Abstract
Persistence of horizontal cell (HC) light responses in extracellular solutions containing low Ca2+ plus divalent cations to block Ca2+ currents (ICa) has been attributed to Ca2+-independent neurotransmission. Using a retinal slice preparation to record both ICa and light responses, we demonstrate that persistence of HC responses in low [Ca2+]o can instead be explained by a paradoxical increase of Ca2+ influx into photoreceptor terminals arising from surface charge-mediated shifts in ICa activation. Consistent with this explanation, application of Zn2+ or Ni2+ caused a hyperpolarizing block of HC light responses that was relieved by lowering [Ca2+]o. The same concentrations of Zn2+ and Ni2+ reduced the amplitude of ICa at the rod dark potential and this reduction was relieved by a hyperpolarizing shift in voltage dependence induced by lowering [Ca2+]o. Block of ICa by Mg2+, which has weak surface charge effects, was not relieved by low [Ca2+]o. Recovery of HC responses in low [Ca2+]o was assisted by enhancement of rod light responses. To bypass light stimulation, OFF bipolar cells were stimulated by steps to −40 mV applied to presynaptic rods during simultaneous paired recordings. Consistent with surface charge theory, the post-synaptic current was inhibited by Zn2+ and this inhibition was relieved by lowering [Ca2+]o. Nominally divalent-free media produced inversion of HC light responses even though rod light responses remained hyperpolarizing; HC response inversion can be explained by surface charge-mediated shifts in ICa. In summary, HC light responses modifications induced by low divalent cation solutions can be explained by effects on photoreceptor light responses and membrane surface charge without necessitating Ca2+-independent neurotransmission. Furthermore, these results suggest that surface charge effects accompanying physiological changing divalent cation levels in the synaptic cleft may provide a means for modulating synaptic output from photoreceptors.
Keywords: retina, horizontal cell, Zn2+, Ni2+, Mg2+
Abbreviations: HC, horizontal cell; HEPES, N-2-hydroxyethylpiperazine-N′2-ethanesulfonic acid; ICa, Ca2+current; PSC, post-synaptic current; TPEN, N,N,N′, N′-tetrakis(2-pyridylmethyl)ethylenediamine
The possibility of Ca2+ independent exocytosis has been invoked to explain the observation that reducing [Ca2+]o while simultaneously applying divalent cations known for their Ca2+-channel blocking action did not successfully abolish transmission from photoreceptors to second order horizontal cells and, in fact, sometimes even caused an increase in transmitter release (Schwartz 1986, 1987; Umino and Watanabe, 1987). However, these earlier studies did not address the possibility that changes in the divalent ion composition of the extracellular medium might influence Ca2+ channel gating by their actions on negative surface charges present on the external face of the plasma membrane. In a series of subsequent investigations carried out in the turtle and salamander retina, Piccolino et al. (1996, 1999) provided evidence that by altering surface charge, lowering [Ca2+]o may in fact promote Ca2+ influx into the synaptic terminals of photoreceptor cells, even in the presence of divalent cations used to block Ca2+ currents (ICa). This finding provided an alternative explanation for the persistence of post-synaptic responses in the presence of low Ca2+ media (Schwartz, 1986, 1987; Umino and Watanabe, 1987) that did not require discarding the classical hypothesis of Ca2+-dependent exocytosis. Unlike most spiking neurons whose resting membrane potentials are below the threshold for ICa, the relatively depolarized resting potential of photoreceptors allows to remain ICa continuously active in darkness and thus particularly susceptible to surface charge-mediated shifts in activation (Piccolino et al., 1998).
A problem with the previous experiments used to establish a role for surface charge in effects of low Ca2+ media is that synaptic transmission and photoreceptor Ca2+ influx were evaluated in different preparations: synaptic transmission was investigated in retinal eyecups by recording the light responses of postsynaptic neurons (mainly horizontal cells, HCs) whereas Ca2+ influx in photoreceptors was investigated in isolated cell preparations. Although these different preparations produced qualitatively similar results consistent with surface charge theory, the ionic concentrations needed to produce similar effects in the two systems were quite different. For example, in isolated photoreceptor cells, perfusion with moderately low Ca2+ solutions caused a shift in the activation of ICa toward hyperpolarized potentials (as predicted by surface charge theory) leading to an increase of Ca2+ influx into photoreceptors at their physiological potential, but application of a nominally Ca2+ free solution produced a complete suppression of ICa (an effect likely due to the decreased gradient for Ca2+ influx; see Piccolino et al., 1999). On the other hand, in the eyecup preparation, use of a nominally Ca2+ free solution did not suppress the post-synaptic response but produced a depolarizing shift in HC membrane potential attributable to an increase in photoreceptor ICa at the resting membrane potential of this cell in darkness. HC light responses persisted in the eyecup preparation even after prolonged exposure to nominally zero Ca2+ solutions. Furthermore, concentrations of Zn2+, Ni2+ and Co2+ capable of inducing a hyperpolarizing block of HCs in the eyecup that could be relieved by low Ca2+ solutions caused a complete block of ICa in isolated photoreceptors. These discrepancies were attributed to the difficulty in equilibrating the ionic concentration of the extracellular spaces in the outer plexiform layer of the eyecup preparation with the concentration of the superfusate (Dmitriev et al., 1999).
A second factor that complicated interpretation of earlier experiments arises from the fact that lowering extracellular Ca2+ causes photoreceptors to depolarize and enhances their light responses (Bertrand et al., 1978; Cervetto et al., 1988; Piccolino et al., 1996, 1998). Thus, independent of their actions on synaptic transmission, effects of low Ca2+ solutions on presynaptic membrane potential and light response amplitude can also contribute to the persistence of HC light responses.
In view of these concerns, we undertook a more direct test of the hypothesis that effects of low Ca2+ media on post-synaptic responses at the first retinal synapse reflect changes in membrane surface charge. To address concentration discrepancies between isolated cell and eyecup preparations, we used a single preparation, the retinal slice preparation from tiger salamander, to compare effects of various divalent cation test solutions on rod photoreceptor ICa and on the resulting post-synaptic responses. To address potential complications introduced by presynaptic effects of low divalent cation solutions on membrane potential and light responses, we compared rod photoreceptor responses with post-synaptic responses of HCs and bipolar cells, often by simultaneously recording both the pre- and post-synaptic responses. In another experimental approach, we bypassed the need for light stimulation entirely by testing the effects of low divalent cation solutions on post-synaptic currents (PSCs) evoked in OFF bipolar cells by depolarizing steps applied to simultaneously recorded presynaptic rods. Our study compared effects of Zn2+ and Ni2+, which exert potent surface charge effects, with effects of Mg2+, which has weak surface charge effects (Blaustein and Goldman, 1968; Hille et al., 1975). Another reason for our interest in Zn2+ is that this ion is concentrated in photoreceptor terminals and may be co-released with glutamate in synaptic vesicles (Akagi et al., 2001; Wu et al., 1993; Rosenstein and Chappell, 2003). The results of the present study show that changes in HC responses induced by the low divalent test solutions can be explained by presynaptic effects on ICa and photoreceptor light response without invoking Ca2+-independent neurotransmission. Furthermore, the potent surface charge effects exerted by divalent cations are consistent with the suggestion that effects on membrane surface charge produced by physiological changes in divalent cation levels in the synaptic cleft may provide a means for modulating synaptic output from photoreceptors.
