, food and water intake increased following surgical removal of the ovaries in female rats (Tarttelin and Gorski, 1971; Blaustein and Wade, 1976). When ovariectomized rats were treated with estradiol benzoate, food intake decreased (Blaustein and Wade, 1976). Estradiol treatment was also associated with a decrease in feeding behavior in ovariectomized female rhesus monkeys (Czaja, 1978). Although alterations in food intake that occur across the menstrual and estrous cycles, or through direct hormonal manipulation (e.g., ovariectomy, estradiol benzoate treatment) appear to reflect the effects of estradiol (Tarttelin and Gorski, 1971; Wade, 1975; Blaustein and Wade, 1976), progesterone can antagonize the effects of estradiol under some conditions (Wade, 1975). In ovariectomized female rats, estradiol-related suppression of feeding behavior was completely blocked by concurrent treatment with progesterone (Wade, 1975). However, progesterone administration did not reverse the reduction in feeding observed in estradiol-treated ovariectomized female rhesus monkeys (Czaja, 1978).
There have been relatively few studies of the influence of menstrual/estrous cycle phase or gender on operant response-contingent food acquisition. Female rhesus monkeys that self-administered low to moderate doses of alcohol showed a consistent decrease in food-maintained responding at mid-cycle (Mello et al., 1986). Gender comparisons in rodents have not shown consistent differences between males and females. Although male rats acquired operant responding on a Fixed Ratio 1 (FR1) schedule maintained by liquid food (Ensure) faster than females, once food-maintained responding was stable, there were no gender differences in responding for a range of liquid food concentrations on a Fixed Ratio 5 (FR5) schedule (Caine et al., 2004). Male and female rats also did not differ in food acquisition on a progressive-ratio schedule (van Hest et al., 1988).
To evaluate the contribution of spontaneous changes in ovarian steroid hormones to patterns of food intake, we examined the effects of menstrual cycle phase, defined by fluctuations in progesterone and estradiol, on food-maintained operant responding in female cynomolgus monkeys. Cynomolgus monkeys were studied, because their menstrual cycles are very similar to those of humans in length (approximately 28 days) and patterns of ovarian steroid hormone changes (Goodman and Hodgen, 1983). Male cynomolgus monkeys were studied under identical conditions to determine if there are gender differences in food-maintained operant responding over time. This approach differs from most previous preclinical studies in which spontaneous eating behavior was observed in animals given access to food that was not contingent upon an operant response (Tarttelin and Gorski, 1971; Wade, 1975; Blaustein and Wade, 1976; Czaja, 1978; Rosenblatt et al., 1980). In the present study, acquisition of a preferred food (banana-flavored pellets) was maintained on a progressive-ratio schedule. This schedule was selected because preclinical studies have indicated that progressive-ratio schedules provide a sensitive measure for detecting ovarian hormone effects and gender differences in drug-maintained responding (Roberts et al., 1989; Donny et al., 2000; Roth and Carroll, 2003). Under progressive-ratio schedules, the dependent measure is the break point, defined as highest ratio (in a series of increasing ratios) of responses reached to acquire a single reinforcer. The progressive-ratio break point also provides a measure of the motivation to obtain a reinforcer and the reinforcing strength of that particular substance (e.g., palatable food, drugs) (Richardson and Roberts, 1996; Stafford et al., 1998; Foltin and Evans, 2001; Negus and Mello, 2003).
Methods
Subjects
Subjects were four adult female (A5438, A5542, A7406 and A5040) and three adult male (A3514, AP4T and A6809) cynomolgus monkeys (Macaca fascicularis), and all were experimentally naïve at the beginning of the experiment. Female monkeys weighed between 2.46 (± 0.02) kg and 2.94 (± 0.03) kg (χ̄ = 2.63 ± 0.56), and the male monkeys weighed between 2.8 (± 0) kg and 3.5 (± 0) kg (χ̄ = 3.15 ± 0.35). There were no significant differences in body weight between males and females. The monkeys were not food deprived, and water was continuously available. Monkeys were fed a diet of fresh fruit, vegetables, and Monkey Lab Diet Jumbo biscuits (60.5 kcal/biscuit) (PMI Feeds, Inc., St. Louis, MO) after the operant session each day. The female monkeys received 2 biscuits per day, and the male monkeys received 3 biscuits per day. During operant sessions, monkeys had the opportunity to respond for a preferred food, banana-flavored pellets (1 g) (3.2 kcal/pellet) (P.J. Noyes, Co., Lancaster, NH), as described below. Monkeys were maintained on a 12 hr light/dark cycle (lights on at 7:00 a.m.). The monkeys were adapted to the laboratory environment and blood collection procedures (described below) for at least 3 months or until normal ovulatory menstrual cycles were observed. Environmental enrichment was provided by access to puzzle feeders, mirrors, chew toys, and music.
Each monkey’s health was monitored on a regular basis by the staff veterinarian. The veterinary observations and animal care procedures were documented and entered into each animal's health record. All of the procedures for the present experiment were conducted in accordance with the guidelines promulgated by the Committee on the Care and Use of Laboratory Animals, the National Research Council, and the U.S.D.A. The experiment was approved by the Institutional Animal Care and Use Committee (IACUC), constituted in accordance with National Institute of Health (NIH) regulations.
