Abstract
Neurotrophic factors are agents with a promising ability to retard progression of neurodegenerative diseases and are effective in slowing photoreceptor degeneration in animal models of retinitis pigmentosa. Here we report a human clinical trial of a neurotrophic factor for retinal neurodegeneration. In this Phase I safety trial, human ciliary neurotrophic factor (CNTF) was delivered by cells transfected with the human CNTF gene and sequestered within capsules that were surgically implanted into the vitreous of the eye. The outer membrane of the encapsulated cell implant is semipermeable to allow CNTF to reach the retina. Ten participants received CNTF implants in one eye. When the implants were removed after 6 months, they contained viable cells with minimal cell loss and gave CNTF output at levels previously shown to be therapeutic for retinal degeneration in rcd1 dogs. Although the trial was not powered to form a judgment as to clinical efficacy, of seven eyes for which visual acuity could be tracked by conventional reading charts, three eyes reached and maintained improved acuities of 10–15 letters, equivalent to two- to three-line improvement on standard Snellen acuity charts. A surgically related choroidal detachment in one eye resulted in a transient acuity decrease that resolved with conservative management. This Phase I trial indicated that CNTF is safe for the human retina even with severely compromised photoreceptors. The approach to delivering therapeutic proteins to degenerating retinas using encapsulated cell implants may have application beyond disease caused by genetic mutations.
Keywords: clinical trial, neurodegeneration, retinitis pigmentosa, photoreceptor, macular degeneration
Retinitis pigmentosa (RP) describes a set of neurodegenerative retinal diseases that cause the death of photoreceptor cells and lead to progressive vision loss and blindness. More than 39 genetic loci and genes have been implicated in monogenic forms of RP (1), underscoring the complexity of its pathogenic mechanisms. With the exception of vitamin A nutritional supplementation (2), no treatments have been shown to be effective across the range of these disorders. Regardless of the initial causative genetic defect, the end result is photoreceptor cell death. The multiplicity of mechanisms stimulated a search for therapeutic agents that are effective in slowing photoreceptor death regardless of the causative genetic mutation.
Intervention studies have indicated the possibility of using neurotrophic factors as therapeutic agents for RP (3). Specifically, ciliary neurotrophic factor (CNTF) is effective at retarding retinal degeneration in at least 13 animal RP models, including rd-PDE6b mice (4, 5), rds-peripherin mice (4, 6–10), transgenic rats expressing P23H or S334ter mutant rhodopsin (7, 10), Rdy cats (11), rcd1-PDE6b dogs (7), rhodopsin-knockout mice (9), and rd/rd mice, nr/nr mice, and Q334ter rhodopsin transgenic mice (4).
CNTF also appears effective for retarding cellular and functional losses in neurodegenerative diseases of the CNS. In mouse models of amyotrophic lateral sclerosis, CNTF significantly reduced motor neuron loss (12, 13), improved motor function, and increased survival time (14). CNTF neuroprotection and functional rescue was also reported in Huntington’s disease models in rat (15–17) and primate (18, 19).
CNTF was first identified as a survival factor in studies involving ciliary ganglion neurons in the chick eye (20). CNTF is a member of the IL-6 family of cytokines (21–23) and acts through a heterotrimeric receptor complex composed of CNTF receptor α plus two signal-transducing transmembrane subunits, leukemia inhibitory factor receptor β and glycoprotein gp130 (24). CNTF receptor α is located on Müller glial membranes (25, 26) and on rod and cone photoreceptors (27–31).
Despite the promise of CNTF in treating neurodegenerative disorders, it has not been evaluated for clinical use in human eyes because of the lack of an effective sustained delivery system. Like the blood–brain barrier, the blood–retinal barrier (32) restricts access from the blood stream to the neural retina tissue. Circumventing these barriers is one of the major challenges for long-term sustained delivery of proteins to the retina and CNS. Encapsulated cell technology offers a promising approach to overcoming this challenge. Preclinical studies using encapsulated cell-based CNTF delivery provided photoreceptor protection in a dose-dependent manner when implanted into the eye of the rcd1 dog with a cGMP-PDE6b mutation (7). The implants were loaded with human retinal pigment epithelium cells that had been transfected with the CNTF gene to produce CNTF protein in situ. The semipermeable membrane allows CNTF to diffuse out and nutrients to diffuse in but prevents the attack by the host immune system, thereby providing a sustainable supply of the rescue factor over an extended time and possibly for years. In addition, the encapsulated cell implants can be retrieved from the eye at any time, providing an additional level of safety. We now report the outcome of a completed Phase I trial that evaluated the safety of CNTF delivered over a 6-month period by encapsulated cell capsules implanted into human eyes.
Results
Primary Outcome
All 10 participants completed the full 6-month study implantation period. Subjects 1–5 received lower-dose implants and subjects 6–10 received higher-dose implants. No implant was rejected or extruded, and severe ocular inflammation did not occur. No ocular or systemic complications ensued that met predetermined protocol-defined adverse outcomes. No lens opacities that affected visual function developed during the implant period. No abnormal serum/urine chemistry, hematology, or urinalysis of clinical Grade II or higher occurred. Participant serum samples were collected multiple times during the implant period, and none had detectable levels of CNTF or antibodies to either CNTF or NTC-201 cells. Only minor fibrosis developed around the surgical implant site. Five eyes receiving the CNTF implant had intermittent low-grade anterior ocular inflammation during the trial, evidenced by transient trace cells and flare in the anterior chamber. None were at a level that warranted treatment, and one had similar findings in the fellow control eye. All participants started with grade 1+ vitreous cells commonly associated with RP, and this remained constant throughout the study period.
