Abstract
In response to invasion by microbial pathogens, host defense mechanisms get activated by both the innate and adaptive arms of the immune responses. TNF (tumor necrosis factor) is a potent proinflammatory cytokine expressed by activated macrophages and lymphocytes that induces diverse cellular responses that can vary from apoptosis to the expression of genes involved in both early inflammatory and acquired immune responses. A wide spectrum of microbes has acquired elegant mechanisms to overcome or deflect the host responses mediated by TNF. For example, modulatory proteins encoded by multiple families of viruses can block TNF and TNF-mediated responses at multiple levels, such as the inhibition of the TNF ligand or its receptors, or by modulating key transduction molecules of the TNF signaling pathway. Bacteria, on the other hand, tend to modify TNF-mediated responses specifically by regulating components of the TNF signaling pathway. Investigation of these diverse strategies employed by viral and bacterial pathogens has significantly advanced our understanding of both host TNF responses and microbial pathogenesis. This review summarizes the diverse microbial strategies to regulate TNF and how such insights into TNF modulation could benefit the treatment of inflammatory or autoimmune diseases.
Introduction
Metazoans have developed a variety of reactive mechanisms to control invading pathogens. On the other hand, microbial invaders such as viruses, bacteria, and intracellular parasites have co-evolved with their hosts to counteract the innate and adaptive responses mounted by the host. Of the many host pathways activated by pathogen invasion, pro-inflammatory cytokines play particularly significant roles in orchestrating both the early and late host responses. TNF is one such pleiotropic pro-inflammatory cytokine that plays an important role in diverse host responses such as septic shock, induction of other cytokines, cell proliferation, differentiation, necrosis, and apoptosis. TNF is expressed as either a membrane-bound or secreted ligand mainly by activated macrophages, lymphocytes, natural killer cells, and epithelial cells. Three classes of TNFs have been identified: TNFα (here called TNF), lymphotoxin-α (LT-α), and LT-β, all of which are bioactive as trimers. A TNF protein superfamily that exhibits 15%–20% identity to each other now comprises at least 20 members [1,2]. Many of the TNF-induced cellular responses are mediated by either one of the two known TNF receptors (TNFR), TNFR1 (p60), and TNFR2 (p80), both of which also belong to a larger superfamily of receptors, consisting of nearly 30 members [1,3].
The TNFR superfamily members fall into three major groups, death domain (DD)-containing receptors, decoy receptors, and TNF receptor-associated factor (TRAF) binding receptors [1]. DD-containing TNFRs (such as FAS, TNFR1, and DR3) can activate caspase cascades via DD-containing signaling intermediates, leading to apoptosis. Receptors that lack DD, such as TNFR2, contain motifs that recruit TRAF proteins. Both TNFR1 and TNFR2 and many other TNFR family members activate NF-κB (nuclear factor-κB) which is associated with cellular activation, differentiation, cytokine production, and survival signaling [1,3,4]. The TNFR superfamily members are all type I transmembrane proteins characterized by the presence of one to six hallmark cysteine-rich domains. Some members of the TNFR superfamily (FAS, TNFR1, and TNFR2) preassemble on the cell surface prior to ligand binding using the N-terminal pre-ligand binding assembly domain (PLAD) [5].
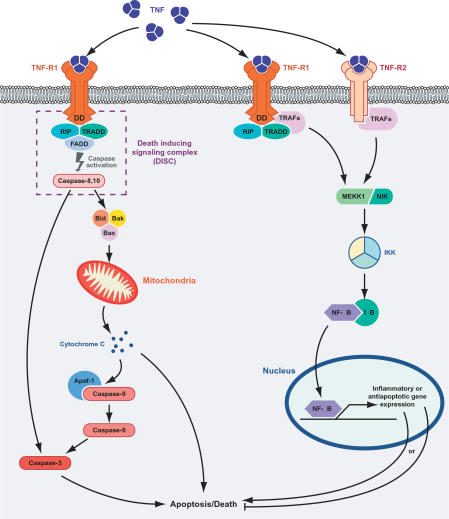
TNF can induce either an NF-κB-mediated survival (and proinflammatory) pathway or an apoptotic response depending on the cellular context (Figure 1). TNFR1 is thought to initiate the majority of TNF-mediated biological activities. The TNF ligand homotrimer binds to the extracellular domain of the receptor, which induces TNFR1 trimer conformational changes and the activation of the intracellular signaling pathway. TNFR1 ligand engagement leads to the release of the inhibitory protein silencer of death domains (SODD) from TNFR1 intracellular DD [6,7]. Release of SODD allows binding of TRADD (TNFR1-associated death domain protein) to the DD and recruits additional adapter proteins such as RIP1 (receptor interacting protein), TRAF2, and cIAP1 (cellular inhibitor of apoptosis) to form complex I. Complex I transduces signals leading to NF-κB translocation to the nucleus. Later, RIP1, TRADD, and TRAF2 dissociate from TNFR1 and recruit FADD (FAS-associated death domain protein) and caspase 8 to form complex II. In the absence of NF-κB activity from complex I, complex II can initiate caspase-8 activation, which leads to cell death [8,9]. On the other hand, NF-κB inhibits cell death through upregulation of antiapoptotic genes such as cellular FLICE-like inhibitory protein (c-FLIP), cIAP1, cIAP2, TRAF1, and TRAF2, which are recruited to complex II and inhibit caspase activation [10].
Figure 1. TNF-Mediated Death and Survival Pathways.
TNF-mediated death and survival pathways are activated following interaction with the TNFRs. The apoptotic pathway is activated through TNFR1 by forming the DISC, which activates caspase-8. Activated caspase-8 or −10 then activates the proapoptotic Bcl-2 family members, which leads to cell death by releasing cytochrome c from mitochondria and loss of MMP. The NF-κB-mediated survival pathway is activated by both TNFR1 and TNFR2. Association of TRAFs with these receptors activate signaling proteins like NIK (NF-κB inhibitor kinase) and MEKK1 (MAPK kinase 1), which activate the inhibitor of NF-κB (IkB) kinase (IKK) signalosome complex. IKK phosphorylates IkB, resulting in the degradation of the inhibitor. The free NF-κB than translocates to nucleus to induce the expression of inflammatory or antiapoptotic genes.
TNFR2 does not contain a cytoplasmic death domain and cannot directly engage the apoptotic machinery, and thus its precise involvement in TNF-mediated cell death is controversial. It can enhance the cell death signal of TNFR1, possibly through TRAF2 degradation and enhanced recruitment of FADD and RIP to TNFR1 [11]. TNFR2 also plays an important role in antiviral response by inducing cellular necrosis [12,13]. TNFR2 itself can induce TNF-dependent apoptosis and cell death, as demonstrated using cytotoxic T lymphocytes from TNFR1 knockout animals, possibly by recruitment of FADD to TNFR2 via RIP1 and TRAF2 together with some still-unidentified adapter molecules [14].
The TNF-induced NF-κB–mediated survival pathway can be activated independently by either TNFR1 or TNFR2 [15]. In response to TNF, complex I signals through some other scaffolding and signaling proteins, such as NIK (NF-κB inducing kinase) and MEKK1 (MAPK kinase-1), which converge on the IκB kinase (IKK) signalosome complex and activate NF-κB. Depending on stimuli such as viral or bacterial infection, exposure to proinflammatory cytokines, mitogens, growth factors, and stress inducing agents, IKK-α/IKK-β can phosphorylate IκB, resulting in proteolytic degradation of the inhibitor and translocation of NF-κB to the nucleus [16]. NF-κB enhances cell survival by upregulating expression of antiapoptotic genes such as members of the Bcl2 family (Bcl-xL and A1/Bfl-1), cellular inhibitors of apoptosis (c-IAP1, c-IAP2, and XIAP), TRAF1 and TRAF2, and the FLICE-inhibitory protein cFLIP. In some cases, activation of NF-κB is also associated with induction of apoptosis by enhancing expression of proapoptotic cytokines [17,18]
Inhibition and Modulation of TNF by Viruses
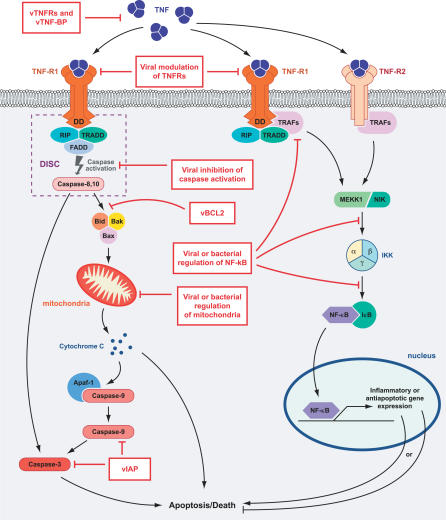
TNF orchestrates powerful anti-microbial responses by a variety of mechanisms, including the direct killing of infected cells (cytolysis), induction of apoptosis, inhibition of intracellular pathogen replication, and upregulation of other diverse host responses. Using TNF or TNFRs-deficient mice, it has been demonstrated that they are essential for survival of infections with bacterial pathogens such as Listeria monocytogens, Mycobacterium tuberculosis, M. avium, Salmonella typhimurium, intracellular parasites such as Leishmania major or Trypanosoma cruzi, and viruses such as herpes simplex virus (HSV-1), mouse cytomegalovirus (MCMV), or lymphocytic choriomeningitis virus (LCMV)[2,4,13]. TNF and TNFR signaling pathway is required for differentiation of T cells, induction of cytokines and chemokines, recruitment of leukocytes, and development of granulomas that are capable of controlling virulent bacterial infection. Deficiency of TNF or TNFR network delays granuloma formation and affects several other components of the innate and adaptive immune system including activation of dendritic cells, natural killer cells, and differentiation of T and B cells. Thus, TNF and TNFR network provided powerful selection pressure for viruses and other pathogens to evolve strategies to combat the TNF-mediated responses to infection. As illustrated in Figure 2, many viruses have acquired strategies to neutralize TNF by targeting almost every step of TNF biology, ranging from direct binding and inhibition of the ligand or receptor, to modulation of various downstream signaling events [19,20]. Table 1 represents a spectrum of the viral factors that inhibit TNF or modulate TNF signaling.
