Abstract
The intermingling of larval functional neurons with adult-specific neurons during metamorphosis contributes to the development of the adult Drosophila brain. To better understand this process, we characterized the development of a dorsal cluster (DC) of Atonal-positive neurons that are born at early larval stages but do not undergo extensive morphogenesis until pupal formation. We found that Baboon(Babo)/dSmad2-mediated TGF-β signaling, known to be essential for remodeling of larval functional neurons, is also indispensable for proper morphogenesis of these adult-specific neurons. Mosaic analysis reveals slowed development of mutant DC neurons, as evidenced by delays in both neuronal morphogenesis and atonal expression. We observe similar phenomena in other adult-specific neurons. We further demonstrate that Babo/dSmad2 operates autonomously in individual neurons and specifically during the late larval stage. Our results suggest that Babo/dSmad2 signaling prior to metamorphosis may be widely required to prepare neurons for the dynamic environment present during metamorphosis.
Keywords: metamorphosis, neuronal morphogenesis, TGF-β
Introduction
Neural circuits can be highly plastic but we do not know much about the molecular mechanisms that underlie changes of neuronal connections. The nervous systems of holometabolous insects undergo extensive reorganization during metamorphosis enabling drastic behavioral changes. Many processes contribute to this complex phenomenon, including neurogenesis, cell death, and the modification of persistent neurons (reviewed in Levine et al, 1995). In these animals, neuroblasts (Nbs) appear to have two neurogenic periods: an initial brief period during embryogenesis to produce neurons involved in larval behavior and a second prolonged phase during larval growth that results in a much larger set that underlies adult behavior (Booker and Truman, 1987; Truman and Bate, 1988; Prokop and Technau, 1991; Truman et al, 2004). Some larval neurons, like mushroom body (MB) γ neurons (Technau and Heisenberg, 1982; Armstrong et al, 1998; Lee et al, 1999), persist to the adult stage but their neurites are remodeled during metamorphosis (for a review, see Truman, 1990; Weeks and Levine, 1990); and many adult-specific neurons extend primary neurites soon after they are born but wait until metamorphosis to complete their extension to adult specific synaptic targets (Booker and Truman, 1987; Truman et al, 2004). Metamorphosis presents these neurites with a complex, dynamic landscape and, therefore, timely executions of early developmental programs in neurons are crucial for their later maturation.
Extensive studies over the past decades have demonstrated that the steroid hormone 20-hydroxyecdysone (hereafter referred to as ecdysone) acts to transform the central nervous system (CNS) from larval to adult form (Levine et al, 1995). The ecdysone receptor gene, EcR, encodes three receptors: EcR-A, EcR-B1, and EcR-B2 that contain the same DNA-binding domain and ligand-binding domain, differing only in the N-terminal regions (Talbot et al, 1993). Different EcR isoforms are differentially expressed in neurons undergoing different developmental changes during metamorphosis (Robinow et al, 1993; Truman et al, 1994). Most larval neurons show high levels of EcR-B1 expression at the start of metamorphosis, a time when they lose larval features in response to ecdysone (Truman et al, 1994). In the larval MBs, EcR-B1 is abundantly expressed in γ neurons but largely absent from α′/β′ neurons and is essential for remodeling of γ neurons (Lee et al, 2000). In addition to the ecdysone pathway, the TGF-β/Activin type I receptor, Babo, and its downstream signaling molecule, dSmad2 (Smad on X), play essential roles in the remodeling of MB γ neurons (Zheng et al, 2003). Interestingly, Babo/dSmad2-mediated TGF-β signaling is apparently required for high-level expression of EcR-B1 in the larval functional neurons that are designated to undergo remodeling during early metamorphosis (Zheng et al, 2003).
The evolutionarily conserved transforming growth factor-β (TGF-β) superfamily has been shown to be involved in aspects of cell differentiation, cell growth, and various morphogenetic processes during development (for a review, see Kingsley, 1994; Derynck and Feng, 1997). Current evidence supports the idea that TGF-β signaling mediates distinct developmental processes through transcriptional regulation of various target genes (reviewed in Derynck et al, 1998). Basically, secreted TGF-β ligands bring TGF-β type I receptor together with TGF-β type II receptors, leading to phosphorylation of receptor-regulated Smad (R-Smad) proteins (reviewed in Heldin et al, 1997). The phosphorylated R-Smad proteins then translocate to the nucleus and regulate gene expression after being incorporated into transcriptional complexes (e.g., Chen et al, 1997; Labbe et al, 1998; Zhang et al, 1998; Yanagisawa et al, 1999).
In Drosophila, homologues for almost all of the different classes of TGF-β-like signaling components have been identified (reviewed in Raftery and Sutherland, 1999). With respect to the putative Drosophila Activin pathway, Babo and Punt are recognized as the TGF-β type I and type II receptor, respectively, based on their structural and ligand binding properties (Childs et al, 1993; Wrana et al, 1994). However, the other type II receptor, Wishful thinking (Wit), initially characterized as a bone morphogenetic protein (BMP)-type receptor (Baarends et al, 1994; Kawabata et al, 1995; Aberle et al, 2002; Marques et al, 2002) can mediate Babo-dependent neuronal remodeling as well (Zheng et al, 2003). In addition, dSmad2 is identified because of its significant homology (∼70%) to vertebrate Smad2/3 (Brummel et al, 1999; Das et al, 1999). It also has been reported that Babo can complex with and subsequently phosphorylate dSmad2, which suggests dSmad2 as Babo's direct downstream effecter (Brummel et al, 1999; Das et al, 1999). However, Babo/dSmad2's complete repertoire of physiological functions remains unclear. Analysis of babo null mutants as well as babo germline clones reveals that babo homozygous mutant organisms are delayed in development. For instance, babo mutant larvae are small in length and exhibit a severe reduction in the size of the brain hemispheres (Brummel et al, 1999; Shyamala and Bhat, 2002; Zheng et al, 2003). Characterization of gene expression by in situ hybridization further shows that the Babo receptor and its candidate ligand, dActivin, exist widely in various tissues (Brummel et al, 1999; Das et al, 1999). These observations suggest that Babo and dSmad2 play broad and critical roles in the development of diverse tissues.