A shallow choroidal detachment was observed in the study eye of participant 004 two weeks after the longer, lower-dose CNTF device was implanted. The choroidal detachment shifted the implant into the visual axis and diminished the acuity. Topical corticosteroids were given, and the choroidal detachment resolved before explant surgery, with visual acuity returning nearly to baseline levels. This eye developed a nuclear cataract 4.5 months after the capsule was removed. Participant 010 developed a small peripheral, inferotemporal, posterior subcapsular cataract at month 6, which did not affect visual acuity.
Visual Acuity
Three participants (001, 003, and 005) with extremely limited baseline acuity of <20/800 in the study eyes reported that their visual perception did not diminish during the study, even though we could not document this with formal visual acuity measurements. All participants with exception of 004 were enthusiastic about their participation, and several gave subjective, anecdotal reports of “brighter vision,” “more vivid color perception” or “sharper vision.” Such reports, although interesting, must be qualified by possible placebo effects associated with open label studies.
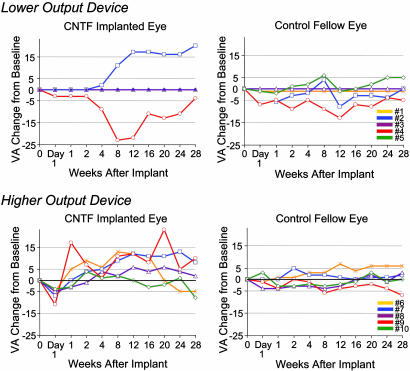
Three of seven study eyes in which ETDRS (Early Treatment of Diabetic Retinopathy Study) visual acuities could be tracked recovered substantial acuity from baseline (Fig. 1), whereas fellow eye acuities were essentially unchanged. These acuity gains were still evident 6 months after CNTF implants were removed. Participant 002 could not read any letters on the four initial examinations of baseline and at 1 day and 1 and 2 weeks after implant. On subsequent examinations, he gradually distinguished an increasing number of acuity letters, and, by week 12, he read 17 letters. One month after explant surgery (week 28), participant 002 read 20 letters (i.e., equivalent to a four-line improvement on a Snellen acuity chart). He maintained a 15-letter improvement when examined 6 months after the implant was removed.
Fig. 1.
Visual acuity (VA) changes of the study eyes and fellow control eyes of the 10 participants over the 6-month implant period grouped by lower-dose implants (Phase IA) and higher-dose implants (Phase IB). Letter acuity changes are relative to the preimplant baseline acuity (designated as zero) and are not the absolute baseline level. Three participants in Phase IA had no measurable acuity perception throughout the trial and remained at zero throughout. Time 0 is the baseline visit, explant was at week 24, and week 28 was the 1-month postremoval examination.
Participants 007 and 009 showed the greatest acuity improvements during the implant period among those receiving higher-dose capsules, with increases of 13 and 23 letters, respectively. Both individuals retained ≥10-letter improvement 6 months after the implant was removed. Participant 006 initially gained 11–13 letters during implant but then decreased to 5 letters below baseline by the end of the study. The remaining two participants (008 and 010) in the higher output group had negligible acuity changes from baseline. Overall, the five participants with higher-dose capsules had a mean visual acuity increase of 3.4 letters from baseline in study eyes and a median of 4.0 letters at week 28 (1 month after implant removal), whereas the mean acuity of the fellow control eyes was unchanged at 0.2 letters, and median was −1.0 letter at week 28.
Electroretinogram (ERG)
Only participant 007 had a measurable flicker ERG, which was 2.05, 2.45, and 2.00 μV (mean ± SD, 2.17 ± 0.25; n = 3) at screening and baseline and at 1 month after explant, respectively. At 1, 3, and 6 months during the implant period, the ERGs were 0.79, 0.98, and 1.25 μV (mean ± SD, 1.01 ± 0.23; n = 3). The ERG of the nonimplant fellow eye varied over a similarly limited range of 3.41–3.98 μV (mean ± SD, 3.68 ± 0.22; n = 6). The ERG responses of the study eye were significantly smaller during the implant period (P = 0.004; two-tailed, two-sample t test), which mirrors the ERG reductions noted in preclinical animal studies (8, 33). However, despite the ERG reduction, visual acuity continued to improve during the implantation period, and was 5, 12, and 13 letters above baseline at 4, 12, and 24 weeks, respectively.
Evaluation of CNTF NT-501 Implant After Removal
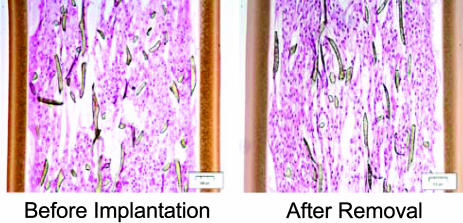
All 10 implants were examined immediately after they were surgically removed at 6 months, and all contained viable, apparently healthy, cells throughout (Fig. 2). On postexplant testing, the lower dose capsules produced 0.28 ± 0.07 ng/day (n = 5), and higher-dose capsules produced 1.53 ± 0.54 ng/day (n = 5), or an ≈5-fold dose difference (Table 1). Cell numbers were counted in three representative NT-501–6A.02 devices from the preimplant lots and from three representative devices removed after the Phase-IB 6-month implant period. The preimplant sections contained 542 ± 69 cells per field (n = 3), whereas the postexplant sections contained 532 ± 50 cells per field (n = 3), indicating minimal cell loss over the 6-month implantation period. No histological evidence of inflammation was found in any of the 10 devices. The 15-nm pore size of the device membrane is ≈1,000-fold smaller than the macrophage size of 10–20 μm, and no macrophage cells were evident on postremoval microscopy of any of the 10 devices.