Menstrual Cycle Phase Monitoring
Phases of the menstrual cycle were verified by measuring levels of estradiol and progesterone, and successive cycles were defined by the onset of menstruation. Each female monkey was observed every day to determine the onset and duration of menstrual bleeding, and vaginal swabs were also used in some monkeys. Venous blood samples (2.0 ml) for analysis of progesterone and estradiol levels were collected from the femoral vein under light ketamine sedation (3–5 mg/kg) twice each week (Mondays and Thursdays). It is well established that even daily administration of ketamine does not disrupt the menstrual cycle in non-human primates (Channing et al., 1977).
Data Analysis by Menstrual Cycle Phase
Progressive-ratio break points were analyzed for 3 consecutive days at each of four phases of the menstrual cycle: 1) the early follicular phase, 2) the mid-follicular phase, 3) the mid-luteal phase, and 4) the late luteal phase. The early follicular phase was defined as days 3–5 after the onset of menses on cycle day 1. The mid-follicular phase was defined as days 8–10 after the onset of menses. The mid-luteal phase for each cycle was defined by the peak progesterone level. The late luteal phase was defined as the last 3 days of the cycle before the onset of the next menstruation. Of the 21 menstrual cycles studied, 14 cycles were ovulatory and 7 cycles were anovulatory. Mid-luteal progesterone levels were used to indicate the presence or absence of ovulation (Mello et al., 1997). Menstrual cycles were considered ovulatory if peak progesterone levels during the luteal phase were ≥ 6 ng/ml and/or peak estradiol levels during the peri-ovulatory phase were ≥ 200 pg/ml (Mello et al., 1997). In male monkeys, food-maintained responding was analyzed across successive months, and 1 month was equivalent to one menstrual cycle. Days 3–5, 8–10, 20–22, and 28–30 were selected for analysis to correspond to the hormonally defined phases of the menstrual cycle in females.
Plasma Hormone Analyses
Data are reported for the analysis of estradiol and progesterone in plasma. Plasma concentration of 17β-estradiol (E2) was determined in duplicate using a direct, double-antibody radioimmunoassay (RIA) (ICN Biomedicals, Inc., Costa Mesa, CA). The protocol was modified as follows: prior to analysis, the plasma samples were extracted, then reconstituted in zero standard. The assay sensitivity was 4.6 pg/ml and the intra- and interassay confidence intervals were 9.3% and 12.3%, respectively. Plasma progesterone was determined in duplicate using a direct, double-antibody RIA kit (ICN Biomedicals, Inc., Costa Mesa, CA). The assay sensitivity was 0.12 ng/ml and the intra- and interassay confidence intervals were 7.3% and 9.8%, respectively.
Apparatus
Each monkey was individually housed in a ventilated stainless steel chamber (64 x 64 x 79 cm) equipped with an operant panel (28 x 28 cm) mounted on the front wall. The panel consisted of 3 circular translucent response keys (6.4 cm in diameter) arranged in a horizontal row 2.54 cm apart and 3.2 cm from the top of the panel. When food pellets were available, the center response key was transilluminated by red stimulus lights (Superbright LEDs; Fairchild Semiconductor, San Jose, CA). Each chamber was equipped with a pellet dispenser (Model G5210; Gerbrands, Arlington, MA). Progressive-ratio schedule parameters were controlled with microprocessors and MED Associates software (Georgia, VT).
Operant Schedule Requirements
Monkeys were trained to respond for banana-flavored food pellets (1g) on a fixed-ratio (FR) schedule of reinforcement. Initially, subjects were trained on an FR 1 schedule, and a maximum of 20 food pellets was available during daily 2 hr sessions (11:00 a.m. to 1:00 p.m.). A 30 sec time out followed the delivery of each pellet, and during this time, the response key was dark and responding had no scheduled consequences. After monkeys obtained the maximum number of food pellets available for 3 consecutive days, the FR value was increased by increments of 2 responses. If a monkey obtained fewer than 10 reinforcers for 3 consecutive days, the FR value was decreased by 2 responses. This progression continued until monkeys obtained the maximum number of available reinforcers under an FR 20 schedule for 3 consecutive sessions.
After monkeys met this criterion for food-maintained responding, they were placed on a progressive-ratio schedule of reinforcement. The progressive-ratio schedule consisted of systematic increases in the response requirement necessary to obtain a single food-pellet. Each ratio increased by 0.05 log units and the progression of response requirements was as follows: 20, 22, 25, 28, 32, 36, 40, 45, 50, 56, 63, 71, 80, 89, 100, 112, 126, 142, 159, 178, 200, 224, 252, 282, 317, 356, 399, 448, 502, 563…..5632. We found that this progression was effective in maintaining food and cocaine self-administration in a previous study (Negus and Mello, 2003). The progressive-ratio schedule sessions were run 7 days a week starting at 11:00 a.m. Each session ended after a 1-h limited hold had elapsed since a food pellet was delivered. Food self-administration under the progressive-ratio schedule was studied over a 10-month period in females. After food-maintained responding under the progressive-ratio schedule was stable in male monkeys, data were collected for 5 months.
