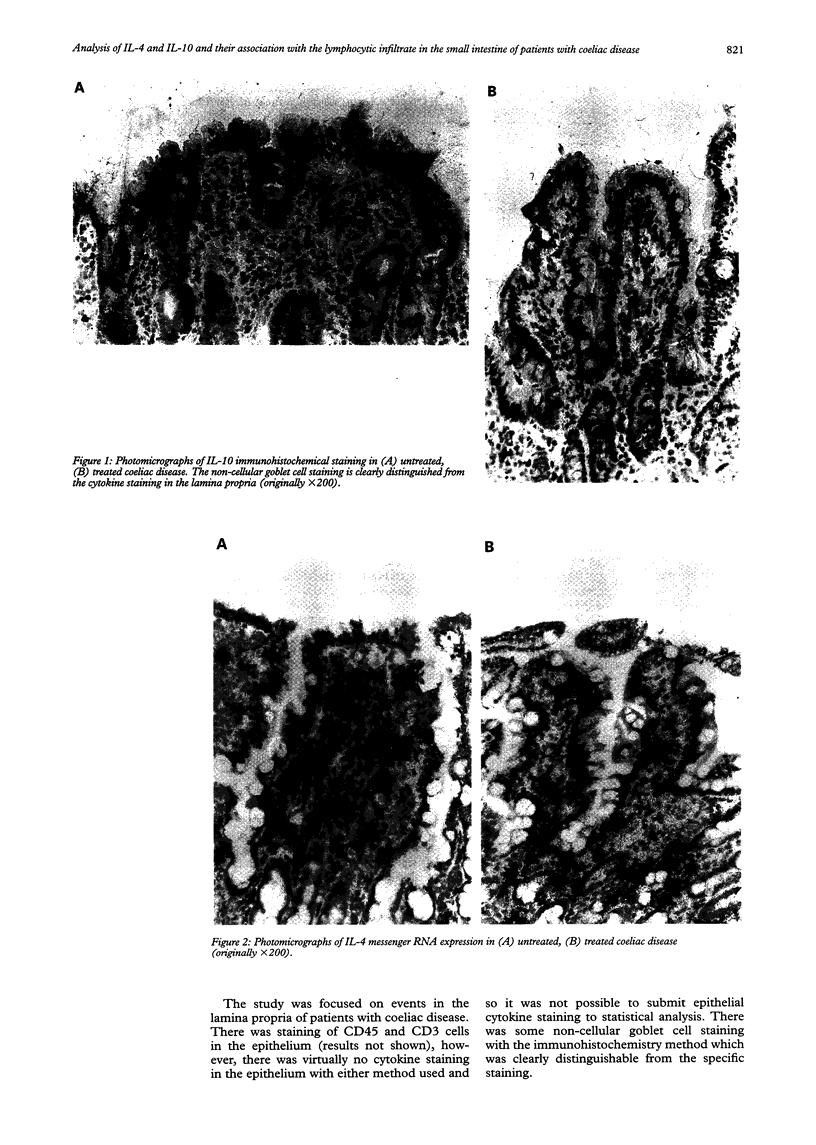
Abstract
BACKGROUND: Concentrations of pro-inflammatory cytokines are raised in the small intestine of patients with coeliac disease after ingestion of gluten but there are equivalent data on interleukin-4 (IL-4) and interleukin-10 (IL-10) producing cells. These cytokines are known to exert important regulatory effects on pro-inflammatory cytokine production from lymphocytes and macrophages. AIMS: To investigate whether there is a primary deficiency of IL-4 and IL-10 producing cells and their site of production in the small intestine of patients with coeliac disease in relation to the changes in inflammatory cell infiltrate. PATIENTS: Jejunal biopsy specimens from patients with coeliac disease (11 untreated, 10 treated) and nine disease controls were studied. METHODS: Immunohistochemical staining of sections for IL-4 and IL-10 cytokines and the cell phenotypic markers CD3 (T lymphocytes) and CD45 (total inflammatory cell infiltrate) was carried out using monoclonal antibodies. Expression of IL-4 and IL-10 messenger RNA was detected by in situ hybridisation with oligonucleotide probe cocktails for each cytokine. RESULTS: IL-4 and IL-10 mRNA and protein were detected in the lamina propria of treated and untreated coeliac patients and disease controls but not in the epithelium. A significant increase in the number of CD45 (p < 0.005) and CD3 (p < 0.05) positive cells was found in the lamina propria of patients with untreated coeliac disease compared with treated coeliac patients and disease controls but there were no differences in IL-4 or IL-10 between these groups with either method. CONCLUSIONS: There is no primary deficiency of IL-4 and IL-10 producing cells in the small intestine of patients with coeliac disease. Detectable concentrations of IL-4 and IL-10 were found in control patients which suggests that these cytokines are involved in normal mucosal immunoregulation. The increased number of T lymphocytes but not IL-4 or IL-10 producing cells in the lamina propria of patients with untreated than in those with treated disease suggests not only that the lamina propria is the major mucosal compartment for cytokine production but that newly recruited mucosal T lymphocytes are directed to a predominant Th1 and not a Th2 cytokine response in coeliac patients on a diet containing gluten.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Karttunnen R., Breese E. J., Walker-Smith J. A., MacDonald T. T. Decreased mucosal interleukin-4 (IL-4) production in gut inflammation. J Clin Pathol. 1994 Nov;47(11):1015–1018. doi: 10.1136/jcp.47.11.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso A. Th1 and Th2 subsets: paradigms lost? Immunol Today. 1995 Aug;16(8):374–379. doi: 10.1016/0167-5699(95)80004-2. [DOI] [PubMed] [Google Scholar]
- Kontakou M., Przemioslo R. T., Sturgess R. P., Limb A. G., Ciclitira P. J. Expression of tumour necrosis factor-alpha, interleukin-6, and interleukin-2 mRNA in the jejunum of patients with coeliac disease. Scand J Gastroenterol. 1995 May;30(5):456–463. doi: 10.3109/00365529509093307. [DOI] [PubMed] [Google Scholar]
- Kontakou M., Przemioslo R. T., Sturgess R. P., Limb G. A., Ellis H. J., Day P., Ciclitira P. J. Cytokine mRNA expression in the mucosa of treated coeliac patients after wheat peptide challenge. Gut. 1995 Jul;37(1):52–57. doi: 10.1136/gut.37.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontakou M., Sturgess R. P., Przemioslo R. T., Limb G. A., Nelufer J. M., Ciclitira P. J. Detection of interferon gamma mRNA in the mucosa of patients with coeliac disease by in situ hybridisation. Gut. 1994 Aug;35(8):1037–1041. doi: 10.1136/gut.35.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharzik T., Stoll R., Lügering N., Domschke W. Circulating antiinflammatory cytokine IL-10 in patients with inflammatory bowel disease (IBD). Clin Exp Immunol. 