EXPERIMENTAL PROCEDURES
Retinal slice preparation
Aquatic tiger salamanders (Ambystoma tigrinum) 15–25 cm in length were handled according to protocols approved for the ethical use of animals by the Institutional Animal Care and Use Committee at the University of Nebraska Medical Center. Every effort was made to minimize the number of animals used and their suffering. After rapid decapitation, an eye was enucleated and the anterior segment with lens removed. The resulting eyecup was cut into thirds and a section was placed vitreal side down on a piece of filter paper (2×5 mm, Type AAWP, 0.8 μm pores; Millipore, Bedford, MA, USA). After the retina adhered to the filter paper, the retina was isolated under chilled amphibian superfusate. The retina and filter paper were cut into 125 μm slices using a razor blade (121–6; Ted Pella Inc., Redding, CA, USA) tissue chopper (Stoelting, Wood Dale, IL, USA). Retinal slices were rotated 90° to permit viewing of the retinal layers when placed under a water immersion objective (40×, 0.7 NA) and viewed on an upright fixed stage microscope (Olympus BHWI, Tokyo, Japan). In experiments on light responses, dissection was performed under infrared illumination using GenIII image intensifiers (Nitemate NAV3; Litton Industries, Tempe, AZ, USA) mounted on the microscope and slices were viewed for recording using a CCD camera (Watec 502H; Rock House Products, Middletown, NY, USA).
Solutions were applied by a single-pass, gravity-feed perfusion system, which delivered medium to the slice chamber at a rate of approximately 1 ml/min. The normal amphibian superfusate that bathed the slices contained (in mM): 111 NaCl, 2.5 KCl, 2 CaCl2, 0.5 MgCl2, 10 HEPES, 5 glucose (pH 7.8). During photoreceptor recordings of ICa, the superfusate was switched to a solution containing (in mM): 95 NaCl, 2.5 KCl, 2 CaCl2, 0.5 MgCl2, 10 HEPES, 5 glucose, 10 tetraethylammonium chloride, 5 CsCl (pH 7.8). The osmolarity was measured with a vapor pressure osmometer (Wescor, Logan, UT, USA) and adjusted, if necessary, to 242±5 mOsm. Solutions were continuously bubbled with 100% O2. Divalent ion test solutions were prepared by reducing Ca2+ and Mg2+ or adding Zn2+, Ni2+, or additional Mg2+ to the low divalent or control superfusate solutions.
Electrophysiology
Patch pipettes were pulled on a PP-830 vertical puller (Narishige USA, East Meadow, NY, USA) from borosilicate glass pipettes (1.2 mm O.D., 0.9 mm I.D., with internal filament; World Precision Instruments, Sarasota, FL, USA) and had tips of approximately 1 μm O.D. with tip resistances of 10–15 MΩ. For light response recordings, pipettes were filled with a solution containing (in mM): 54 KCl, 61.5 KCH3SO4 (Pfaltz and Bauer, Waterbury, CT, USA), 3.5 NaCH3SO4, 10 HEPES (pH 7.2). For ICa recordings, pipettes were filled with a solution containing (in mM): 54 CsCl, 61.5 CsCH3SO3, 3.5 NaCH3SO4, 10 HEPES (pH 7.2). The osmolarity of both solutions was adjusted, if necessary, to 242±5 mOsm. To maintain endogenous second messenger signaling pathways, most recordings were obtained using the perforated patch method of whole cell recording with gramicidin (Kyrozis and Reichling, 1995). Gramicidin was mixed in ethanol at a concentration of 5 mg/ml, vortexed briefly, and then added to the pipette electrolyte solution to achieve a final concentration of 5 μg/ml. To increase the yield of cells, some paired pre- and post-synaptic recordings were done using ruptured patch techniques with a pipette solution containing (in mM): 48 Cs gluconate, 42 CsCl, 9.4 TEACl, 1.9 mM MgCl2, 9.4 mM MgATP, 0.5 mM GTP, 0.5 mM EGTA, 32.9 mM HEPES (pH 7.2). Acceptable access resistances for voltage clamp recordings were ≤40 MΩ. Responses were acquired with a Multiclamp amplifier (Axon Instruments Inc., Union City, CA, USA) using PClamp 8 software (Axon Instruments).
Rods were identified visually by their characteristic outer segments. Bipolar and HC cell bodies are in the distal half of the inner nuclear layer but HC cell bodies are typically more oblong in shape. Physiologically, HCs can be distinguished from bipolar cells by the presence of prominent inward-rectifying voltage-dependent currents in the former and prominent outward-rectifying currents in the latter. ON and OFF bipolar cells can be distinguished from one another by their inward and outward light-evoked currents, respectively. Bipolar cells were voltage clamped at −50 mV, HCs at −40 mV and rods at −70 mV. Charging curves of rods and bipolar cells were fit by single exponentials indicating that they form single electrotonic compartments. HC light responses were recorded in current clamp.
Light stimuli
Light stimuli were generated using a 50 W halogen lamp focused onto a fiberoptic and then reflected through a beam splitter into the condenser path of the microscope. Wratten gel neutral density filters were used to decrease light intensity of the white light stimulus.
RESULTS
A note on surface charge theory
To facilitate interpretation of our experiments, we begin with a brief discussion of the mechanisms of surface charge mediated effects of divalent cations on excitable membrane and why photoreceptor synapses are particularly susceptible to these effects. Surface charge theory, formulated by Gouy (1910) and Chapman (1913) to account for electrostatic interactions on a planar surface separating fixed and mobile charges, has been profitably applied to biological membranes. In this theory, divalent cations screen and bind the negative charges present on the extracellular surface of the cell membrane thereby altering the electric field across the membrane which induces shifts in the activation curves of voltage gated channels (see Hille et al., 1975; McLaughlin et al., 1971; Green and Andersen, 1991; and Piccolino et al., 1998 for more detailed explanations). Lowering the extracellular concentration of divalent cations reduces surface charge screening, which in turn reduces the potential drop within the membrane bilayer and thus shifts the activation of voltage-dependent channels to more negative potentials. Conversely, raising the extracellular concentration of divalent cations increases surface charge screening and thereby shifts the activation of voltage-dependent channels to more positive potentials. Cations differ in their interfering action on surface charge with ions like Zn2+, Ni2+ or Co2+ being particularly effective while other ions such as Cd2+, Mn2+ or Mg2+ have relatively modest action.
The effects of divalent cations on membrane surface charge are particularly evident at the synapse between photoreceptors and second order cells because of the tonic nature of this synapse. Photoreceptors continuously release glutamate in darkness when their membrane is relatively depolarized (−35 to −45 mV; Dowling and Ripps, 1973; Cervetto and Piccolino, 1974; Kaneko and Shimazaki, 1975). However, only a small fraction of pre-synaptic Ca2+ channels are open at the dark resting potential because the Ca2+ current (ICa) begin to activate only at potentials more depolarized than −50 mV (Bader et al., 1982; Corey et al., 1984; Lasater and Witkovsky, 1991; Piccolino et al., 1996; Thoreson et al., 2003). When photoreceptors hyperpolarize as a consequence of light absorption, the fraction of open channels decreases. Since the window of superposition between the physiological range of photoreceptor potentials and ICa activation is relatively narrow, even a small shift in the activation curve for ICa (e.g. due to a change in divalent cation concentration), can have large effects on glutamate release. A modest rightward shift, induced by an increase in divalent cation concentrations, can cause complete closure of Ca2+ channels in darkness, while a decrease in divalent cation concentrations (including Ca2+) can cause a left-ward shift in ICa leading to a robust increase in the number of open Ca2+ channels.
