Abstract
Caenorhabditis elegans shows chemoattraction to 0.1–200 mM NaCl, avoidance of higher NaCl concentrations, and avoidance of otherwise attractive NaCl concentrations after prolonged exposure to NaCl (gustatory plasticity). Previous studies have shown that the ASE and ASH sensory neurons primarily mediate attraction and avoidance of NaCl, respectively. Here we show that balances between at least four sensory cell types, ASE, ASI, ASH, ADF and perhaps ADL, modulate the response to NaCl. Our results suggest that two NaCl-attraction signalling pathways exist, one of which uses Ca2+/cGMP signalling. In addition, we provide evidence that attraction to NaCl is antagonised by G-protein signalling in the ASH neurons, which is desensitised by the G-protein-coupled receptor kinase GRK-2. Finally, the response to NaCl is modulated by G-protein signalling in the ASI and ADF neurons, a second G-protein pathway in ASH and cGMP signalling in neurons exposed to the body fluid.
Keywords: behavioural plasticity, C. elegans, NaCl, sensory signalling, taste
Introduction
Salt taste is essential for ion and water homeostasis; however, our understanding of the molecular mechanisms of salt perception is relatively limited (Lindemann, 2001). In rodents and Drosophila, salts are thought to be primarily sensed by cation influx through degenerin/epithelial Na+ channels (DEG/ENaC), leading to membrane depolarisation (Lindemann, 2001; Liu et al, 2003). In humans, the contribution of ENaC channels is less pronounced and salts are thought to be detected by a vanilloid receptor-1 (TRPV1) variant, a member of the transient receptor potential (TRP) cation channel family (Lyall et al, 2004). We use the nematode Caenorhabditis elegans to unravel the molecular mechanisms that mediate the response to NaCl.
C. elegans shows chemoattraction to low NaCl and avoidance of high NaCl concentrations (Ward, 1973; Dusenbery et al, 1975; Culotti and Russell, 1978). Chemoattraction to salts is mediated by four pairs of amphid sensory neurons (ADF, ASE, ASG and ASI), of which the ASE cells are most important (Bargmann and Horvitz, 1991). Several genes involved in salt detection have been identified. These include the guanylate cyclase DAF-11, the cGMP-gated cation channel subunits TAX-2 and TAX-4, and the calcineurin A subunit TAX-6, suggesting that C. elegans uses cGMP and Ca2+ signalling in salt detection (Coburn and Bargmann, 1996; Komatsu et al, 1996; Birnby et al, 2000; Kuhara et al, 2002). C. elegans avoids NaCl concentrations above 200 mM (RK Hukema and G Jansen, unpublished results). This response is thought to be due to a general avoidance of high osmotic strength. The ASH sensory neurons play a pivotal role in this process (Bargmann et al, 1990). Osmotic avoidance requires the Gα protein ODR-3, the TRP channel subunits OSM-9 and OCR-2, the cytoplasmic protein OSM-10, the G-protein-coupled receptor kinase GRK-2 and glutamatergic neurotransmission, involving the glutamate receptors GLR-1 and NMR-1, the glutamate transporter EAT-4 and the proprotein convertase EGL-3 (Colbert et al, 1997; Berger et al, 1998; Roayaie et al, 1998; Hart et al, 1999; Mellem et al, 2002; Tobin et al, 2002; Fukuto et al, 2004).
The behavioural response to salts is plastic and depends on various cues, including exposure time and salt concentration. We use a new chemotaxis assay to analyse both salt detection and its plasticity (Figure 1A; Wicks et al, 2000; Jansen et al, 2002). In the plasticity assay, animals are washed in a buffer containing 100 mM NaCl and subsequently analysed for chemotaxis. These pre-exposed animals show avoidance of otherwise attractive NaCl concentrations. This behaviour is time and concentration dependent, reversible and partially salt specific. Thus far, two proteins have been shown to contribute to this behaviour, the TRP channel subunit OSM-9 and the Gγ protein GPC-1 (Jansen et al, 2002). The finding that prolonged exposure to NaCl results in avoidance suggests a mechanism that involves more than adaptation or desensitisation. We propose a model in which the behavioural response to salt involves a balance between attraction and avoidance, as has been described for the regulation of the response to benzaldehyde, aggregation behaviour and the integration of sensory signals (Nuttley et al, 2001; de Bono et al, 2002; Ishihara et al, 2002). We call this behaviour gustatory plasticity.
Figure 1.
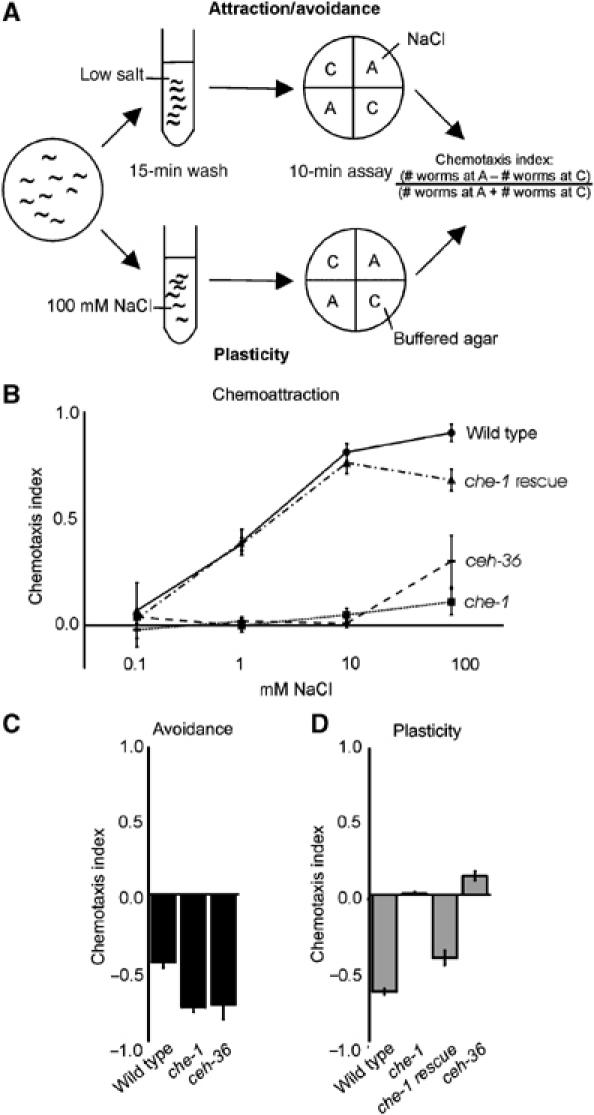
Both chemoattraction to NaCl and gustatory plasticity require the ASE neurons. (A) Worms are washed in a low-salt buffer or a buffer containing 100 mM NaCl and tested for chemotaxis. The assay plates contain two quadrants with only buffered agar (C) and two quadrants with buffered agar and NaCl (A). We use 0.1, 1, 10 or 100 mM NaCl to test for chemoattraction, 1 M NaCl to test for avoidance and 25 mM NaCl in the gustatory plasticity assay. The distribution of the animals over the quadrants is determined after 10 min, and the chemotaxis index is calculated. (B) ASE function is required for the detection of NaCl, since chemotaxis to NaCl was abolished in che-1 and ceh-36 mutants (P<0.001). Chemotaxis could be restored by introduction of a che-1 transgene (che-1 rescue, P<0.001). (C) che-1 and ceh-36 animals strongly avoided 1 M NaCl. (D) che-1 and ceh-36 animals showed no significant response to NaCl after pre-exposure to 100 mM NaCl (P<0.001 compared to wild-type animals). The che-1 defect could be rescued by introduction of a che-1 transgene (che-1 rescue). In all figures: panels show mean±s.e.m.; n⩾4 for all assays. Open bars indicate chemotaxis to 25 mM NaCl; grey bars indicate the response to 25 mM NaCl after pre-exposure to 100 mM NaCl; black bars show the response to 1 M NaCl.
In this paper, we show that four pairs of amphid sensory neurons and neurons exposed to the body fluid are involved in gustatory plasticity. Our results suggest that two signalling pathways mediate attraction to NaCl, likely in the ASE neurons. One of these pathways involves cGMP and Ca2+ signalling. We show that attraction to NaCl is antagonised by a G-protein-signalling pathway in the ASH neurons, which is desensitised by GRK-2. Furthermore, the response to NaCl is modulated by G-protein signalling in the ASI and ADF neurons, an additional G-protein pathway in the ASH neurons and cGMP signalling in neurons exposed to the body fluid.
