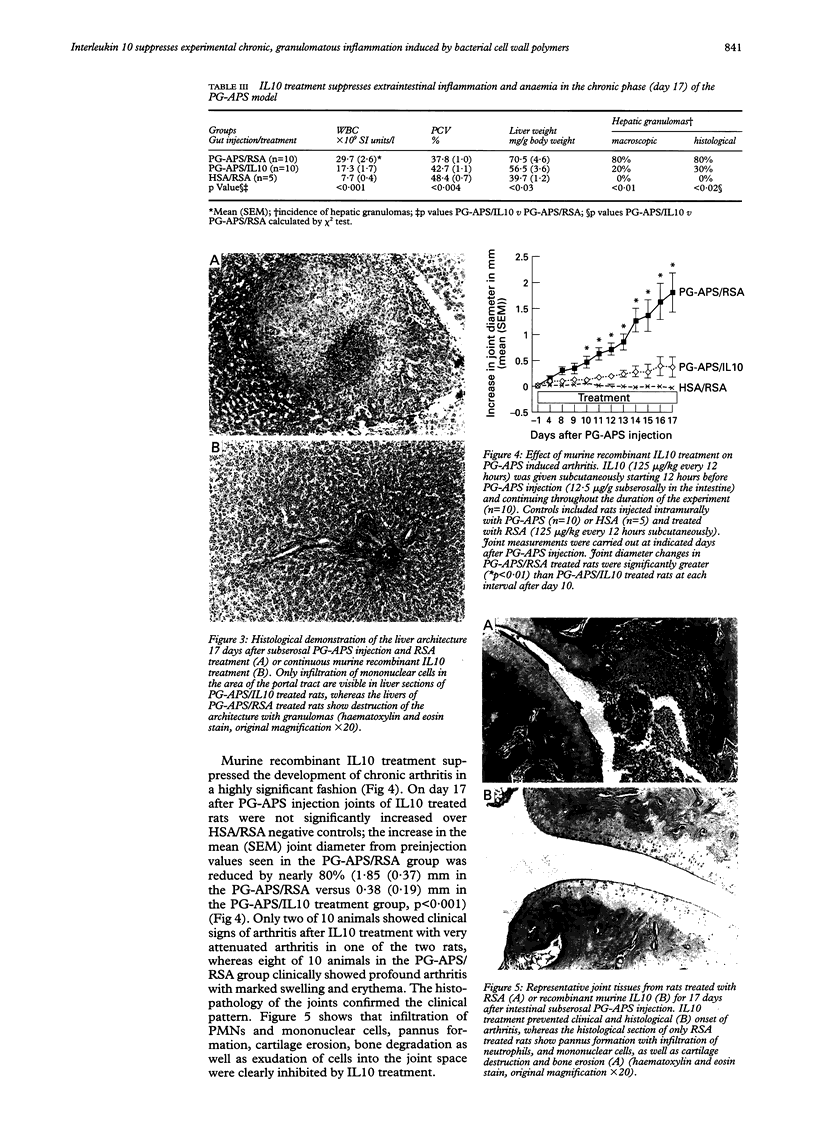
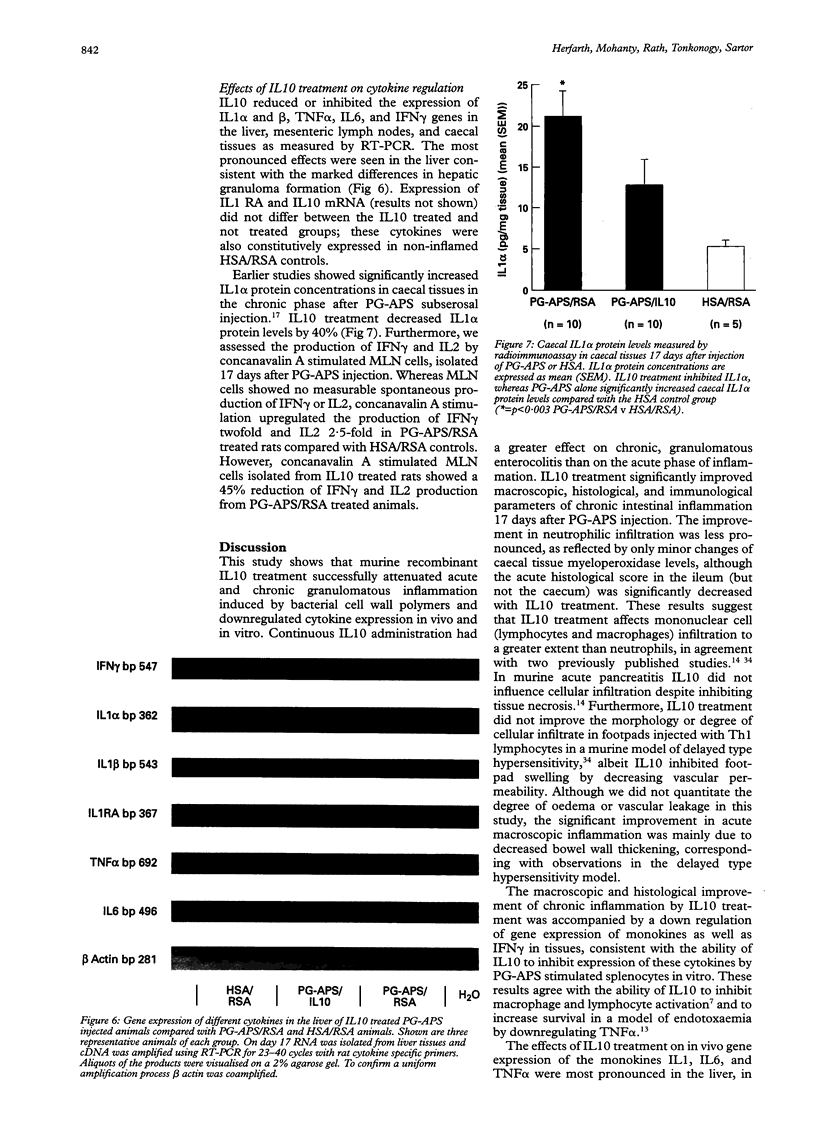
Abstract
BACKGROUND AND AIMS: Interleukin 10 (IL10) inhibits monocyte/macrophage and T lymphocyte effector functions. This study examined the effect of systemically administered IL10 on acute and chronic granulomatous enterocolitis, hepatitis, and arthritis in a rat model. METHODS: Lewis rats were injected intramurally with streptococcal peptidoglycan-polysaccharide (PG-APS) polymers. Beginning 12 hours before PG-APS injection, rats were treated daily with subcutaneous murine recombinant IL10 or vehicle for three or 17 days. RESULTS: IL10 attenuated acute enterocolitis in a dose dependent fashion (p < 0.01). Protective effects were more profound in the chronic granulomatous phase with decreased enterocolitis and markedly inhibited leucocytosis, hepatic granulomas, and chronic erosive arthritis (p < 0.001). IL10 downregulated tissue IL1, IL6, tumour necrosis factor alpha, and interferon gamma gene expression, consistent with the in vitro effects of IL10 on PG-APS-stimulated splenocytes. Caecal IL1 protein concentrations and IL2 and interferon gamma secretion by in vitro stimulated mesenteric lymph nodes were downregulated in IL10 treated animals. CONCLUSIONS: These results indicate that exogenous IL10 can inhibit experimental granulomatous inflammatory responses and suggest that IL10 treatment could be an effective new therapeutic approach in human disorders such as Crohn's disease, rheumatoid arthritis, and sarcoidosis.