Here we demonstrate that, prior to metamorphosis, Babo/dSmad2-mediated TGF-β signaling plays central roles in governing postmitotic development of adult-specific neurons. Normal Babo/dSmad2 activity assures prompt morphological differentiation of two types of adult-specific neurons, including dorsal cluster (DC) neurons and ellipsoid body (EB) neurons. Furthermore, knocking out genes in controlled manners reveals that Babo/dSmad2 operates autonomously in individual neurons and specifically during the late larval stage. Taken together with our previous findings, we suggest a broad requirement of stage-specific TGF-β signaling in neurons prior to fly brain metamorphosis. Interestingly, we further observe that several components of the Babo/dSmad2-mediated signaling pathway are differentially involved in neuronal remodeling versus neuronal morphogenesis, potentially explaining some TGF-β′s versatility.
Results
Characterization of DC neurons for genetic mosaic analysis of de novo neuronal development in the postembryonic brains
Directed by a 3.6-kb genomic fragment upstream of the atonal (ato) open reading frame, ato-GAL4-dependent expression of UAS-markers permits selective labeling of three clusters of neurons in each lobe of the Drosophila adult brain (Hassan et al, 2000). Among them, DC neurons have their cell bodies located at the protocerebrum–optic lobe junctions (Figure 1C–F, small arrows) and extensively innervate the optic lobes (Figure 1C–F). Like other adult-specific neurons, DC neurons undergo limited morphogenesis in the larval brains. Their primary bundles of neurites traverse the entire central brain but fail to extend into the developing optic lobes before pupal formation (Hassan et al, 2000). Initial characterization of DC neurons involved labeling of bilaterally overlapping neurites and, therefore, provided limited insight into the individual DC neurons' projection patterns. Before undertaking mosaic analysis of gene functions in DC neurons, we sought to determine their projection patterns, elucidate their connectivity by distinguishing dendrites from axons, and monitor the morphogenesis of DC neurons through development.
Figure 1.
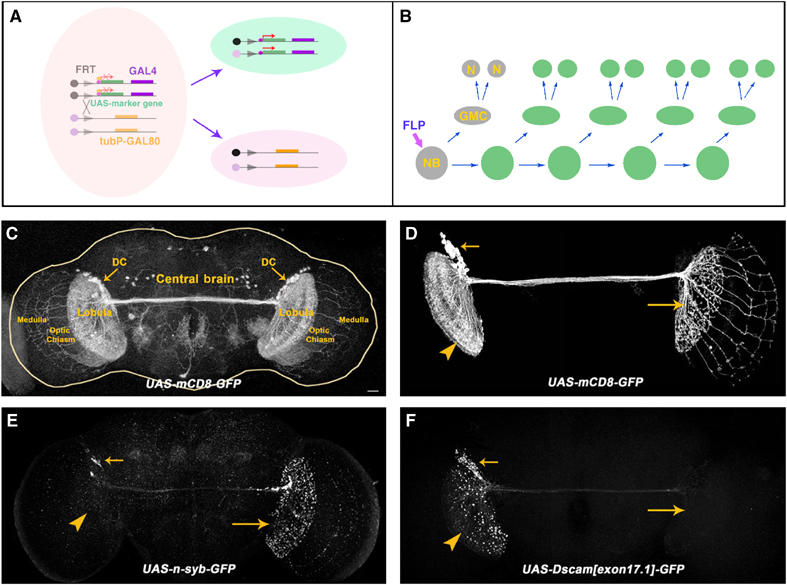
MARCM analysis of DC neurons. (A, B) Schematic drawings showing the essence of the MARCM system. FLP-mediated mitotic recombination between two FRT sites (triangles) leads to loss of the repressor transgene (tubP-GAL80) in one of the daughter cells (A), and hence GAL4-dependent expression of the marker transgene in the GAL80-minus cell as well as its progeny. For instance, loss of GAL80 in an Nb may give rise to a MARCM-labeled multicellular clone (B). (C) ato-GAL4-driven expression of mCD8-GFP permits labeling of the paired DC neurons' entire morphologies. Note the trans-central brain fascicle, dense elaboration in the lobulas, and patterned projections in the medullas. (D–F) Composite confocal images of adult DC Nb clones that are respectively labeled by mCD8-GFP (D), Syb-GFP (E), and Dscam[exon 17.1]-GFP (F). Note restriction of the ipsilateral elaboration to the lobular complex while extension of 13 or so long neurites through the chiasm to the contralateral medulla in [D], selective targeting of Syb-GFP to the contralateral neurites in [E], and an accumulation of Dscam[exon 17.1]-GFP on the ipsilateral side in [F]. Genotypes: (C) UAS-mCD8-GFP/+;ato-GAL4/+; (D) hs-FLP,FRTG13,tubP-GAL80/FRTG13,UAS-mCD8-GFP;ato-GAL4/+; (E) FRTG13/hs-FLP,FRTG13,tubP-GAL80;ato-GAL4/UAS-n-syb-GFP; (F) FRTG13,UAS-Dscam[exon17.1]-GFP/hs-FLP,FRTG13,tubP-GAL80;ato-GAL4/+. The scale bar in this and all the following images equals 20 μm.
MARCM (Lee and Luo, 1999) permits specific labeling of a subset of GAL4-positive cells based on lineage (Figure 1A and B). The bilaterally symmetrical DC neurons are likely derived from paired but independent Nbs; and we reasoned that visualizing one clone of neurons at a time would allow us to resolve their projection patterns. Following mitotic recombination during early larval development, we frequently obtained unilateral clones of DC neurons. Our initial analysis was focused on the large multicellular Nb clones that were generated at the 1st-instar larval stage. Consistent with the notion of only one lineage per hemisphere, all the Nb clones look alike and apparently consist of all the DC neurons that reside in one brain lobe (Figure 1, compare C with D). In contrast with labeling DC neurons bilaterally, marking of one Nb clone of DC neurons reveals asymmetrical patterns of neurite elaboration in the paired optic lobes (Figure 1D, arrowhead versus big arrow). While ipsilateral neurites densely elaborate within the lobular complex (Figure 1D, arrowhead), the projections that traverse the entire central brain innervate the contralateral lobular complex and further extend through the chiasm and into the medulla (Figure 1D, big arrow). Our clonal analysis unambiguously revealed the asymmetrical morphological specializations and encouraged us to characterize further the DC neurons' polarity.