Results
Antagonistic inputs from three pairs of sensory neurons modulate gustatory behaviour
Sensation of attractive NaCl concentrations is primarily mediated by the ASE neurons (Bargmann and Horvitz, 1991). To confirm this role of the ASE cells, we tested the behaviour of che-1 and ceh-36 mutant animals. che-1 mutants lack functional ASE neurons due to mutation of a zinc-finger protein similar to the Drosophila GLASS transcription factor (Uchida et al, 2003). che-1 is predominantly expressed in the ASE neurons; only occasionally expression was observed in other neurons (Uchida et al, 2003). ceh-36 encodes an Otx-related homeodomain protein expressed in the ASE and the AWC neurons, which specifies the identities of these neurons (Lanjuin et al, 2003). Both che-1 and ceh-36 mutant animals showed no significant chemotaxis to NaCl (0.1–100 mM; Figure 1B). In contrast, these animals showed wild type or even increased avoidance of 1 M NaCl (Figure 1C). Surprisingly, che-1 and ceh-36 animals showed no response to NaCl after pre-exposure to 100 mM NaCl (Figure 1D). The chemotaxis defects observed in che-1 animals could be rescued by introduction of the wild-type che-1 gene as a transgene (Figure 1B and D). Our results confirm that the ASE neurons are essential for chemotaxis to NaCl, also in our assay, and that these cells are not required for avoidance of 1 M NaCl (Bargmann and Horvitz, 1991; Uchida et al, 2003). Importantly, our results indicate that signalling via the ASE neurons is essential for gustatory plasticity.
To identify additional cells involved in gustatory plasticity, we used the Gγ subunit, GPC-1. gpc-1 mutants showed reduced avoidance of, or even attraction to, NaCl after pre-exposure (Jansen et al, 2002). Interestingly, gpc-1 is not expressed in the ASE neurons, but it is expressed in three other pairs of neurons involved in salt perception (Jansen et al, 2002): the ASI cells, which have a minor function in attraction to salts (Bargmann and Horvitz, 1991), and the ADL and ASH nociceptive neurons (Bargmann et al, 1990). Introduction of the wild type gpc-1 gene as a transgene in gpc-1 animals could restore the response to NaCl after pre-exposure (Figure 2A; Jansen et al, 2002). However, we never obtained full rescue of the defect. Since overexpression of gpc-1 induced a gustatory plasticity defect (Jansen et al, 2002), we surmise that probably the levels of GPC-1 are crucial for the behavioural response. Specific expression of GPC-1 in the ASI or ASH neurons partially restored avoidance after pre-exposure in gpc-1 mutants (Figure 2A). Expression of GPC-1 both in the ASI and ASH neurons did not yield better rescue (results not shown). These results suggest that ASI and ASH do not have additive functions; however, since gpc-1 expression levels seem a crucial determinant in this behaviour, these experiments are not conclusive. Expression of GPC-1 in the ADL and ASH nociceptive neurons resulted in better rescue than expression in the ASH neurons alone (Figure 2A), suggesting that GPC-1 probably also functions in ADL. Expression of gpc-1 in the ASE neurons did not restore gustatory plasticity (Figure 2A), suggesting that GPC-1 does not function non-cell-autonomously. Taken together, our findings indicate that GPC-1 acts both in the ASI and ASH neurons, and probably also in the ADL neurons, to modulate the response to NaCl. Avoidance of 1 M NaCl is not affected in gpc-1 animals, suggesting that separate pathways exist in the nociceptive neurons for osmotic avoidance and the modulation of the response to attractive NaCl concentrations.
Figure 2.
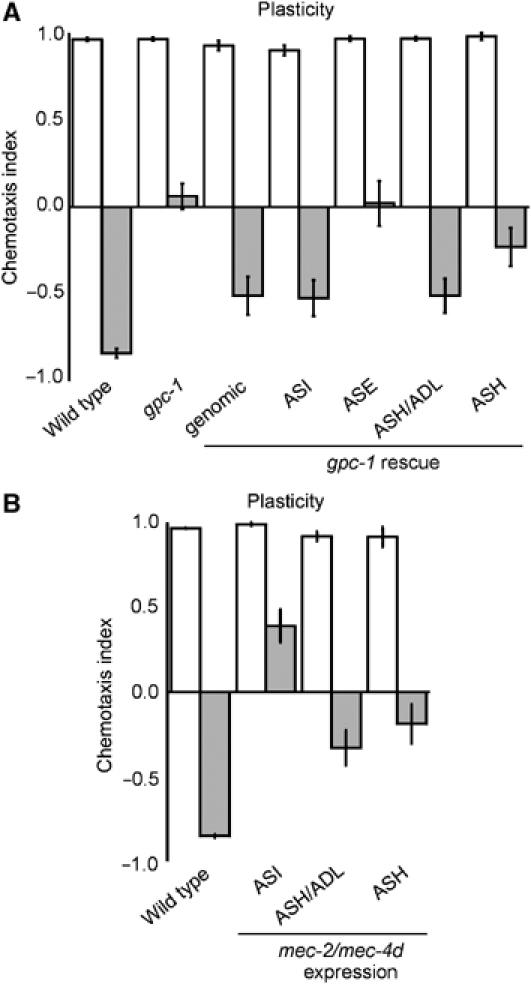
Identification of sensory neurons involved in gustatory plasticity. (A) Avoidance after pre-exposure could be restored by introducing the wild-type gpc-1 gene in gpc-1 animals (genomic), or by expressing gpc-1 in the ASI, ASH or ASH and ADL neurons (P<0.01). Expression in ASE did not rescue the plasticity defect (P>0.05). (B) Expression of mec-4d/mec-2 constructs in the ASI neurons, in the ASH/ADL nociceptive neurons or the ASH neurons significantly impaired gustatory plasticity (P<0.01).
To validate the importance of the ASI, ADL and ASH neurons in gustatory plasticity, we expressed a dominant mutant DEG/ENaC channel MEC-4d in specific neurons to inactivate these cells (Harbinder et al, 1997). To increase the efficacy, we coexpressed mec-2 constructs, since MEC-2 has been shown to increase MEC-4d activity (Goodman et al, 2002). Expression of the mec-4d/mec-2 constructs in the ASI neurons strongly affected gustatory plasticity, while expression in the ASH or ADL cells only mildly interfered with plasticity (Figure 2B). Expression of the mec-4d/mec-2 constructs in the ASI, ASH or ADL neurons did not affect salt detection (Figure 2B, results not shown). The mec-4d/mec-2 data confirm that the ASI, ASH and probably ADL neurons are involved in gustatory plasticity.
Our results indicate that gustatory plasticity is the result of integration of input from three to four pairs of amphid neurons. Signalling via the ASE cells is essential for both attraction to salt and avoidance after pre-exposure. However, the ASE neurons are not involved in avoidance of 1 M NaCl. This response is mediated by the ASH neurons (Bargmann et al, 1990). Avoidance after pre-exposure involves cues from the ASI and ASH cells, and probably the ADL neurons.
G-protein signalling in the ADF neurons modulates gustatory plasticity
Our finding that the Gγ subunit GPC-1 plays a role in gustatory plasticity prompted us to test the behaviour of mutants of the Gα subunits expressed in the gustatory neurons. None of the sensory Gα mutants showed a defect in chemotaxis to NaCl (Table I). However, two Gα mutants showed impaired gustatory plasticity: GPA-1 and ODR-3 (Figure 3A and B).
Table 1.