Data Analysis
Food-maintained responding was compared with non-parametric statistics for each of four menstrual cycle phases (i.e., early follicular, mid-follicular, mid-luteal, and late luteal) and between males and females. Data were averaged for 3 days at each cycle phase. The main dependent measure was the progressive-ratio break point, defined as the highest ratio of responses completed in a session (Negus and Mello, 2003). Progesterone and estradiol levels were also measured, and the relationship to daily progressive-ratio break points was examined. Friedman’s test was used to determine the effects of menstrual cycle phase on progressive-ratio break points for each female monkey, and for the males using days that corresponded sequentially to the days of the females’ phases. Dunn’s test was used for post hoc analyses. The Kruskal-Wallis test and the Mann Whitney U test with Dunn’s post-test were used to examine overall gender differences in progressive-ratio break points. Spearman correlations (r) were performed to determine if progesterone, or estradiol, were significantly correlated with progressive-ratio break points in monkeys.
Results
Ovarian Steroid Hormone Levels and Progressive-Ratio Break Points Across Successive Ovulatory Menstrual Cycles (Figures 1, 2 and 3)
Figure 1.
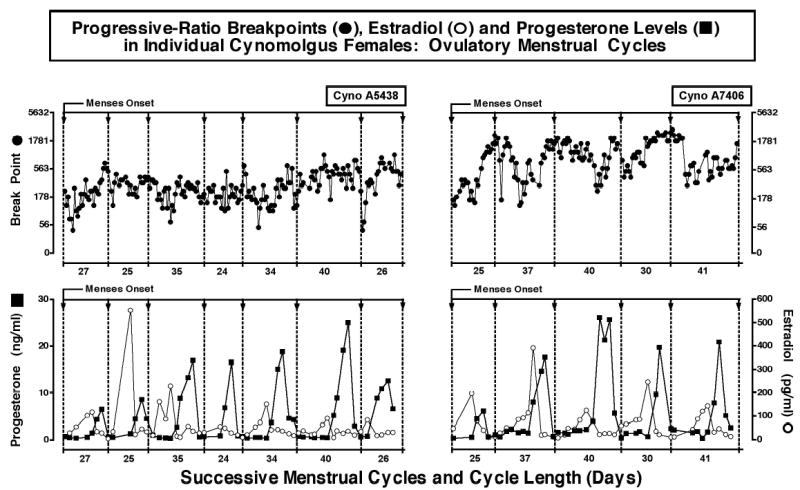
Progressive-ratio break points and plasma estradiol and progesterone levels across successive ovulatory menstrual cycles in two female monkeys. Data are shown for seven ovulatory menstrual cycles in monkey A5438 (left panel) and for five ovulatory menstrual cycles in monkey A7406 (right panel). Abscissae: Cycle length (days) across successive ovulatory menstrual cycles. Progressive-ratio break points for each day of the cycle are shown in the top panels (filled circles). Break points are shown as the final response ratio on the left ordinate. Estradiol levels (pg/ml) (open circles) and progesterone levels (ng/ml) (filled squares) for successive ovulatory menstrual cycles are shown in the bottom panels. The solid, downward pointing arrows along the top of each panel indicate the onset of menses, and the beginning of a new menstrual cycle. The progesterone and estradiol level data points in the bottom panels are temporally concordant with the progressive-ratio data point in the top panel.
Figure 2.
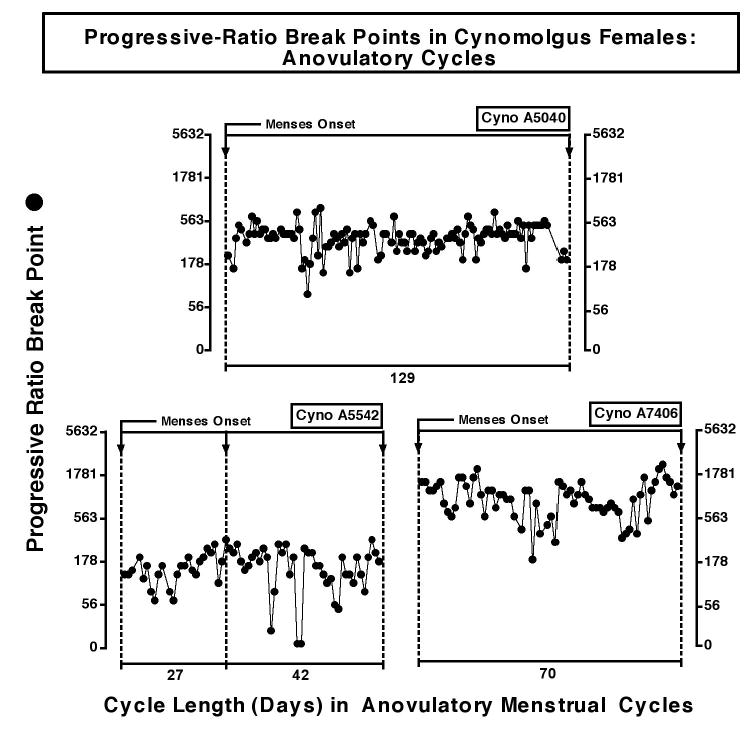
Progressive-ratio break points during anovulatory cycles in three female monkeys. One anovulatory cycle of 129 days is shown for monkey A5040; two anovulatory cycles of 27 and 42 days are shown for monkey A5542 and one anovulatory cycle of 70 days is shown for monkey A7406. Abscissae: Cycle length (days). Ordinates: Progressive-ratio break points for each day of the cycle are shown as the final response ratio. The solid, downward pointing arrows along the top of each panel indicate the onset of menses, and the beginning of a new menstrual cycle.