In insect brains, most interneurons are unipolar and lone primary neurites frequently migrate long distances to form axon arbors while dendrite-like branches are often extended into nearer neuropils (Strausfeld, 1976). Consistent with this basic architecture, we observe several phenomena suggesting that the DC neurons' ipsilateral and contralateral projections are dendrites and axons, respectively. First, numerous fine branches versus multiple discrete bouton-like structures are evident in the ipsilateral (Figure 1D, arrowhead) versus the contralateral (Figure 1D, big arrow) elaboration. Second, when UAS-markers were used to label MARCM clones, we saw selective targeting of GFP-tagged Synaptobrevin (a presynaptic marker; Ito et al, 1998) to the contralateral processes (Figure 1E, big arrow) versus an accumulation of Dscam[exon 17.1]-GFP (a dendritic marker; Wang et al, 2004) on the ipsilateral side (Figure 1F, arrowhead). These results collectively argue that the ipsilaterally targeted neurites that are well restricted to the lobular complex are dendrites (postsynaptic) while the contralaterally located arbors that may extend to the medulla are axons (presynaptic).
As previously reported, DC neurons do not undergo extensive morphogenesis until pupal formation (Hassan et al, 2000). To study the morphological development of DC neurons, we examined analogously generated Nb clones of DC neurons at various pupal stages. Following pupal formation, we observe immediate defasciculation of the axonal processes at the terminus of the traversing DC bundle (Figure 2A and B, arrows). The primary dendrites and the extending axons then gradually elaborate into fan-shaped lobules by 24 h after pupal formation (APF) (Figure 2C, arrowhead and arrow). In the following 24 h, symmetrical enlargement of both the dendritic (Figure 2D and E, arrowheads) and axonal (Figure 2D and E, arrows) lobules occurs. Meanwhile, certain axonal projections migrate through the chiasm and acquire stereotyped arbors within the developing medulla (Figure 2D and E, arrows). It appears that innervation of the medulla is completed by 72 h APF (Figure 2F, arrow).
Figure 2.
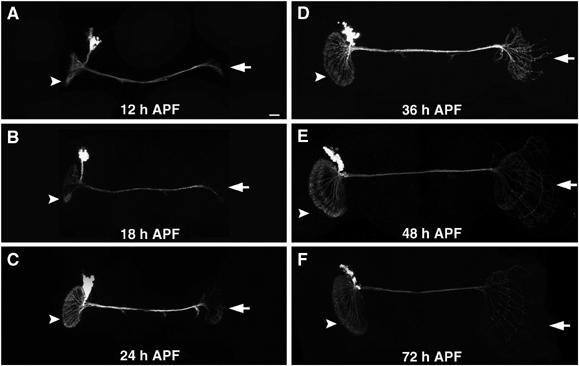
Morphogenesis of DC Nb clones during pupal development. Composite confocal images of analogously generated DC Nb clones that were fixed at various pupal stages. Note the expansion of the paired lobular elaborations (arrowheads) through development and appearance of medulla-targeting neurites (arrows) in the contralateral optic lobe around 36 h after pupal formation (36 h APF). Genotypes: hs-FLP,FRTG13,tubP-GAL80/FRTG13,UAS-mCD8-GFP;ato-GAL4/+.
Requirement of Babo/dSmad2/Punt for proper morphogenesis of DC neurons
Previously, we have shown a requirement for Babo/dSmad2 in the remodeling of the MB γ neurons during early metamorphosis (Zheng et al, 2003). Prompted by the observation that dActivin, a putative ligand for the Babo TGF-β type I receptor, is broadly expressed in the late larval brain (Zheng et al, 2003), we wondered whether Babo/dSmad2-mediated TGF-β signaling governs additional neuronal developmental processes during postembryonic development of the Drosophila brain. Through MARCM analysis of Babo/dSmad2 as well as the Punt TGF-β type II receptor, we found that normal TGF-β signaling is essential for proper morphogenesis of DC neurons.
DC Nb clones homozygous for babo [9], dSmad2 [1], or punt [Δ61] mutations were analogously generated in otherwise heterozygous organisms. When examined at the adult stage, all of them exhibit drastic morphological defects despite containing normal numbers of cells. Compared with wild-type clones, mutant neurons poorly innervate the contralateral optic lobe (Figure 3). The axons often stall at the contralateral protocerebrum–optic lobe junction and are occasionally repelled back to the central brain (Figure 3B–D, big arrowheads). In addition, we sometimes observe neurites that wander away and/or ectopically branch in the process of traversing the central brain (Figure 3B and C, big arrows). By contrast, we detect less obvious abnormalities on the ipsilateral side; and they frequently involve aberrant aggregation of neurites (Figure 3B–D, open arrows) and overextension of processes (Figure 3B and C, small arrowheads). Finally, we notice that defects of punt clones (Figure 3D) are less severe than those of babo and dSmad2 clones (Figure 3B and C). Nevertheless, these loss-of-function mosaic experiments demonstrate that DC neurons require babo, dSmad2 and punt for the extension of axons towards and across the protocerebrum–optic lobe junction.
Figure 3.
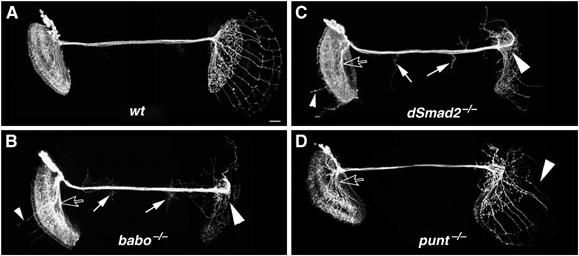
DC Nb clones homozygous for babo/dSmad2/punt exhibit drastic morphogenetic defects. Composite confocal images of DC Nb clones of various genotypes in mosaic adult brains. As compared with the wild-type clone (A), clones homozygous for a babo (B), dSmad2 (C), or punt (D) mutation display various anomalies, including arrest of most axons at the protocerebrum–optic lobe junction (big arrowheads), overextension of neurites from the ipsilateral lobular complex (small arrowheads), ectopic projections in the central brain (big arrows), and aberrant fasciculation of processes (open arrows). Genotype: (A) hs-FLP,FRTG13,tubP-GAL80/FRTG13,UAS-mCD8-GFP;ato-GAL4/+; (B) hs-FLP,FRTG13,tubP-GAL80/FRTG13,babo9,UAS-mCD8-GFP;ato-GAL4/+; (C) FRT19A,tubP-GAL80,hs-FLP/FRT19A,dSmad21,UAS-mCD8-GFP;ato-GAL4/+; (D) ato-GAL4,UAS-mCD8-GFP/hs-FLP,UAS-mCD8-GFP;FRT82B,punt[Δ61]/FRT82B,tubP-GAL80.