Full text
PDF


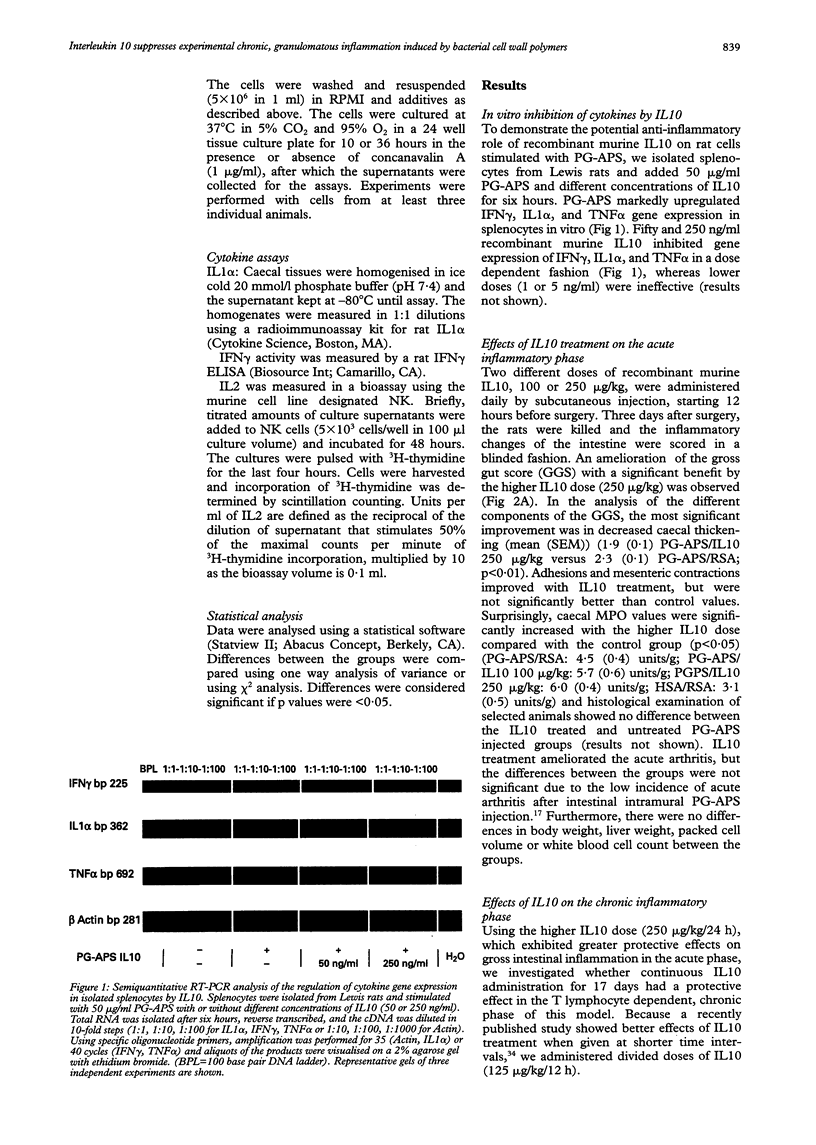
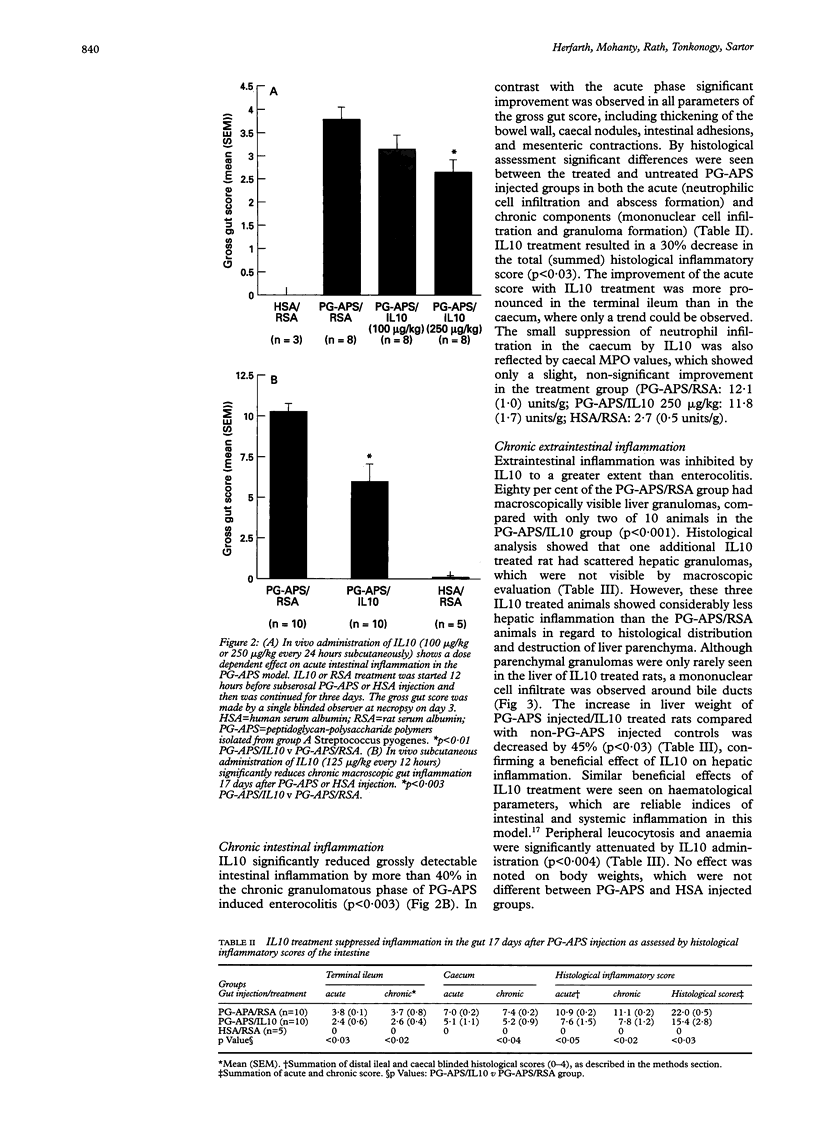



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. B., Malone D. G., Wahl S. M., Calandra G. B., Wilder R. L. Role of the thymus in streptococcal cell wall-induced arthritis and hepatic granuloma formation. Comparative studies of pathology and cell wall distribution in athymic and euthymic rats. J Clin Invest. 1985 Sep;76(3):1042–1056. doi: 10.1172/JCI112057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson M. A., Maclaren N. K. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med. 1994 Nov 24;331(21):1428–1436. doi: 10.1056/NEJM199411243312107. [DOI] [PubMed] [Google Scholar]
- Bogdan C., Nathan C. Modulation of macrophage function by transforming growth factor beta, interleukin-4, and interleukin-10. Ann N Y Acad Sci. 1993 Jun 23;685:713–739. doi: 10.1111/j.1749-6632.1993.tb35934.x. [DOI] [PubMed] [Google Scholar]
- Bogdan C., Paik J., Vodovotz Y., Nathan C. Contrasting mechanisms for suppression of macrophage cytokine release by transforming growth factor-beta and interleukin-10. J Biol Chem. 1992 Nov 15;267(32):23301–23308. [PubMed] [Google Scholar]
- Brandes M. E., Allen J. B., Ogawa Y., Wahl S. M. Transforming growth factor beta 1 suppresses acute and chronic arthritis in experimental animals. J Clin Invest. 1991 Mar;87(3):1108–1113. doi: 10.1172/JCI115073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassatella M. A., Meda L., Gasperini S., Calzetti F., Bonora S. Interleukin 10 (IL-10) upregulates IL-1 receptor antagonist production from lipopolysaccharide-stimulated human polymorphonuclear leukocytes by delaying mRNA degradation. J Exp Med. 1994 May 1;179(5):1695–1699. doi: 10.1084/jem.179.5.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassatella M. A., Meda L., Gasperini S., D'Andrea A., Ma X., Trinchieri G. Interleukin-12 production by human polymorphonuclear leukocytes. Eur J Immunol. 1995 Jan;25(1):1–5. doi: 10.1002/eji.1830250102. [DOI] [PubMed] [Google Scholar]
- Chernoff A. E., Granowitz E. V., Shapiro L., Vannier E., Lonnemann G., Angel J. B., Kennedy J. S., Rabson A. R., Wolff S. M., Dinarello C. A. A randomized, controlled trial of IL-10 in humans. Inhibition of inflammatory cytokine production and immune responses. J Immunol. 1995 May 15;154(10):5492–5499. [PubMed] [Google Scholar]