Figure 3.
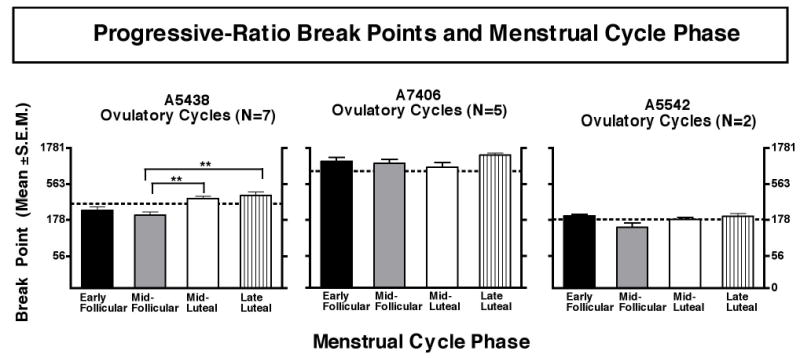
Progressive-ratio break points and menstrual cycle phase. Abscissae: Phase of the menstrual cycle. Ordinates: Progressive-ratio break points (mean ± S.E.M.) for each phase of the menstrual cycle. Each data point represents an average of 7 ovulatory cycles for A5438, 5 ovulatory cycles for A7406, and 2 ovulatory cycles for A5542. Statistical analysis (Friedman’s repeated measures ANOVA for non-parametric data with Dunn’s post tests) indicated a significant main effect of menstrual cycle phase on final ratio in female A5438 (Friedman statistic = 15.9, p < 0.01). The asterisks (*) indicate that the final ratios in the mid-luteal and late-luteal phases were significantly higher than in the mid-follicular phase (**p < 0.001). The dashed lines mark the average final ratio for each monkey.
Figure 1 shows the progressive-ratio break points and the corresponding progesterone and estradiol levels across successive ovulatory cycles for female monkeys. Menstrual cycles varied in length from 25 to 41 days. It is apparent that there was considerable variability in progressive-ratio break points within and between phases of the menstrual cycles. The peak in progesterone levels in the mid-luteal phase of each cycle indicates that ovulation had occurred at mid-cycle. Pre-ovulatory increases in estradiol were not always detected due to the sampling frequency. For comparison, progressive ratio break points during anovulatory cycles are shown in Figure 2. Again, response-contingent food intake under a progressive ratio schedule did not show any consistent patterns.
Figure 3 shows progressive-ratio break points as a function of menstrual cycle phase for three individual female monkeys during ovulatory menstrual cycles. The three females differed in progressive-ratio break points across the ovulatory menstrual cycles (p < 0.001). Average break points of 298 ± 18, 853 ± 85 and 181 ± 9 were measured in individual monkeys. There was a significant main effect of menstrual cycle phase on progressive-ratio break points in one female (A5438) (p < 0.01). Her break points were significantly higher during the mid- and late luteal phases than during the mid-follicular phase of the menstrual cycle ( p< 0.01). There was no significant main effect of menstrual cycle phase on progressive-ratio break points in the other two females. Data for anovulatory cycles in these monkeys are not shown. A fourth female had only anovulatory cycles, and her data are not included.
Gender and Progressive-Ratio Break Points (Figure 4)
Figure 4.
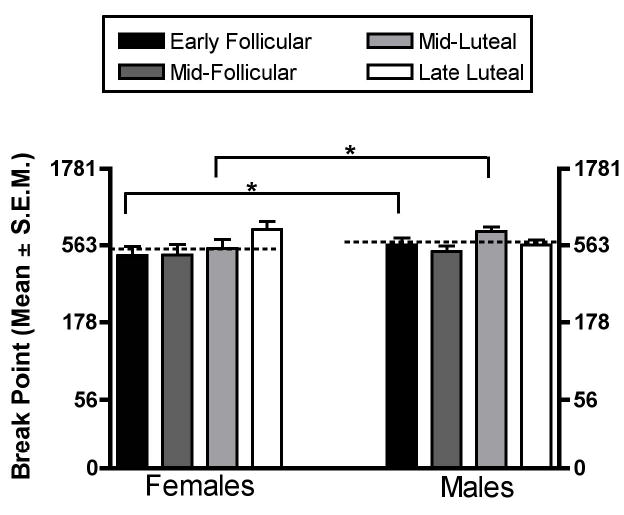
Progressive-ratio break points for female monkeys for each phase of the menstrual cycle and for males for the days that corresponded to the menstrual cycle phases in females. Progressive-ratio break points are shown as the final response ratio on the left ordinate. Each data point represents an average of 14 ovulatory menstrual cycles in females and 4 pseudo cycles in males. The medians were significantly different (p < 0.0001) according to Kruskal-Walllis test for unpaired groups. The asterisk (*) indicates that break points were significantly lower during the early-follicular and mid-luteal phase in females than in males (Dunn’s post tests, (p < 0.05)).