DC neurons need Punt specifically during the third instar stage
In addition to controlling cell growth and differentiation, some morphogens, including members of the TGF-β superfamily, may be involved in directly guiding neuronal growth cone migration (e.g. Colavita et al, 1998; Augsburger et al, 1999; Butler and Dodd, 2003). Two ways that Babo/dSmad2 signaling may be involved in the morphogenesis of DC neurons are through governing cell differentiation or guiding growth cone migrations. Given that gross maturation and elaborative morphogenesis of DC neurites occur in two distinct developmental stages, we reasoned that determining when TFG-β signaling is required should help distinguish these two scenarios. One well-characterized punt allele, punt[135-22(ts)], is temperature sensitive (Simin et al, 1998). By analysis of punt[135-22(ts)] clones that had developed at 16°C (permissive) or 25°C (restrictive) at various stages, we sought to determine when DC neurons require Punt for proper morphogenesis.
Following clone induction, mosaic organisms were reared at the permissive or restrictive temperature for various durations, depending on when deprivation of Punt was desired. First, we observe normal numbers of processes in the chiasm region in the punt[135-22(ts)] clones that developed continuously at 16°C (Figure 4A, top panel). In contrast, after being cultured at 25°C through development, punt[135-22(ts)] clones have fewer processes reaching the chiasm (Figure 4A, bottom panel). Second, we performed a set of experiments to block signaling in a stage-specific manner by moving mosaic organisms back and forth between the permissive and the restrictive temperature (Figure 4B). We quantified the severity of phenotypes by counting the number of axons reaching the contralateral chiasm/medulla (Figure 4C). Interestingly, only after culturing 3rd-instar larvae at the restrictive temperature did we see significant reduction in the numbers of chiasm-targeted axons. For instance, transient disruption of Punt activity at the 3rd-instar stage reduces the number from 13 to 6 (Figure 4; compare Condition 1 to Condition 6). Furthermore, the number of chiasm-targeted axons can be increased to 11 from 5 with transient Punt activity at the 3rd-instar stage (Figure 4; compare Condition 9 to Condition 2). These results indicate that Babo/dSmad2-mediated TGF-β signaling is required by DC neurons during their initial development, suggesting the involvement of Babo/dSmad2/Punt in differentiation of DC neurons rather than direct guidance of DC axons.
Figure 4.
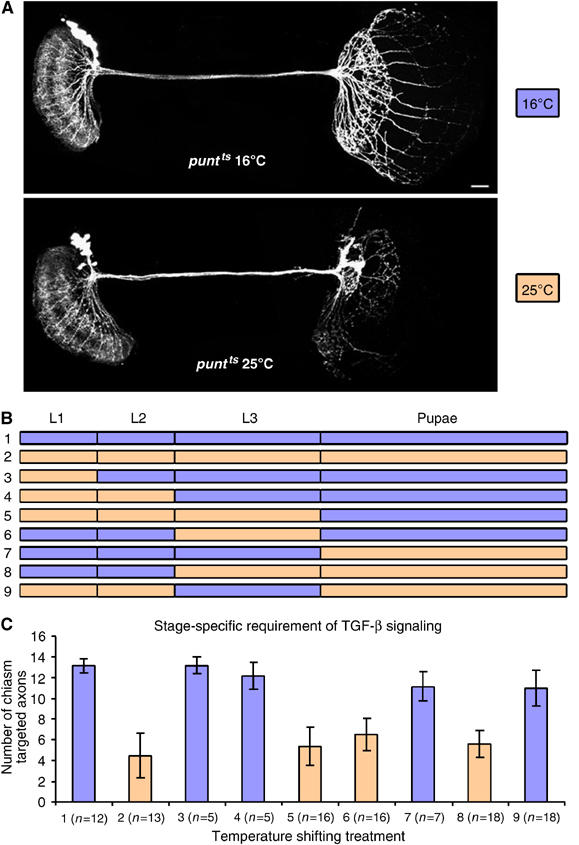
Stage-specific requirement of Punt for proper morphogenesis of DC neurons. (A) punt[135-22(ts)] DC Nb clones developed continuously at a permissive (top) or restrictive (bottom) temperature. Note that 13 (top) versus two (bottom) neurites reached the contralateral chiasm/medulla. (B) Schematic drawing showing various experimental conditions. After clone induction, animals were cultured at either 16°C (blue) or 25°C (orange) at various developmental stages. For instance, mosaic organisms, initially developed at 16°C, were transferred to 25°C since pupal formation in Condition 7. (C) Statistical analysis of the numbers of chiasm targeted neurites per punt[135-22(ts)] DC Nb clone following culture through different conditions, as shown in (B). As compared with Condition 1, 3, 4, 7, and 9 (blue), statistically fewer chiasm projections are observed in Condition 2, 5, 6, and 8 (orange). Genotype: ato-GAL4,UAS-mCD8-GFP/hs-FLP,UAS-mCD8-GFP;FRT82B,punt[135-22(ts)]/FRT82B,tubP-GAL80.