Figure 2. Different Strategies for Inhibiting TNF by Pathogens.
Pathogens have evolved diverse strategies to target almost every step of TNF biology. Virus-encoded proteins inhibit TNF-mediated responses by directly binding to TNF with secreted soluble decoy TNFR (vTNFRs) and vTNFBPs, downregulating the cellular death receptors, interacting with the TNFR-associated factors, blocking caspase activation, and regulating the apoptotic checkpoint function of mitochondria. Viruses also regulate the pathways leading to TNF-mediated activation of NF-κB. Bacteria and other pathogens can express proteins that regulate TNF-mediated responses by activating or inhibiting NF-κB at different levels of signaling that range from the death receptor to nuclear localization of NF-κB.
Table 1.
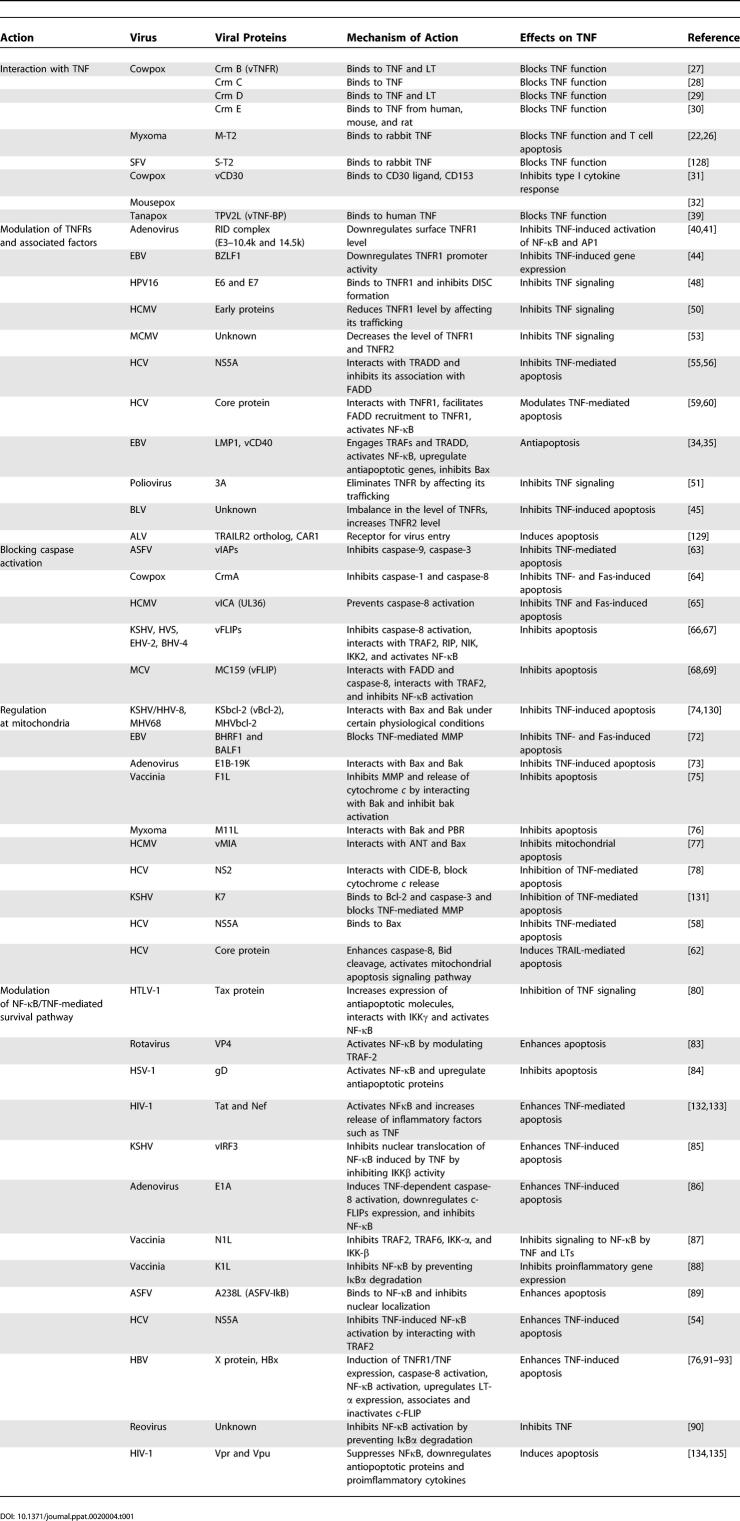
Viral Inhibition and Modulation of TNF
Viral TNFR Homologues
The first-identified TNF inhibition strategy deployed by viruses was revealed by the discovery of encoded soluble TNFRs homologs which function by binding and sequestering extracellular TNF. Among these vTNFRs are the T2-like family members encoded by Leporipoxviruses and the cytokine response modifier (Crm) family members encoded by Orthopoxviruses [21,22]. The myxoma-virus-encoded M-T2 protein is a glycosylated, dimeric, secreted protein that specifically inhibits rabbit TNF [23,24]. The pathogenicity of myxoma virus in domestic rabbits is attenuated when the M-T2 gene is deleted from the viral genome [25]. The intracellular form of M-T2 protein is also able to inhibit apoptosis in virus-infected lymphocytes. The first two N-terminal cysteine-rich domains are responsible for anti-apoptotic properties, while at minimum the first three cysteine-rich domains are required to inhibit TNF [26]. Related TNFR orthlogs, designated CrmB, CrmC, CrmD, and CrmE, have been characterized from members of the orthopoxvirus genus [27–30]. Another poxvirus encoded ortholog of TNFR family members, vCD30, has been identified in cowpox and mousepox viruses [31,32]. vCD30 binds to CD30L/CD153 with high affinity, inhibits the ability of CD30L to signal via cell surface CD30, and also inhibits type I cytokine responses in a murine model of antigen-induced granuloma [32].
Epstein-Barr virus (EBV) latent infection membrane protein 1 (LMP1) mimics a constitutively activated TNFR member, CD40. LMP1 is essential for EBV conversion of infected B lymphocytes into perpetually proliferating lymphoblasts and is expressed in EBV-associated lymphoproliferative disease, Hodgkin disease, and nasopharyngeal carcinoma [33]. Two motifs of LMP1 located in the carboxy-terminal cytoplasmic domain, designated CTAR1 (C-terminal activation domain) and CTAR2, activate both canonical (IKKβ-dependent) and noncanonical (NIK/IKKα-dependent) NF-κB pathways by engaging different TRAFs. [34]. This activation mediates the antiapoptotic activity of LMP-1 by upregulation of the expression of antiapoptotic factors such as Bcl-2, Bfl-1, and A20, which are potent inhibitors of Bax [35].
Some viruses exploit TNFR family members as receptors to enter into cells. HSV-1 glycoprotein D (gD) interacts with HVEM (herpes virus entry mediator) to enter into resting T cells, monocytes, and immature dendritic cells [36]. A TNFR ortholog (UL 144 orf) encoded by human CMV, which is closest in sequence to the extracellular domain of human HVEM and TRAILR2, binds BTLA (B and T lymphocyte attenuator) and inhibits T cell proliferation by mimicking the inhibitory cosignaling function of HVEM [37]. Recently, another TNFR family member, designated equine lentivirus receptor-1, has been identified as cellular receptor for the entry of equine infectious anemia virus, a member of lentiviruses family, into monocytes and macrophages [38].
Viral TNF-Binding Proteins
A new class of TNF binding protein has been recently identified from Tanapox virus, a member of the Yatapoxvirus genus of poxviruses [39]. This vTNF-BP is encoded by the TPV gene 2L, and related orthologs are present in other members of the Yatapoxvirus (YLDV and YMTV), swinepox, and deerpoxvirus. The 2L protein exhibits some sequence identity (25%) to the α1, α2, and α3 domains of the cellular MHC class I molecules, but, unlike the cellular counterpart, lacks a transmembrane domain. TPV-2L binds to human TNF with very high affinity (Kd = 43 pM), but this protein failed to interact with any other human cytokine or TNF from other species. The discovery of this novel group of vTNF-BPs suggests that there could still be unidentified classes of cellular TNF-BPs, too.
Modulation of TNF Receptors and Associated Factors by Viruses
The adenovirus early transcription region 3 (E3) encodes at least seven proteins, five of which block the acquired or innate immune response. Three of these, Ad E3–14.7K, Ad E3–10.4K, and Ad E3–14.5K, impose inhibitory effects on the TNF pathway [40]. Two of these proteins, 10.4K (RIDα) and 14.5K (RIDβ), form a heterotrimeric complex in the plasma membrane known as RID (receptor internalization and degradation), which inhibits signaling through TNFR1 [40]. RID downregulates surface TNFR1 levels by reducing the assembly of TNFR1 signaling complex and thus inhibiting TNF induced activation of NF-κB. In terms of the NF-κB pathway, RID blocks the association of members of the IKK complex, as well as the protein kinase RIP, with the TNFR1 [40]. In a recent study it has been demonstrated that RIDβ directly interacts with TNFR1, and its tyrosine sorting motif plays a major role in downregulation of TNFR1 by a clathrin-dependent process involving μ2 and dynamin, followed by degradation of TNFR1 via an endosomal/lysosomal pathway [41]. In addition to TNFR1, RID can degrade other death receptors such as Fas, and in conjunction with E3–6.7K protein it can also degrade TRAIL receptor 2 [42,43].