Fig. 2.
Histology in longitudinal section of a CNTF device after removal at 6 months from the study eye of a Phase-IB participant. Histology of a comparable device that was not implanted is shown for comparison. NTC-201 cells are evident at approximately equal density on the poly(ethylene terephthalate) yarn scaffold. No macrophages were found in any explanted device. Shown are 4-μm-thick sections embedded in glycol methacrylate and stained with hematoxylin and eosin. (Magnification, ×10.)
Table 1.
CNTF release levels for the 10 capsules after removal
| Participant | CNTF output, ng/day |
|---|---|
| Phase IA | |
| 001 | 0.32 |
| 002 | 0.38 |
| 003 | 0.24 |
| 004 | 0.20 |
| 005 | 0.24 |
| Mean ± SD | 0.28 ± 0.07 |
| Phase IB | |
| 006 | 0.68 |
| 007 | 1.85 |
| 008 | 1.80 |
| 009 | 1.3 |
| 010 | 2.0 |
| Mean ± SD | 1.53 ± 0.54 |
The implant duration for all participants was 6 months.
Discussion
This clinical trial tested CNTF delivered to human eyes as a potential therapy for retinal neurodegeneration. The trial involved a dose escalation design, with the higher dose approximately five times that of the lower dose. The safety results were encouraging in that no systemic or ocular complications ensued that met predetermined protocol-defined adverse outcomes with the exception of a single shallow choroidal detachment in one eye receiving the lower-dose device. This eye developed a nuclear cataract 4.5 months after the implant was removed that was interpreted as likely secondary to mechanical insults related to the surgeries rather than to effects of CNTF. RP eyes can develop nuclear cataract (6–15%), although less commonly than posterior subcapsular cataract (PSC) (40–50%) (34, 35). Preclinical toxicology studies of these NT-501 CNTF devices showed that some animals developed PSC lens changes, but none occurred after the implant suture technique was optimized. In the present clinical trial, none of the three other participants with natural lenses (one receiving a lower-dose implant and two receiving higher-dose implants) developed visually significant cataract during the study or over the 18-month follow-up period to date, although participant 010 had a small peripheral PSC near the implant wound.
Three of seven study eyes for which acuity could be tracked showed increases of 10–15 letters over baseline, equivalent to 2–3 lines of conventional Snellen acuity, and these increases were maintained when they were examined again 6 months after the implants had been removed. An upward but variable trend in visual acuity was also observed in the other study eyes, whereas visual acuity of the fellow eyes remained virtually unchanged. Although the sample size was small and this was not a placebo-controlled trial, the observed visual acuity improvements of some participants is intriguing because spontaneous acuity improvement runs counter to the normal course of retinal photoreceptor neurodegenerative disease.
The diminished ERG response of participant 007 during the CNTF capsule implant period mirrors ERG reductions observed in some animal models treated with CNTF, particularly in higher doses. All animal models consistently demonstrate prolonged photoreceptor survival with CNTF, but the ERG component was inconsistent (6, 8–10). Intraocular adenovirus-mediated CNTF gene transfer significantly increased the ERG scotopic a-waves and b-waves in the rds mouse (6). However, other examples of CNTF gene transfer using adenovirus-associated vectors caused no ERG change (8–10) or decreased all ERG components of the scotopic a-waves and b-waves and the photopic b-wave (8, 33). The route of administration and level of protein expression may play a role (8). NT-501 capsules implanted into normal rabbits secreted CNTF at twice the levels used in this present human trial, but this did not reduce the rabbit scotopic ERG responses and indeed augmented responses to the dimmest stimuli, although the photopic b-wave responses to dim stimuli were reduced slightly (36). The functional implication of reduced ERG responses with CNTF is not clear. But the observation remains that visual acuity of participant 007 increased concurrent with the ERG reduction. A similar observation of increased acuity but reduced ERG was reported for intraocular injection of INF-α2b in human for neovascular age-related macular degeneration (37). CNTF-induced ERG suppression appears to be transient and reversible in animals (R. Wen, personal communication), again mirroring the ERG amplitude recovery for participant 007 after removal of the CNTF implant.