Development of babo mutant DC neurons is delayed prior to the onset of extensive morphogenesis
Since Babo/dSmad2-mediated TGF-β signaling is likely required during the 3rd instar stage, we examined DC neurons' initial morphogenesis to provide insights about the mutants' primary pathological changes. Mitotic recombination was induced in 1st-instar larvae; and we then looked for DC Nb clones at various later larval stages. Briefly, when cultured at 25°C, tiny wild-type clones could be found as early as 2.5 days after larval hatching (ALH; Figure 5A). These clones contain small numbers of cell bodies with neurites that extend ventrally before turning medially toward the protocerebral midline (Figure 5A). Extending axons cross the midline around 3.5 days ALH (Figure 5B, arrow) and some reach the contralateral protocerebrum–optic lobe junction by 4 days ALH (Figure 5C). Meanwhile, dendrites branch out at the turning point (Figure 5B, arrowhead) and gradually elaborate into a bud-like structure (Figure 5D, arrowhead). In addition, we see a steady increase in the numbers of ato-GAL4-positive cells in the developing clones until the late wandering larval stage (Figure 5A′–D′). Interestingly, with reference to the wild-type development, we observe a delay in the initial development of babo mutant DC neurons. First, we do not detect any babo mutant DC clones until 3 days ALH (data not shown; Figure 5F), which is roughly one-half day late compared with normal onset of ato-GAL4 in DC neurons. Second, mutant clones undergo minimal morphogenesis during larval development and remain tiny with few ato-GAL4-positive cells even in the wandering larval brains (Figure 5G and G′). Third, the slowly migrating neurites finally cross the protocerebral midline around 18 h APF (Figure 5I) and reach the contralateral protocerebrum–optic lobe junction one day APF (Figure 5J); but the initial part of the resulting trajectory appears abnormal, as evidenced by a shortened vertical segment and defasciculation/misrouting of neurites (Figure 5J, arrow). Nevertheless, mutant clones ultimately contain similar numbers of cell bodies as wild-type clones (compare Figure 5J′ with E′), suggesting that development of DC neurons is delayed but not blocked by loss of Babo/dSmad2 function. This significant delay in the initial morphogenesis may interfere secondarily with later elaboration of DC neurites in the optic lobes. Taken together with the finding that Punt is specifically required at the 3rd-instar stage, these results demonstrate involvement of TGF-β signaling in governing timely development of DC neurons prior to metamorphosis.
Figure 5.
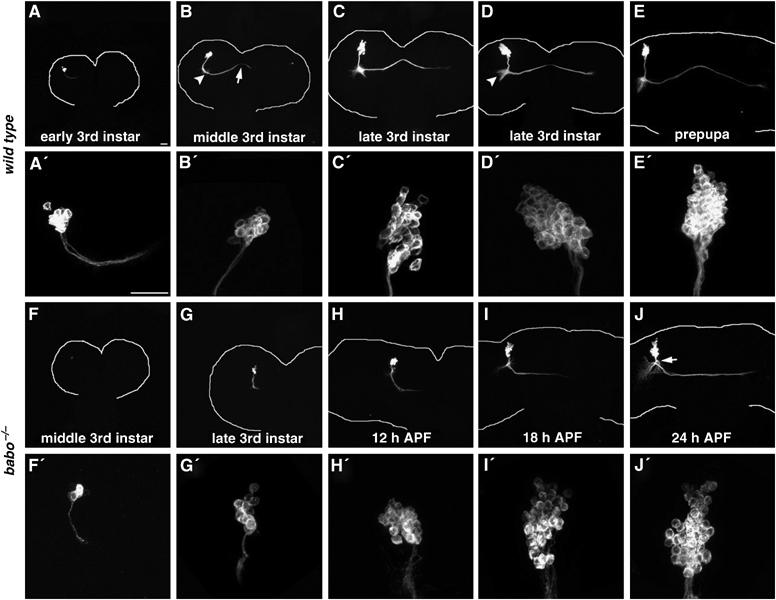
Larval development of babo mutant DC neurons is retarded. DC Nb clones that are wild-type (A–E) or homozygous for babo9 (F–J) were fixed at different developmental stages. Close-up views of individual clones' cell body regions are shown right underneath. Note the differences in the numbers of cell bodies and the extents of neurite migration between wild type and mutant clones during late larval development (e.g. compare (C′) & (C) to (G′) & (G), respectively). In addition, misrouting of mutant neurites becomes evident by 24 h APF (arrow in (J)). Also, note midline crossing of wild-type DC axons (arrow in (B)) and sprouting of DC dendrites (arrowhead in (B)) followed by formation of the DC dendritic bud (arrowhead in (D)). White curves outline brain morphologies. Genotype: (A–E) hs-FLP,FRTG13,tubP-GAL80/FRTG13,UAS-mCD8-GFP;ato-GAL4/+; (F–J) hs-FLP,FRTG13,tubP-GAL80/FRTG13,babo9,UAS-mCD8-GFP;ato-GAL4/+.
Babo/dSmad2 acts cell-autonomously in post-mitotic neurons to promote morphogenesis
Two possible mechanisms of action may explain why babo mutant Nb clones appear late and their development is delayed compared to the surrounding tissues. First, Babo might act in the precursors to assure efficient production of DC neurons. Second, Babo could operate in individual DC neurons to promote their postmitotic development, including expression of ato-GAL4 and initial morphogenesis. To elucidate the underlying mechanism(s), we sought to generate single-cell/two-cell clones of DC neurons in which genes of interest were not removed until the last one or two mitoses. If Babo were largely required in the DC Nbs, one would expect different (more subtle or none at all) phenotypes in single-cell/two-cell clones of babo mutant DC neurons as compared to Nb clones. In addition, learning when single-cell/two-cell clones can be generated provides insight into the DC neurons' timing of birth. If most of the DC neurons were born before the Punt requirement (the 3rd-instar stage), it seems unlikely that Babo regulates neurogenesis in the developing larval brain.
Typical neurogenesis involves the derivation of two postmitotic neurons from a ganglion mother cell (GMC) following each asymmetric division of one Nb; and Nbs repeat asymmetric divisions to establish multicellular lineages (Figure 6A). Thus, to generate single-cell/two-cell clones that consist of the neurons of interest, one needs to induce mitotic recombination during birth of the neurons or their GMCs. With respect to DC neurons, we found that brief induction of mitotic recombination around the 2nd- and early 3rd-instar stage efficiently yielded single-cell/two-cell clones of DC neurons. This observation implies that the majority of DC neurons are born before the requirement of Punt at the 3rd-instar stage. Analysis of wild-type single-cell/two-cell clones further reveals the individual DC neurons' basic patterns of neurite elaboration. First, one DC neuron gives rise to one dendrite and one axon, both of which remain unbranched until innervation of specific optic lobe subdomains (Figure 6B–D). Second, the dendrite and axon derived from the same DC neuron project at similar angles when turning into the optic lobes (Figure 6B–D). Third, sister neurons exhibit almost overlapping elaboration (Figure 6D) while separate single-cell clones often innervate distinct subdomains (Figure 6B). Fourth, different subsets of axons innervate the lobular complex (Figure 6C and D; 52%, n=42) versus the medulla (Figure 6B; 48%, n=42); and it appears that late-derived axons selectively target to the medulla (Figure 6B). These principles greatly facilitate phenotypic analysis of babo mutant single-cell/two-cell clones of DC neurons. First, mutant dendrites may arrive at their correct targets but mutant axons fail to extend into the contralateral optic lobe (Figure 6E–G). They mostly stall at the contralateral protocerebrum–optic lobe junction (Figure 6E–G, arrows; 70%, n=37) and sometimes appear to be repelled back into the central brain (Figure 6E, arrow; 16%, n=37). Second, misrouting of neurites can occur at the beginning of the trajectories (Figure 6F–G, arrowheads; 19%, n=37). These phenotypes mirror what we observe in mutant Nb clones (compare Figure 6 with Figure 3); and, thus, one can ascribe mutant Nb clones' abnormalities to loss of TGF-β signaling in postmitotic neurons. Taken together, we suggest that Babo/dSmad2-mediated TGF-β signaling acts cell-autonomously in individual DC neurons to promote their postmitotic development.