The EBV-encoded immediate-early gene product BZLF1 (also called Zta, ZEBRA, or EB1) prevents cellular responses to TNF, including TNF-induced cell death [44]. During reactivation of the EBV lytic cycle, BZLF1 reduces TNF-R1 promoter activity and thus downregulates TNF-R1 protein expression levels. Mutational analysis of BZLF1 revealed that inhibition of TNF-R1 promoter activity requires both the transactivation and the DNA binding domains of BZLF1, suggesting that BZLF1 may bind to and activate the promoter of a gene that encodes a repressor of the TNF-R1 promoter [44]. Bovine leukemia virus (BLV), a type C retrovirus, induces TNFR2, but not TNFR1, by a yet unknown mechanism in PBMC from BLV-infected cattle, which results in resistance to TNF-induced apoptosis, possibly by activating antiapoptotic genes in an NF-κB–dependent fashion [45].
Small DNA viruses, such as human papillomaviruses (HPVs) are the major cause of cervical cancer (>90%) and a significant number of head and neck cancers [46]. They infect various human epithelial tissues, and have acquired mechanisms to inhibit TNF-induced apoptosis. HPV16 encoded two oncogene products, E6 and E7, which can stimulate cell cycle progression by binding to the tumor suppressor proteins or negative regulator of the cell cycle p53 and retinoblastoma (Rb) protein, respectively [47]. Inactivation of these proteins leads to deregulated entry of cells into S phase and maintenance of a favourable environment for viral DNA replication. Both E6 and E7 can also associate with other proteins involved in cell proliferation and apoptosis. HPV16 E6 protein binds to TNFR1 and affects the transmission of pro-apoptotic signals triggered by TNF [48]. E6 binds to the C-terminal 41 amino acids of TNFR1 and inhibits binding of TRADD to TNFR1 and thereby blocks formation of the death-inducing signaling complex (DISC). This inhibition subsequently blocks transmission of apoptotic signals by inhibiting the activation of initiator caspases such as caspase 8. E6-mediated protection against TNF-induced apoptosis occurs in cells of different species (mouse and human) and tissues (fibroblast, osteosarcoma, and histiocyte/monocyte). Both E6 and E7 of HPV16 increased the transcription of cIAP1 and cIAP2 by upregulation of NF-κB–expression and confer resistance to TNF in human keratinocytes [49].
Human and murine CMV have also developed mechanisms to evade a TNF-induced antiviral state by dysregulating TNFRs. HCMV infection of THP1 cells reduced the level of TNFR1 on the cell surface by accumulating the receptor pool in the trans-Golgi network [50]. Time course analysis and drug inhibition studies suggest that viral early gene products may target trafficking of TNFR1 [50]. Poliovirus noncapsid protein 3A also affects the intracellular trafficking of TNFR and induces TNF resistance by eliminating TNFRs from the plasma membrane [51]. However, 3A-protein–mediated inhibition of ER to Golgi traffic of TNFR was limited to poliovirus and coxsackievirus B3 [51,52]. MCMV infection of bone marrow-derived macrophages inhibited TNF-induced ICAM-1 surface expression and mRNA expression in infected cells via expression of immediate early and/or early viral genes [53]. MCMV infection blocked TNF-induced nuclear translocation of NF-κB, which decreased the level of both TNFR1 and TNFR2.
The hepatitis C virus (HCV) nonstructural protein 5A (NS5A) is a multifunctional phosphoprotein that utilizes multiple mechanisms to inhibit both extrinsic and intrinsic apoptotic stimuli [54]. Using NS5A transgenic mice, it has been demonstrated that NS5A interacts with TRADD, inhibiting its association with FADD and TNF-mediated apoptosis, resulting in persistent infection [55,56]. NS5A also binds to the TNFR1 signaling complex through its interaction with TRAF2, and subsequently inhibits TRAF2-dependent NF-κB activation, thereby sensitizing the cells to TNF-induced cytotoxicity. However, the sensitivity of cells expressing NS5A to TNF was not affected [57]. The inhibition of intrinsic apoptotic signals is mediated by the putative BH (Bcl-2 homology) domain of NS5A, which allows it to bind to the pro-apoptotic protein Bax, rendering cells refractile to certain pro-apoptotic agonists [58]. HCV core protein has been reported as both inducer and inhibitor of TNF-mediated apoptosis. In some human and mouse cell lines, HCV core protein interacts with TNFR1 or LTβR and activates the NF-κB pathway [59]. Core protein can also facilitate FADD recruitment to TNFR1 and sensitize cells to TNF-induced apoptosis [60]. However, HCV core-protein–mediated suppression of TNF-induced apoptosis has also been reported [61]. One recent study demonstrated that HCV core protein also induces TRAIL-mediated apoptosis in Huh7 cells through sequential induction of DISC formation [62].
Viral Inhibition of TNF-Induced Apoptosis
Activation of caspases and release of cytochrome c from the mitochondria generally lead to the induction of apoptosis and cell death. A number of viral proteins have been shown to inhibit caspases or intervening at the mitochondrial checkpoint to prevent TNF-mediated apoptosis. Proteins that inhibit caspase activation include vIAP from ASFV, CrmA from poxvirus, vICA from HCMV, vFLIPs from several γ-herpesviruses, and its ortholog MC159 from MCV [63–69]. One group of antiapoptotic proteins known as vBcl-2 from γ-herpesviruses, adenoviruses, and poxviruses inhibit proapoptotic Bax and Bak, and block mitochondrial apoptosis. Some viruses that lack vBcl-2 instead encode mitochondria-localized protein such as F1L from vaccinia, M11L from myxoma, and vMIA from HCMV, which inhibit apoptosis by preventing depolarization of the mitochondrial membrane potential (MMP) and stopping the release of cytochrome c [70–78].
Modulation of NF-κB by Viruses
NF-κB is a critical regulator of the immediate early pathogen response, playing an important role in promoting inflammation, and in the regulation of cell proliferation, activation and survival [79]. NF-κB thus provides an attractive target to various microbial pathogens for modulating host TNF-mediated events.
The Tax transactivator oncoprotein of human T cell leukemia virus type I (HTLV-I) persistently activates NF-κB signaling pathways, resulting in the deregulation of cellular gene expression and immortalization of HTLV-I-infected T cells [80]. Tax interacts with IKK-γ and stimulates the catalytic activity of IKK-α and IKK-β, which degrades IκB and enhances the activation of NF-κB [81]. Tax also interacts with and blocks tristetraprolin repressor, an inhibitor of TNF expression, and indirectly increases TNF expression in macrophages [82]. Rotavirus capsid protein VP4 contains a conserved TRAF-binding motif, and is responsible for NF-κB activation and the inhibition of TNF-mediated death signaling by engaging the TRAF2-NIK signaling pathway [83]. HSV-1 encoded gD inhibits TNF-mediated apoptosis in the U937 monocytoid cells by activation of NFκB and upregulation of some of the downstream antiapoptotic proteins, such as FLIP and cIAP2 [84].
Viruses also can inhibit NF-κB activation by different methods, which can result in increased sensitivity to TNF-induced apoptosis. KSHV-encoded viral interferon regulatory factor 3 (vIRF3) inhibits the activation of NF-κB induced by TNF [85]. In 293T cells, vIRF3 inhibits IKKβ activity, resulting in reduced IκB phosphorylation and inhibition of NF-κB activity and thus sensitizes cells to TNF-induced apoptosis [85]. Adenovirus E1A protein also sensitizes cells to TNF-mediated apoptosis. E1A inhibits c-FLIPs expression, which results in TNF-dependent caspase-8 activation in the DISC [86]. Vaccinia virus encoded protein N1L, a viral virulence factor, inhibits signaling to NF-κB via both TNF and LT. This N1L-mediated inhibition of NF-κB occurs by association with IKK-γ and inhibition of IKK-α and IKK-β [87]. N1L also inhibits IRF3 signaling and thus might play a broad role as viral immunomodulator of innate immunity. Another vaccinia encoded protein, K1L, inhibits NF-κB activation by preventing IκBα degradation, probably by interfering directly with IKK to prevent phosphorylation or indirectly by hampering kinases that act upstream of IKK [88]. ASFV A238L, which is an ankyrin-repeat–containing homolog of host IκB (ASFV-IκB), binds to NF-κB following degradation of host IκB and inhibits the nuclear transportation of NF-κB [89]. In contrast, Reovirus induces apoptosis by regulating NF-κB at two distinct levels. In human epithelial HEK293 cells, reovirus activates NF-κB to induce apoptosis early after infection. At later times, the reovirus inhibits NF-κB activation by preventing degradation of IκBα and inhibiting TNF-mediated apoptosis [90].
Induction of apoptosis in HBV-infected hepatocytes is mediated by the viral X protein (HBx). HBx-dependent activation of p38MAP kinase and JNK pathways leads to the activation of both TNFR1/TNF and Fas/FasL [91]. HBx also binds and inactivates c-FLIP and activates NF-κB through induction of LTα and TNF, both of which induce apoptosis [92,93].