Overview of strains tested for salt perception
| Gene | Reference | Function | Taste | Plasticity |
|---|---|---|---|---|
| arr-1 (ok401) | Fukuto et al (2004) | Arrestin | WT | −0.12±0.17*** |
| ceh-36 (ks86) | Lanjuin et al (2003) | Transcription factor | m*** | 0.13±0.03*** |
| che-1 (p679) | Uchida et al (2003) | Transcription factor | m | 0.01±0.02*** |
| cnb-1 (jh103) | Bandyopadhyay et al (2002) | Calcineurin B | m* | 0.74±0.04** |
| daf-11 (m47) | Birnby et al (2000) | Gyanylate cyclase | WT | WT |
| dgk-1 (sy428) | Hadju-Cronin et al (1999); Nurrish et al (1999) | Diacylglycerol kinase | WT | 0.72±0.05*** |
| (nu62) | WT | 0.27±0.10*** | ||
| egl-4 (ky95) | Daniels et al (2000) | PKG | WT | 0.06±0.06*** |
| (ok1105) | WT | −0.06±0.08*** | ||
| fat-1 (wa9) | Watts and Browse (2002) | ω-3 fatty acyl desaturase | WT | −0.02±0.16** |
| fat-3 (wa22) | Watts and Browse (2002) | δ-6 fatty acid desaturase | WT | 0.20±0.06*** |
| fat-4 (wa14) | Watts and Browse (2002) | δ-5 fatty acid desaturase | WT | 0.31±0.09*** |
| (ok958) | WT | 0.10±0.03*** | ||
| gcy-32 (ok995) | Coates and de Bono (2002) | Guanylate cyclase | WT | WT |
| gcy-35 (ok769) | Cheung et al (2004) | Gyanylate cyclase | WT | −0.10±0.10* |
| gpa-1 (pk15) | Jansen et al (1999) | Gα subunit | WT | 0.17±0.06** |
| gpa-2 (pk16) | Zwaal et al (1997) | Gα subunit | WT | WT |
| gpa-3 (pk35) | Zwaal et al (1997) | Gα subunit | WT | WT |
| gpa-4 (pk381) | Jansen et al (1999) | Gα subunit | WT | WT |
| gpa-10 (pk362) | Jansen et al (1999) | Gα subunit | WT | WT |
| gpa-11 (pk349) | Jansen et al (1999) | Gα subunit | WT | WT |
| gpa-13 (pk1270) | Jansen et al (1999) | Gα subunit | WT | WT |
| gpa-14 (pk347) | Jansen et al (1999) | Gα subunit | WT | WT |
| gpa-15 (pk477) | Jansen et al (1999) | Gα subunit | WT | WT |
| grk-2 (rt97) | Fukuto et al (2004) | GRK | m*** | −0.61±0.06***,# |
| itr-1 (sa73) | Baylis et al (1999); Dal Santo et al (1999) | IP3 receptor | WT | 0.82±0.06*** |
| ncs-1 (qa406) | Gomez et al (2001) | Ca2+ sensor | m** | −0.13±0.14** |
| ocr-1 (ok132) | Tobin et al (2002) | TRPV channel | WT | −0.20±0.08*** |
| (ak46) | WT | −0.25±0.14*** | ||
| ocr-2 (ak47) | Tobin et al (2002) | TRPV channel | WT | −0.18±0.12*** |
| odr-3 (n1605) | Roayaie et al (1998) | Gα subunit | WT | 0.85±0.04*** |
| osm-9 (ky10) | Colbert et al (1997) | TRPV channel | WT | 0.06±0.05** |
| pdl-1 (gk157) | Smith and Pilgrim, personal communication | Phosphodiesterase | WT | 0.13±0.09** |
| tax-2 (p671) | Coburn and Bargmann (1996) | cGMP-gated channel | m*** | 0.83±0.04*** |
| tax-4 (p678) | Komatsu et al (1996) | cGMP-gated channel | m*** | 0.83±0.03*** |
| tax-6 (p675) | Kuhara et al (2002) | Calcineurin A | m*** | 0.64±0.05*** |
| In all, 32 mutant strains were tested for chemotaxis to 0.1, 1, 10 and 100 mM NaCl (taste) or chemotaxis to 25 mM NaCl after pre-exposure to 100 mM NaCl (plasticity) and their responses were compared to wild-type animals: *P<0.05; **P<0.01; ***P<0.001; unc=locomotion defect; these strains did not perform well in the assay; #pre-exposure for only 5 min, response stronger than wild types. WT=wild type. | ||||
Figure 3.
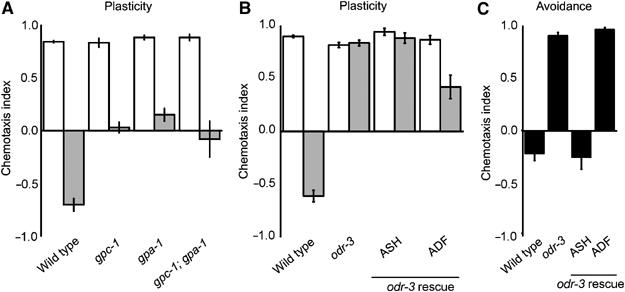
Gα subunits GPA-1 and ODR-3 modulate gustatory plasticity. (A) GPA-1 is involved in gustatory plasticity (P<0.0001) and functions in the same genetic pathway as GPC-1, since gpa-1 gpc-1 animals showed the same response as the single mutants (P>0.05). (B) Also, ODR-3 is involved in gustatory plasticity (P<0.0001). Avoidance after pre-exposure could be restored by expressing ODR-3 in the ADF neurons (P<0.0001), but not by expressing it in the ASH neurons (P>0.05). (C) ODR-3 signalling mediates avoidance of 1 M NaCl (P<0.0001). Avoidance could be restored by expressing ODR-3 in the ASH neurons (P<0.0001), but not by expressing it in the ADF neurons (P>0.05).
Thus far, no function has been described for GPA-1 (Jansen et al, 1999). We found that gpa-1 animals have a defect in gustatory plasticity, whereas they showed wild-type avoidance of 1 M NaCl (results not shown). The behaviour of gpa-1 animals was very similar to that of gpc-1 animals. Moreover, like gpc-1, gpa-1 is expressed in the ASI and nociceptive neurons (Jansen et al, 1999), suggesting that GPA-1 might function together with GPC-1 in these neurons. To test this hypothesis, we generated a gpa-1 gpc-1 double mutant. The behaviour of the double mutant was not significantly different from the behaviour of the two single mutants (Figure 3A), indicating that gpa-1 and gpc-1 function in the same genetic pathway that modulates gustatory plasticity.
The Gα subunit ODR-3 is essential for olfaction and chemoavoidance, and is expressed in the AWA, AWB and AWC olfactory neurons and in the ADF and ASH neurons (Roayaie et al, 1998). odr-3 animals showed a very strong gustatory plasticity defect (Figure 3B). To test if this is caused by the osmotic avoidance defect of odr-3 animals, we tested if expression of the odr-3 gene in the ASH neurons could restore gustatory plasticity. Expression of ODR-3 in the ASH neurons of odr-3 animals fully restored avoidance of 1 M NaCl (Figure 3C), confirming that ODR-3 in the ASH neurons is essential for this response (Roayaie et al, 1998). However, avoidance after pre-exposure could not be restored by expressing odr-3 in the ASH neurons (Figure 3B), indicating that ODR-3-mediated signalling in the ASH neurons is not sufficient for gustatory plasticity. Next, we tested if the ADF neurons are involved in gustatory plasticity, by expressing the odr-3 gene in these neurons. ADF specific expression of odr-3 restored avoidance after pre-exposure (Figure 3B), but did not restore avoidance of 1 M NaCl (Figure 3C). Expression of odr-3 in both ASH and ADF did not yield better rescue, but reproduced the ASH-specific rescue of avoidance of 1 M NaCl and ADF-specific rescue of gustatory plasticity (results not shown). Hence, ODR-3 functions in two separate processes. It mediates avoidance of 1 M NaCl in the ASH neurons, and mediates a signal in the ADF neurons that modulates gustatory plasticity.
Excessive avoidance in grk-2 animals counterbalances chemoattraction to NaCl
Our results indicate that G proteins play an important role in gustatory plasticity. Several mechanisms that modulate G-protein signal transduction exist, providing a molecular basis for behavioural plasticity. One of these mechanisms involves G-protein-coupled receptor kinases (GRKs) and arrestins. Studies in mammals and Drosophila have shown that GRKs inhibit G-protein signalling by phosphorylating activated G-protein-coupled receptors, followed by arrestin binding and receptor internalisation (Ferguson, 2001). In addition to this classical function, recent studies indicate a role for GRKs and arrestins as signal transduction molecules (Perry and Lefkowitz, 2002; Kim et al, 2005; Ren et al, 2005). The C. elegans genome contains two GRKs, grk-1 and grk-2, and one arrestin gene, arr-1 (Fukuto et al, 2004). Loss of function of grk-2 impairs the response to attractive odorants and several repellents, including odorants, quinine and 1–4 M glycerol, but seems to have no or little involvement in olfactory adaptation. These results suggest that GRK-2 functions as a signal transduction molecule in the nociceptive and olfactory neurons (Fukuto et al, 2004). The C. elegans arrestin, arr-1, is required for adaptation and recovery from adaptation to various odorants (Palmitessa et al, 2005). We determined the role of GRK-2 and ARR-1 in gustatory plasticity.
Loss of function of grk-2 severely impaired chemotaxis to NaCl (Figure 4A). However, grk-2 animals did show avoidance after pre-exposure (Figure 4B), indicating that these animals can detect NaCl. When these animals were only briefly pre-exposed to NaCl, they even showed enhanced avoidance after pre-exposure (Figure 4C). Since the ASE cells are essential for both salt taste and gustatory plasticity, we surmised that GRK-2 does not function in the ASE neurons, but in the nociceptive neurons instead. We first tested the response of grk-2 animals to 1 M NaCl. Surprisingly, grk-2 animals showed strong avoidance of 1 M NaCl (Figure 4D), in contrast to the previously reported defect in avoidance of 1–4 M glycerol (Fukuto et al, 2004). These results suggest that different signalling routes exist for avoidance of high concentrations of glycerol and NaCl.