The effects of CNTF on cone photoreceptor protection and macular function have not been investigated extensively, but the positive acuity changes in participants 002, 007, and 009 cause one to consider whether neurotrophic factors might improve visual function in some cases of advanced RP with atrophic macular degeneration. A biologically plausible hypothesis can be proposed. Performance of visual acuity tasks require relatively few cone photoreceptors (38), as was deduced from experimental modeling of visual perception in human Stargardt macular degeneration, which indicated that a 90% loss of foveal cone photoreceptors was still commensurate with 20/50–20/100 acuity (39). The small number of cone photoreceptors required for visual acuity tasks was also demonstrated by an individual with RP from a rhodopsin P23H mutation who, shortly before death, retained 20/50 acuity despite the >60% loss of para-foveal cones and severe shortening of the remaining outer segments on postmortem ocular histology (40). Furthermore, for at least some animal genetic models of photoreceptor degeneration and loss, a clear relationship exists between the number of photoreceptor nuclei remaining and the progressive shortening of the photoreceptor outer segments (41). At some stage of degeneration, short outer segments will severely limit photon catch such that the remaining photoreceptor cells will become functionally unresponsive under ordinary light levels and will no longer contribute to visual acuity. Rod photoreceptors of the rhodopsin knockout rho−/− mouse are evidence of this, because they produce no visual pigment and do not elaborate any outer segments, but the rod cell soma survive for many weeks, despite their nonfunctional status for vision (42). Consequently, it is biologically plausible that CNTF may improve human visual acuity by eliciting sufficient metabolic activity in damaged cone photoreceptors to allow them to resume contributing to visual tasks. Evidence that CNTF augments cellular activity of gene expression is found in the nuclear DNA uncoiling observed in photoreceptor cells of mice (8) and rabbits (36) treated with high-dose CNTF. Such increased photoreceptor nuclear and metabolic activity from CNTF could underlie the observation from this Phase I trial that several of the implanted eyes showed a trend of better acuity on a letter recognition task compared with fellow control eyes. This hypothesis cannot readily be evaluated in nonverbal animal models, because biologically subtle improvements that would support improved visual function are below the detection sensitivity of standard laboratory ERG functional studies and probably are below the level of change detectable by retinal structural measurements. All of these observations support a biological rationale for proposing further studies to learn whether CNTF can rescue visual function in atrophic macular degeneration when delivered by means of the encapsulated cell implants.
Effective delivery of neurotrophic molecules to target sites in the CNS and the eye has proven to be a formidable task because of the barrier properties of the brain and eye. Despite promising results in short-term animal preclinical studies, few if any proteins have become successful therapeutics for human CNS or eye disorders. A clinical trial of systemically administered CNTF for amyotrophic lateral sclerosis is a good example. In this trial, despite delivery of high systemic doses, CNTF was undetectable in the CNS, and no therapeutic benefit was identified. In addition, the high peripheral CNTF levels were associated with major side effects, including fever, fatigue, and blood chemistry changes, that were consistent with activation of the acute phase response (43, 44). One reason for these disappointing results may relate to the difficulty in achieving adequate concentrations of drug at the appropriate site. In contrast to systemic administration, a continuous and site-specific delivery system, as described in this trial, should optimize the pharmacokinetics of potential therapeutic agents to the eye.
Encapsulated cell implants provide an attractive alternative to the conventional means of administration, because a wide range of therapeutic agents can be engineered into cells to address a broad range of medical applications. Mammalian cell-produced protein, freshly synthesized and released within the target site in situ, is therapeutically more potent than purified recombinant factors (45) and therefore reduces the dose requirement. Th low dose requirement and the limited distribution volume of the eye and CNS, along with the presence of the blood–retinal and blood–brain barriers, minimize the potential for systemic toxicity of protein released by encapsulated cell device delivery. Because the cell-containing implants can be retrieved an important layer of safety is added to their use.
This trial indicates the safety and promising utility of encapsulated cell delivery as a mode of administration of protein therapeutics to the eye. The results raise the intriguing possibility that CNTF may improve visual acuity in some eyes with advanced RP and atrophic macular degeneration. At the end of the 6-month implantation duration, all explanted capsules contained viable cells that secreted CNTF at expected levels that were therapeutic in the rcd1 dog study (7). Because pharmacokinetic data on preclinical studies showed continued CNTF production out to 1 year and beyond (46), encapsulated cell implants may provide a longer-term therapeutic release that will facilitate efficacy studies in retinal and macular neurodegenerative diseases. These results, coupled with robust implant performance, provide the basis for considering the next stages of human trials of CNTF delivered by encapsulated cell implants.
Materials and Methods
This prospective nonrandomized Phase I study was conducted at a single center and used an open-label dose-escalation format to investigate the safety of human CNTF delivered by encapsulated cell devices that were surgically implanted into one eye each of 10 participants who had vision loss from degeneration of retinal photoreceptors. The implantation period was 6 months, and all devices were then removed. The protocol was formulated and conducted by investigators of the National Eye Institute and carried out in the intramural clinical facilities of the National Institutes of Health. The CNTF encapsulated cell devices were provided by Neurotech USA under a National Institutes of Health Clinical Trials Agreement.
CNTF Implant
The CNTF-secreting, encapsulated cell implants, designated NT-501 (Neurotech USA), are 1 mm in diameter and are constructed of a semipermeable polymer outer membrane with 15-nm pores. The implants contain an internal poly(ethylene terephthalate) yarn scaffold that supports human mammalian cells. These cells (designated NTC-201) derived originally from human retinal pigment epithelium cell line ARPE-19 (catalog no. CRL-2302; American Type Culture Collection) and were genetically engineered to produce human CNTF. CNTF was targeted for secretion by fusing the genomic murine Ig signal peptide in frame to the 5′ end of the hCNTF gene. Two separate transfections with this construct yielded two independent cell lines that released CNTF at different output rates. The two lines were designated NTC-201-10 and NTC-201-6A and released CNTF at rates of 250 and 800 ng per 1 × 106 cells per day in vitro, respectively. The implants for the first five participants (NT-501-10) that released lower amounts of CNTF were 11 mm long (including the titanium anchoring loop) and were loaded with 435,000 cells from the lower-CNTF-expressing line NTC-201-10. The implants for the second five participants were engineered to be shorter (6 mm), although they released a greater amount of CNTF. These 6-mm-long devices (NT-501-6A.02) were loaded with 203,000 cells from the higher-CNTF-expressing NTC-201-6A line. CNTF output was established empirically and involves the intrinsic CNTF delivery rate of the cell line and the number of CNTF-secreting cells loaded into the capsule. Shorter implants are preferable to avoid blocking the optical axis of the eye and to increase the safety of surgical implantation and removal.