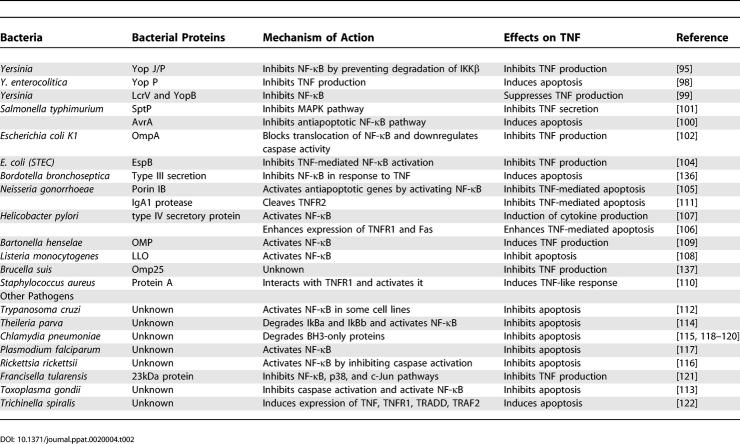
Modulation of TNF by Bacteria and Parasites
Bacterial mechanisms of inhibiting TNF-mediated responses differ significantly from that of viruses. To date, no bacterial or parasite protein has been reported that directly binds and inhibits TNF, TNFR, or their associated factors. Instead, they rely on indirect mechanisms to modulate TNF signaling, for example by reducing the synthesis of inflammatory mediators, cytokines, and the modulation of signaling pathways such as NF-κB or MAPK (Figure 2). Table 2 lists some of the known TNF modulating factors produced by bacteria and other nonviral pathogens.
Table 2.
Modulation of TNF by Bacteria and Parasites
Bacterial pathogens generally mediate their interactions with host cells via preformed soluble small-molecule or peptide effectors secreted by type III secretion system, a multicomponent translocation apparatus that spans the bacterial cell wall from the interior of the bacterium into the external environment [94]. The best-studied effector proteins are from Yersinia, and known as Yops (Yersinia outer proteins). Among the six Yop effector proteins, YopP/YopJ is involved in the inhibition of NF-κB and MAPK pathways, which results in the downregulation of cytokines such as TNF, chemokines, and adhesion molecules. This then inhibits the recruitment and activation of macrophages and natural killer cells to the site of infection [95]. YopJ is a cysteine protease (structurally related to the ubiquitin-like protease family of proteins) that can remove K63- and K48-linked polyubiquitin chains and inhibit proteasomal degradation of 1κBα, resulting in inhibition of NFκB signaling. YopJ also removes ubiquitin moieties from TRAF2 and TRAF6 [96,97]. Recently it has been demonstrated that YopP from Yersinia enterocolitica also induces apoptosis in murine dendritic cells and inhibits TNF production [98]. Two other type III secretory proteins from Yersinia pestis, Low calcium response V (LcrV) or V antigen and YopB inhibit production of TNF from murine peritoneal macrophages by inhibiting the transcription factor NF-κB after LPS treatment [99].
An ortholog of YopJ, AvrA from Salmonella typhimurium, also demonstrates potent inhibitory action towards the NF-κB pathway but does not seem to affect MAPK activation [100]. Unlike YopJ, AvrA in HeLa epithelial cells potently inhibits TNF-induced activation of the NF-κB pathway by inhibiting translocation of the p65 subunit of NF-κB. Another type III secretion protein, SptP from S. typhimurium, is also involved in host modulation involving the MAPK pathway by inhibiting Raf activation, which ultimately attenuates the secretion of TNF from infection-activated macrophages [101].
Escherichia coli K1 outer membrane protein A (OmpA) in infected monocytes suppresses the production of cytokines such as TNF by inhibiting IκB phosphorylation and blocking the translocation of NF-κB to the nucleus [102]. OmpA also induces expression of antiapoptotic protein Bcl-xL to promote the survival of monocytes and macrophages [103]. Suppression of NF-κB activation and downregulation of genes involved in inflammatory and immune responses is the most common mechanism used by other pathogenic E. coli strains. The Shiga toxin-producing E. coli (STEC), enterohemorrhagic E. coli, and enteropathogenic E. coli all interfere with NF-κB–activation initiated by TNF. EspB (E. coli–secreted protein B), a component of the type III secretion system, is also involved in the inhibition of NF-κB activation and proinflammatory cytokine production [104].
Some bacterial proteins activate NF-κB, which inhibits TNF-mediated apoptosis by upregulation of antiapoptotic genes, or induces TNF production to enhance apoptosis. The outer membrane protein, porin, from Neisseria gonorrhoeae, increases the transcription of several host antiapoptotic genes, including bfl-1, cox-2, and c-IAP-2, by the activation of NF-κB, and thus protects human urethral epithelial cells from apoptosis [105]. Infection with Helicobacter pylori, the main causative agent of chronic active type B gastritis, enhances the expression of TNFR1 and Fas and induces apoptosis of gastric epithelial cells [106]. Proteins encoded by the cag pathogenicity island of H. pylori are required for NF-κB activation, which enhances the production of TNF and other pro-apoptotic cytokines [107]. Listeria monocytogenes virulence protein Listeriolysin O (LLO) and Outer membrane protein (OMP) from Bartonella henselae both activate NF-κB and induce antiapoptotic signaling, which prolongs bacterial survival [108,109].
Bacteria can also use host TNFRs to mediate pathogenicity. A recent study has demonstrated that TNFR1 is a receptor for protein A from Staphylococcus aureus, a pathogen associated with pneumonia and sepsis. Activation of TNFR1 by protein A induces TNF-like responses which are associated with the pathogenesis of staphylococcal pneumonia [110]. Extracellular IgA1 protease from Neisseria gonorrhoeae is capable of inhibiting the TNF-mediated apoptosis of the human myelo-monocytic cell line U937 [111]. This proteolytic enzyme cleaves TNFRII but not TNFRI. Since TNFRII also can activate NFκB and induces apoptosis, inactivation of TNFRII could lead to direct inhibition of apoptosis.
Like virus and bacteria, parasites can also modulate NF-κB function and regulate host immune responses. They can either activate NF-κB by degrading the IκB or inhibit NF-κB activation by blocking the degradation of IκB. Protozoan parasites such as Trypanosoma cruzi, Toxoplasma gondii, Theileria parva, and other pathogens such as Chlamydia pneumoniae, Plasmodium falciparum, and Rickettsia rickettsii all activate NF-κB and inhibit apoptosis to enhance parasite replication [112–117]. Recent studies demonstrated that Chlaydia infection results in the degradation of BH3-only proteins such as Bim, Puma, Bad, Bik, Bmf, Noxa, and tBid, which inhibit proapoptotic Bax and Bak [118–120]. Degradation of these proapoptotic factors leads to protection of infected cells against apoptotic stimuli such as TNF [120]. A 23 kDa protein, which is upregulated during intracellular infection from the intracellular pathogen Francisella tularensis, inhibits TNF secretion from the murine macrophage like cell line J774A.1 by blocking the degradation of IκB and inhibiting NF-κB [121]. It has been demonstrated that the intracellular parasite nematode Trichinella spiralis can induce expression of TNF, TNFR1, TRADD, caspase-3, caspase-8, TRAF-2, and RIP in infected muscle cells, resulting in induction of either apoptosis or the transformation of muscle cells to nurse cells [122].
Anti-TNF Therapy: Clues from Pathogens?
Although TNF plays a major role in growth regulation, cell differentiation, and response to microbial infections, its inappropriate overexpression has been implicated in the pathogenesis of a wide spectrum of human disorders, such as autoimmunity (e.g., multiple sclerosis, rheumatoid arthritis, inflammatory bowel disease), allergy, septic shock, allograft rejection, and insulin resistance. TNF derived from mast cells also plays a crucial role in initiation of inflammation, particularly in the case of rheumatoid arthritis [123]. TNF may also exert tumor-promoting activity [124]. A recent study has demonstrated that the PLAD domain of TNFR1 is critical in TNF response, because mutations in PLAD reduce NF-κB activation and cause TNFR-associated periodic syndrome, an autoinflammatory syndrome [125]. Protein therapeutics containing only the PLAD domain can effectively prevent TNFR signaling and potently inhibit arthritis [126].
Many approaches have been investigated to inhibit TNF activity for the treatment of various inflammatory/autoimmune diseases (e.g., rheumatoid arthritis, Crohn disease, and inflammatory bowel disease). The currently commercially available TNF antagonists are infliximab (a chimeric mouse/human monoclonal anti-TNF antibody), etanercept (a soluble fusion protein combining two p75 TNFRs with an Fc fragment of human IgG1), and adalimumab (a humanized monoclonal anti-TNF antibody). Although they have shown to be partially effective in clinical trails, still more needs to be learned in terms of the biology of TNF. These current inhibitors need to be delivered at high doses, and some adverse events have been reported, so that the long-term safety of all these molecules is not thoroughly understood [127]. The investigation of TNF inhibition mechanisms by pathogens may provide novel therapeutic insights. In particular, TNF inhibitors derived from viral pathogens, which operate at relatively low concentration within the infected host, offer new therapeutic strategies for reducing the pathologic consequences of excessive TNF expression in inflammatory disorders.
Acknowledgments
We thank Doris Hall for assistance with the manuscript preparation.