- Cromartie W. J., Craddock J. G., Schwab J. H., Anderle S. K., Yang C. H. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977 Dec 1;146(6):1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkema R., van der Meide P. H., Dubbeld M., Caspers M., Wubben J., Schellekens H. Cloning, expression, and purification of rat IFN-gamma. Methods Enzymol. 1986;119:453–464. doi: 10.1016/0076-6879(86)19065-3. [DOI] [PubMed] [Google Scholar]
- Ding L., Shevach E. M. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. J Immunol. 1992 May 15;148(10):3133–3139. [PubMed] [Google Scholar]
- Eisenberg S. P., Brewer M. T., Verderber E., Heimdal P., Brandhuber B. J., Thompson R. C. Interleukin 1 receptor antagonist is a member of the interleukin 1 gene family: evolution of a cytokine control mechanism. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5232–5236. doi: 10.1073/pnas.88.12.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Madden K. B., Cheever A. W., Katona I. M., Morris S. C., Gately M. K., Hubbard B. R., Gause W. C., Urban J. F., Jr Effects of interleukin 12 on immune responses and host protection in mice infected with intestinal nematode parasites. J Exp Med. 1994 May 1;179(5):1563–1572. doi: 10.1084/jem.179.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewe M., Gausling R., Gyufko K., Hoffmann R., Decker K. Regulation of the mRNA expression for tumor necrosis factor-alpha in rat liver macrophages. J Hepatol. 1994 Jun;20(6):811–818. doi: 10.1016/s0168-8278(05)80154-0. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Benoit J. N., Granger D. N. Assessment of leukocyte involvement during ischemia and reperfusion of intestine. Methods Enzymol. 1990;186:729–742. doi: 10.1016/0076-6879(90)86172-r. [DOI] [PubMed] [Google Scholar]
- Gérard C., Bruyns C., Marchant A., Abramowicz D., Vandenabeele P., Delvaux A., Fiers W., Goldman M., Velu T. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med. 1993 Feb 1;177(2):547–550. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. H., Ahern M. J., Smith M. D., Finlay-Jones J. J. Comparison of the suppressive effects of interleukin-10 and interleukin-4 on synovial fluid macrophages and blood monocytes from patients with inflammatory arthritis. Immunology. 1995 Apr;84(4):536–542. [PMC free article] [PubMed] [Google Scholar]
- Kasama T., Strieter R. M., Lukacs N. W., Lincoln P. M., Burdick M. D., Kunkel S. L. Interleukin-10 expression and chemokine regulation during the evolution of murine type II collagen-induced arthritis. J Clin Invest. 1995 Jun;95(6):2868–2876. doi: 10.1172/JCI117993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. K., Torrance D. S., Picha K. S., Mohler K. M. Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol. 1992 Oct 1;149(7):2496–2505. [PubMed] [Google Scholar]