Figure 4.
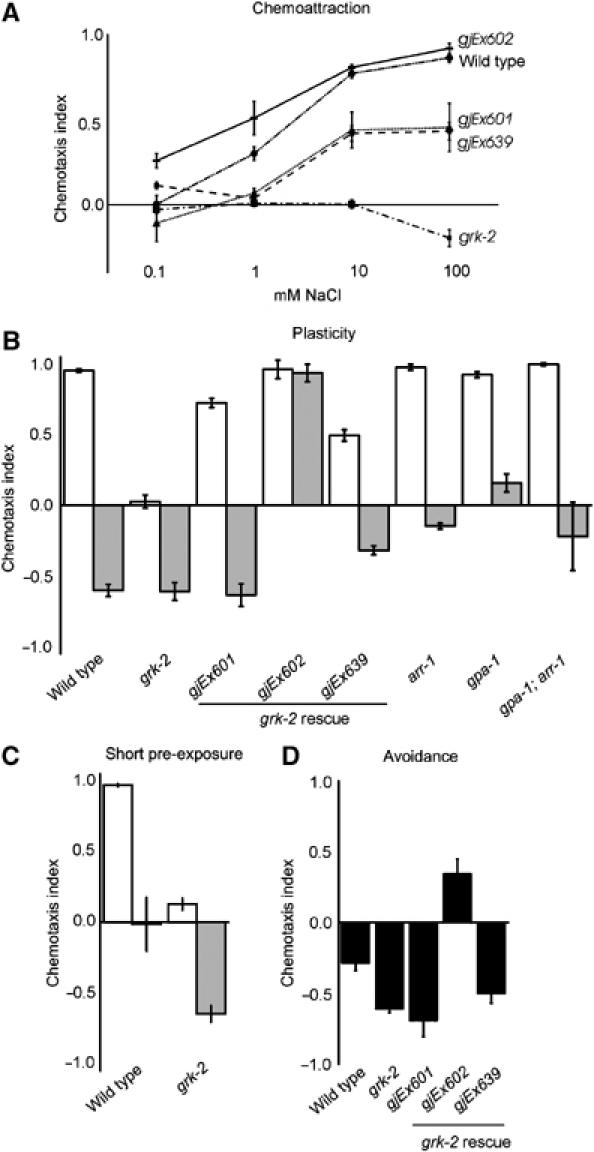
Excessive avoidance in grk-2 animals counterbalances chemoattraction to NaCl. (A) Chemotaxis to 0.1–100 mM NaCl was impaired in grk-2 animals (P<0.0001). The grk-2 defect could be restored by expressing GRK-2 in the nociceptive neurons in strains grk-2 gjEx601 (P<0.05), gjEx602 (P<0.001) and gjEx639 (P<0.001). (B) grk-2 mutants showed no chemoattraction to 25 mM NaCl, but strong avoidance after pre-exposure to 100 mM NaCl. Expression of GRK-2 in the nociceptive neurons restored chemoattraction in grk-2 gjEx601 animals (P<0.001) and grk-2 gjEx639 animals ((P<0.0001). grk-2 gjEx602 animals showed even better rescue of chemoattraction (P<0.0001), but impaired avoidance after pre-exposure (P<0.0001). Mutation of arr-1 significantly reduced avoidance after pre-exposure (P<0.001), to a similar level as loss of gpa-1. The behaviour of the gpa-1 arr-1 double mutant suggests that both genes function in the same pathway (P>0.05). (C) grk-2 animals showed enhanced avoidance after pre-exposure to NaCl for only 5 min (P<0.001). (D) grk-2 animals showed strong avoidance of 1 M NaCl, which was not affected in grk-2 gjEx601 and gjEx639 animals (P>0.05), but impaired in grk-2 gjEx602 animals (P<0.001).
We next tested if specific expression of grk-2 in the nociceptive neurons ADL and ASH could restore chemotaxis of the grk-2 mutants. Indeed, a srb-6∷grk-2 construct restored the response to 1–100 mM NaCl partially (gjEx601 and two other strains) or fully (gjEx602) (Figure 4A). Probably, gjEx601 animals did not express sufficient GRK-2 in the nociceptive neurons for full rescue, since avoidance of 1 M NaCl was not restored (Figure 4C). In contrast, gjEx602 animals probably overexpressed GRK-2, diminishing the avoidance responses (Figure 4A and D), and restoring chemoattraction. To further confirm GRK-2 function in ASH, we also used an osm-10∷grk-2 construct to express grk-2 in the ASH and ASI neurons. This construct could also restore the NaCl chemoattraction defect of grk-2 animals (gjEx639 in Figure 4A, B and D). In addition, specific expression of grk-2 in the ASH neurons could restore avoidance of octanol and 1 M glycerol (results not shown). In contrast, expression of GRK-2 in interneurons, but not the sensory neurons, using a glr-1∷grk-2 construct did not restore chemotaxis to NaCl (results not shown). Taken together, our results suggest that GRK-2 modulates the strength of the NaCl avoidance signal mediated by the nociceptive neurons. Such a function fits with a classical function for GRK-2 in desensitisation of the NaCl avoidance-signalling pathway (Figure 7), suggesting that GRK-2 has two different functions in separate signalling pathways in the ASH cells. Moreover, these results show that signals derived from the nociceptive neurons counterbalance chemoattraction to NaCl mediated by the ASE neurons.
Figure 7.
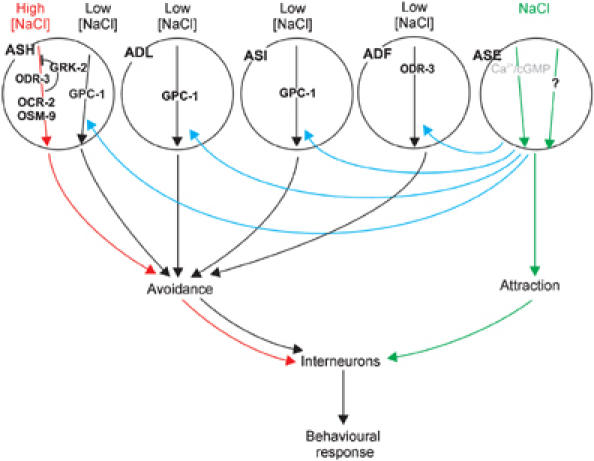
Model for NaCl detection and its plasticity in C. elegans. NaCl is detected by the ASE sensory neurons (green arrows), probably using cGMP/Ca2+ signalling and an unknown signalling pathway. Attraction is antagonised by avoidance mediated by the ASH neurons (red arrows), using ODR-3, OSM-9, OCR2 and the inhibitor GRK-2. We propose that, upon prolonged exposure to attractive NaCl concentrations, an ASE-derived signal sensitises the ASH, ADL, ASI and ADF neurons, resulting in avoidance of normally attractive NaCl concentrations. We have shown that the ADF neurons use ODR-3, and the ASI neurons, a second pathway in the ASH neurons and probably the ADL neurons use GPC-1. Integration of these signals ultimately leads to the behavioural response of the animal.
Mutation of arr-1 reduced avoidance after pre-exposure, while chemoattraction or avoidance responses were not affected (Figure 4B, results not shown). These results are not in agreement with a function of ARR-1 in receptor or channel internalisation in the ASE or nociceptive neurons. arr-1 animals rather displayed behavioural deficits comparable to those observed in gpc-1 and gpa-1 animals. gpa-1 arr-1 double-mutant animals indeed showed the same response after pre-exposure as the two single mutants (Figure 4B), indicating that these genes function in the same genetic pathway. arr-1 is however broadly expressed in the nervous system of C. elegans (Fukuto et al, 2004; Palmitessa et al, 2005), which makes it unclear in which cells ARR-1 functions in gustatory plasticity.
Gustatory plasticity requires cGMP signalling in the body cavity neurons
We next studied the role of downstream signalling molecules in gustatory plasticity. Frequently used second messengers are cGMP and Ca2+. cGMP can be generated by guanylate cyclases, and it can activate other signalling molecules such as cGMP-gated channels or protein kinases (PKG). Previous studies have shown that cGMP signalling plays an important role in chemotaxis to NaCl. Mutation of the guanylate cyclase DAF-11 and the cGMP-gated channel subunits TAX-2 and TAX-4 abolish chemotaxis to NaCl (Coburn and Bargmann, 1996; Komatsu et al, 1996; Birnby et al, 2000). Surprisingly, mutation of daf-11 did not affect chemotaxis to NaCl in our assay (Figure 5A), whereas mutation of tax-2 and tax-4 strongly affected chemotaxis to 0.1–10 mM NaCl. However, these latter mutants still showed chemoattraction to 100 mM NaCl (Figure 5A). Our results confirm that cGMP signalling is involved in chemotaxis to NaCl, although it is unclear which proteins activate the TAX-2/TAX-4 channel. In addition, our results suggest that another pathway exists for the detection of NaCl, which functions in parallel to the cGMP pathway.