Effect of divalent cations on rod ICa
Zn2+ exerts potent surface charge effects (Blaustein and Goldman, 1968; Hille et al., 1975) and is concentrated in photoreceptor terminals (Qian et al., 1997; Wu et al., 1993; Akagi et al., 2001). Fig. 1A shows the current/voltage (I–V) profile of ICa measured in a rod using a ramp voltage protocol (0.5 mV/ms, −90 to +60 mV) under control conditions with 2 mM Ca2+ and 0.5 mM Mg2+ (black trace). The I–V curve was fit with a Boltzmann function corrected for driving force (dashed green line) and the V50 found to be −26.6 mV. Addition of 5 μM Zn2+ (Fig. 1A, red trace) for 2 min produced a rightward shift of the I–V curve (V50=−18.4 mV) and a slight reduction in the peak amplitude of ICa. Reducing the concentration of Ca2+ to. 0.5 mM and Mg2+ to 0 mM in the presence of Zn2+ caused a leftward shift (V50=−28.0 mV) and further reduction in the peak amplitude of ICa (Fig. 1A, blue trace). We typically began perfusion with the low Ca2+ solution before Zn2+ had attained its full steady state effect since continued application of Zn2+ (or other blocking cations) and the low Ca2+ solution for more than 4 min blocked ICa completely. As discussed later, this block of ICa was also accompanied by a block of post-synaptic responses. Mg2+ has very weak surface charge effects relative to Ca2+ so the left shift observed after lowering both Ca2+ and Mg2+ levels is almost completely due to the effects of lowering Ca2+ on surface charge (Blaustein and Goldman, 1968; Hille et al., 1975; Hagiwara and Byerly, 1981). Partial recovery of ICa was obtained after returning to control medium. Complete recovery of ICa amplitude from effects of low divalent solutions required extremely long washout times. After recovery, ICa often showed a voltage dependence that was more positive than control. Similar effects were observed in all 20 rods tested with this solution.
Fig. 1.

Effects of divalent cations on ICa. Control traces (black traces) were fit with a Boltzmann function adjusted for driving force (green dashed line). Red traces show ICa recorded 1–2 min after applying Zn (5 μM, A), Ni2+ (5 μM, B), or Mg2+ (4 mM, C). Blue traces show ICa recorded 1–2 min after beginning superfusion with low Ca2+ media (0.5 mM Ca2+, 0 mM Mg2+) in the continued presence of Zn2+ (A), Ni2+ (B) or Mg2+ (C).
Like Zn2+, Ni2+ also exerts potent surface charge effects (Blaustein and Goldman, 1968; Hille et al., 1975). Accordingly, addition of Ni2+ (5 μM; red trace) to the perfusion solution (Fig. 1B) caused a depolarizing shift in V50 from −29.1 to −20.1 mV with no change in the peak amplitude of ICa. Reducing [Ca2+]o to 0.5 mM and Mg2+ to 0 mM in the presence of Ni2+ caused a left shift in the activation curve (V50=24.7 mV; blue trace) and reduced the peak amplitude of ICa by >50%. Similar results were obtained in seven experiments.
A quite different action was exerted on rod ICa by Mg2+ (N=9), a cation which has only a slight screening action on surface charges (Blaustein and Goldman, 1968; Hille et al., 1975; Hagiwara and Byerly, 1981). We had to increase Mg2+ to 4 mM from its normal concentration of 0.5 mM to obtain a detectable effect on ICa. Although this concentration was nearly 1000-fold higher than the concentration of Zn2+ used in Fig. 1A, 4 mM Mg2+ produced only a modest rightward shift in the I–V curve with the V50 shifting from −29.1 to −24.2 mV (Fig. 1C; red trace). The peak amplitude of ICa was also slightly reduced by this concentration of Mg2+. As the Ca2+ concentration was lowered from 2 to 0.5 mM, ICa continued its rightward activation shift (V50=−21.2 mV; blue trace), in contrast to the leftward shifts observed in the presence of Ni2+ and Zn2+. The continued rightward shift in this experiment may reflect slowly accumulating effects of the elevated Mg2+ concentration on surface charge. Lowering Ca2+ in the presence of 4 mM Mg2+ also caused a pronounced decrease in the amplitude of ICa. This is largely the result of reducing the number of Ca2+ ions available to flow through the channel but may also reflect an increasingly efficacious block of Ca2+ channels by Mg2+ as [Ca2+]o decreases (McDonald et al., 1994).
Fig. 4.

Effects of Mg2+ (4 mM) and low Ca2+ on light-evoked voltage responses in a rod and HC. Responses were evoked by a near saturating flash in control medium, after addition of Mg2+, 2 min after lowering Ca2+ to 0.5 mM in the continued presence of 4 mM Mg2+, and following return to control solution. The dark resting membrane potentials of both cells in control medium are indicated by dashed horizontal lines.
To predict the impact of these changes in ICa on synaptic transmission from photoreceptors, we examined the effects on ICa at −35 mV, near the dark resting potential (indicated by the dotted vertical lines in Fig. 1). In Fig. 1A, the rightward shift in ICa produced by adding Zn2+ diminished ICa at −35 mV which would be expected to diminish the release of glutamate. Although lowering [Ca2+]o to 0.5 mM reduced the peak amplitude of the current by approximately 50%, the left shift in activation enhanced the amplitude of ICa at −35 mV which should enhance synaptic output. The effects of Ni2+ illustrated in Fig. 1B show a similar picture. The rightward shift in ICa produced by addition of Ni2+ reduced ICa at −35 mV, but lowering Ca2+ and Mg2+ levels caused a leftward shift that, despite the reduction in peak amplitude, enhanced ICa at −35 mV. In contrast to effects with Zn2+ and Ni2+, lowering Ca2+ levels in the presence of 4 mM Mg2+ produced only a further decrease in ICa without a compensatory leftward shift (Fig. 1C). These results lead to the prediction that light responses in HCs should be inhibited by Zn2+, Ni2+ and, to a lesser extent, Mg2+. However, surface charge effects of lowering [Ca2+]o should restore light responses in the presence of Zn2+ and Ni2+ but not Mg2+.
Effect of divalent cations on rod and HC light responses
To test predictions of surface charge effects of Zn2+, Ni2+ and Mg2+ in control and low Ca2+ solutions, we recorded light responses from second order retinal neurons, primarily HCs. The far left top of Fig. 2 shows the light response of a HC in current clamp under control conditions. Application of Zn2+ (5 μM) caused the HC to hyperpolarize and greatly reduced its light response, consistent with the reduction in glutamate release from photoreceptors predicted from Fig. 1A. Lowering Ca2+ and Mg2+ levels in the presence of Zn2+ rapidly depolarized the cell and restored the light response (Fig. 2) as expected from a leftward shift in ICa (Fig. 1A). The HC membrane potential in this test solution often reached a level above the dark potential recorded in control superfusate. The recovery of the light response observed in low Ca2+ solution persisted for some minutes after which there was a reduction in the light response accompanied by membrane hyperpolarization (Fig. 2). Further perfusion with the low Ca2+ solution produced a complete block of HC light responses (not shown). This secondary block probably reflects continuing competitive block of ICa by Zn2+ since, as described above, there was also a large decrease in ICa when we perfused for >4 min with low Ca2+ and Zn2+ solutions. Similar effects of Zn2+ on HC responses were observed in 10 cells.
Fig. 2.