The progressive-ratio break points reached by male monkeys were significantly higher than break points reached by female monkeys (p < 0.01). Figure 4 also shows break points of males and females as a function of cycle phase. As noted earlier, data from males were analyzed for month-long pseudo-cycles, with days 3–5 corresponding to the early follicular phase, days 8–10 to the mid-follicular phase, days 19–20 to the mid-luteal phase and the final 3 days of the month to the late luteal phase. Progressive-ratio break points were significantly lower during the early-follicular phase and the mid-luteal phase in females than in males (p < 0.05).
Ovarian Hormone Levels and Progressive-Ratio Break Points (Figure 5)
Figure 5.
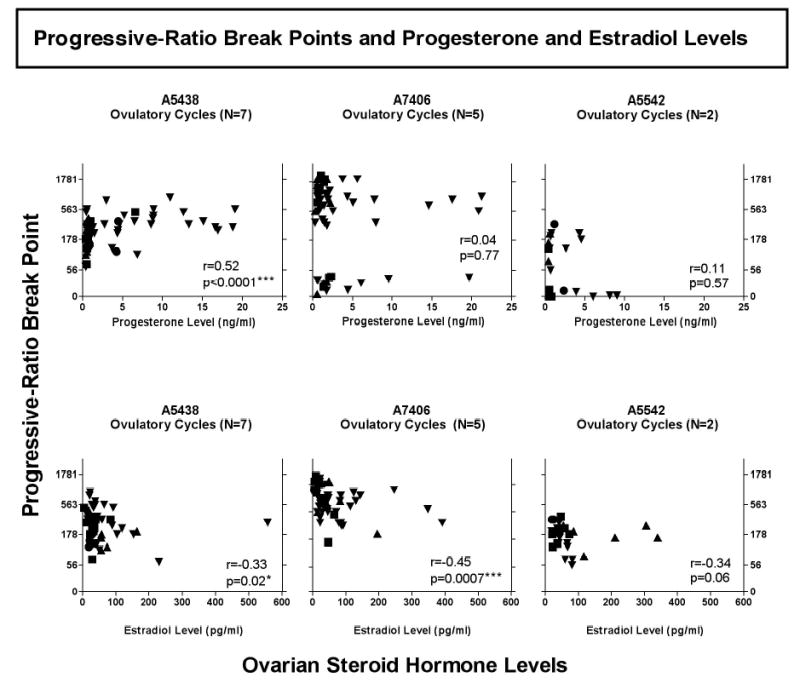
Correlations between progressive-ratio break points and plasma progesterone (ng/ml) and estradiol (pg/ml) levels during each phase of the menstrual cycle in females. Abscissae: Progesterone (top panel) and estradiol (bottom panel) levels. Progressive-ratio break points are shown as the final response ratio on the left ordinate. Each point represents the hormone level and the corresponding break point during the early follicular (▪), mid-follicular (▴), mid-luteal (▾), and late luteal phase (•) for each female monkey. The asterisks indicate that analysis with Spearman correlation showed a significant positive relationship between progesterone level (ng/ml) and break point in A5438 (Spearman r=0.52; p < 0.0001) and a significant negative relationship between estradiol level (pg/ml) and break point in A5438(r=0.33, P=0.02) and in A7406 (r=0.45, p=0.0007).
In Figure 5, the relationship between progesterone and estradiol levels and corresponding progressive-ratio break points for each phase of the menstrual cycle is shown for the three female monkeys that had ovulatory cycles. There was a significant positive relationship between progesterone level and break point in monkey A5438 (p < 0.0001), but not in the other females. In addition, a significant negative relationship between estradiol levels and break point was observed in monkeys A7406 (p = 0.0007 and A5438 (p = 0.02), but not in the third female.
Discussion
The present study examined the effects of gender and ovarian hormone fluctuations across phases of the menstrual cycle on food-maintained responding under a progressive-ratio schedule. Male and female cynomolgus monkeys were studied under identical conditions to determine if there were gender differences in food-maintained responding. Although males reached higher progressive-ratio break points for banana-flavored pellets than females, no consistent effects of menstrual cycle phase on food-maintained responding in females were detected. Only one of four female monkeys had higher progressive-ratio break points during the mid- and late luteal phase, consistent with previous clinical and preclinical studies. The relation of these findings to previous studies is discussed below.