Figure 5.
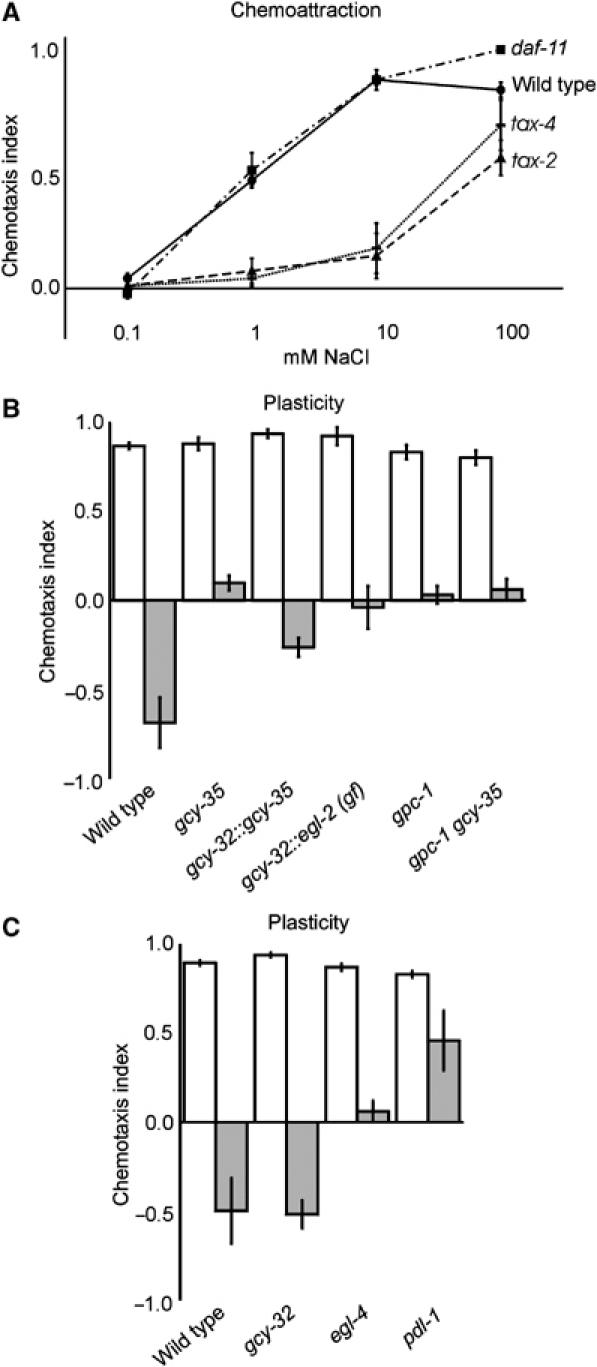
Gustatory plasticity requires cGMP. (A) Salt detection was not affected in daf-11 animals (P>0.05), but significantly impaired in tax-2 and tax-4 animals (P<0.01 at 1 and 10 mM NaCl). These mutants still responded to 100 mM NaCl. (B) Mutation of gcy-35 affected gustatory plasticity (P<0.001). This defect could be rescued by expression of gcy-35 in the AQR, PQR and URX neurons (P<0.0001). Expression of a gcy-32∷egl-2(gf) construct to inhibit the activity of AQR, PQR and URX neurons reduced avoidance after pre-exposure (P<0.05). Analysis of a gpc-1 gcy-35 double-mutant strain indicated that these genes function in the same genetic pathway in gustatory plasticity (P>0.05 compared to the single mutants). (C) Mutation of the cGMP signalling molecules egl-4 (P<0.001) and pdl-1 (P<0.01) affected gustatory plasticity, whereas mutation of gcy-32 did not (P>0.05).
Mutations in tax-2 and tax-4 also abolish avoidance after pre-exposure (results not shown). However, since these proteins play important roles in chemotaxis to NaCl, we cannot conclude whether TAX-2 and TAX-4 function in gustatory plasticity. Analysis of other cGMP signalling molecules did indicate a role of cGMP signalling in gustatory plasticity. Mutations in the cGMP-dependent kinase EGL-4 and the phosphodiesterase delta-like protein PDL-1 affected gustatory plasticity (Figure 5C). EGL-4 is expressed in a large number of neurons (Fujiwara et al, 2002; Hirose et al, 2003) and PDL-1, responsible for cGMP breakdown, shows pan-neuronal expression (J Smith and D Pilgrim, personal communication). Hence, further cell-specific rescue and epistasis analysis are needed to determine the specific functions of these proteins.
We also identified a guanylate cyclase that plays a role in gustatory plasticity. Mutations in the soluble guanylate cyclase gcy-35 affected this behaviour (Figure 5B), but did not affect chemoattraction or avoidance of high NaCl concentrations. gcy-35 is expressed, among others, in the body cavity neurons AQR, PQR and URX (Cheung et al, 2004; Gray et al, 2004). These neurons are directly exposed to the pseudocoelomic body fluid (White et al, 1986) and regulate social feeding or aggregation behaviour (Coates and de Bono, 2002). We tested whether GCY-35 functions in these body cavity neurons during gustatory plasticity by specific expression of gcy-35 in these neurons. Indeed, avoidance after pre-exposure was restored in gcy-35 animals expressing a gcy-32∷gcy-35 construct (Figure 5B). We did not obtain full rescue, suggesting that gcy-35 might also function in other cells in gustatory plasticity.
To confirm that the AQR, PQR and URX cells are involved in gustatory plasticity, we expressed a gain-of-function mutant form of the eag-type potassium channel EGL-2 in these neurons, using a gcy-32∷egl-2(gf) construct. Expression of this construct has been shown to inhibit neuronal activity (Coates and de Bono, 2002). We found that expression of EGL-2(gf) in the AQR, PQR and URX neurons significantly reduced avoidance after pre-exposure to 100 mM NaCl, without affecting chemotaxis (Figure 5B, results not shown). These results show that these body cavity neurons mediate a signal that modulates the response to NaCl.
The phenotype of the gcy-35 animals was very comparable to that of the arr-1, gpa-1 and gpc-1 animals. To test if also gcy-35 functions in the same genetic pathway, we tested the behaviour of gpc-1 gcy-35 double-mutant animals. The double-mutant strain behaved as the two single mutants (Figure 5B). Therefore, we hypothesise that cGMP signalling via gcy-35 in the AQR, PQR and/or URX neurons functions in the same genetic pathway as gpc-1, gpa-1 and arr-1 to modulate the response to NaCl after prolonged exposure.
Gustatory plasticity requires Ca2+ signalling
We also tested if Ca2+ signalling is involved in gustatory plasticity. Ca2+ levels in the cell can be increased by influx via TRP channels or cGMP-gated channels, and by depletion of intracellular stores after inositol (1,4,5) trisphosphate (IP3)-receptor activation. Increased Ca2+ levels can subsequently activate various downstream effector molecules, including calcineurin and the neuronal calcium sensor NCS-1. Previous studies have shown that Ca2+ signalling is important for chemotaxis to NaCl, since mutation of the cGMP-gated channel TAX-2/TAX-4, which upon activation leads to Ca2+ influx, affects chemotaxis to NaCl (Coburn and Bargmann, 1996; Komatsu et al, 1996; Figure 5A). In addition, it was shown that mutation of the calcineurin A subunit TAX-6 affects chemotaxis to NaCl (Kuhara et al, 2002). Calcineurin is a phosphatase consisting of two subunits A and B, encoded by tax-6 and cnb-1, respectively (Bandyopadhyay et al, 2002; Kuhara et al, 2002). We tested tax-6 and cnb-1 mutants in our assays, as well as ncs-1 mutants, which lack a functional neuronal calcium sensor 1 (Gomez et al, 2001). All three mutants showed strongly reduced chemotaxis to low NaCl concentrations (0.1–10 mM), but strong or even wild-type responses to 100 mM NaCl (Figure 6A). These mutants also showed defects in avoidance after pre-exposure; however, because of their function in chemotaxis, their role in gustatory plasticity remains unclear.
Figure 6.