Figure 6.
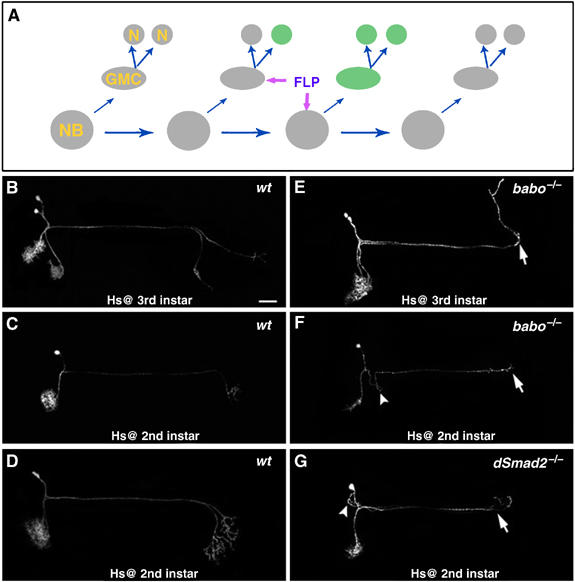
Single-cell mosaic analysis of DC neurons. (A) Schematic drawing of the derivation of a single-cell or two-cell clone following FLP-mediated mitotic recombination in a dividing GMC versus Nb. (B–G) Single-cell/two-cell clones of DC neurons were obtained following stage-specific induction of mitotic recombination. As compared with wild-type clones (B–D), babo mutant DC neurons (E–G) fail to extend axons (arrows) into the contralateral optic lobe and their initial trajectories can be kinky (arrowheads). Genotype: (B–D) hs-FLP,FRTG13,tubP-GAL80/FRTG13,UAS-mCD8-GFP;ato-GAL4/+; (E-F) hs-FLP,FRTG13,tubP-GAL80/FRTG13,babo9,UAS-mCD8-GFP;ato-GAL4/+; (G) FRT19A,tubP-GAL80,hs-FLP/FRT19A,dSmad21,UAS-mCD8-GFP;ato-GAL4/+.
Involvement of Babo/dSmad2 in morphogenesis of other adult-specific neurons
Given their broad expression patterns, we wondered whether Babo and dSmad2 are analogously involved in governing morphological differentiation of other adult-specific neurons. Using GAL4-EB1, we have characterized a subset of ellipsoid body (EB) neurons (Wang et al, 2002). The EB is one of the four neuropils of the adult-specific central complex, which lays at the junction of the two brain lobes. To study roles of Babo in the GAL4-EB1-positive neurons, we generated and examined MARCM-labeled babo mutant GAL4-EB1-positive neurons in otherwise heterozygous organisms. Interestingly, both Nb (Figure 7, compare K with E) and single-cell clones (Figure 7, compare L with F, and compare M with G) of babo mutant EB neurons exhibit similar abnormal axon arborization patterns within the EB neuropils. Mutant axons correctly navigate and initiate arborization inside the EB. But their arbors fail to converge properly at the midline (Figure 7L and M), resulting in a deformed EB ring (Figure 7K). These phenotypes suggest that Babo/dSmad2 signaling is required for proper morphogenesis of postmitotic EB neurons. We further examined EB Nb clones through development and detected a significant delay in mutant neurons' morphogenesis. Around 24 h APF, several wild-type EB neurons start to express GAL4-EB1, accompanied by rudimentary elaboration of EB axons (Figure 7A). During the following 2 days, additional neurons become positive for GAL4-EB1 and a wheel-like structure ultimately forms in wild-type clones (Figure 7B–D). Compared with the wild-type development, babo mutant clones are about 12 h late in their development. They become positive for GAL4-EB1 at 36 h APF (Figure 7H), exhibit neurite elaboration patterns reminiscent of 12-h younger wild-type controls (Figure 7, compare H with A, compare I with B, and compare J with C), and acquire a deformed ring by 72 h APF (Figure 7J). Therefore, Babo is autonomously required by both DC and EB neurons to promote their postmitotic development, suggesting general involvement of Babo/dSmad2-mediated TGF-β signaling in governing larval development of adult-specific Drosophila neurons.
Figure 7.
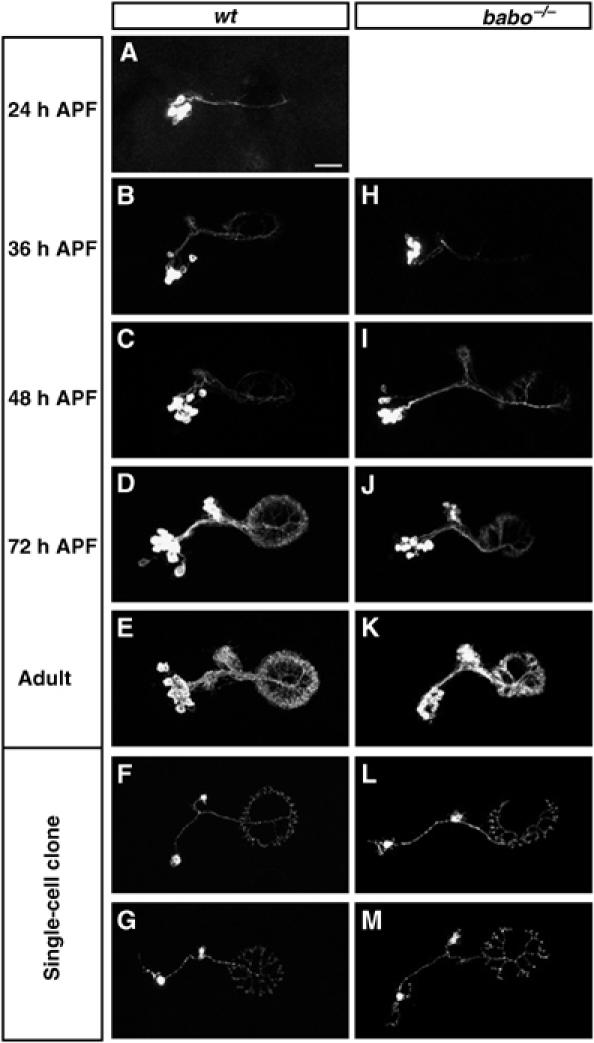
Autonomous requirement of Babo for proper development of postmitotic EB neurons. MARCM clones of EB neurons examined at various stages. Compared with controls (A–G), babo mutant clones (H–M) are slow in initial morphogenesis ((H) to (J), compared with (A) to (D)) and fail to elaborate fully in the ellipsoid body (compare (K) to (E)). Note similar morphogenetic defects in single-cell clones of babo mutant EB neurons (compare (L) & (M) to (F) & (G), respectively). Genotype: (A–G) hs-FLP,FRTG13,tubP-GAL80/FRTG13,UAS-mCD8-GFP;GAL4-EB1/+; (H–M) hs-FLP,FRTG13,tubP-GAL80/FRTG13,babo9,UAS-mCD8-GFP;GAL4-EB1/+.