Effects of Zn2+ (5 μM) and low Ca2+ on light-evoked voltage responses in a simultaneously recorded HC (top row) and rod (bottom row). Responses were evoked by a near saturating flash in control medium (left column), after addition of Zn2+ (2nd column), 3 min after lowering Ca2+ to 0.5 mM and Mg2+ to 0 mM in the continued presence of Zn2+ (third column), and 6 min after lowering Ca2+ and Mg2+ in the presence of Zn2+ (right column). The dark resting membrane potentials of both cells in control medium are indicated by dashed horizontal lines.
In addition to actions on photoreceptor ICa, alterations in the divalent cation makeup of the superfusate can also influence photoreceptor light responses which would in turn affect HC light responses. To compare pre- and post-synaptic light responses directly, we obtained simultaneous paired recordings from both rods and HCs. Effects of Zn2+ on pre- and post-synaptic light responses during such a paired recording are illustrated in Fig. 2. As shown at the bottom of Fig. 2, the peak hyperpolarizing excursion of the rod light response was not greatly altered by application of Zn2+ although the dark resting membrane potential of the rod hyperpolarized slightly from −47.8 mV to −48.4 mV after application of Zn2+. This small membrane hyperpolarization would slightly reduce ICa and could thus contribute, along with effects of Zn2+ on surface charge, to the hyperpolarization of the HC membrane in darkness (Fig. 2, top left). Following application of the low Ca2+ solution the rod depolarized to −44.4 mV. This depolarization would increase ICa activation and should therefore also increase depolarization of the HC. Similar effects of Zn2+ on rod light responses were observed in four rods during paired recordings with HCs and three more independently recorded rods.
In addition to its presynaptic effects, Zn2+ can inhibit AMPA receptors on fish HCs (Zhang et al., 2002). However, AMPA-evoked currents were inhibited only 2% by 3 μM Zn2+ suggesting that the concentration of 5 μM Zn2+ used in the present experiments should produce little inhibition of AMPA receptors. Consistent with this conclusion, we found that inward currents evoked in HCs by l-glutamate (0.1 mM) bath applied in the presence of cyclothiazide (0.1 mM) to inhibit AMPA receptor desensitization were not reduced by application of 5 μM Zn2+ (n=4). Thus, the effects of Zn2+ on HC light responses in the present experiments appear to be largely due to its presynaptic actions.
We also tested the effects of Ni2+ on simultaneously recorded pre- and post-synaptic light responses. Like Zn2+, Ni2+ caused the HC to hyperpolarize and its light response to diminish (Fig. 3). Addition of a low Ca2+ solution in the presence of Ni2+ relieved this hyperpolarizing block. The amplitude of rod light responses did not change appreciably during the experiment, but Ni2+ hyperpolarized the rod slightly from −42.3 mV to −44 mV which would contribute to hyperpolarization of the HC. Following application of the low [Ca2+]o medium, the rod resting potential returned to near the dark potential recorded in control solution (−42.1 mV). This slight depolarization may contribute slightly to recovery of the HC light response produced by low Ca2+ solution but cannot fully account for the very large increase in the HC response relative to that in control conditions (n=3 in paired recordings; n=5 in single recorded rods).
Fig. 3.

Effects of Ni2+ (5 μM) and low Ca2+ on light-evoked voltage responses in a simultaneously recorded HC (top row) and rod (bottom row). Responses were evoked by a near saturating flash in control medium, after addition of Ni2+, and 2–3 min after lowering Ca2+ to 0.5 mM and Mg2+ to 0 mM in the continued presence of Ni2+. The dark resting membrane potentials of both cells in control medium are indicated by dashed horizontal lines.
In contrast to what was observed with Zn2+ and Ni2+, low Ca2+ solutions were poorly effective in relieving the HC light response block produced by application of media containing high Mg2+. As shown in Fig. 4, application of 4 mM Mg for 3 min hyperpolarized the HC and diminished its light response by more than half (n=4). The same solution hyperpolarized a rod (recorded in a separate experiment) but did not diminish the amplitude of its response. Lowering Ca2+ to 0.5 mM caused the rod to depolarize and its light response to increase significantly (n=5). Since was further diminished by this solution ICa (Fig. 1C), we conclude that the increase in rod light responses is largely responsible for the small increase in HC responses seen in the same condition. In the experiment illustrated in Fig. 4, the low Ca2+ solution was applied after the light response had diminished by 73% as a consequence of Mg2+ application. If Mg2+ application was continued for a longer time, complete block of the HC response was obtained (not shown). In contrast to results with Ni2+ or Zn2+, application of a low Ca2+ solution was unable to relieve the complete or nearly complete block of the HC response with Mg2+.
Divalent cation effects on post-synaptic currents (PSCs) in OFF bipolar cells
To prevent divalent cation effects on rod membrane potential and circumvent the need for light stimulation, we tested Zn2+ and low Ca2+ solutions on voltage-clamped rods while recording the PSCs in voltage-clamped OFF bipolar cells. PSCs were recorded from OFF bipolar cells because one can maintain better voltage clamp in this cell type than in HCs which are strongly coupled to one another. The rod was held at a potential of −70 mV, below the activation threshold for ICa. To mimic light offset, the rod was depolarized by a 100 ms step to −40 mV (Fig. 5). In control conditions, this stimulus evoked an inward PSC of 20 pA in the OFF bipolar cell. In the presence of Zn2+ (5 μM), the PSC was diminished to 7 pA. However, the PSC recovered to control levels after lowering Ca2+ to 0.25 mM and Mg2+ to 0 mM in the presence of Zn2+. Similar results were seen with five cell pairs. Although divalent cation effects on the dark potential and light responses of rods contribute to recovery of post-synaptic light responses, the finding of Fig. 5 that the PSC recovered fully after application of the low Ca2+ solution indicates that much of the recovery of the post-synaptic light response produced by application of low Ca2+ solution in the presence of Zn2+ is due to presynaptic effects independent of changes in rod membrane potential.
Fig. 5.
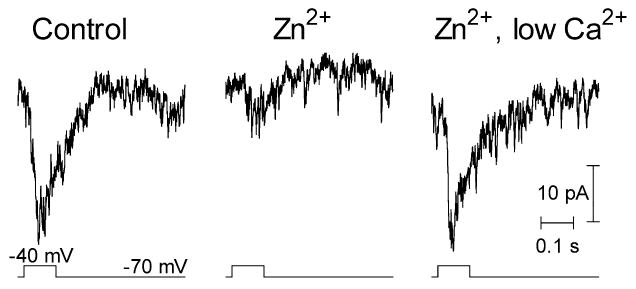
PSCs evoked in an OFF bipolar cell by moderate depolarizing voltage steps (−70 to −40 mV, 100 ms) applied to a presynaptic rod during paired whole cell recordings. Responses are shown in control medium, after addition of Zn2+ (5 μM), and after lowering Ca2+ to 0.5 mM and Mg2+ to 0 mM in the continued presence of Zn2+.
Inverted light responses
In agreement with previous studies in fish and turtle retina (Rowe, 1987; Normann et al., 1988; Piccolino et al., 1999), we found that reducing divalent cation levels can sometimes produce inverted (i.e. depolarizing) light responses in HCs of the salamander retinal slice. Fig. 6 shows an experiment performed while recording simultaneously from a synaptically coupled rod and HC. Reducing Ca2+ to 0.25 mM and Mg2+ to 0 mM caused a large depolarization of the HC and an increase in its light response. Ca2+ and Mg2+-free solution with EGTA (2 mM) was then applied for 3–4 min (not shown). After switching from this solution to one containing 0 Ca2+ and 0 Mg2+ but lacking EGTA, the same light stimulus now evoked a depolarizing, rather than hyperpolarizing, response in the HC. The rod membrane potential was substantially more depolarized and its light response had diminished in amplitude but nonetheless remained hyperpolarizing. Increasing the intensity of the light flash by 0.6 log units increased the hyperpolarizing amplitude of the rod response. However, in the HC, increasing the stimulus intensity did not simply increase the amplitude of the response but instead converted the depolarizing response observed at a lower intensity into a complex triphasic response. Similar results were obtained with four simultaneously recorded pairs of cells (three rod-HC pairs, one rod-OFF bipolar cell pair). Inversions were also observed in two more independently recorded HCs.