Response-Contingent vs Spontaneous Food Acquisition
Menstrual Cycle Phase
In the present study, a progressive-ratio schedule was used to determine if a highly palatable food (i.e., banana-flavored pellet) is more reinforcing during certain phases of the menstrual cycle. This operant schedule has been useful for detecting gender and estrous cycle effects on drug-maintained responding in rodents (Roberts et al., 1989; Donny et al., 2000; Roth and Carroll, 2003). Moreover, progressive ratio schedules provide a measure of motivation for the reinforcer (Richardson and Roberts, 1996; Stafford et al., 1998; Negus and Mello, 2003). Most preclinical investigations of the influence of the menstrual/estrous cycle on feeding behavior have used spontaneous intake of standard laboratory chow as the dependent measure (Tarttelin and Gorski, 1971; Wade, 1975; Blaustein and Wade, 1976; Czaja, 1978; Rosenblatt et al., 1980). We are aware of only one other study that used operant response-contingent self-administration of a preferred food to examine the effects of menstrual cycle phase on food intake in female rhesus monkeys (Mello et al., 1986). In that study, female monkeys that self-administered low to moderate amounts of alcohol consistently self-administered less food at mid-cycle than during menstruation; however, this difference in food-maintained responding was significant only in the moderate alcohol dose group (Mello et al., 1986). The female monkeys used in the present study were drug naïve, so there was no effect of prior drug exposure on the menstrual cycle and/or on food-maintained responding.
The methods used to identify menstrual cycle phase in the present study also differed from previous studies of menstrual cycle phase and feeding behavior in female monkeys. In previous studies, the phase of the menstrual cycle usually was estimated by visual observation of the vaginal opening and perineal area for signs of menstruation and by counting days from the onset of menses (Czaja, 1975; Czaja and Goy, 1975). When plasma estradiol and progesterone levels were analyzed, this was done for only one, or at most two, complete menstrual cycles in female rhesus monkeys (Czaja, 1978; Rosenblatt et al., 1980). In the present study, progesterone and estradiol analyses were used to determine each phase of the menstrual cycle over several successive cycles in female cynomolgus monkeys. Moreover, samples were collected every four days to maximize the possibility that luteal phase progesterone increases would be detected. Progesterone elevations usually persist for 6 to 8 days (Mello and Mendelson, 2002).
Only menstrual cycles in which ovulation could be inferred from a mid-luteal phase increase in progesterone (≥ 6 ng/ml) were used in the final data analysis (Mello et al., 1997). Despite careful attention to classification of the 21 menstrual cycles as ovulatory or anovulatory, and hormonal verification of menstrual cycle phases, significant cycle phase-dependent changes in food-maintained responding were detected in only one female. Higher progressive-ratio break points during the luteal phase of the menstrual cycle are consistent with the pattern usually reported in studies of spontaneous feeding in rhesus monkeys (Czaja, 1978; Rosenblatt et al., 1980) and bulimic women (Gladis and Walsh, 1987; Price et al., 1987; Lester et al., 2003). The absence of cycle-dependent changes in progressive-ratio break points for food during ovulatory cycles in the present study suggests that operant measures of food-maintained responding may be less sensitive to modulation by menstrual cycle phase than observations of spontaneous feeding.
Other Methodological Considerations
The substantial differences between operant response-contingent food acquisition and the various methods used to measure spontaneous food intake limit meaningful comparisons. However, it was somewhat surprising that the combination of rigorous behavioral methods (progressive ratio) with hormonal verification of menstrual cycle phase did not result in detection of robust menstrual cycle phase effects on feeding behavior. Although different progressive-ratio parameters may have yielded a different pattern of responding, there is considerable evidence that break points maintained by food, as well as by cocaine, are relatively independent of the starting ratio or the step size in the ratio progression (Rowlett et al., 1996; Stafford and Branch, 1998; Negus and Mello, 2003). It is also unlikely that a simple fixed ratio schedule would have yielded different results. Systematic analyses of the effects of multiple doses of an anorectic drug, d-amphetamine, on the acquisition of preferred foods have shown that responding was disrupted on both progressive-ratio and fixed-ratio schedules (Foltin and Evans, 2001). Moreover, food-maintained responding on a progressive-ratio schedule was not disrupted by lower doses of d-amphetamine than responding maintained on a fixed-ratio schedule; rather, d-amphetamine produced dose-related decreases in food-maintained responding under both schedule conditions (Foltin and Evans, 2001). By analogy, multiple doses of estradiol and progesterone occur across an ovulatory menstrual cycle, and it is difficult to argue that a fixed ratio schedule would be more sensitive to hormonal effects than a progressive-ratio schedule.
In the present study, monkeys were not food deprived, and were fed after each daily operant session. The consistently high progressive ratio break points attained suggest that daily feeding did not decrease the reinforcing efficacy of a preferred food, banana-flavored pellets. Moreover, the range and stability of food-maintained progressive-ratio break points in cynomolgus males in the present study was comparable to that observed under saline treatment conditions in rhesus males in our previous study (Negus and Mello, 2003).