Abbreviations
- BLV
bovine leukemia virus
- c-FLIP
cellular FLICE-like inhibitory protein
- cIAP1
cellular inhibitor of apoptosis
- CMV
cytomegalovirus
- Crm
cytokine response modifier
- DD
death-domain
- DISC
death-inducing signaling complex
- E3
early transcription region 3
- EBV
Epstein-Barr virus
- EspB
(E. coli–secreted protein B)
- FADD
FAS-associated death domain protein
- HCV
hepatitis C virus
- HPV
human papillomavirus
- HSV-1
herpes simplex virus 1
- HTLV-I
human T cell leukemia virus type I
- HVEM
herpes virus entry mediator
- LcrV
Low calcium response
- LLO
Listeriolysin O
- LMP1
latent infection membrane protein 1
- MMP
mitochondrial membrane potential
- OMP
outer membrane protein
- OmpA
outer membrane protein A
- PLAD
pre-ligand binding assembly domain
- RID
receptor internalization and degradation
- RIP
receptor interacting protein
- SODD
silencer of death domains
- TNF
tumor necrosis factor
- TNFR
TNF receptors
- TRADD
(TNFR1-associated death domain protein
- TRAF
TNF receptor-associated factor
- vIRF3
viral interferon regulatory factor 3
- Yops
Yersinia outer proteins
Footnotes
Funding. GM holds a Canada Research Chair in Molecular Virology and a Howard Hughes Medical Institute Scholarship.
Competing interests. GM is a co-founder of VIRON Therapeutics, Inc.
References
- Aggarwal BB. Signalling pathways of the TNF superfamily: A double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- Ware CF. Network communications: Lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 2005;23:787–819. doi: 10.1146/annurev.immunol.23.021704.115719. [DOI] [PubMed] [Google Scholar]
- Mocellin S, Rossi CR, Pilati P, Nitti D. Tumor necrosis factor, cancer and anticancer therapy. Cytokine Growth Factor Rev. 2005;16:35–53. doi: 10.1016/j.cytogfr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Hehlgans T, Pfeffer K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: Players, rules and the games. Immunology. 2005;115:1–20. doi: 10.1111/j.1365-2567.2005.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan FK, Chun HJ, Zheng L, Siegel RM, Bui KL, et al. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science. 2000;288:2351–2354. doi: 10.1126/science.288.5475.2351. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Woronicz JD, Liu W, Goeddel DV. Prevention of constitutive TNF receptor 1 signaling by silencer of death domains. Science. 1999;283:543–546. doi: 10.1126/science.283.5401.543. [DOI] [PubMed] [Google Scholar]
- Takada H, Chen NJ, Mirtsos C, Suzuki S, Suzuki N, et al. Role of SODD in regulation of tumor necrosis factor responses. Mol Cell Biol. 2003;23:4026–4033. doi: 10.1128/MCB.23.11.4026-4033.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- Muppidi JR, Tschopp J, Siegel RM. Life and death decisions: Secondary complexes and lipid rafts in TNF receptor family signal transduction. Immunity. 2004;21:461–465. doi: 10.1016/j.immuni.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: Induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- Chan FK, Lenardo MJ. A crucial role for p80 TNF-R2 in amplifying p60 TNF-R1 apoptosis signals in T lymphocytes. Eur J Immunol. 2000;30:652–660. doi: 10.1002/1521-4141(200002)30:2<652::AID-IMMU652>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Chan FK, Shisler J, Bixby JG, Felices M, Zheng L, et al. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem. 2003;278:51613–51621. doi: 10.1074/jbc.M305633200. [DOI] [PubMed] [Google Scholar]
- Suresh M, Gao X, Fischer C, Miller NE, Tewari K. Dissection of antiviral and immune regulatory functions of tumor necrosis factor receptors in a chronic lymphocytic choriomeningitis virus infection. J Virol. 2004;78:3906–3918. doi: 10.1128/JVI.78.8.3906-3918.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depuydt B, van Loo G, Vandenabeele P, Declercq W. Induction of apoptosis by TNF receptor 2 in a T-cell hybridoma is FADD dependent and blocked by caspase-8 inhibitors. J Cell Sci. 2005;118:497–504. doi: 10.1242/jcs.01640. [DOI] [PubMed] [Google Scholar]
- Thommesen L, Laegreid A. Distinct differences between TNF receptor 1– and TNF receptor 2–mediated activation of NFkappaB. J Biochem Mol Biol. 2005;38:281–289. doi: 10.5483/bmbrep.2005.38.3.281. [DOI] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: The control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Benedict CA. Viruses and the TNF-related cytokines, an evolving battle. Cytokine Growth Factor Rev. 2003;14:349–357. doi: 10.1016/s1359-6101(03)00030-3. [DOI] [PubMed] [Google Scholar]
- Benedict CA, Banks TA, Ware CF. Death and survival: Viral regulation of TNF signaling pathways. Curr Opin Immunol. 2003;15:59–65. doi: 10.1016/s0952-7915(02)00018-3. [DOI] [PubMed] [Google Scholar]
- Cunnion KM. Tumor necrosis factor receptors encoded by poxviruses. Mol Genet Metab. 1999;67:278–282. doi: 10.1006/mgme.1999.2878. [DOI] [PubMed] [Google Scholar]
- Xu X, Nash P, McFadden G. Myxoma virus expresses a TNF receptor homolog with two distinct functions. Virus Genes. 2000;21:97–109. [PubMed] [Google Scholar]
- Schreiber M, Rajarathnam K, McFadden G. Myxoma virus T2 protein, a tumor necrosis factor (TNF) receptor homolog, is secreted as a monomer and dimer that each bind rabbit TNFalpha, but the dimer is a more potent TNF inhibitor. J Biol Chem. 1996;271:13333–13341. doi: 10.1074/jbc.271.23.13333. [DOI] [PubMed] [Google Scholar]
- Schreiber M, McFadden G. The myxoma virus TNF-receptor homologue inhibits TNFα in a species specific fashion. Virology. 1994;204:692–705. doi: 10.1006/viro.1994.1585. [DOI] [PubMed] [Google Scholar]
- Upton C, Macen JL, Schreiber M, McFadden G. Myxoma virus expresses a secreted protein with homology to the tumor necrosis factor receptor gene family that contributes to viral virulence. Virology. 1991;184:370–382. doi: 10.1016/0042-6822(91)90853-4. [DOI] [PubMed] [Google Scholar]
- Schreiber M, Sedger L, McFadden G. Distinct domains of M-T2, the myxoma virus TNF receptor homolog, mediate extracellular TNF binding and intracellular apoptosis inhibition. J Virol. 1997;71:2171–2181. doi: 10.1128/jvi.71.3.2171-2181.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu FQ, Smith CA, Pickup DJ. Cowpox virus contains two copies of an early gene encoding a soluble secreted form of the Type II TNF receptor. Virology. 1994;204:343–356. doi: 10.1006/viro.1994.1539. [DOI] [PubMed] [Google Scholar]
- Smith CA, Hu FQ, Smith TD, Richards CL, Smolak P, et al. Cowpox virus genome encodes a second soluble homologue of cellular TNF receptors, distinct from CrmB, that binds TNF but not LT alpha. Virology. 1996;223:132–147. doi: 10.1006/viro.1996.0462. [DOI] [PubMed] [Google Scholar]
- Loparev VN, Parsons JM, Knight JC, Panus JF, Ray CA, et al. A third distinct tumor necrosis factor receptor of orthopoxviruses. Proc Natl Acad Sci U S A. 1998;95:3786–3791. doi: 10.1073/pnas.95.7.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva M, Alcami A. CrmE, a novel soluble tumor necrosis factor receptor encoded by poxviruses. J Virol. 2001;75:226–233. doi: 10.1128/JVI.75.1.226-233.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panus JF, Smith CA, Ray CA, Smith TD, Patel DD, et al. Cowpox virus encodes a fifth member of the tumor necrosis factor receptor family: A soluble, secreted CD30 homologue. Proc Natl Acad Sci U S A. 2002;99:8348–8353. doi: 10.1073/pnas.122238599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva M, Smith P, Fallon PG, Alcami A. Inhibition of type 1 cytokine-mediated inflammation by a soluble CD30 homologue encoded by ectromelia (mousepox) virus. J Exp Med. 2002;196:829–839. doi: 10.1084/jem.20020319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam N, Sugden B. CD40 and its viral mimic, LMP1: Similar means to different ends. Cell Signal. 2003;15:9–16. doi: 10.1016/s0898-6568(02)00083-9. [DOI] [PubMed] [Google Scholar]
- Luftig M, Prinarakis E, Yasui T, Tsichritzis T, Cahir-McFarland E, et al. Epstein-Barr virus latent membrane protein 1 activation of NF-kappaB through IRAK1 and TRAF6. Proc Natl Acad Sci U S A. 2003;100:15595–15600. doi: 10.1073/pnas.2136756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm T, Schneider S, Naschberger E, Huber J, Guenzi E, et al. EBV latent membrane protein-1 protects B cells from apoptosis by inhibition of BAX. Blood. 2005;105:3263–3269. doi: 10.1182/blood-2004-07-2752. [DOI] [PubMed] [Google Scholar]