Fig. 6.
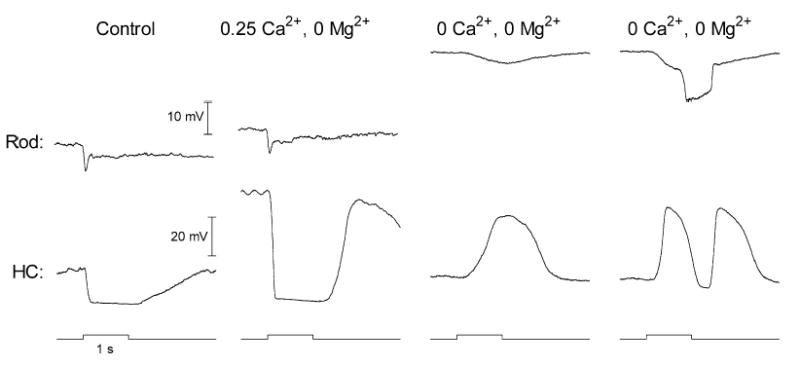
Inverted and triphasic light-evoked voltage responses obtained in an HC while simultaneously recording the light-evoked responses in a presynaptic rod. Responses evoked by a near saturating flash in control medium, in low Ca2+ medium (0.25 mM Ca2+, 0 mM Mg2+), and in nominally Ca2+-free medium (0 mM Ca2+, 0 mM Mg2+). The responses shown in the rightmost column were also obtained in the Ca2+-free medium but evoked with a light flash that was 0.6 log units brighter than the responses in the previous column. Before applying the nominally divalent cation-free medium, the retinal slice was perfused for 3–4 min with a Ca2+- and Mg2+-free medium containing EGTA (2 mM). Note that although lowering the divalent cation concentration can produce inverted or triphasic responses in the HC, rod responses remain purely hyperpolarizing.
The simultaneous paired recording in Fig. 6 shows that the inverted or triphasic responses of HCs are not due to inverted or triphasic responses in rods since rod responses remained purely hyperpolarizing throughout the experiment. Although the control responses of the rod in Fig. 6 appeared quite normal, lowering extracellular Ca2+ produced a smaller membrane depolarization and lesser change in the amplitude of the light response than was typically observed. As shown in Fig. 7A, lowering extracellular Ca2+ more often produced a strong membrane potential depolarization and a large increase in the amplitude of the light response (see also Bertrand et al., 1978; Cervetto et al., 1988; Piccolino et al., 1996, 1998).
Fig. 7.
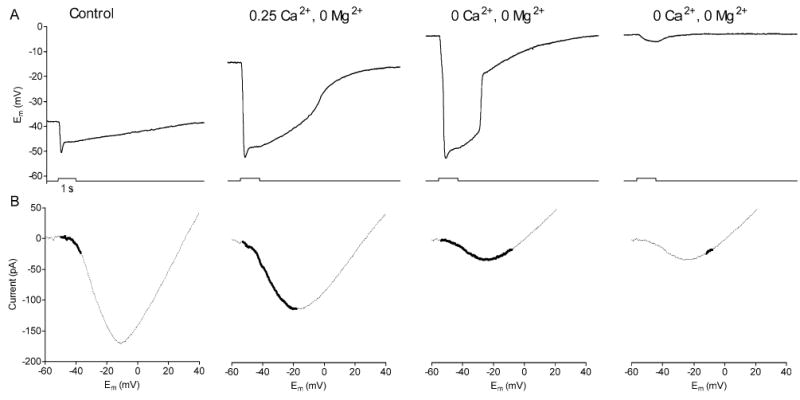
A comparison of the rod light response (A) and ICa (B) during superfusion with control medium, low Ca2+ medium (0.25 mM Ca2+, 0 mM Mg2+), and nominally divalent free solution (0 Ca2+, 0 Mg2+). The first three light responses in A were obtained using an identical white light stimulus while the fourth response was obtained after attenuating the flash by l.0 log unit. The thick lines superimposed on each I/V plot in B show the excursion of the rod membrane potential during the light response.
To explain the genesis of inverted post-synaptic responses, we compared the effects of low Ca2+ test solutions on the rod membrane potential (Fig. 7A), rod light response (Fig. 7A) and rod (Fig. 7B). The thick line segments ICa overlaid on in each panel of Fig. 7B denote the rod ICa potential excursions during light. The first three panels show rod responses obtained with an identical light stimuli while the fourth response was obtained using a light flash that was 1.0 log unit less intense. In control superfusate, the rod’s resting potential was −37 mV and the light flash caused the cell to hyperpolarize to −51 mV. Thus, in control conditions, the light stimulus fully closed the small fraction of Ca2+ channels that were active in darkness. This light-evoked reduction in ICa would reduce glutamate release leading to a light-evoked hyperpolarization in the HC. Application of the 0.25 mM Ca2+, 0 Mg2+ solution caused the dark resting potential of the rod to depolarize to −18 mV. This solution also caused a leftward shift in ICa (due to surface charge effects) accompanied by a decrease in its peak amplitude (due to a reduction in the concentration of the charge carrier). Despite a reduction in the peak amplitude of ICa, the leftward activation shift plus the membrane depolarization combined to greatly increase the number of open Ca2+ channels in darkness leading to a much larger post-synaptic light response (e.g. see Fig. 6).
Reducing Ca2+ to 0 mM depolarized the rod and shifted ICa further to the left so that its peak (approximately −25 mV) was now at a more hyperpolarized level than the rod’s dark potential (approximately −8 mV). This solution also further reduced peak amplitude of ICa. Because the peak of ICa is below the level of the resting potential, a dim light stimulus which evokes a weak hyperpolarizing response in the rod (from −8 to −12 mV; Fig. 7A, far right) causes an increase in ICa (Fig. 7B, far right). The resulting light-evoked increase in glutamate release can thus account for the purely depolarizing response evoked in the HC by the weaker light flash in Fig. 6. Application of a brighter light caused the rod in Fig. 7A to hyperpolarize from −8 to −53 mV. As with the dimmer light flash, the initial portion of this hyperpolarizing excursion would increase ICa leading to an initial increase in glutamate release and depolarization of the HC. However, with the brighter flash, the rod membrane potential continues below the peak of ICa resulting in a reduction in glutamate release which in turn leads to a hyperpolarizing response in the HC. As the rod membrane potential depolarizes at light offset, ICa first increases and then decreases as the membrane potential passes to the right of the peak of ICa, leading to the second depolarizing component in the HC response. Thus, by combining changes in rod membrane potential with shifts in the voltage dependence of rod ICa produced by low Ca2+ test solutions (Fig. 7), we can account for both the inverted and triphasic responses of post-synaptic neurons illustrated in Fig. 6.