Gender comparisons of food-maintained responding
Relatively few studies have used operant procedures to examine possible gender differences in food-maintained responding. In one study, male and female rats did not differ in the number of food reinforcers acquired under progressive-ratio schedules (van Hest et al., 1988). In another study, male rats met the acquisition criterion for food-maintained responding faster than female rats; but there were no gender differences in operant responding for a range of palatable liquid food concentrations (Caine et al., 2004). We were also unable to detect gender differences in cocaine-maintained responding in rats trained to self-administer food before exposure to cocaine (Caine et al., 2004). We concluded that the behavioral history, the schedule of reinforcement (FR5) and the unit dose of cocaine were important determinants of our findings, and that gender differences in cocaine self-administration are not consistently observed across a wide range of conditions (Caine et al., 2004). The amount of previous training on simple fixed ratio and progressive-ratio schedules may have contributed to the stable food-maintained responding observed in these cynomolgus monkeys. Although males reached significantly higher progressive ratio break points than females, these effects were relatively small. The gender differences observed may have reflected the fact that males were larger than females, even though their weights did not differ significantly. It is unlikely that these differences in progressive ratio break points were related to gender differences in hormonal status. One limitation of the current study is that the effects of exogenous hormone administration and/or gonadectomy were not examined. As noted earlier, studies in female rodents reported that ovariectomy and ovarian hormone replacement reliably influenced spontaneous food intake (Tarttelin and Gorski, 1971; Wade, 1975; Blaustein and Wade, 1976; Eckel et al., 2000). Further research will be necessary to clarify the conditions under which ovarian steroid hormones may influence food-maintained behavior in non-human primates.
Acknowledgments
The authors would like to thank Kevin Costa, Peter Fivel, Maureen Kelly and Kyle Rheaume for their technical assistance. This research was supported in part by grants R01 DA14670, T32 DA07252, and K05 DA00101 from the National Institute on Drug Abuse, NIH.
References
- DSM-IV: Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Press; 1997.
- Ainslie DA, Morris MJ, Wittert G, Turnbull H, Proietto J, Thorburn AW. Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptide Y. Int J Obes Relat Metab Disord. 2001;25:1680–1688. doi: 10.1038/sj.ijo.0801806. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Wade GN. Ovarian influences on the meal patterns of female rats. Physiol Behav. 1976;17:201–208. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- Buffenstein R, Poppitt SD, McDevitt RM, Prentice AM. Food intake and the menstrual cycle: a retrospective analysis, with implications for appetite research. Physiol Behav. 1995;58:1067–1077. doi: 10.1016/0031-9384(95)02003-9. [DOI] [PubMed] [Google Scholar]
- Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, Mello NK. Effect of Gonadectomy and Gonadal Hormone Replacement on Cocaine Self-Administration in Female and Male Rats. Neuropsychopharmacology 2004:1–14. [DOI] [PubMed]
- Channing CP, Fowler S, Engel B, Vitek K. Failure of daily injections of ketamine HCl to adversely alter menstrual cycle length, blood estrogen and progesterone levels in the rhesus monkey. Proc. Soc. Exp. Biol. Med. 1977;155:615–619. doi: 10.3181/00379727-155-39862. [DOI] [PubMed] [Google Scholar]
- Czaja JA. Food rejection by female rhesus monkeys during the menstrual cycle and early pregnancy. Physiol Behav. 1975;14:579–587. doi: 10.1016/0031-9384(75)90185-7. [DOI] [PubMed] [Google Scholar]
- Czaja JA. Ovarian influences on primate food intake: assessment of progesterone actions. Physiol Behav. 1978;21:923–928. doi: 10.1016/0031-9384(78)90167-1. [DOI] [PubMed] [Google Scholar]
- Czaja JA, Goy RW. Ovarian hormones and food intake in female guinea pigs and rhesus monkeys. Horm Behav. 1975;6:329–349. doi: 10.1016/0018-506x(75)90003-3. [DOI] [PubMed] [Google Scholar]
- Dalvit SP. The effect of the menstrual cycle on patterns of food intake. Am J Clin Nutr. 1981;34:1811–1815. doi: 10.1093/ajcn/34.9.1811. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology (Berl) 2000;151:392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- Dye L, Blundell JE. Menstrual cycle and appetite control: implications for weight regulation. Hum Reprod. 1997;12:1142–1151. doi: 10.1093/humrep/12.6.1142. [DOI] [PubMed] [Google Scholar]
- Eckel LA, Houpt TA, Geary N. Spontaneous meal patterns in female rats with and without access to running wheels. Physiol Behav. 2000;70:397–405. doi: 10.1016/s0031-9384(00)00278-x. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Evans SM. The effects of d-amphetamine on responding for candy and fruit drink using a fixed ratio and a progressive ratio schedule of reinforcer delivery. Pharm. Biochem. Behav. 2001;69:125–131. doi: 10.1016/s0091-3057(01)00496-8. [DOI] [PubMed] [Google Scholar]
- Freeman ME. The neuroendocrine control of the ovarian cycle of the rat. In: Knobil E and Neill JD eds. The physiology of reproduction. New York: Raven Press, Ltd.; 1994. p 613–658.