Protocol Design
The trial and reviews were conducted according to the guidelines of the Declaration of Helsinki. The protocol was approved by the Institutional Review Board of the National Institute on Alcohol Abuse and Alcoholism. The CNTF implants have not previously been implanted in humans, and a strict monitoring plan was developed and implemented by an independent Data and Safety Monitoring Committee. All participants gave knowledgeable signed consent before collecting data and initiating therapy. These CNTF implants contain genetically engineered cells, and the protocol was reviewed by the National Institutes of Health Recombinant DNA Advisory Committee (June 18, 2003).
The trial was conducted in two phases, with five participants in Phase IA receiving lower-dose capsules and five in Phase IB receiving higher-dose capsules. Surgical implantation was spaced to allow monitoring of the earlier participants. Participants received a preimplantation baseline examination and were examined at 1 day after surgery and at 1, 2, 4, 8, 12, 16, 20, and 24 weeks after implantation. The CNTF implants were surgically removed 1 day after the 24-week examination (i.e., nominally at 6 months after implantation), and the participants were then examined 1 month later at 28 weeks.
All participants were >18 years of age and had a clinical diagnosis of advanced RP with photoreceptor degeneration established by reduced visual acuity, visual field constriction, night blindness, marked reduction of rod and cone ERG responses, and presence of intraretinal “bone-spicule” pigment on clinical examination. None had glaucoma, retinal inflammatory disease, macular edema, or herpes simplex virus of the eye. None were on treatment for diabetes or cancer, and none were pregnant. None had cataract. Five participants previously had cataract surgery in the study eye, four had received intraocular lens implants, and one remained aphakic. Additional selection criteria are given in Supporting Appendix 1, which is published as supporting information on the PNAS web site.
Ten participants were enrolled between October 21, 2003 and October 25, 2004. Eight participants were male, and two were female, ages 30–71 years. Eight were Caucasian, one was African American, and one was Asian. The family histories indicated that six were simplex isolates, one was autosomal recessive, one was autosomal dominant, and two were X-linked recessive. Genetic screening began after trial enrollment and thus far has identified a choroideremia gene mutation in participant 007. As a consequence of selecting all participants with diminished visual acuity, the majority of participants had atrophic macular degeneration changes in addition to retinal changes typical for RP, as seen in the representative trial participant in Fig. 3.
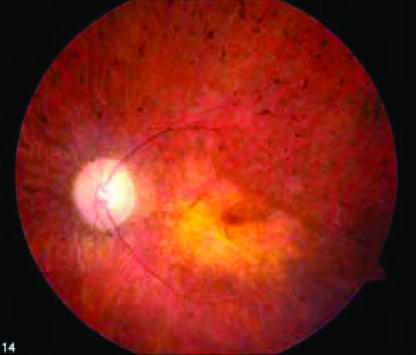
Fig. 3.
Ocular fundus photograph of CNTF trial participant 001 shows clinical features representative of all 10 participants who have peripheral retina pigmentary changes typical for retinitis pigmentosa plus a central yellowish region of atrophic macular degeneration that accounts for the reduced visual acuities.
Visual acuity was tested by using multiple ETDRS charts under standard conditions (47). The first two participants had visual acuity of 20/400 or less, and the remaining eight had 20/100 or less in the study eye, with the same or better in the fellow eye. Eligibility also required visual field constriction to a <40° diameter on Goldmann perimetry with the large, V4e isopter white target (48) and photopic 30-Hz, flicker ERG responses of ≤2 μV to standard stimuli (49).
Primary Outcome
The primary outcome was ocular safety after implantation of the CNTF capsule. The main safety metrics were based on clinical slit-lamp examination of the anterior segment and vitreous for inflammatory haze and cells (50), and on ophthalmoscopy, plus testing of visual acuity and visual fields. The following adverse outcomes were specifically designated in the trial protocol.
Rejection or extrusion of the NT-501 implant.
Serious adverse events, potentially implant-related, such as severe inflammation or abnormal findings on serum chemistry, hematology, urinalysis, or urine chemistry out of range or indicating clinical chemistry toxicity of Grade II or higher.
Formation of systemic antibodies to CNTF or to NTC-201.
Symptoms of immune disorder or allergy.
Periimplant fibrosis blocking the visual axis or affecting the lens or with potential to contribute to retinal detachment.
Development or progression of lens opacity that impacted vision during the trial.
Substantial reduction of function from baseline indicated by (i) a more than three-line decrease in the best corrected visual acuity or reduction to no light perception, (ii) a ≥50% reduction in ERG amplitude, or (iii) a ≥50% reduction in visual field measured by the sum of meridian scores.