To consistently observe inverted responses in solutions lacking Ca2+ and Mg2+, we found that it was necessary to apply Ca2+-free solutions containing EGTA for a short period. EGTA can chelate Zn2+ as well as Ca2+, but light response inversions were not observed after applying a selective chelator for Zn2+, TPEN (N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine) suggesting that it was the removal of Ca2+, not Zn2+, by EGTA that was critical for generating inverted responses. We hypothesize that use of EGTA depleted the reservoir of Ca2+ ions electrostatically bound to negative charges on the membrane surface (McLaughlin, 1989; Morris and Krnjevic, 1981; Piccolino and Pignatelli, 1996; Piccolino et al., 1999).
DISCUSSION
The central results of this study can be explained by the effects of divalent cations on membrane surface charge. Divalent cations such as Ca2+, Ni2+ and Zn2+ screen and bind negative charges present on the membrane surface producing a decrease in external surface negativity and thereby increasing the strength of the electric field within the membrane. Removal of divalent cations reduces the intramembrane electric field and thus allows ICa to activate at potentials which appear to be more hyperpolarized when measured with conventional techniques between the intracellular and extracellular bulk solutions. As a consequence, the activation threshold is lowered. Our results showed that Zn2+ and Ni2+ caused a rightward shift in ICa that reduced ICa amplitude at the dark resting membrane potential and inhibited post-synaptic light responses of HCs. Lowering Ca2+ levels in the presence of Zn2+ or Ni2+ caused a leftward shift in ICa that enhanced ICa at the dark potential and restored HC light responses. Unlike Zn2+ and Ni2+ which exert strong surface charge effects, Mg2+ has weak surface charge effects (Hagiwara and Byerly, 1981). Accordingly, a high concentration of Mg2+ was required to produce even a small shift in ICa activation. In the presence of 4 mM Mg2+, application of a low Ca2+ solution did not cause a leftward shift in ICa but rather a continued reduction in amplitude and further rightward shift. It seems likely that this was due to a continuing accumulation of Mg2+ at the membrane surface that continues to cause surface charge effects even as Ca2+ levels are diminishing. Unlike previous studies comparing results from turtle eyecup and isolated photoreceptors from salamander retina in which there was considerable disagreement in the concentrations of ions required to produce observable effects, the results of the present study show a good concordance between the effects of divalent cations on light responses of HCs and effects on ICa which substantiates the role of surface charge.
The observed enhancement of photoreceptor light responses in low Ca2+ solutions is consistent with previous studies (e.g. Bertrand et al., 1978; Cervetto et al., 1988; Piccolino et al., 1996, 1998). Lowering [Ca2+]o causes a decrease in [Ca]i through the action of the Na+/Ca2+ exchanger (Cervetto et al., 1988; Rispoli et al., 1996; McNaughton, 1991) and this reduction in [Ca2+]i promotes the opening of cGMP-gated cation channels in the outer segment (Koch and Stryer, 1998; Hsu and Molday, 1993; Ebrey and Koutalos, 2001). This sequence of events can account for the depolarization and increase in the amplitude of the light response observed after lowering extracellular Ca2+. The increase in the photoreceptor light responses can, in turn, explain how application of a low Ca2+ solution caused partial recovery of HC light responses in the presence of Mg2+ despite the absence of a leftward shift in ICa. The enhancement of rod light responses by low Ca2+ solution in experiments with Zn2+ and Ni2+ was also likely to have contributed to the recovery of HC responses with these ions. However, the enhancement of pre-synaptic responses in low Ca2+ solutions accounts for only a small part of the enhancement of post-synaptic light responses. For example, in experiments with Ni2+, application of low Ca2+ media increased the size of the rod response to near that observed in control medium but the size of the HC response was increased well beyond that observed in control. Furthermore, in experiments which bypassed the need for light stimulation, application of low Ca2+ solutions restored the PSC evoked by depolarizing steps to −40 mV applied to rods following application of Zn2+, indicating that much of the recovery of the post-synaptic response is the result of changes downstream from the rod light response.
We focused on recording the light responses from rods because dark-adapted HCs of the salamander retinal slice preparation are strongly rod-dominated (Thoreson et al., 2002). Cone inputs were likely to have contributed to the observed post-synaptic light responses but the effects of surface charge on ICa appear to be similar in cones and rods (Piccolino et al., 1996).
The present results show that effects of low divalent cation solutions on photoreceptor light responses and membrane surface charge are sufficient to account for observed modifications in HC light responses. Furthermore, Ca2+ influx appears necessary to maintain synaptic transmission since rod ICa and HC light responses were blocked completely when low Ca2+ solutions and/or divalent cations were perfused for long periods. These results suggest little or no role for Ca2+-independent neurotransmission at the photoreceptor synapse.
A clear understanding of the dramatic effects of divalent cations on ICa and synaptic transmission is not only important for resolving prior experimental results (Schwartz, 1986, 1987; Umino and Watanabe, 1987; Rowe, 1987; Normann et al., 1988) but also for understanding how physiological variations in divalent cation levels in the retina can regulate ICa. Extracellular Ca2+ in the retina can change significantly with adaptation level (Gallemore et al., 1994). Extracellular Ca2+ levels can be increased by extrusion of Ca2+ ions from photoreceptor synaptic terminals (Morgans et al., 1998) and decreased by influx through tonically active voltage-gated Ca2+ channels (Rabl and Thoreson, 2002). Zn2+ is colocalized with glutamate in synaptic vesicles in photoreceptor terminals (Qian et al., 1997; Wu et al., 1993; Akagi et al., 2001). The potent surface charge effects of Zn2+ described in the present study suggest that even a modest increase in [Zn2+]o as the result of release from photoreceptor terminals could modulate synaptic transmission. It has been suggested that Zn2+ levels can rise above 300 μM in glutamatergic synaptic clefts with intense activity in the hippocampus (Assaf and Chung, 1984). The precise concentration of zinc attained in the synaptic cleft of photoreceptors is unclear but it is probably much lower than this concentration since we found that 20 μM Zn2+ caused a complete block of HC responses and prolonged application of zinc at a concentration above 10 μM can be toxic to the rabbit retina (Ugarte and Osborne, 2001). Application of the Zn2+ chelator, TPEN (10 μM), did not affect rod ICa (not shown) but it is likely that prolonged superfusion of retinal slices will deplete them of Zn2+ by washing away endogenous Zn2+following its release into the synaptic cleft. Further experiments in isolated retinae or eyecup preparations might help to clarify this point.
Divalent cations exert a particularly strong influence on surface charge (Hille et al., 1975; Piccolino et al., 1998; Baldridge et al., 1998), but surface potential can also be affected by monovalent ions. For example, the voltage dependence of ICa is altered by changes in pH (Barnes and Bui, 1991; Hille, 2001; Barnes et al., 1993) which can fluctuate with the physiological state of the retina (Chesler and Kaila, 1992; Yamamoto et al., 1992). ICa activation can also be shifted by changes in the levels of physiological anions (e.g. phosphate; Thoreson and Stella, 2000). The modulation of ICa activation by changes in surface charge produced by physiological changes in the levels of divalent and monovalent ions may provide a means for the fine regulation of neurotransmitter release. Activation shifts in ICa have been proposed to be the basis for the negative feedback from HCs to cones evoked by illumination of the receptive field surround (Verweij et al., 1996). In cone feedback, the shift in ICa has been attributed to ephaptic feedback involving hemigap junctions in the HC membrane (Kamermans et al., 2001). But changes in the extracellular ionic environment, particularly divalent cation levels and pH can produce similar shifts in activation. Indeed, recent experiments support the possibility that the feedback action of HCs is mediated by local changes of pH in the synaptic cleft via a surface charge effect (Hirasawa and Kaneko, 2003).