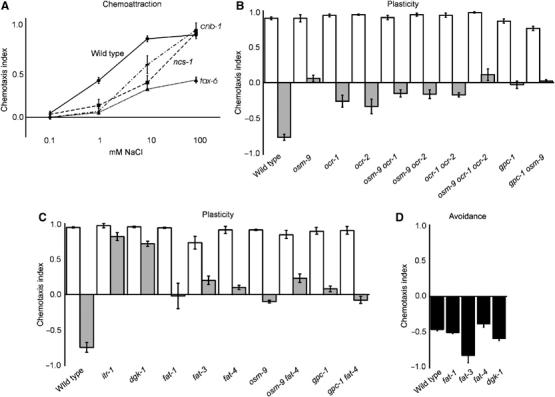
Gustatory plasticity requires Ca2+ signalling. (A) Salt detection was significantly impaired in tax-6, cnb-1 and ncs-1 animals (P<0.01 at 1 and 10 mM NaCl). These mutants still responded to 100 mM NaCl. (B) TRP channel mutants osm-9, ocr-1 and ocr-2 showed defects in plasticity (P<0.001). TRP-channel double mutants showed similar defects to the single mutants (P>0.05). The TRP-channel triple mutant showed a very small additive effect of inactivation of all three subunits (P<0.05, compared to ocr-1 and ocr-2 animals). The TRP channels function in the same genetic pathway as gpc-1, since the response of the gpc-1 osm-9 double did not differ significantly from the single mutants (P>0.05). (C) itr-1, dgk-1, fat-1, fat-3 and fat-4 showed defects in plasticity (P<0.001). FAT-4 functions in the same pathway as OSM-9 and GPC-1, since no significant difference could be detected between double and single mutants (P>0.05). (D) Avoidance of 1 M NaCl was not reduced in fat-1, fat-3, fat-4 or dgk-1 animals.
The similarities of the NaCl chemotaxis phenotypes of the tax-2, tax-4 and tax-6 animals suggested that these genes might function in the same pathway. The expression patterns of these genes overlap only in the ASE neurons (Coburn and Bargmann, 1996; Komatsu et al, 1996; Kuhara et al, 2002). Since the ASE neurons are essential for NaCl detection, it is likely that the cGMP and Ca2+ signalling molecules for the detection of NaCl act in the ASE neurons. However, at present, we cannot rule out a function for these proteins in other sensory neurons or interneurons. Moreover, it is unclear which signals activate the cGMP/Ca2+ NaCl detection pathway.
Besides cGMP-gated channels, also TRP channels mediate Ca2+ influx. We have previously shown a function for the TRPV channel subunit OSM-9 in gustatory plasticity (Jansen et al, 2002). Here we show that also the OCR-1 and OCR-2 TRPV channel subunits are involved (Figure 6B). The responses of the three TRPV channel mutants did not differ significantly from each other, although the defect seems a little more severe in the osm-9 animals (Figure 6B). In addition, the behaviour of doubles between these three mutants did not differ significantly from the single mutants (Figure 6B), suggesting that these three subunits function in the same genetic pathway. In the ocr-1, ocr-2, osm-9 triple mutant, we observed a very small additive effect of inactivation of all three TRP channel subunits compared to the ocr-1 and ocr-2 single mutants (Figure 6B).
The plasticity defects of the TRPV channel mutants were very similar to the defects of the gpa-1, gpc-1, arr-1 and gcy-35 mutants. The behaviour of gpc-1 osm-9 double-mutant animals was not significantly different from the single mutants (Figure 6B), suggesting that also the TRPV channel subunits function in this genetic gustatory plasticity pathway. The expression patterns of these TRPV genes are restricted to subsets of sensory neurons, overlapping with neurons that play a role in gustatory plasticity: ASH and ADL (Colbert et al, 1997; Tobin et al, 2002).
TRP channels can be activated in various ways, including by phospholipase C (PLC)-dependent mechanisms (Montell et al, 2002). Activation of PLC by G proteins leads to the production of IP3 and diacylglycerol (DAG). IP3 can activate IP3 receptors, resulting in Ca2+ release from intracellular stores, which can activate TRP channels. TRP channels can also be activated by DAG, or its derivatives, polyunsaturated fatty acids (PUFAs; Kahn-Kirby et al, 2004). We tested if these signalling molecules are involved in gustatory plasticity.
Mutation of both the IP3 receptor ITR-1 (Baylis et al, 1999; Dal Santo et al, 1999) and the DAG kinase DGK-1, essential for reduction of DAG levels (Hadju-Cronin et al, 1999; Nurrish et al, 1999), strongly reduced avoidance after pre-exposure, but did not affect chemoattraction to 0.1–100 mM NaCl or avoidance of 1 M NaCl (Figure 6C and D, results not shown). This indicates that IP3 and DAG signalling are only involved in the plasticity response. It is possible that these second messengers activate TRP channels, but they could also affect other signalling routes such as the Goα/Gqα network, which is involved in gustatory plasticity as well (RK Hukema and G Jansen, unpublished results).
To test if PUFAs are involved in gustatory plasticity, we tested three lipid desaturase mutants that affect PUFA synthesis, fat-1, fat-3 and fat-4 (Watts and Browse, 2002). Recently, strong avoidance defects were described for fat-3 mutant animals, including a defect in avoidance of 1 M glycerol; mild or no defects were observed for fat-1 or fat-4 animals (Kahn-Kirby et al, 2004). In our assays, the PUFA synthesis mutants showed no defect in avoidance of 1 M NaCl (Figure 6D), again indicating that separate pathways exist for the detection of 1 M glycerol and 1 M NaCl. However, all three mutants showed aberrant behaviour in our gustatory plasticity assay (Figure 6C), indicating that PUFAs play a role in this process.
Since the PUFA synthesis mutants showed a very comparable behaviour to gpa-1, gpc-1, arr-1, osm-9 and gcy-35 animals, we analysed the behaviour of gpc-1 fat-4 and osm-9 fat-4 double mutants. The behaviour of these double mutants did not differ significantly from the behaviour of the single mutants, indicating that all these genes function in the same genetic pathway (Figure 6C).
Discussion
Model for gustatory plasticity in C. elegans
We can discriminate three responses of C. elegans to NaCl: first, chemotaxis to NaCl concentrations between 0.1 and 200 mM, second, avoidance of NaCl above 200 mM, and third, avoidance of otherwise attractive NaCl concentrations (25 mM) after pre-exposure to 100 mM NaCl. Data presented here and by others indicate that these responses require input from at least four chemosensory neurons and the neurons exposed to the body fluid. We propose a model to explain the different gustatory responses (Figure 7).
The ASE neurons are essential for chemotaxis to low NaCl concentrations (green arrows in Figure 7). Chemoattraction is antagonised by avoidance, mediated by the ASH neurons (red arrows in Figure 7). The ASH neurons are not activated at low NaCl concentrations, but become activated at high NaCl concentrations (Hilliard et al, 2004). It is unclear where the ASE and ASH derived signals are integrated and where the choice between attraction and avoidance is made. It is also not known why avoidance is preferred over attraction at NaCl concentrations above 200 mM. Perhaps ASE signalling is blocked at high salt concentrations, or alternatively, preference for avoidance might be the default state, for example, determined by the wiring of the nervous system. Our model suggests that also the ADF, ADL and ASI neurons are not or are only weakly activated at low NaCl concentrations. We propose that upon prolonged exposure to 100 mM NaCl the ASE neurons produce a signal (blue arrows in Figure 7) that sensitises the ADF, ADL, ASI and ASH neurons, resulting in avoidance of otherwise attractive NaCl concentrations (black arrows in Figure 7). We suggest that the ASE neurons signal via the body cavity neurons, AQR, PQR and URX, but it is also possible that the body cavity neurons function downstream of the ADF, ADL, ASI and ASH neurons.
At present, it is not clear which environmental signals modulate the response to NaCl. However, our results show that gustatory plasticity depends on salt concentration and exposure time (Jansen et al, 2002). In addition, the involvement of the guanylate cyclase GCY-35 suggests that perhaps oxygen levels might play a role (Gray et al, 2004). Finally, our preliminary data suggest that also food signals modulate gustatory plasticity (RK Hukema and G Jansen, unpublished data). In line with these results, Saeki et al (2001) have reported that chemotaxis to NaCl is drastically decreased when C. elegans are starved on plates that contain NaCl.
Based on the cellular circuit described above, expression patterns and our cell-specific rescue experiments, we can place several molecules in our model (Figure 7). We propose that stimulation of the ASE neurons by low NaCl concentrations activates a cGMP and Ca2+ signalling pathway and another unknown pathway. Avoidance of 1 M NaCl, mediated by the ASH neurons, requires the Gα subunit ODR-3 and the TRP channel subunits OSM-9 and OCR-2, and is inhibited by GRK-2. ODR-3 also functions in the ADF neurons in gustatory plasticity, where it might either transduce the avoidance signal, or alternatively transduce the ASE-derived sensitising signal.