1995 Jun;100(3):452–456. doi: 10.1111/j.1365-2249.1995.tb03721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993 Oct 22;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Kühn R., Rajewsky K., Müller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991 Nov 1;254(5032):707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- Lundin K. E., Scott H., Hansen T., Paulsen G., Halstensen T. S., Fausa O., Thorsby E., Sollid L. M. Gliadin-specific, HLA-DQ(alpha 1*0501,beta 1*0201) restricted T cells isolated from the small intestinal mucosa of celiac disease patients. J Exp Med. 1993 Jul 1;178(1):187–196. doi: 10.1084/jem.178.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T., Spencer J. Evidence that activated mucosal T cells play a role in the pathogenesis of enteropathy in human small intestine. J Exp Med. 1988 Apr 1;167(4):1341–1349. doi: 10.1084/jem.167.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. W., O'Garra A., de Waal Malefyt R., Vieira P., Mosmann T. R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mowat A. M., Widmer M. B. A role for IL-4 in immunologically mediated enteropathy. Clin Exp Immunol. 1995 Jan;99(1):65–69. doi: 10.1111/j.1365-2249.1995.tb03473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. S., Madri J., Tite J., Carding S. R., Bottomly K. MHC control of CD4+ T cell subset activation. J Exp Med. 1989 Dec 1;170(6):2135–2140. doi: 10.1084/jem.170.6.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessner M., Volk B. A. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR). Clin Exp Immunol. 1995 Sep;101(3):428–435. doi: 10.1111/j.1365-2249.1995.tb03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen E. M., Gjertsen H. A., Jensen K., Brandtzaeg P., Lundin K. E. Gluten activation of peripheral blood T cells induces a Th0-like cytokine pattern in both coeliac patients and controls. Clin Exp Immunol. 1996 Feb;103(2):295–303. doi: 10.1046/j.1365-2249.1996.d01-611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen E. M., Lundin K. E., Krajci P., Scott H., Sollid L. M., Brandtzaeg P. Gluten specific, HLA-DQ restricted T cells from coeliac mucosa produce cytokines with Th1 or Th0 profile dominated by interferon gamma. Gut. 1995 Dec;37(6):766–776. doi: 10.1136/gut.37.6.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przemioslo R. T., Kontakou M., Nobili V., Ciclitira P. J. Raised pro-inflammatory cytokines interleukin 6 and tumour necrosis factor alpha in coeliac disease mucosa detected by immunohistochemistry. Gut. 1994 Oct;35(10):1398–1403. doi: 10.1136/gut.35.10.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przemioslo R. T., Lundin K. E., Sollid L. M., Nelufer J., Ciclitira P. J. Histological changes in small bowel mucosa induced by gliadin sensitive T lymphocytes can be blocked by anti-interferon gamma antibody. Gut. 1995 Jun;36(6):874–879. doi: 10.1136/gut.36.6.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revised criteria for diagnosis of coeliac disease. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Arch Dis Child. 1990 Aug;65(8):909–911. doi: 10.1136/adc.65.8.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S., Heinig T., Panzer U., Reinking R., Bouchard A., Stahl P. D., Raedler A. Impaired response of activated mononuclear phagocytes to interleukin 4 in inflammatory bowel disease. Gastroenterology. 1995 Jan;108(1):21–33. doi: 10.1016/0016-5085(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Schreiber S., Heinig T., Thiele H. G., Raedler A. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterology. 1995 May;108(5):1434–1444. doi: 10.1016/0016-5085(95)90692-4. [DOI] [PubMed] [Google Scholar]
- Soloway P., Fish S., Passmore H., Gefter M., Coffee R., Manser T. Regulation of the immune response to peptide antigens: differential induction of immediate-type hypersensitivity and T cell proliferation due to changes in either peptide structure or major histocompatibility complex haplotype. J Exp Med. 1991 Oct 1;174(4):847–858. doi: 10.1084/jem.174.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenson W. F. Interleukin-4 hyporesponsiveness in inflammatory bowel disease: immune defect or physiological response? Gastroenterology. 1995 Jan;108(1):284–286. doi: 10.1016/0016-5085(95)90034-9. [DOI] [PubMed] [Google Scholar]