The observation that many physiological ions can influence ICa voltage dependence by their effects on surface charge may also help to explain why there is such a strong misalignment between the rod dark potential and the peak of ICa. The rod resting potential is near the foot of the ICa activation curve and thus only a small portion of is ICa active in darkness. This low activation level helps to prevent an excessive influx of Ca2+ which could be extremely dangerous for the photoreceptor cell and could stimulate an increase in glutamate release that would saturate postsynaptic receptors. However, the same small Ca2+ influx could be obtained by using a much smaller number of Ca2+ channels but whose peak activation corresponded to the value of the dark membrane potential (Piccolino et al., 1998). But if this were the case, it would be much easier for changes in surface charge to shift the peak of ICa to potentials more negative than the resting potential, leading to an inversion of the postsynaptic response such as those illustrated in Fig. 6. This would have catastrophic consequences for vision if it were to occur under physiological conditions. By maintaining the membrane potential near the foot of the ICa activation curve, the possibility of such an occurrence becomes extremely rare. Consistent with this, we found that it was only possible to obtain inverted and triphasic responses under very particular experimental circumstances (e.g. during perfusion with solutions lacking Ca2+ and Mg2+ and only after we had previously applied EGTA).
In recent years the mechanisms involved in the release of synaptic transmitter have been the object of intense experimental study at both the biophysical and biochemical level. However, these studies have concentrated mostly on the intramembrane and intracellular processes involved in presynaptic function, with relatively little attention given to the extracellular environment. As suggested by the present results, ionic changes at the external face of the synaptic membrane produced by various physiological (or pathological) processes can have an important influence on synaptic transmission via surface charge effects (as well as other mechanisms). Surface charge modulation imparted by changes in the extracellular ionic environment might have particular significance for morphologically complex structures where different pre- and postsynaptic elements converge in close association within a limited space (as occurs at photoreceptor synapses in the retina). In these situations, transmitter release at a given terminal might change not only as consequence of ionic modifications arising from the activity of that particular terminal, but also as the result of the activity of nearby synapses and other physiological processes. Surface charge modulation may thus provide another means for the control and modulation of interneuronal communication.
Acknowledgments
This study was supported by NEI (EY-10542), Gifford Foundation, Lion’s Clubs, and Research to Prevent Blindness. The authors thank Eric Bryson for his technical assistance.
References
- Akagi T, Kaneda M, Ishii K, Hashikawa T. Differential subcellular localization of zinc in the rat retina. J Histochem Cytochem. 2001;49:87–96. doi: 10.1177/002215540104900109. [DOI] [PubMed] [Google Scholar]
- Assaf SY, Chung SH. Release of endogenous zinc from brain tissue during activity. Nature. 1984;308:734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- Bader CR, Bertrand D, Schwartz EA. Voltage-activated and calcium-activated currents studied in solitary rod inner segments from the salamander retina. J Physiol Lond. 1982;331:253–284. doi: 10.1113/jphysiol.1982.sp014372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldridge HW, Kurenny DE, Barnes S. Calcium-sensitive calcium influx in photoreceptor inner segments. J Neurophysiol. 1998;79:3012–3018. doi: 10.1152/jn.1998.79.6.3012. [DOI] [PubMed] [Google Scholar]
- Barnes S, Merchant V, Mahmud F. Modulation of transmission gain by protons at the photoreceptor output synapse. Proc Natl Acad Sci USA. 1993;90:10081–10085. doi: 10.1073/pnas.90.21.10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S, Bui Q. Modulation of calcium-activated chloride current via pH-induced changes of calcium channel properties in cone photoreceptors. J Neurosci. 1991;11:4015–4023. doi: 10.1523/JNEUROSCI.11-12-04015.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D, Fuortes MG, Pochobradsky J. Action of EGTA and high calcium on the cones in the turtle retina. J Physiol. 1978;275:419–437. doi: 10.1113/jphysiol.1978.sp012198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein MP, Goldman DE. The action of certain polyvalent cations on the voltage-clamped lobster axon. J Gen Physiol. 1968;51:279–291. doi: 10.1085/jgp.51.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L, Piccolino M. Synaptic transmission between photoreceptors and horizontal cells in the turtle retina. Science. 1974;183:417–419. doi: 10.1126/science.183.4123.417. [DOI] [PubMed] [Google Scholar]
- Cervetto L, Menini A, Rispoli G, Torre V. The modulation of the ionic selectivity of the light-sensitive current in isolated rods of the tiger salamander. J Physiol. 1988;406:181–198. doi: 10.1113/jphysiol.1988.sp017375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DL. A contribution to the theory of electrocapillarity. Philos Mag. 1913;25:475–481. [Google Scholar]
- Chesler M, Kaila K. Modulation of pH by neuronal activity. Trends Neurosci. 1992;15:396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- Corey DP, Dubinsky JM, Schwartz EA. The calcium current in inner segments of rods from the salamander (Ambystoma tigrinum) retina. J Physiol. 1984;354:557–575. doi: 10.1113/jphysiol.1984.sp015393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev A, Pignatelli A, Piccolino M. Resistance of retinal extracellular space to Ca++ level decrease: implications for the synaptic effects of divalent cations. J Neurophysiol. 1999;82:283–289. doi: 10.1152/jn.1999.82.1.283. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Ripps H. Effect of magnesium on horizontal cell activity in the skate retina. Nature. 1973;242:101–103. doi: 10.1038/242101a0. [DOI] [PubMed] [Google Scholar]
- Ebrey T, Koutalos Y. Vertebrate photoreceptors. Prog Retin Eye Res. 2001;20:49–94. doi: 10.1016/s1350-9462(00)00014-8. [DOI] [PubMed] [Google Scholar]
- Gallemore RP, Li JD, Govardovskii VI, Steinberg RH. Calcium gradients and light-evoked calcium changes outside rods in the intact cat retina. Vis Neurosci. 1994;11:753–761. doi: 10.1017/s0952523800003059. [DOI] [PubMed] [Google Scholar]
- Gouy M. Sur la constitution de la charge electrique a la surface d’un electrolyte. J Physiol Theor Appl (Paris) 1910;9:457–468. [Google Scholar]
- Green WN, Andersen OS. Surface charges and ion channel function. Annu Rev Physiol. 1991;53:341–359. doi: 10.1146/annurev.ph.53.030191.002013. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Byerly L. Calcium channels. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hille B (2001) Ionic channels of excitable membranes. Sunderland, MA: Sinauer Associates.