Secondary Outcome
Secondary outcomes were functional measures of best-corrected visual acuity using letter perception on ETDRS charts (for which each five letters equates to one line change on a traditional Snellen reading chart), visual fields by Goldmann perimetry, and photopic 32-Hz, flicker ERG (49). These participants had limited vision from advanced RP, which precluded obtaining highly reproducible visual field data for serial tracking. Fundus photography and optical coherence tomography (51) were obtained. Optical coherence tomography scans revealed thin retinas with abnormally low optical density indicative of photoreceptor changes associated with retinitis pigmentosa.
Surgery
Participants received 1 mg·kg−1·day−1 oral prednisone for 1 week before intraocular implantation, which required ≈15 min to perform under retrobulbar anesthesia of 0.75% bupivacaine at a 1:1 mixture with 4% lidocaine. The implant was inserted through a 2.0-mm sclerotomy made 3.75 mm posterior to the limbus in the inferotemporal quadrant and anchored with a single suture. A subconjunctival antibiotic injection of 100 mg of cefazolin was given at the conclusion of surgery, and topical 1% prednisolone acetate and ciproflaxocin drops were given daily over the following week. The implants were surgically removed after 6 months with a modified vitrectomy procedure after reopening the wound and dissecting any fibrous attachments or residual vitreous adherence. Clinical care after removing the CNTF implant was similar to that after the implant procedure. Minor to moderate fibrosis was found around the implant attachment point and did not impact ocular function.
Serum Antibodies to CNTF and NTC-201 Cells
Participant serum samples were collected at preimplantation baseline, at 1, 2, 8, 12, and 24 weeks after implantation, and again 1 month after devices were removed (study week 28). The serum anti-hCNTF antibody titer was determined by an anti-hCNTF ELISA by incubating participant serum on a plate coated with hCNTF (R & D Systems), and the signal was detected by a secondary antibody of horseradish peroxidase-conjugated donkey anti-human IgG (Jackson ImmunoResearch). Titers for serum antibodies against the NTC-201 cells were determined by ELISA by incubating participant serum on a plate coated with NTC-201 cells for 16 h and then probing with horseradish peroxidase-conjugated donkey anti-human IgG (Jackson ImmunoResearch).
Evaluation of NT-501 Implant After Removal
The implants were removed from all 10 eyes at 6 months and evaluated by function (CNTF output) and morphology (histology). Immediately upon removal, the devices were placed into human endothelial serum-free conditioned medium (GIBCO/BRL) at 37°C, 5% CO2, 95% humidity for 24 h, and the rate of CNTF secretion was determined with a commercial ELISA kit (R & D Systems). The CNTF standard was prepared according to the package insert, and all standard and sample dilutions were performed in duplicate. Capsules were then fixed in 4% paraformaldehyde for 1 h and embedded in methacrylate. Ten consecutive longitudinal sections 4 μm thick were cut from the center of each capsule and stained with hematoxylin and eosin, and cells were counted in light microscopy images at ×10 magnification.
Supplementary Material
Acknowledgments
We thank Drs. Ruben Adler, Gustavo Aguirre, Jean Bennett, Alan Bird, Alan Laties, Matthew LaVail, and Jose Sahel for thoughtful input before trial initiation; Drs. Deborah Carper, Frederick Ferris, and Santa Tumminia for assistance during the trial; and Dr. Neal Oden for providing statistical consultation. Gordon Byrnes, M.D. (Retina Group of Washington, Rockville, MD), surgically implanted the first device. Patrick Lopez, C.O.T., and Leanne Reuter, C.O.A., collected visual function data. Diane Litten, R.N., B.S.N., and Renee Gaiter, R.N., B.S.N., were trial coordinators. Juanita Marner helped with manuscript preparation, and Cathy Geer helped with figure formatting. This work was supported by National Institutes of Health Intramural Program Funding for Human Protocol 03-EI-0234 and by Contract N01-EY-1-2113.
Abbreviations
- RP
retinitis pigmentosa
- CNTF
ciliary neurotrophic factor
- ERG
electroretinogram
Footnotes
Conflict of interest statement: W.T. is employed by Neurotech USA, which provided the CNTF encapsulated cell implants under a National Institutes of Health Clinical Trials Agreement.