Components of the Babo/dSmad2-mediated signaling pathway are differentially involved in neuronal remodeling versus neuronal morphogenesis
Although TGF-β/Activin signaling is apparently required for both remodeling of larval-functional neurons and temporal morphogenesis of adult-specific neurons during similar developmental stages, our observations indicate that components of this pathway are differentially involved in the two developmental processes. First, the babo gene encodes two isoforms, Babo-a and Babo-b, which are different only in their ligand-binding domains (Wrana et al, 1994; Brummel et al, 1999). Interestingly, ectopic expression of Babo-a (Figure 8C), but not Babo-b (Figure 8D), in babo mutant γ neurons fully rescues MB remodeling defects. However, individual expression of UAS-babo-a and UAS-babo-b equally and significantly rescue the defects of DC neurons (Figure 8H and I) and coexpression of both isoforms does not further improve the rescue. Incomplete rescue in the DC neurons may be because UAS-babo-a/b expression is later than required due to the delayed onset of ato-GAL4 (Figure 5). Second, homozygous medea[13] (Xu et al, 1998) mutant clones display intermediate defects in DC neurons (Figure 8J), but do not exhibit remodeling defects in MB γ neurons (Figure 8E). Third, EcR-B1 expression is upregulated by the expression of two mutually redundant TGF-β type II receptors, Punt and Wit (Zheng et al, 2003), and disrupting either alone does not cause any detectable MB remodeling defects (Figure 8F and G) (Zheng et al, 2003). Although less severe than those of babo/dSmad2 mutant clones, defects are observed in punt mutant DC clones (Figure 3D). In contrast, wit mutant DC clones appear grossly normal (Figure 8K).
Figure 8.
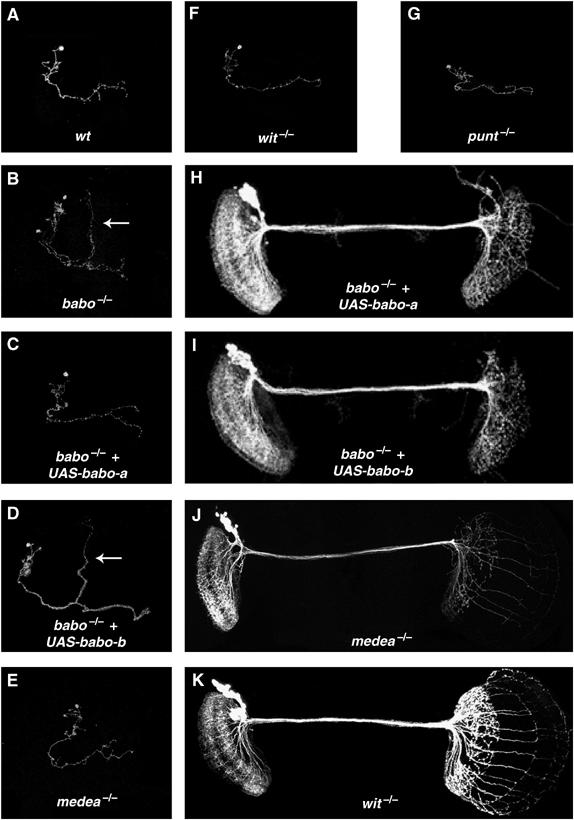
Components of the Babo/dSmad2-mediated signaling pathway are differentially involved in neuronal remodeling versus neuronal morphogenesis. (A–G) Single-cell clones of MB γ neurons of various genotypes in mosaic adult brains. As compared with the wild type (A), babo homozygous mutant γ neurons (B) retained their larval-characteristic dorsal projections (arrows) into the adult stage. Such defective remodeling of γ neurons was rescued in (C) but not in (D), while normal remodeling occurred in (E), (F), and (G). (H–K) DC Nb clones of various genotypes in adult mosaic brains. As compared with Figure 3B, expression of either UAS-babo-a (H) or UAS-36abo-b (I) partially rescued babo mutant DC neurons' morphogenetic defects. In addition, the medea clone (J) but not the wit clone (K) exhibits neurite morphogenetic defects. Genotype: (A) hs-FLP/X;FRTG13,UAS-mCD8-GFP,GAL4-201Y/FRTG13,tubP-GAL80; (B) hs-FLP/X;FRTG13,babo9,UAS-mCD8-GFP,GAL4-201Y/FRTG13,tubP-GAL80; (C) hs-FLP/UAS-babo-a;FRTG13,babo9,UAS-mCD8-GFP,GAL4-201Y/FRTG13,tubP-GAL80; (D) hs-FLP/UAS-babo-b;FRTG13,babo9,UAS-mCD8-GFP,GAL4-201Y/FRTG13,tubP-GAL80; (E) hs-FLP,UAS-mCD8-GFP/X;FRT82B, medea13/FRT82B, tubP-GAL80;GAL4-OK107/+; (F) hs-FLP,UAS-mCD8-GFP/X;FRT82B,punt[Δ61]/FRT82B,tubP-GAL80;GAL4-OK107/+; (G) hs-FLP,UAS-mCD8-GFP/X;wit[G15], FRT[2A]/tub-P,GAL80,FRT[2A];GAL4-OK107/+; (H) UAS-babo-a/+; hs-FLP, FRTG13, tubP-GAL80/FRTG13,babo9,UAS-mCD8-GFP;ato-GAL4/+; (I) UAS-babo-b/+; hs-FLP, FRTG13,tubP-GAL80/FRTG13,babo9,UAS-mCD8-GFP;ato-GAL4/+; (J) hs-FLP/X; UAS-mCD8-GFP, ato-GAL4/+; FRT82B, medea13/FRT82B, tubP-GAL80; (K) hs-FLP/X; UAS-mCD8-GFP, ato-GAL4/+; wit[G15], FRT[2A]/tub-P,GAL80, FRT[2A].