- Hille B, Woodhull AM, Shapiro BI. Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions, and pH. Philos Trans R Soc Lond B Biol Sci. 1975;270:301–318. doi: 10.1098/rstb.1975.0011. [DOI] [PubMed] [Google Scholar]
- Hirasawa H, Kaneko A. pH changes in the invaginating synaptic cleft mediate feedback from horizontal cells to cone photoreceptors by modulating Ca2+ channels. J Gen Physiol. 2003;122:657–671. doi: 10.1085/jgp.200308863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y, Molday RS. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature. 1993;361:76–79. doi: 10.1038/361076a0. [DOI] [PubMed] [Google Scholar]
- Kamermans M, Fahrenfort I, Schultz K, Janssen-Bienhold U, Sjoerdsma T, Weiler R. Hemichannel-mediated inhibition in the outer retina. Science. 2001;292:1178–1180. doi: 10.1126/science.1060101. [DOI] [PubMed] [Google Scholar]
- Kaneko A, Shimazaki H. Effect of external ions on the synaptic transmission from photoreceptor to horizontal cells in the carp retina. J Physiol. 1975;252:509–522. doi: 10.1113/jphysiol.1975.sp011155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KW, Stryer L. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature. 1998;334:64–66. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- Kyrozis A, Reichling DB. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. J Neurosci Methods. 1995;57:27–35. doi: 10.1016/0165-0270(94)00116-x. [DOI] [PubMed] [Google Scholar]
- Lasater EM, Witkovsky P. The calcium current of turtle cone photoreceptor axon terminals. Neurosci Res Suppl. 1991;15:S165–S173. [PubMed] [Google Scholar]
- McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. The electrostatic properties of membranes. Annu Rev Biophys Biophys Chem. 1989;18:113–136. doi: 10.1146/annurev.bb.18.060189.000553. [DOI] [PubMed] [Google Scholar]
- McLaughlin SG, Szabo G, Eisenman G. Divalent ions and the surface potential of charged phospholipid membranes. J Gen Physiol. 1971;58:667–687. doi: 10.1085/jgp.58.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton PA. Fundamental properties of the Na-Ca exchange: an overview. Ann NY Acad Sci. 1991;639:2–9. doi: 10.1111/j.1749-6632.1991.tb17284.x. [DOI] [PubMed] [Google Scholar]
- Morgans CW, El Far O, Berntson A, Wassle H, Taylor WR. Calcium extrusion from mammalian photoreceptor terminals. J Neurosci. 1998;18:2467–2474. doi: 10.1523/JNEUROSCI.18-07-02467.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris ME, Krnjevic K. Slow diffusion of Ca++ in the rat’s hippocampus. Can J Physiol Pharmacol. 1981;59:1022–1025. doi: 10.1139/y81-156. [DOI] [PubMed] [Google Scholar]
- Normann RA, Perlman I, Anderton PJ. Modulation of cone-to-horizontal-cell signal transmission in the turtle retina by magnesium ions. Brain Res. 1988;443:95–100. doi: 10.1016/0006-8993(88)91602-2. [DOI] [PubMed] [Google Scholar]
- Piccolino M, Byzov AL, Kurennyi DE, Pignatelli A, Sappia F, Wilkinson M, Barnes S. Low-calcium-induced enhancement of chemical synaptic transmission from photoreceptors to horizontal cells in the vertebrate retina. Proc Natl Acad Sci USA. 1996;93:2302–2306. doi: 10.1073/pnas.93.6.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolino M, Pignatelli A. Calcium-independent synaptic transmission: artifact or fact? Trends Neurosci. 1996;19:120–125. doi: 10.1016/s0166-2236(96)80017-8. [DOI] [PubMed] [Google Scholar]
- Piccolino M, Pignatelli A, Rakotobe LA. Calcium independent release of neurotransmitter in the retina: a “Copernican” viewpoint change. Prog Retin Eye Res. 1998;18:1–38. doi: 10.1016/s1350-9462(98)00015-9. [DOI] [PubMed] [Google Scholar]
- Piccolino M, Vellani V, Rakotobe LA, Pignatelli A, Barnes S, McNaughton P. Manipulation of synaptic sign and strength with divalent cations in the vertebrate retina: pushing the limits of tonic, chemical neurotransmission. Eur J Neurosci. 1999;11:4134–4138. doi: 10.1046/j.1460-9568.1999.00842.x. [DOI] [PubMed] [Google Scholar]
- Qian H, Li L, Chappell RL, Ripps H. GABA receptors of bipolar cells from he skate retina: actions of zinc on GABA-mediated membrane currents. J Neurophysiol. 1997;78:2402–2412. doi: 10.1152/jn.1997.78.5.2402. [DOI] [PubMed] [Google Scholar]
- Rabl K, Thoreson WB. Calcium-dependent inactivation and depletion of synaptic cleft calcium ions combine to regulate rod calcium currents under physiological conditions. Eur J Neurosci. 2002;16:2070–2077. doi: 10.1046/j.1460-9568.2002.02277.x. [DOI] [PubMed] [Google Scholar]
- Rispoli G, Navangione A, Vellani V. Turnover rate and number of Na+-Ca2+, K+ exchange sites in retinal photoreceptors. Ann NY Acad Sci. 1996;779:346–355. doi: 10.1111/j.1749-6632.1996.tb44806.x. [DOI] [PubMed] [Google Scholar]
- Rosenstein FJ, Chappell RL. Endogenous zinc as a retinal neuromodulator: evidence from the skate (Raja erinacea) Neurosci Lett. 2003;345:81–84. doi: 10.1016/s0304-3940(03)00472-5. [DOI] [PubMed] [Google Scholar]
- Rowe JS. Effects of external calcium on horizontal cells in the superfused goldfish retina. Neurosci Res Suppl. 1987;6:S147–163. doi: 10.1016/0921-8696(87)90014-4. [DOI] [PubMed] [Google Scholar]
- Schwartz EA. Synaptic transmission in amphibian retinae during conditions unfavourable for calcium entry into presynaptic terminals. J Physiol. 1986;376:411–428. doi: 10.1113/jphysiol.1986.sp016160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz EA. Depolarization without calcium can release gamma-aminobutyric acid from a retinal neuron. Science. 1987;238:350–355. doi: 10.1126/science.2443977. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Stella SL. Anion modulation of calcium current voltage dependence and amplitude in salamander rods. Biochim Biophys Acta. 2000;1464:142–150. doi: 10.1016/s0005-2736(99)00257-6. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Stella SL, Jr, Bryson EI, Clements J, Witkovsky P. D2-like dopamine receptors promote interactions between calcium and chloride channels that diminish rod synaptic transfer in the salamander retina. Vis Neurosci. 2002;19:235–247. doi: 10.1017/s0952523802192017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Tranchina D, Witkovsky P. Kinetics of synaptic transfer from rods and cones to horizontal cells in the salamander retina. Neuroscience. 2003;122:785–798. doi: 10.1016/j.neuroscience.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Ugarte M, Osborne NN. Zinc in the retina. Prog Neurobiol. 2001;64:219–249. doi: 10.1016/s0301-0082(00)00057-5. [DOI] [PubMed] [Google Scholar]
- Umino O, Watanabe K. Decline of blocking effect of cobalt ions on transmission from photoreceptors to horizontal cells during its prolonged application. Neurosci Lett. 1987;82:291–296. doi: 10.1016/0304-3940(87)90271-0. [DOI] [PubMed] [Google Scholar]
- Verweij J, Kamermans M, Spekreijse H. Horizontal cells feed back to cones by shifting the cone calcium-current activation range. Vision Res. 1996;36:3943–3953. doi: 10.1016/s0042-6989(96)00142-3. [DOI] [PubMed] [Google Scholar]
- Wu SM, Qiao X, Noebels JL, Yang XL. Localization and modulatory actions of zinc in vertebrate retina. Vision Res. 1993;33:2611–2626. doi: 10.1016/0042-6989(93)90219-m. [DOI] [PubMed] [Google Scholar]
- Yamamoto F, Borgula GA, Steinberg RH. Effects of light and darkness on pH outside rod photoreceptors in the cat retina. Exp Eye Res. 1992;54:685–697. doi: 10.1016/0014-4835(92)90023-l. [DOI] [PubMed] [Google Scholar]
- Zhang DQ, Ribelayga C, Mangel SC, McMahon DG. Suppression by zinc of AMPA receptor-mediated synaptic transmission in the retina. J Neurophysiol. 2002;88:1245–1251. doi: 10.1152/jn.2002.88.3.1245. [DOI] [PubMed] [Google Scholar]