- Montgomery RI, Warner MS, Lum BJ, Spear PG. Herpes simplex virus entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- Cheung TC, Humphreys IR, Potter KG, Norris PS, Shumway HM, et al. Evolutionarily divergent herpesviruses modulate T cell activation by targeting the herpesvirus entry mediator cosignaling pathway. Proc Natl Acad Sci U S A. 2005;102:13218–13223. doi: 10.1073/pnas.0506172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Jin S, Jin J, Li F, Montelaro RC. A tumor necrosis factor receptor family protein serves as a cellular receptor for the macrophage-tropic equine lentivirus. Proc Natl Acad Sci U S A. 2005;102:9918–9923. doi: 10.1073/pnas.0501560102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti CR, Paulose-Murphy M, Singh R, Qin J, Barrett JW, et al. A secreted high-affinity inhibitor of human TNF from Tanapox virus. Proc Natl Acad Sci U S A. 2003;100:4831–4836. doi: 10.1073/pnas.0737244100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler SP, Chin YR, Horwitz MS. Inhibition of tumor necrosis factor (TNF) signal transduction by the adenovirus group C RID complex involves downregulation of surface levels of TNF receptor 1. J Virol. 2004;78:13113–13121. doi: 10.1128/JVI.78.23.13113-13121.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin YR, Horwitz MS. Mechanism for removal of tumor necrosis factor receptor 1 from the cell surface by the adenovirus RIDalpha/beta complex. J Virol. 2005;79:13606–13617. doi: 10.1128/JVI.79.21.13606-13617.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefson AE, Hermiston TW, Lichtenstein DL, Colle CF, Tripp RA, et al. Forced degradation of Fas inhibits apoptosis in adenovirus-infected cells. Nature. 1998;392:726–730. doi: 10.1038/33712. [DOI] [PubMed] [Google Scholar]
- Lichtenstein DL, Doronin K, Toth K, Kuppuswamy M, Wold WS, et al. Adenovirus E3–6.7K protein is required in conjunction with the E3-RID protein complex for the internalization and degradation of TRAIL receptor 2. J Virol. 2004;78:12297–12307. doi: 10.1128/JVI.78.22.12297-12307.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison TE, Mauser A, Klingelhutz A, Kenney SC. Epstein-Barr virus immediate-early protein BZLF1 inhibits tumor necrosis factor alpha-induced signaling and apoptosis by downregulating tumor necrosis factor receptor 1. J Virol. 2004;78:544–549. doi: 10.1128/JVI.78.1.544-549.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnai S, Usui T, Ikeda M, Kohara J, Hirata T, et al. Imbalance of tumor necrosis factor receptors during progression in bovine leukemia virus infection. Virology. 2005;339:239–248. doi: 10.1016/j.virol.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Mandic A, Vujkov T. Human papillomavirus vaccine as a new way of preventing cervical cancer: A dream or the future? Ann Oncol. 2004;15:197–200. doi: 10.1093/annonc/mdh043. [DOI] [PubMed] [Google Scholar]
- Doorbar J. The papillomavirus life cycle. J Clin Viro. 2005;S25:S7–S15. doi: 10.1016/j.jcv.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Filippova M, Song H, Connolly JL, Dermody TS, Duerksen-Hughes PJ. The human papillomavirus 16 E6 protein binds to tumor necrosis factor (TNF) R1 and protects cells from TNF-induced apoptosis. J Biol Chem. 2002;277:21730–21739. doi: 10.1074/jbc.M200113200. [DOI] [PubMed] [Google Scholar]
- Yuan H, Fu F, Zhuo J, Wang W, Nishitani J, et al. Human papillomavirus type 16 E6 and E7 oncoproteins upregulate c-IAP2 gene expression and confer resistance to apoptosis. Oncogene. 2005;24:5069–5078. doi: 10.1038/sj.onc.1208691. [DOI] [PubMed] [Google Scholar]
- Baillie J, Sahlender DA, Sinclair JH. Human cytomegalovirus infection inhibits tumor necrosis factor alpha (TNF-alpha) signaling by targeting the 55-kilodalton TNF-alpha receptor. J Virol. 2003;77:7007–7016. doi: 10.1128/JVI.77.12.7007-7016.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neznanov N, Kondratova A, Chumakov KM, Angres B, Zhumabayeva B, et al. Poliovirus protein 3A inhibits tumor necrosis factor (TNF)-induced apoptosis by eliminating the TNF receptor from the cell surface. J Virol. 2001;75:10409–10420. doi: 10.1128/JVI.75.21.10409-10420.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe SS, Dodd DA, Kirkegaard K. Inhibition of cellular protein secretion by picornaviral 3A proteins. Virology. 2005;337:18–29. doi: 10.1016/j.virol.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Popkin DL, Virgin, HW IV. Murine cytomegalovirus infection inhibits tumor necrosis factor alpha responses in primary macrophages. J Virol. 2003;77:10125–10130. doi: 10.1128/JVI.77.18.10125-10130.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald A, Harris M. Hepatitis C virus NS5A: Tales of a promiscuous protein. J Gen Virol. 2004;85:2485–2502. doi: 10.1099/vir.0.80204-0. [DOI] [PubMed] [Google Scholar]
- Majumder M, Ghosh AK, Steele R, Zhou XY, Phillips NJ, et al. Hepatitis C virus NS5A protein impairs TNF-mediated hepatic apoptosis, but not by an anti-FAS antibody, in transgenic mice. Virology. 2002;294:94–105. doi: 10.1006/viro.2001.1309. [DOI] [PubMed] [Google Scholar]
- Miyasaka Y, Enomoto N, Kurosaki M, Sakamoto N, Kanazawa N, et al. Hepatitis C virus nonstructural protein 5A inhibits tumor necrosis factor-alpha-mediated apoptosis in Huh7 cells. J Infect Dis. 2003;188:1537–1544. doi: 10.1086/379253. [DOI] [PubMed] [Google Scholar]
- Park KJ, Choi SH, Choi DH, Park JM, Yie SW, et al. 1Hepatitis C virus NS5A protein modulates c-Jun N-terminal kinase through interaction with tumor necrosis factor receptor-associated factor 2. J Biol Chem. 2003;278:30711–30718. doi: 10.1074/jbc.M209623200. [DOI] [PubMed] [Google Scholar]
- Chung YL, Sheu ML, Yen SH. Hepatitis C virus NS5A as a potential viral Bcl-2 homologue interacts with Bax and inhibits apoptosis in hepatocellular carcinoma. Int J Cancer. 2003;107:65–73. doi: 10.1002/ijc.11303. [DOI] [PubMed] [Google Scholar]
- Zhu N, Khoshnan A, Schneider R, Matsumoto M, Dennert G, et al. Hepatitis C virus core protein binds to the cytoplasmic domain of tumor necrosis factor (TNF) receptor 1 and enhances TNF-induced apoptosis. J Virol. 1998;72:3691–3697. doi: 10.1128/jvi.72.5.3691-3697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N, Ware CF, Lai MM. Hepatitis C virus core protein enhances FADD-mediated apoptosis and suppresses TRADD signaling of tumor necrosis factor receptor. Virology. 2001;283:178–187. doi: 10.1006/viro.2001.0896. [DOI] [PubMed] [Google Scholar]
- Marusawa H, Hijikata M, Chiba T, Shimotohno K. Hepatitis C virus core protein inhibits Fas- and tumor necrosis factor alpha-mediated apoptosis via NF-kappaB activation. J Virol. 1999;73:4713–4720. doi: 10.1128/jvi.73.6.4713-4720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou AH, Tsai HF, Wu YY, Hu CY, Hwang LH, et al. Hepatitis C virus core protein modulates TRAIL-mediated apoptosis by enhancing Bid cleavage and activation of mitochondria apoptosis signaling pathway. J Immunol. 2005;174:2160–2166. doi: 10.4049/jimmunol.174.4.2160. [DOI] [PubMed] [Google Scholar]
- Nogal ML, Gonzalez DB, Rodriguez C, Cubelos B, Carracosa AL, et al. African swine fever virus IAP homologue inhibits caspase activation and promotes cell survival in mammalian cells. J Virology. 2001;75:2535–2543. doi: 10.1128/JVI.75.6.2535-2543.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari M, Dixit VM. Fas- and tumor necrosis factor-induced apoptosis is inhibited by the poxvirus crmA gene product. J Biol Chem. 1995;270:3255–3260. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- Skaletskaya A, Bartle LM, Chittenden T, McCormick AL, Mocarski ES et al. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc Natl Acad Sci U S A. 2001;98:7829–7834. doi: 10.1073/pnas.141108798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome M, Tschopp J. Regulation of lymphocyte proliferation and death by FLIP. Nat Rev Immunol. 2001;1:50–58. doi: 10.1038/35095508. [DOI] [PubMed] [Google Scholar]
- Matta H, Chaudhary PM. Activation of alternative NF-kappa B pathway by human herpes virus 8-encoded Fas-associated death domain-like IL-1 beta-converting enzyme inhibitory protein (vFLIP) Proc Natl Acad Sci U S A. 2004;101:9399–9404. doi: 10.1073/pnas.0308016101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey TL, Bertin J, Siegel RM, Wang GH, Leonardo MJ, et al. Binding of FADD and Caspase-8 to molluscun contagiosum virus MC159 is not sufficient for its antiapoptotic function. J Virol. 2002;76:697–706. doi: 10.1128/JVI.76.2.697-706.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murao LE, Shisler JL. The MCV MC159 protein inhibits late, but not early, events of TNF-alpha-induced NF-kappaB activation. Virology. 2005;340:255–264. doi: 10.1016/j.virol.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Boya P, Pauleau AL, Poncet D, Gonzalez-Polo RA, Zamzami N, et al. Viral proteins targeting mitochondria: Controlling cell death. Biochim Biophys Acta. 2004;1659:178–189. doi: 10.1016/j.bbabio.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Cuconati A, White E. Viral homologs of BCL-2 role of apoptosis in the regulation of virus infection. Genes Dev. 2002;16:2465–2478. doi: 10.1101/gad.1012702. [DOI] [PubMed] [Google Scholar]