- Kossmann T., Manthey C. L., Brandes M. E., Morganti-Kossmann M. C., Ohura K., Allen J. B., Mergenhagen S. E., Wahl S. M. Kupffer cells express type I TGF-beta receptors, migrate to TGF-beta and participate in streptococcal cell wall induced hepatic granuloma formation. Growth Factors. 1992;7(1):73–83. doi: 10.3109/08977199209023939. [DOI] [PubMed] [Google Scholar]
- Kwon J., Chung I. Y., Benveniste E. N. Cloning and sequence analysis of the rat tumor necrosis factor-encoding genes. Gene. 1993 Oct 15;132(2):227–236. doi: 10.1016/0378-1119(93)90200-m. [DOI] [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993 Oct 22;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Li L., Elliott J. F., Mosmann T. R. IL-10 inhibits cytokine production, vascular leakage, and swelling during T helper 1 cell-induced delayed-type hypersensitivity. J Immunol. 1994 Nov 1;153(9):3967–3978. [PubMed] [Google Scholar]
- Lichtman S. N., Wang J., Schwab J. H., Lemasters J. J. Comparison of peptidoglycan-polysaccharide and lipopolysaccharide stimulation of Kupffer cells to produce tumor necrosis factor and interleukin-1. Hepatology. 1994 Apr;19(4):1013–1022. [PubMed] [Google Scholar]
- Locksley R. M. Th2 cells: help for helminths. J Exp Med. 1994 May 1;179(5):1405–1407. doi: 10.1084/jem.179.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey C. L., Kossmann T., Allen J. B., Corcoran M. L., Brandes M. E., Wahl S. M. Role of Kupffer cells in developing streptococcal cell wall granulomas. Streptococcal cell wall induction of inflammatory cytokines and mediators. Am J Pathol. 1992 May;140(5):1205–1214. [PMC free article] [PubMed] [Google Scholar]
- McCall R. D., Haskill S., Zimmermann E. M., Lund P. K., Thompson R. C., Sartor R. B. Tissue interleukin 1 and interleukin-1 receptor antagonist expression in enterocolitis in resistant and susceptible rats. Gastroenterology. 1994 Apr;106(4):960–972. doi: 10.1016/0016-5085(94)90755-2. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R. Properties and functions of interleukin-10. Adv Immunol. 1994;56:1–26. [PubMed] [Google Scholar]
- Neurath M. F., Fuss I., Kelsall B. L., Stüber E., Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995 Nov 1;182(5):1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida T., Nishino N., Takano M., Sekiguchi Y., Kawai K., Mizuno K., Nakai S., Masui Y., Hirai Y. Molecular cloning and expression of rat interleukin-1 alpha cDNA. J Biochem. 1989 Mar;105(3):351–357. doi: 10.1093/oxfordjournals.jbchem.a122667. [DOI] [PubMed] [Google Scholar]