In addition, we have identified a genetic pathway that mediates gustatory plasticity. This pathway involves the Gγ GPC-1, the Gα GPA-1, the arrestin ARR-1, the TRPV channel subunits OSM-9, OCR-1 and OCR-2, PUFA signalling (FAT-4) and the guanylate cyclase GCY-35. It is unclear if these proteins function in the same cells, but our and previously published data are consistent with a function for GPC-1 in the ASI, ASH and perhaps ADL neurons (based on our rescue experiments), GPA-1 and OSM-9 in these same neurons (based on expression patterns; Colbert et al, 1997; Jansen et al, 1999), OCR-1 in ADL and OCR-2 in ADL, ASH and ADF (Tobin et al, 2002) and GCY-35 in the AQR, PQR and URX body cavity neurons (based on rescue experiments). We cannot discriminate if these proteins transduce the avoidance response or function in a pathway that mediates the ASE-derived sensitising signal. As indicated, part of this model is based on previously described expression patterns. Most of these expression patterns have been derived using GFP reporter constructs. These constructs may not fully represent the gene's expression pattern. Therefore, additional cell-specific rescue experiments will be needed to confirm our model.
Materials and methods
Strains, genetics and germline transformation
Strains used in this work are listed in Table I. Wild-type C. elegans were strain Bristol N2. Germline transformation and transgene integration were performed as described (Mello et al, 1991). We used an elt-2∷GFP construct (30 ng/μl) as coinjection marker (Fukushige et al, 1999). Rescue of che-1, gpc-1, odr-3 and grk-2 and cell inactivation using mec-4d and mec-2 or egl-2(gf) were tested using five or more transgenic strains for each clone injected at various concentrations (1–100 ng/μl). Although cell-specific expression of mec-2/mec-4d or egl-2gf did affect gustatory plasticity, we did not observe neuronal degeneration.
Molecular biology
Details of plasmid construction are available on request. Promoters used for cell-specific rescue or cell inactivation were: flp-5 (ASE; Li et al, 1999), gpa-4 (ASI; Jansen et al, 1999), gpa-11 (ADL, ASH; Jansen et al, 1999), gpa-13 (ADF, ASH, AWC; Jansen et al, 1999), sra-6 (ASH, ASI faint; Troemel et al, 1995), srb-6 (ADL, ASH; Troemel et al, 1995), glr-1 (17 classes of neurons; Hart et al, 1995; Maricq et al, 1995) and srh-142 (ADF; Sagasti et al, 1999). Expression patterns were confirmed using GFP-fusion constructs driven by the same promoter.
Behavioural assays
Chemotaxis towards NaCl and gustatory plasticity were assessed as described before (Wicks et al, 2000; Jansen et al, 2002), using 0.1, 1, 10 or 100 mM or 1 M NaCl; after pre-exposure to 100 mM NaCl, animals were tested for chemotaxis to 25 mM NaCl. Gustatory plasticity was assayed after 15 min pre-exposure; enhanced plasticity was tested by pre-exposing only for 5 min. All newly identified gustatory plasticity mutants were tested for recovery, by using a 5-min wash in CTX buffer, after the 15-min pre-exposure in CTX buffer containing 100 mM NaCl. All mutants showed wild-type levels of recovery (results not shown). A chemotaxis index was calculated: (A−C)/(A+C), where A is the number of worms at the quadrants with NaCl and C is the number of worms at the quadrants without attractant. Statistical significance was determined using the two-tailed t-test. Error bars represent the s.e.m.
Acknowledgments
We thank T Stiernagle, the Caenorhabditis Genetics Center and the C. elegans Knock-out Consortium, C Bargmann, A Hart and N L'Etoile for C. elegans strains; C Bargmann, M de Bono, J McGhee, A Hart and C Li for constructs; H Fukuto, D Ferkey and A Hart, D Karow, J Gray, C Bargmann and M Marletta, J Smith and D Pilgrim for the communication of unpublished results; J Watts for suggestions; K Ackema, H Lans and J Zareno for discussions and comments on the manuscript. This work was funded by the Centre for Biomedical Genetics and the Royal Netherlands Academy of Sciences. GJ is a Royal Netherlands Academy of Sciences Fellow.
References
- Bandyopadhyay J, Lee J, Lee JI, Yu JR, Jee C, Cho JH, Jung S, Lee MH, Zannoni S, Singson A, Kim do H, Koo HS, Ahnn J (2002) Calcineurin, a calcium/calmodulin-dependent protein phosphatase, is involved in movement, fertility, egg laying, and growth in Caenorhabditis elegans. Mol Biol Cell 13: 3281–3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI, Horvitz HR (1991) Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 7: 729–742 [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Thomas JH, Horvitz HR (1990) Chemosensory cell function in the behavior and development of Caenorhabditis elegans. Cold Spring Harb Symp Quant Biol 55: 529–538 [DOI] [PubMed] [Google Scholar]
- Baylis HA, Furuichi T, Yoshikawa F, Mikoshiba K, Sattelle DB (1999) Inositol 1, 4, 5-trisphosphate receptors are strongly expressed in the nervous system, pharynx, intestine, gonad and excretory cell of Caenorhabditis elegans and are encoded by a single gene (itr-1). J Mol Biol 294: 467–476 [DOI] [PubMed] [Google Scholar]
- Berger AJ, Hart AC, Kaplan JM (1998) Gαs-Induced neurodegeneration in Caenorhabditis elegans. J Neurosci 18: 2871–2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnby DA, Link EM, Vowels JJ, Tian H, Colacurcio PL, Thomas JH (2000) A transmembrane guanylyl cyclase (DAF-11) and Hsp90 (DAF-21) regulate a common set of chemosensory behaviors in Caenorhabditis elegans. Genetics 155: 85–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung BH, Arellano-Carbajal F, Rybicki I, de Bono M (2004) Soluble guanylate cyclases act in neurons exposed to the body fluid to promote C. elegans aggregation behavior. Curr Biol 14: 1105–1111 [DOI] [PubMed] [Google Scholar]
- Coates JC, de Bono M (2002) Antagonistic pathways in neurons exposed to body fluid regulate social feeding in Caenorhabditis elegans. Nature 419: 925–929 [DOI] [PubMed] [Google Scholar]
- Coburn CM, Bargmann CI (1996) A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron 17: 695–706 [DOI] [PubMed] [Google Scholar]
- Colbert HA, Smith TL, Bargmann CI (1997) OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci 17: 8259–8269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culotti JG, Russell RL (1978) Osmotic avoidance defective mutants of the nematode Caenorhabditis elegans. Genetics 90: 243–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Santo P, Logan MA, Chisholm AD, Jorgensen EM (1999) The inositol trisphosphate receptor regulates a 50-s behavioral rhythm in C. elegans. Cell 98: 757–767 [DOI] [PubMed] [Google Scholar]
- Daniels SA, Ailion M, Thomas JH, Sengupta P (2000) egl-4 acts through a transforming growth factor-β. SMAD pathway in Caenorhabditis elegans to regulate multiple neuronal circuits in response to sensory cues. Genetics 156: 123–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bono M, Tobin DM, Davis MW, Avery L, Bargmann CI (2002) Social feeding in Caenorhabditis elegans is induced by neurons that detect aversive stimuli. Nature 419: 899–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusenbery DB, Scheridan RE, Russell RL (1975) Chemotaxis-defective mutants of the nematode Caenorhabditis elegans. Genetics 80: 297–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SS (2001) Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev 53: 1–24 [PubMed] [Google Scholar]
- Fujiwara M, Sengupta P, McIntire SL (2002) Regulation of body size and behavioral state of C. elegans by sensory perception and the EGL-4 cGMP-dependent protein kinase. Neuron 36: 1091–1102 [DOI] [PubMed] [Google Scholar]
- Fukushige T, Hendzel MJ, Bazett-Jones DP, McGhee J (1999) Direct visualization of the elt-2 gut-specific GATA factor binding to a target promoter inside the living Caenorhabditis elegans. Proc Natl Acad Sci USA 96: 11883–11888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuto HS, Ferkey DM, Apicella AJ, Lans H, Sharmeen T, Chen W, Lefkowitz RJ, Jansen G, Schafer WR, Hart AC (2004) G protein-coupled receptor kinase function is essential for chemosensation in C. elegans. Neuron 42: 581–593 [DOI] [PubMed] [Google Scholar]