References
- 1.Hims M. M., Diager S. P., Inglehearn C. F. Dev. Ophthalmol. 2003;37:109–125. doi: 10.1159/000072042. [DOI] [PubMed] [Google Scholar]
- 2.Berson E. L., Rosner B., Sandberg M. A., Hayes K. C., Nicholson B. W., Weigel-DiFranco C., Willett W. Arch. Ophthalmol. 1993;111:761–772. doi: 10.1001/archopht.1993.01090060049022. [DOI] [PubMed] [Google Scholar]
- 3.Faktorovich E. G., Steinberg R. H., Yasumura D., Matthes M. T., LaVail M. M. Nature. 1990;347:83–86. doi: 10.1038/347083a0. [DOI] [PubMed] [Google Scholar]
- 4.LaVail M. M., Yasumura D., Matthes M. T., Lau-Villacorta C., Unoki K., Sung C. H., Steinberg R. H. Invest. Ophthalmol. Vis. Sci. 1998;39:592–602. [PubMed] [Google Scholar]
- 5.Cayouette M., Gravel C. Hum. Gene Ther. 1997;8:423–430. doi: 10.1089/hum.1997.8.4-423. [DOI] [PubMed] [Google Scholar]
- 6.Cayouette M., Behn D., Sendtner M., Lachapelle P., Gravel C. J. Neurosci. 1998;18:9282–9293. doi: 10.1523/JNEUROSCI.18-22-09282.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tao W., Wen R., Goddard M. B., Sherman S. D., O’Rourke P. J., Stabila P. F., Bell W. J., Dean B. J., Kauper K. A., Budz V. A., et al. Invest. Ophthalmol. Vis. Sci. 2002;43:3292–3298. [PubMed] [Google Scholar]
- 8.Bok D., Yasumura D., Matthes M. T., Ruiz A., Duncan J. L., Chappelow A. V., Zolutukhin S., Hauswirth W., LaVail M. M. Exp. Eye Res. 2002;74:719–735. doi: 10.1006/exer.2002.1176. [DOI] [PubMed] [Google Scholar]
- 9.Liang F. Q., Dejneka N. S., Cohen D. R., Krasnoperova N. V., Lem J., Maguire A. M., Dudus L., Fisher K. J., Bennett J. Mol. Ther. 2001;3:241–248. doi: 10.1006/mthe.2000.0252. [DOI] [PubMed] [Google Scholar]
- 10.Liang F. Q., Aleman T. S., Dejneka N. S., Dudus L., Fisher K. J., Maguire A. M., Jacobson S. G., Bennett J. Mol. Ther. 2001;4:461–472. doi: 10.1006/mthe.2001.0473. [DOI] [PubMed] [Google Scholar]
- 11.Chong N. H., Alexander R. A., Waters L., Barnett K. C., Bird A. C., Luthert P. J. Invest. Ophthalmol. Vis. Sci. 1999;40:1298–1305. [PubMed] [Google Scholar]
- 12.Sagot Y., Tan S. A., Baetge E., Schmalbruch H., Kato A. C., Aebischer P. Eur. J. Neurosci. 1995;7:1313–1322. doi: 10.1111/j.1460-9568.1995.tb01122.x. [DOI] [PubMed] [Google Scholar]
- 13.Aebischer P., Kato A. C. Eur. Neurol. 1995;35:65–68. doi: 10.1159/000117095. [DOI] [PubMed] [Google Scholar]
- 14.Lindsay R. M. Neurobiol. Aging. 1994;15:249–251. doi: 10.1016/0197-4580(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 15.Emerich D. F., Lindner M. D., Winn S. R., Chen E. Y., Frydel B. R., Kordower J. H. J. Neurosci. 1996;16:5168–5181. doi: 10.1523/JNEUROSCI.16-16-05168.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regulier E., Pereira de Almeida L., Sommer B., Aebischer P., Deglon N. Hum. Gene Ther. 2002;13:1981–1990. doi: 10.1089/10430340260355383. [DOI] [PubMed] [Google Scholar]
- 17.de Almeida L. P., Zala D., Aebischer P., Deglon N. Neurobiol. Dis. 2001;8:433–446. doi: 10.1006/nbdi.2001.0388. [DOI] [PubMed] [Google Scholar]
- 18.Emerich D. F., Winn S. R., Hantraye P. M., Peschanski M., Chen E. Y., Chu Y., McDermott P., Baetge E. E., Kordower J. H. Nature. 1997;386:395–399. doi: 10.1038/386395a0. [DOI] [PubMed] [Google Scholar]
- 19.Mittoux V., Joseph J. M., Conde F., Palfi S., Dautry C., Poyot T., Bloch J., Deglon N., Ouary S., Nimchinsky E. A., et al. Hum. Gene Ther. 2000;11:1177–1187. doi: 10.1089/10430340050015220. [DOI] [PubMed] [Google Scholar]
- 20.Adler R., Landa K. B., Manthorpe M., Varon S. Science. 1979;204:1434–1436. doi: 10.1126/science.451576. [DOI] [PubMed] [Google Scholar]
- 21.Varon S., Manthorpe M., Adler R. Brain Res. 1979;173:29–45. doi: 10.1016/0006-8993(79)91093-x. [DOI] [PubMed] [Google Scholar]
- 22.Bazan J. F. Neuron. 1991;7:197–208. doi: 10.1016/0896-6273(91)90258-2. [DOI] [PubMed] [Google Scholar]
- 23.Helfand S. L., Smith G. A., Wessells N. K. Dev. Biol. 1976;50:541–547. doi: 10.1016/0012-1606(76)90174-3. [DOI] [PubMed] [Google Scholar]
- 24.Stahl N., Yancopoulos G. D. J. Neurobiol. 1994;25:1454–1466. doi: 10.1002/neu.480251111. [DOI] [PubMed] [Google Scholar]