- Kawanishi M, Tada-Oikawa S, Kawanishi S. Epstein-Barr virus BHRF1 functions downstream of Bid cleavage and upstream of mitochondrial dysfunction to inhibit TRAIL-induced apoptosis in BJAB cells. Biochem Biophys Res Commun. 2002;297:682–687. doi: 10.1016/s0006-291x(02)02261-1. [DOI] [PubMed] [Google Scholar]
- Sundararajan R, White E. E1B 19K blocks Bax oligomerization and tumor necrosis factor alpha-mediated apoptosis. J Virol. 2001;75:7506–7516. doi: 10.1128/JVI.75.16.7506-7516.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangappa S, van Dyk LF, Jewett TJ, Speck SH, Virgin HW IV. Identification of the in vivo role of a viral bcl-2. J Exp Med. 2002;195:931–940. doi: 10.1084/jem.20011825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasilenko ST, Banadyga L, Bond D, Barry M. The Vaccinia virus F1L protein interacts with the proapoptotic protein Bak and inhibits Bak activation. J Virol. 2005;79:14031–14043. doi: 10.1128/JVI.79.22.14031-14043.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Barrett JW, Nazarian SH, Everett H, Gao X, et al. Myxoma virus M11L prevents apoptosis through constitutive interaction with Bak. J Virol. 2004;78:7097–7111. doi: 10.1128/JVI.78.13.7097-7111.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoult D, Bartle LM, Skaletskaya A, Poncet D, Zamzami N et al. Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria. Proc Natl Acad Sci U S A. 2004;101:7988–7993. doi: 10.1073/pnas.0401897101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdtmann L, Franck N, Lera H, Le Seyec J, Gilot D, et al. The hepatitis C virus NS2 protein is an inhibitor of CIDE-B-induced apoptosis. J Biol Chem. 2003;278:18256–18264. doi: 10.1074/jbc.M209732200. [DOI] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- Harhaj EW, Harhaj NS. Mechanisms of persistent NF-kappaB activation by HTLV-I tax. IUBMB Life. 2005;57:83–91. doi: 10.1080/15216540500078715. [DOI] [PubMed] [Google Scholar]
- Fu DX, Kuo YL, Liu BY, Jeang KT, Giam CZ. Human T-lymphotropic virus type I tax activates I-kappa B kinase by inhibiting I-kappa B kinase-associated serine/threonine protein phosphatase 2A. J Biol Chem. 2003;278:1487–1493. doi: 10.1074/jbc.M210631200. [DOI] [PubMed] [Google Scholar]
- Twizere JC, Kruys V, Lefebvre L, Vanderplasschen A, Collete D, et al. Interaction of retroviral Tax oncoproteins with tristetraprolin and regulation of tumor necrosis factor-alpha expression. J Natl Cancer Inst. 2003;95:1846–1859. doi: 10.1093/jnci/djg118. [DOI] [PubMed] [Google Scholar]
- LaMonica R, Kocer SS, Nazarova J, Dowling W, Geimonen E, et al. VP4 differentially regulates TRAF2 signaling, disengaging JNK activation while directing NF-kappa B to effect rotavirus-specific cellular responses. J Biol Chem. 2001;276:19889–19896. doi: 10.1074/jbc.M100499200. [DOI] [PubMed] [Google Scholar]
- Medici MA, Sciortino MT, Perri D, Amici C, Avitabile E, et al. Protection by herpes simplex virus glycoprotein D against Fas-mediated apoptosis: Role of nuclear factor kappaB. J Biol Chem. 2003;278:36059–36067. doi: 10.1074/jbc.M306198200. [DOI] [PubMed] [Google Scholar]
- Seo T, Park J, Lim C, Choe J. Inhibition of nuclear factor kappaB activity by viral interferon regulatory factor 3 of Kaposi's sarcoma-associated herpesvirus. Oncogene. 2004;23:6146–6155. doi: 10.1038/sj.onc.1207807. [DOI] [PubMed] [Google Scholar]
- Perez D, White E. E1A sensitizes cells to tumor necrosis factor alpha by downregulating c-FLIP S. J Virol. 2003;77:2651–2662. doi: 10.1128/JVI.77.4.2651-2662.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPerna G, Stacks J, Bowie AG, Boyd A, Kowal G, et al. Poxvirus protein N1L targets the I-kB kinase complex, inhibits signaling to NF-kB and IRF3 signaling by toll-like receptors. J Biol Chem. 2004;279:36570–36578. doi: 10.1074/jbc.M400567200. [DOI] [PubMed] [Google Scholar]
- Shisler JL, Jin XL. The vaccinia virus K1L gene product inhibits host NF-kBa degradation. J Virol. 2004;78:3553–3560. doi: 10.1128/JVI.78.7.3553-3560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revilla Y, Callejo M, Rodriguez JM, Culebras E, Nogal ML, et al. Inhibition of nuclear factor kB activation by a virus-encoded IkB-like protein. J Biol Chem. 1998;273:5405–5411. doi: 10.1074/jbc.273.9.5405. [DOI] [PubMed] [Google Scholar]
- Clarke P, Meintzer SM, Moffitt LA, Tyler KL. Two distinct phases of virus-induced nuclear factor kappa B regulation enhance tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in virus-infected cells. J Biol Chem. 2003;278:18092–18100. doi: 10.1074/jbc.M300265200. [DOI] [PubMed] [Google Scholar]
- Wang WH, Gregori G, Hullinger RL, Andrisani OM. Sustained activation of p38 mitogen-activated protein kinase and c-Jun N-terminal kinase pathways by hepatitis B virus X protein mediates apoptosis via induction of Fas/FasL and tumor necrosis factor (TNF) receptor 1/TNF-alpha expression. Mol Cell Biol. 2004;24:10352–10365. doi: 10.1128/MCB.24.23.10352-10365.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Park SG, Lim SO, Jung G. The hepatitis B virus X protein up-regulates lymphotoxin alpha expression in hepatocytes. Biochim Biophys Acta. 2005;1741:75–84. doi: 10.1016/j.bbadis.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Kim KH, Seong BL. Pro-apoptotic function of HBV X protein is mediated by interaction with c-FLIP and enhancement of death-inducing signal. EMBO J. 2003;22:2104–2116. doi: 10.1093/emboj/cdg210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan JE, Collmer A. Type III secretion machines: Bacterial devices for protein delivery into host cells. Science. 1999;284:1322–1328. doi: 10.1126/science.284.5418.1322. [DOI] [PubMed] [Google Scholar]
- Navarro L, Alto NM, Dixon JE. Functions of the Yersinia effector proteins in inhibiting host immune responses. Curr Opin Microbiol. 2005;8:21–27. doi: 10.1016/j.mib.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Zhou H, Monack DM, Kayagaki N, Wertz I, Yin J, et al. Yersinia virulence factor YopJ acts as a deubiquitinase to inhibit NF-(kappa}B activation. J Exp Med. 2005;202:1327–1332. doi: 10.1084/jem.20051194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth K, Xu Z, Mudgett MB, Bao ZQ, Palmer LE, et al. Disruption of signaling by Yersinia effector YopJ, a ubiquitin-like protein protease. Science. 2000;290:1594–1597. doi: 10.1126/science.290.5496.1594. [DOI] [PubMed] [Google Scholar]
- Erfurth SE, Grobner S, Kramer U, Gunst DS, Soldanova I, et al. Yersinia enterocolitica induces apoptosis and inhibits surface molecule expression and cytokine production in murine dendritic cells. Infect Immun. 2004;72:7045–7054. doi: 10.1128/IAI.72.12.7045-7054.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi A, Sharma RK, Batra HV, Tuteja U. Mechanism of rLcrV and rYopB mediated Immunosuppression in murine peritoneal macrophages. Mol Immunol. 2004;41:767–774. doi: 10.1016/j.molimm.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Collier-Hyams LS, Zeng H, Sun J, Tomlinson AD, Bao ZQ, et al. Cutting edge: Salmonella AvrA effector inhibits the key proinflammatory, anti-apoptotic NF-kappa B pathway. J Immunol. 2002;169:2846–2850. doi: 10.4049/jimmunol.169.6.2846. [DOI] [PubMed] [Google Scholar]
- Lin SL, Le TX, Cowen DS. SptP, a Salmonella typhimurium type III-secreted protein, inhibits the mitogen-activated protein kinase pathway by inhibiting Raf activation. Cell Microbiol. 2003;5:267–275. doi: 10.1046/j.1462-5822.2003.t01-1-00274.x. [DOI] [PubMed] [Google Scholar]
- Selvaraj SK, Prasadarao NV. Escherichia coli K1 inhibits proinflammatory cytokine induction in monocytes by preventing NF-kappaB activation. J Leukoc Biol. 2005;78:544–554. doi: 10.1189/jlb.0904516. [DOI] [PubMed] [Google Scholar]
- Sukumaran SK, Selvaraj SK, Prasadarao NV. Inhibition of apoptosis by Escherichia coli K1 is accompanied by increased expression of BclXL and blockade of mitochondrial cytochrome c release in macrophages. Infect Immun. 2004;72:6012–6022. doi: 10.1128/IAI.72.10.6012-6022.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauf N, Chakraborty T. Suppression of NF-kappa B activation and proinflammatory cytokine expression by Shiga toxin-producing Escherichia coli. J Immunol. 2003;170:2074–2082. doi: 10.4049/jimmunol.170.4.2074. [DOI] [PubMed] [Google Scholar]
- Binnicker MJ, Williams RD, Apicella MA. Gonococcal porin IB activates NF-kappaB in human urethral epithelium and increases the expression of host antiapoptotic factors. Infect Immun. 2004;72:6408–6417. doi: 10.1128/IAI.72.11.6408-6417.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasumi K, Tanaka K, Saitoh S, Takagi A, Miwa T, et al. Roles of tumor necrosis factor-alpha-receptor type 1 and Fas in the Helicobacter pylori-induced apoptosis of gastric epithelial cells. J Gastroenterol Hepatol. 2002;17:651–658. doi: 10.1046/j.1440-1746.2002.02757.x. [DOI] [PubMed] [Google Scholar]