- Gomez M, De Castro E, Guarin E, Sasakura H, Kuhara A, Mori I, Bartfai T, Bargmann CI, Nef P (2001) Ca2+ signaling via the neuronal calcium sensor-1 regulates associative learning and memory in C. elegans. Neuron 30: 241–248 [DOI] [PubMed] [Google Scholar]
- Goodman MB, Ernstrom GG, Chelur DS, O'Hagan R, Yao CA, Chalfie M (2002) MEC-2 regulates C.elegans DEG/ENaC channels needed for mechanosensation. Nature 415: 1039–1042 [DOI] [PubMed] [Google Scholar]
- Gray JM, Karow DS, Lu H, Chang AJ, Chang JS, Ellis RE, Marletta MA, Bargmann CI (2004) Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature 430: 317–322 [DOI] [PubMed] [Google Scholar]
- Hadju-Cronin YM, Chen WJ, Patikoglou G, Koelle MR, Sternberg PW (1999) Antagonism between Goα and Gqα in Caenorhabditis elegans: the RGS protein EAT-16 is necessary for Goα signaling and regulates Gqα activity. Genes Dev 13: 1780–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbinder S, Tavernarakis N, Herndon LA, Kinnell M, Xu SQ, Fire A, Driscoll M (1997) Genetically targeted cell disruption in Caenorhabditis elegans. Proc Natl Acad Sci USA 94: 13128–13133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AC, Kass J, Shapiro JE, Kaplan JM (1999) Distinct signaling pathways mediate touch and osmosensory responses in a polymodal sensory neuron. J Neurosci 19: 1952–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AC, Sims S, Kaplan JM (1995) Synaptic code for sensory modalities revealed by C. elegans GLR-1 glutamate receptor. Nature 378: 82–85 [DOI] [PubMed] [Google Scholar]
- Hilliard MA, Apicella AJ, Kerr R, Suzuki H, Bazzicalupo P, Schafer WR (2004) In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. EMBO J 24: 63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose T, Nakano Y, Nagamatsu Y, Misumi T, Ohta H, Ohshima Y (2003) Cyclic GMP-dependent protein kinase EGL-4 controls body size and lifespan in C. elegans. Development 130: 1089–1099 [DOI] [PubMed] [Google Scholar]
- Ishihara T, Iino Y, Mohri A, Mori I, Gengyo-Ando K, Mitani S, Katsura I (2002) HEN-1, a secretory protein with an LDL receptor motif, regulates sensory integration and learning in Caenorhabditis elegans. Cell 109: 639–649 [DOI] [PubMed] [Google Scholar]
- Jansen G, Thijssen KL, Werner P, van der Horst M, Hazendonk E, Plasterk RH (1999) The complete family of genes encoding G proteins of Caenorhabditis elegans. Nat Genet 21: 414–419 [DOI] [PubMed] [Google Scholar]
- Jansen G, Weinkove D, Plasterk RHA (2002) The G-protein gamma subunit gpc-1 of the nematode C. elegans is involved in taste adaptation. EMBO J 21: 986–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn-Kirby AH, Dantzker JL, Apicella AJ, Schafer WR, Browse J, Bargmann CI, Watts JL (2004) Specific polyunsaturated fatty acids drive TRPV-dependent sensory signaling in vivo. Cell 119: 889–900 [DOI] [PubMed] [Google Scholar]
- Kim J, Ahn S, Ren XR, Whalen EJ, Reiter E, Wei H, Lefkowitz RJ (2005) Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci USA 102: 1442–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu H, Mori I, Rhee JS, Akaike N, Ohshima Y (1996) Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron 17: 707–718 [DOI] [PubMed] [Google Scholar]
- Kuhara A, Inada H, Katsura I, Mori I (2002) Negative regulation and gain control of sensory neurons by the C. elegans calcineurin TAX-6. Neuron 33: 751–763 [DOI] [PubMed] [Google Scholar]
- Lanjuin A, VanHoven MK, Bargmann CI, Thompson JK, Sengupta P (2003) Otx/otd homeobox genes specify distinct sensory neuron identities in C. elegans. Dev Cell 5: 621–633 [DOI] [PubMed] [Google Scholar]
- Li C, Kim K, Nelson LS (1999) FMRFamide-related neuropeptide gene family in Caenorhabditis elegans. Brain Res 848: 26–34 [DOI] [PubMed] [Google Scholar]
- Lindemann B (2001) Receptors and transduction in taste. Nature 413: 219–225 [DOI] [PubMed] [Google Scholar]
- Liu L, Leonard AS, Motto DG, Feller MA, Price MP, Johnson WA, Welsh MJ (2003) Contribution of Drosophila DEG/ENaC genes to salt taste. Neuron 39: 133–146 [DOI] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan TH, Alam RI, Russell OF, Malik SA, Bigbee JW, DeSimone JA (2004) The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J Physiol 558: 147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricq AV, Peckol E, Driscoll M, Bargmann CI (1995) Mechanosensory signaling in C. elegans mediated by the GLR-1 glutamate receptor. Nature 378: 78–81 [DOI] [PubMed] [Google Scholar]
- Mellem JE, Brockie PJ, Zheng Y, Madsen DM, Maricq AV (2002) Decoding of polymodal sensory stimuli by postsynaptic glutamate receptors in C. elegans. Neuron 36: 933–944 [DOI] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V (1991) Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J 10: 3959–3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell DJ, Birnbaumer L, Flockerzi V (2002) The TRP channels, a remarkably functional family. Cell 108: 595–598 [DOI] [PubMed] [Google Scholar]
- Nurrish S, Segalat L, Kaplan JM (1999) Serotonin inhibition of synaptic transmission: Goα decreases the abundance of UNC-13 at release sites. Neuron 24: 231–242 [DOI] [PubMed] [Google Scholar]
- Nuttley WM, Harbinder S, van der Kooy D (2001) Regulation of distinct attractive and aversive mechanisms mediating benzaldehyde chemotaxis in Caenorhabditis elegans. Learn Mem 8: 170–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmitessa A, Hess HA, Bany LA, Kim Y-M, Koelle MR, Benovic JL (2005) C. elegans arrestin regulates neural G protein signalling and olfactory adaptation and recovery. J Biol Chem 280: 24649–24662 [DOI] [PubMed] [Google Scholar]
- Perry SJ, Lefkowitz RJ (2002) Arresting developments in hepthahelical receptor signaling and regulation. Trends Cell Biol 12: 130–138 [DOI] [PubMed] [Google Scholar]
- Ren XR, Reiter E, Ahn S, Kim J, Chen W, Lefkowitz RJ (2005) Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci USA 102: 1448–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roayaie K, Crump JG, Sagasti A, Bargmann CI (1998) The Gα protein ODR-3 mediates olfactory and nociceptive function and controls cilium morphogenesis in C. elegans olfactory neurons. Neuron 20: 55–67 [DOI] [PubMed] [Google Scholar]
- Saeki S, Yamamoto M, Iino Y (2001) Plasticity of chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode Caenorhabditis elegans. J Exp Biol 204: 1757–1764 [DOI] [PubMed] [Google Scholar]
- Sagasti A, Hobert O, Troemel ER, Ruvkun G, Bargmann CI (1999) Alternative olfactory neuron fates are specified by the LIM homeobox gene lim-4. Genes Dev 15: 1794–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin D, Madsen D, Kahn-Kirby A, Peckol E, Moulder G, Barstead R, Maricq A, Bargmann C (2002) Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron 35: 307–318 [DOI] [PubMed] [Google Scholar]
- Troemel ER, Chou JH, Dwyer ND, Colbert HA, Bargmann CI (1995) Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell 83: 207–218 [DOI] [PubMed] [Google Scholar]
- Uchida O, Nakano H, Koga M, Oshima Y (2003) The C. elegans che-1 gene encodes a zinc finger transcription factor required for specification of the ASE chemosensory neurons. Development 130: 1215–1224 [DOI] [PubMed] [Google Scholar]
- Ward S (1973) Chemotaxis by the nematode Caenorhabditis elegans: identification of attractants and analysis of the response by use of mutants. Proc Natl Acad Sci USA 70: 817–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JL, Browse J (2002) Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc Natl Acad Sci USA 99: 5854–5859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Southgate JN, Thomson JN, Brenner S (1986) The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B 314: 1–340 [DOI] [PubMed] [Google Scholar]
- Wicks SR, de Vries CJ, van Luenen HG, Plasterk RH (2000) CHE-3, a cytosolic dynein heavy chain, is required for sensory cilia structure and function in Caenorhabditis elegans. Dev Biol 221: 295–307 [DOI] [PubMed] [Google Scholar]
- Zwaal RR, Mendel JE, Sternberg PW, Plasterk RHA (1997) Two neuronal G proteins are involved in chemosensation of the Caenorhabditis elegans dauer-inducing phenormone. Genetics 145: 715–727 [DOI] [PMC free article] [PubMed] [Google Scholar]