- 25.Peterson W. M., Wang Q., Tzekova R., Wiegand S. J. J. Neurosci. 2000;20:4081–4090. doi: 10.1523/JNEUROSCI.20-11-04081.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wahlin K. J., Campochiaro P. A., Zack D. J., Adler R. Invest. Ophthalmol. Vis. Sci. 2000;41:927–936. [PubMed] [Google Scholar]
- 27.Fuhrmann S., Kirsch M., Heller S., Rohrer H., Hofmann H. D. J. Comp. Neurol. 1998;400:244–254. [PubMed] [Google Scholar]
- 28.Valter K., Bisti S., Stone J. Brain Res. 2003;985:169–175. doi: 10.1016/s0006-8993(03)03130-5. [DOI] [PubMed] [Google Scholar]
- 29.Seydewitz V., Rothermel A., Fuhrmann S., Schneider A., DeGrip W. J., Layer P. G., Hofmann H. D. Invest. Ophthalmol. Vis. Sci. 2004;45:655–661. doi: 10.1167/iovs.03-0182. [DOI] [PubMed] [Google Scholar]
- 30.Beltran W. A., Zhang Q., Kijas J. W., Gu D., Rohrer H., Jordan J. A., Aguirre G. D. Invest. Ophthalmol. Vis. Sci. 2003;44:3642–3649. doi: 10.1167/iovs.02-0763. [DOI] [PubMed] [Google Scholar]
- 31.Beltran W. A., Rohrer H., Aguirre G. D. Mol. Vis. 2005;11:232–244. [PubMed] [Google Scholar]
- 32.Pournaras C. J., Donati G. In: Principles and Practice of Ophthalmology. Albert D. M., Jakobiec F. A., editors. Vol. 3. Philadelphia: Saunders; 2000. pp. 1808–1809. [Google Scholar]
- 33.Schlichtenbrede F. C., MacNeil A., Bainbridge J. W., Tschernutter M., Thrasher A. J., Smith A. J., Ali R. R. Gene Ther. 2003;10:523–527. doi: 10.1038/sj.gt.3301929. [DOI] [PubMed] [Google Scholar]
- 34.Heckenlively J. Am. J. Ophthalmol. 1982;93:733–738. doi: 10.1016/0002-9394(82)90469-x. [DOI] [PubMed] [Google Scholar]
- 35.Auffarth G. U., Tetz M. R., Krastel H., Blankenagel A., Volcker H. E. Ophthalmologe. 1997;94:642–646. doi: 10.1007/s003470050175. [DOI] [PubMed] [Google Scholar]
- 36.Bush R. A., Lei B., Tao W., Raz D., Chan C. C., Cox T. A., Santos-Muffley M., Sieving P. A. Invest. Ophthalmol. Vis. Sci. 2004;45:2420–2430. doi: 10.1167/iovs.03-1342. [DOI] [PubMed] [Google Scholar]
- 37.Kertes P. J., Britton W. A., Jr, Leonard B. C. Can. J. Ophthalmol. 1997;32:185–188. [PubMed] [Google Scholar]
- 38.Geller A. M., Sieving P. A. In: Retinal Degeneration. Anderson R. E., Hollyfield J. G., LaVail M. M., editors. New York: Plenum; 1993. pp. 25–34. [Google Scholar]
- 39.Geller A. M., Sieving P. A. Vision Res. 1993;33:1509–1524. doi: 10.1016/0042-6989(93)90144-l. [DOI] [PubMed] [Google Scholar]
- 40.Sieving P. A. In: Ophthalmology. Yanoff M., Duker J. S., editors. St. Louis: Mosby; 2004. pp. 813–823. [Google Scholar]
- 41.Machida S., Kondo M., Jamison J. A., Khan N. W., Kononen L. T., Sugawara T., Bush R. A., Sieving P. A. Invest. Ophthalmol. Vis. Sci. 2000;41:3200–3209. [PubMed] [Google Scholar]
- 42.Humphries M. M., Rancourt D., Farrar G. J., Kenna P., Hazel M., Bush R. A., Sieving P. A., Sheils D. M., McNally N., Creighton P., et al. Nat. Genet. 1997;15:216–219. doi: 10.1038/ng0297-216. [DOI] [PubMed] [Google Scholar]
- 43.Cedarbaum J. M. Clin. Neuropharmacol. 1995;18:500–514. doi: 10.1097/00002826-199512000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Cedarbaum J. M. Clin. Neuropharmacol. 1995;18:515–532. doi: 10.1097/00002826-199512000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Hoane M. R., Puri K. D., Xu L., Stabila P. F., Zhao H., Gulwadi A. G., Phillips H. S., Devaux B., Lindner M. D., Tao W. Exp. Neurol. 2000;162:189–193. doi: 10.1006/exnr.2000.7311. [DOI] [PubMed] [Google Scholar]
- 46.Thanos C. G., Bell W. J., O’Rourke P., Kauper K., Sherman S., Stabila P., Tao W. Tissue Eng. 2004;10:1617–1622. doi: 10.1089/ten.2004.10.1617. [DOI] [PubMed] [Google Scholar]
- 47.Ferris F. L., III, Kassoff A., Bresnick G. H., Bailey I. Am. J. Ophthalmol. 1982;94:91–96. [PubMed] [Google Scholar]
- 48.Anderson D. R., Allison L. M. Testing the Field of Vision. St. Louis: Mosby; 1982. [Google Scholar]
- 49.Sieving P. A., Arnold E. B., Jamison J., Liepa A., Coats C. Invest. Ophthalmol. Vis. Sci. 1998;39:1462–1469. [PubMed] [Google Scholar]
- 50.Nussenblatt R. B., Palestine A. G., Chan C. C., Roberge F. Ophthalmology. 1985;92:467–471. doi: 10.1016/s0161-6420(85)34001-0. [DOI] [PubMed] [Google Scholar]
- 51.Schuman J. S., Puliafito C. A., Fujimoto J. G. Optical Coherence Tomography of Ocular Diseases. Thorofare, NJ: Slack; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.