- Gladis MM, Walsh BT. Premenstrual exacerbation of binge eating in bulimia. Am J Psychiatry. 1987;144:1592–1595. doi: 10.1176/ajp.144.12.1592. [DOI] [PubMed] [Google Scholar]
- Goodman AL, Hodgen GD. The ovarian triad of the primate menstrual cycle. Recent Prog Horm Res. 1983;39:1–73. doi: 10.1016/b978-0-12-571139-5.50005-7. [DOI] [PubMed] [Google Scholar]
- Lester NA, Keel PK, Lipson SF. Symptom fluctuation in bulimia nervosa: relation to menstrual-cycle phase and cortisol levels. Psychol Med. 2003;33:51–60. doi: 10.1017/s0033291702006815. [DOI] [PubMed] [Google Scholar]
- Li ET, Tsang LB, Lui SS. Menstrual cycle and voluntary food intake in young Chinese women. Appetite. 1999;33:109–118. doi: 10.1006/appe.1999.0235. [DOI] [PubMed] [Google Scholar]
- Lissner L, Stevens J, Levitsky DA, Rasmussen KM, Strupp BJ. Variation in energy intake during the menstrual cycle: implications for food-intake research. Am J Clin Nutr. 1988;48:956–962. doi: 10.1093/ajcn/48.4.956. [DOI] [PubMed] [Google Scholar]
- Martini MC, Lampe JW, Slavin JL, Kurzer MS. Effect of the menstrual cycle on energy and nutrient intake. Am J Clin Nutr. 1994;60:895–899. doi: 10.1093/ajcn/60.6.895. [DOI] [PubMed] [Google Scholar]
- Mello NK, Bree MP, Mendelson JH. Alcohol and food self-administration by female Macaque monkeys as a function of menstrual cycle phase. Physiol. & Behav. 1986;36:959–966. doi: 10.1016/0031-9384(86)90460-9. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Cocaine, hormones and behavior: clinical and preclinical studies. In: Pfaff DW, Arnold AP, Etgen AM, Pahrbach SE and Rubin RT eds. Hormones, Brain and Behavior. New York: Academic Press; 2002. p 665–745.
- Mello NK, Mendelson JH, Kelly M, Diaz-Migoyo N, Sholar JW. The effects of chronic cocaine self-administration on the menstrual cycle in rhesus monkeys. J Pharmacol Exp Ther. 1997;281:70–83. [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology (Berl) 2003;167:324–332. doi: 10.1007/s00213-003-1409-y. [DOI] [PubMed] [Google Scholar]
- Pelkman CL, Chow M, Heinbach RA, Rolls BJ. Short-term effects of a progestational contraceptive drug on food intake, resting energy expenditure, and body weight in young women. Am J Clin Nutr. 2001;73:19–26. doi: 10.1093/ajcn/73.1.19. [DOI] [PubMed] [Google Scholar]
- Price WA, Torem MS, DiMarzio LR. Premenstrual exacerbation of bulimia. Psychosomatics. 1987;28:378–379. doi: 10.1016/s0033-3182(87)72511-0. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Rosenblatt H, Dyrenfurth I, Ferin M, Vande Wiele RL. Food intake and the menstrual cycle in rhesus monkeys. Physiol. Behav. 1980;24:447–449. doi: 10.1016/0031-9384(80)90234-6. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology (Berl) 2003. [DOI] [PubMed]
- Rowlett JK, Massey BW, Kleven MS, Woolverton WI. Parametric analysis of cocaine self-administration under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology. 1996;125:361–370. doi: 10.1007/BF02246019. [DOI] [PubMed] [Google Scholar]
- Rubinoff BE, Wurtman J, Rojansky N, Adler D, Stein P, Schenker JG, Brzezinski A. Effects of hormone replacement therapy on weight, body composition, fat distribution, and food intake in early postmenopausal women: a prospective study. Fertil Steril. 1995;64:963–968. doi: 10.1016/s0015-0282(16)57910-2. [DOI] [PubMed] [Google Scholar]
- Solomon SJ, Kurzer MS, Calloway DH. Menstrual cycle and basal metabolic rate in women. Am J Clin Nutr. 1982;36:611–616. doi: 10.1093/ajcn/36.4.611. [DOI] [PubMed] [Google Scholar]
- Stafford D, Branch MN. Effects of step size and break-point criterion on progressive-ratio performance. J. Exp. Annal. Behav. 1998;70:123–128. doi: 10.1901/jeab.1998.70-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology (Berl) 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Tarttelin MF, Gorski RA. Variations in food and water intake in the normal and acyclic female rat. Physiol Behav. 1971;7:847–852. doi: 10.1016/0031-9384(71)90050-3. [DOI] [PubMed] [Google Scholar]
- van Hest A, van Haaren F, van de Poll NE. The behavior of male and female Wistar rats pressing a lever for food is not affected by sex differences in food motivation. Behav. Brain Res. 1988;27:215–221. doi: 10.1016/0166-4328(88)90118-0. [DOI] [PubMed] [Google Scholar]
- Wade GN. Some effects of ovarian hormones on food intake and body weight in female rats. J Comp Physiol Psychol. 1975;88:183–193. doi: 10.1037/h0076186. [DOI] [PubMed] [Google Scholar]