- Northemann W., Braciak T. A., Hattori M., Lee F., Fey G. H. Structure of the rat interleukin 6 gene and its expression in macrophage-derived cells. J Biol Chem. 1989 Sep 25;264(27):16072–16082. [PubMed] [Google Scholar]
- Nudel U., Zakut R., Shani M., Neuman S., Levy Z., Yaffe D. The nucleotide sequence of the rat cytoplasmic beta-actin gene. Nucleic Acids Res. 1983 Mar 25;11(6):1759–1771. doi: 10.1093/nar/11.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W. E. Pleiotropy and redundancy: T cell-derived lymphokines in the immune response. Cell. 1989 May 19;57(4):521–524. doi: 10.1016/0092-8674(89)90121-9. [DOI] [PubMed] [Google Scholar]
- Paul W. E., Seder R. A. Lymphocyte responses and cytokines. Cell. 1994 Jan 28;76(2):241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- Pennline K. J., Roque-Gaffney E., Monahan M. Recombinant human IL-10 prevents the onset of diabetes in the nonobese diabetic mouse. Clin Immunol Immunopathol. 1994 May;71(2):169–175. doi: 10.1006/clin.1994.1068. [DOI] [PubMed] [Google Scholar]
- Powrie F., Leach M. W., Mauze S., Menon S., Caddle L. B., Coffman R. L. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994 Oct;1(7):553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Powrie F., Menon S., Coffman R. L. Interleukin-4 and interleukin-10 synergize to inhibit cell-mediated immunity in vivo. Eur J Immunol. 1993 Sep;23(9):2223–2229. doi: 10.1002/eji.1830230926. [DOI] [PubMed] [Google Scholar]
- Rott O., Fleischer B., Cash E. Interleukin-10 prevents experimental allergic encephalomyelitis in rats. Eur J Immunol. 1994 Jun;24(6):1434–1440. doi: 10.1002/eji.1830240629. [DOI] [PubMed] [Google Scholar]
- Sartor R. B., Cromartie W. J., Powell D. W., Schwab J. H. Granulomatous enterocolitis induced in rats by purified bacterial cell wall fragments. Gastroenterology. 1985 Sep;89(3):587–595. doi: 10.1016/0016-5085(85)90455-x. [DOI] [PubMed] [Google Scholar]
- Sartor R. B. Current concepts of the etiology and pathogenesis of ulcerative colitis and Crohn's disease. Gastroenterol Clin North Am. 1995 Sep;24(3):475–507. [PubMed] [Google Scholar]
- Sartor R. B. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994 Feb;106(2):533–539. doi: 10.1016/0016-5085(94)90614-9. [DOI] [PubMed] [Google Scholar]