- Glocker E, Lange C, Covacci A, Bereswill S, Kist M, et al. Proteins encoded by the cag pathogenicity island of Helicobacter pylori are required for NF-kappaB activation. Infect Immun. 1998;66:2346–2348. doi: 10.1128/iai.66.5.2346-2348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayal S, Lilienbaum A, Join-Lambert O, Li X, Israel A, et al. Listeriolysin O secreted by Listeria monocytogenes induces NF-kappaB signalling by activating the IkappaB kinase complex. Mol Microbiol. 2002;44:1407–1419. doi: 10.1046/j.1365-2958.2002.02973.x. [DOI] [PubMed] [Google Scholar]
- Fuhrmann O, Arvand M, Gohle A, Schmid M, Krull M, et al. Bartonella henselae induces NF-kappaB-dependent upregulation of adhesion molecules in cultured human endothelial cells: Possible role of outer membrane proteins as pathogenic factors. Infect Immun. 2001;69:5088–5097. doi: 10.1128/IAI.69.8.5088-5097.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez MI, Lee A, Reddy B, Muir A, Soong G, et al. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat Med. 2004;10:842–848. doi: 10.1038/nm1079. [DOI] [PubMed] [Google Scholar]
- Beck SC, Meyer TF. IgA1 protease from Neisseria gonorrhoeae inhibits TNFalpha-mediated apoptosis of human monocytic cells. FEBS Lett. 2000;472:287–292. doi: 10.1016/s0014-5793(00)01478-2. [DOI] [PubMed] [Google Scholar]
- Hall BS, Tam W, Sen R, Pereira ME. Cell-specific activation of nuclear factor-kappaB by the parasite Trypanosoma cruzi promotes resistance to intracellular infection. Mol Biol Cell. 2000;11:153–160. doi: 10.1091/mbc.11.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molestina RE, Payne TM, Coppens I, Sinai AP. Activation of NF-kappaB by Toxoplasma gondii correlates with increased expression of antiapoptotic genes and localization of phosphorylated IkappaB to the parasitophorous vacuole membrane. J Cell Sci. 2003;116:4359–4371. doi: 10.1242/jcs.00683. [DOI] [PubMed] [Google Scholar]
- Palmer GH, Machado J, Jr, Fernandez P, Heussler V, Perinat T, et al. Parasite-mediated nuclear factor kappaB regulation in lymphoproliferation caused by Theileria parva infection. Proc Natl Acad Sci U S A. 1997;94:12527–12532. doi: 10.1073/pnas.94.23.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl C, Oswald F, Simnacher U, Weiss S, Marre R, et al. Survival of Chlamydia pneumoniae-infected Mono Mac 6 cells is dependent on NF-kappaB binding activity. Infect Immun. 2001;69:7039–7045. doi: 10.1128/IAI.69.11.7039-7045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi SG, Francis CW, Silverman DJ, Sahni SK. Nuclear factor kappa B protects against host cell apoptosis during Rickettsia rickettsii infection by inhibiting activation of apical and effector caspases and maintaining mitochondrial integrity. Infect Immun. 2003;71:4127–4136. doi: 10.1128/IAI.71.7.4127-4136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachado SD, Gerold P, McConville MJ, Baldwin T, Quilici D, et al. Glycosylphosphatidylinositol toxin of Plasmodium induces nitric oxide synthase expression in macrophages and vascular endothelial cells by a protein tyrosine kinase-dependent and protein kinase C-dependent signaling pathway. J Immunol. 1996;156:1897–1907. [PubMed] [Google Scholar]
- Fischer SF, Vier J, Kirschnek S, Klos A, Hess S, et al. Chlamydia inhibit host cell apoptosis by degradation of proapoptotic BH3-only proteins. J Exp Med. 2004;200:905–916. doi: 10.1084/jem.20040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong F, Pirbhai M, Xiao Y, Zhong Y, Wu Y, et al. Degradation of the proapoptotic proteins Bik, Puma, and Bim with Bcl-2 domain 3 homology in Chlamydia trachomatis-infected cells. Infect Immun. 2005;73:1861–1864. doi: 10.1128/IAI.73.3.1861-1864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying S, Seiffert BM, Hacker G, Fischer SF. Broad degradation of proapoptotic proteins with the conserved Bcl-2 homology domain 3 during infection with Chlamydia trachomatis. Infect Immun. 2005;73:1399–1403. doi: 10.1128/IAI.73.3.1399-1403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telepnev M, Golovliov I, Grundstrom T, Tarnvik A, Sjostedt A. Francisella tularensis inhibits Toll-like receptor-mediated activation of intracellular signalling and secretion of TNF-alpha and IL-1 from murine macrophages. Cell Microbiol. 2003;5:41–51. doi: 10.1046/j.1462-5822.2003.00251.x. [DOI] [PubMed] [Google Scholar]
- Wu Z, Nagano I, Boonmars T, Takahashi Y. Tumor necrosis factor receptor-mediated apoptosis in Trichinella spiralis-infected muscle cells. Parasitology. 2005;131:373–381. doi: 10.1017/s0031182005007663. [DOI] [PubMed] [Google Scholar]
- Choo-Kang BS, Hutchison S, Nickdel MB, Bundick RV, Leishman AJ, et al. TNF-blocking therapies: An alternative mode of action? Trends Immunol. 2005;26:518–522. doi: 10.1016/j.it.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Balkwill F. Tumor necrosis factor or tumor promoting factor? Cytokine Growth Factor Rev. 2002;13:135–141. doi: 10.1016/s1359-6101(01)00020-x. [DOI] [PubMed] [Google Scholar]
- Siebert S, Fielding CA, Williams BD, Brennan P. Mutation of the extracellular domain of tumour necrosis factor receptor 1 causes reduced NF-kappaB activation due to decreased surface expression. FEBS Lett. 2005;579:5193–5198. doi: 10.1016/j.febslet.2005.08.037. [DOI] [PubMed] [Google Scholar]
- Deng GM, Zheng L, Ka-Ming Chan F, Lenardo M. Amelioration of inflammatory arthritis by targeting the pre-ligand assembly domain of tumor necrosis factor receptors. Nat Med. 2005;11:1066–1072. doi: 10.1038/nm1304. [DOI] [PubMed] [Google Scholar]
- Weisman MH. What are the risks of biologic therapy in rheumatoid arthritis? An update on safety. J Rheumatol Suppl. 2002;65:33–38. [PubMed] [Google Scholar]
- Smith CA, Davis T, Wignall JM, Din WS, Farrah T, et al. T2 open reading frame from Shope fibroma virus encodes a soluble form of the TNF receptor. Biochem Biophys Res Commun. 1991;176:335–342. doi: 10.1016/0006-291x(91)90929-2. [DOI] [PubMed] [Google Scholar]
- Brojatsch J, Naughton J, Rolls MM, Zingler K, Young JA. CAR1, a TNFR-related protein, is a cellular receptor for cytopathic avian leukosis-sarcoma viruses and mediates apoptosis. Cell. 1996;87:845–855. doi: 10.1016/s0092-8674(00)81992-3. [DOI] [PubMed] [Google Scholar]
- Cheng EHY, Nicholas J, Bellows DS, Hayward GS, Guo HG, et al. A bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apopotosis but does not heterodimerize with Bax or Bak. Proc Natl Acad Sci U S A. 1997;94:690–694. doi: 10.1073/pnas.94.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HW, Sharp TV, Koumi A, Koentges G, Boshoff C. Characterization of an anti-apoptotic glycoprotein encoded by Kaposi's sarcoma-associated herpesvirus which resembles a spliced variant of human survivin. EMBO J. 2002;21:2602–2615. doi: 10.1093/emboj/21.11.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota-Gomez A, Flores NC, Cruz C, Casullo A, Aw TY, et al. The human immunodeficiency virus-1 Tat protein activates human umbilical vein endothelial cell E-selectin expression via an NF-kappa B-dependent mechanism. J Biol Chem. 2002;277:14390–14399. doi: 10.1074/jbc.M108591200. [DOI] [PubMed] [Google Scholar]
- Olivetta E, Percario Z, Fiorucci G, Mattia G, Schiavoni I, et al. HIV-1 Nef induces the release of inflammatory factors from human monocyte/macrophages: Involvement of Nef endocytotic signals and NF-kappa B activation. J Immunol. 2003;170:1716–1727. doi: 10.4049/jimmunol.170.4.1716. [DOI] [PubMed] [Google Scholar]
- Muthumani K, Choo AY, Hwang DS, Chattergoon MA, Dayes NN, et al. Mechanism of HIV-1 viral protein R-induced apoptosis. Biochem Biophys Res Commun. 2003;304:583–592. doi: 10.1016/s0006-291x(03)00631-4. [DOI] [PubMed] [Google Scholar]
- Akari H, Bour S, Kao S, Adachi A, Strebel K. The human immunodeficiency virus type 1 accessory protein Vpu induces apoptosis by suppressing the nuclear factor kappaB-dependent expression of antiapoptotic factors. J Exp Med. 2001;194:1299–1311. doi: 10.1084/jem.194.9.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuk MH, Harvill ET, Cotter PA, Miller JF. Modulation of host immune responses, induction of apoptosis and inhibition of NF-kappaB activation by the Bordetella type III secretion system. Mol Microbiol. 2000;35:991–1004. doi: 10.1046/j.1365-2958.2000.01785.x. [DOI] [PubMed] [Google Scholar]
- Jubier-Maurin V, Boigegrain RA, Cloeckaert A, Gross A, Alvarez-Martinez MT, et al. Major outer membrane protein Omp25 of Brucella suis is involved in inhibition of tumor necrosis factor alpha production during infection of human macrophages. Infect Immun. 2001;69:4823–4830. doi: 10.1128/IAI.69.8.4823-4830.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]