- Schreiber S., Heinig T., Thiele H. G., Raedler A. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterology. 1995 May;108(5):1434–1444. doi: 10.1016/0016-5085(95)90692-4. [DOI] [PubMed] [Google Scholar]
- Schwab J. H., Anderle S. K., Brown R. R., Dalldorf F. G., Thompson R. C. Pro- and anti-inflammatory roles of interleukin-1 in recurrence of bacterial cell wall-induced arthritis in rats. Infect Immun. 1991 Dec;59(12):4436–4442. doi: 10.1128/iai.59.12.4436-4442.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H. Phlogistic properties of peptidoglycan-polysaccharide polymers from cell walls of pathogenic and normal-flora bacteria which colonize humans. Infect Immun. 1993 Nov;61(11):4535–4539. doi: 10.1128/iai.61.11.4535-4539.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell K. L., Trentham D. E. Pathogenesis of rheumatoid arthritis. Lancet. 1993 Jan 30;341(8840):283–286. doi: 10.1016/0140-6736(93)92627-6. [DOI] [PubMed] [Google Scholar]
- Simon A. K., Seipelt E., Sieper J. Divergent T-cell cytokine patterns in inflammatory arthritis. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8562–8566. doi: 10.1073/pnas.91.18.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimpson S. A., Dalldorf F. G., Otterness I. G., Schwab J. H. Exacerbation of arthritis by IL-1 in rat joints previously injured by peptidoglycan-polysaccharide. J Immunol. 1988 May 1;140(9):2964–2969. [PubMed] [Google Scholar]
- Van Laethem J. L., Marchant A., Delvaux A., Goldman M., Robberecht P., Velu T., Devière J. Interleukin 10 prevents necrosis in murine experimental acute pancreatitis. Gastroenterology. 1995 Jun;108(6):1917–1922. doi: 10.1016/0016-5085(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Van den Broek M. F., Van de Langerijt L. G., Van Bruggen M. C., Billingham M. E., Van den Berg W. B. Treatment of rats with monoclonal anti-CD4 induces long-term resistance to streptococcal cell wall-induced arthritis. Eur J Immunol. 1992 Jan;22(1):57–61. doi: 10.1002/eji.1830220110. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Allen J. B., Wilder R. L., Paglia L., Hand A. R. Bacterial cell wall-induced hepatic granulomas. An in vivo model of T cell-dependent fibrosis. J Exp Med. 1986 Apr 1;163(4):884–902. doi: 10.1084/jem.163.4.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yocum D. E., Allen J. B., Wahl S. M., Calandra G. B., Wilder R. L. Inhibition by cyclosporin A of streptococcal cell wall-induced arthritis and hepatic granulomas in rats. Arthritis Rheum. 1986 Feb;29(2):262–273. doi: 10.1002/art.1780290215. [DOI] [PubMed] [Google Scholar]