It is possible that protein stability varies in different neurons, which may contribute to the difference in the severity of medea or punt phenotypes in MB versus DC neurons. Alternatively, DC neurons may be more sensitive than MB neurons to the protein levels of either Punt or Medea, and thus DC neurons display defects. It is also possible that the requirement of different type II receptors and different isoforms of type I receptor indicates the involvement of different ligands, and different requirement of the co-Smad, Medea, suggests a diversity of involved co-transcriptional factors. Therefore, the specificity of TGF-β/Activin signaling may vary in different cells due to the availability of different ligands and co-factors. It is technically challenging to determine the ligands and co-factors involved in temporal morphogenesis of adult-specific neurons and future study of these molecules will likely help determine the mechanisms underlying the roles of TGF-β/Activin signaling.
Discussion
The evolutionarily conserved TGF-βs and their signaling molecules are involved in diverse biological processes (Kingsley, 1994; Derynck and Feng, 1997). Interestingly, similar signaling cues often elicit different responses in different cells. Consistent with this notion, we implicate Babo/dSmad2 in mediating distinct morphogenetic processes in different neurons, and we further suggest the involvement of widespread TGF-β signaling in post-embryonic fly brain development before the prepupal ecdysone peak (Figure 9A). Given that many vertebrate TGF-β signaling molecules are dynamically present in the postnatal brain (reviewed in Ebendal et al, 1998), it is tempting to speculate that similar mechanisms may help modulate neural circuitry in higher organisms during periods of extensive morphogenesis.
Figure 9.
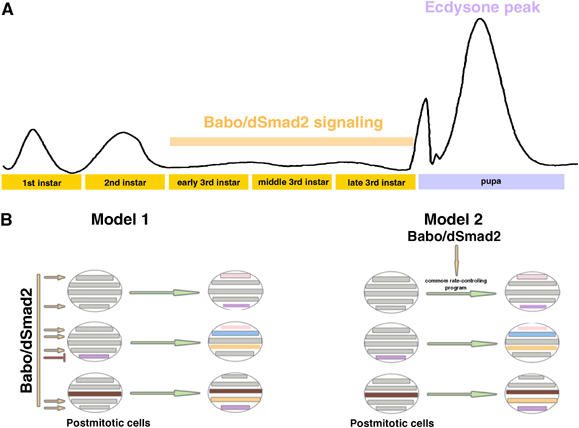
Involvement of Babo/dSmad2 in prepupal development and its possible mechanisms of action. (A) Stage-specific widespread Babo/dSmad2 signaling governs timely differentiation of various tissues prior to the prepual ecdysone peak. (B) Babo/dSmad2 may be directly involved in terminal cell fate determination by regulating different sets of target genes in different types of cells (model 1) or simply govern the rate of cell differentiation via acting through a common rate-controlling program (model 2).
Babo/dSmad2 signaling is involved in temporal morphogenesis of adult-specific neurons
We used spatially and/or temporally controlled genetic manipulations to provide several insights into Babo/dSmad2's roles in postmitotic neuronal morphogenesis. First, mutant clones of interest exhibit similar phenotypes in various mosaic organisms (Figure 3B–D), supporting the cell-autonomous involvement of Babo/dSmad2/Punt and arguing against interference from unlabeled background clones. Second, knocking down Punt at different developmental stages indicated a specific requirement of TGF-β signaling in prewandering larvae (Figure 4B and C), providing an argument against the direct involvement of Babo/dSmad2/Punt in adult-specific neurons' extensive morphogenesis during early metamorphosis. Third, the defects observed in single-cell (Figures 6, 7L and M) versus Nb (Figure 3 and Figure 7K) mutant clones are similar, suggesting a requirement of TGF-β signaling in postmitotic neurons rather than their precursors. Fourth, phenotypic analysis through development reveals a delay in the postmitotic development of mutant neurons (Figures 5 and 7), as evidenced by slow morphogenesis as well as late onset of subtype-specific GAL4 drivers (Figures 5 and 7). Interestingly mutant neurons acquire stereotyped, although abnormal morphologies. For instance, mutant DC axons often stall and occasionally get repelled from the junction between the central brain and the optic lobe (Figure 3). It may be that the optic lobe becomes impermeable to late-arriving DC neurites or that mutant neurons intrinsically lack the ability to penetrate the protocerebral-optic lobe interface. The subtler dendritic phenotypes we observe may be because dendrites have nearby targets and undergo little morphogenesis before pupal formation.
Potential functions of DC neurons in the fly brain
Due to the presence of extensively overlapping neurites, previous studies utilizing dendritic (Clark et al, 1997) and axonal (Callahan and Thomas, 1994) markers were unable to identify the dendrites of DC neurons on the ipsilateral optic lobe (Hassan et al, 2000). To exclude interference from the neurites coming from the opposing side, we created MARCM clones only on one side of the brain and tested several different dendritic and axonal markers (data not shown, Figure 1E and F). We demonstrated the selective targeting of GFP-tagged Synaptobrevin (a presynaptic marker) to the contralateral processes versus an accumulation of Dscam (exon 17.1)-GFP (a dendritic marker) on the ipsilateral side. Thus, DC neurons send their dendrites to the ipsilateral optic lobe and axons to the contralateral one, directly connecting the two optic lobes (Figure 1). It appears that they receive input from the nearby optic lobe and output onto the contralateral lobular complex, chiasm and medulla. Even though dendrites and axons from contralateral DC neurons innervate similar regions of the optic lobes, they may not make direct connections, since they occupy different focal planes in our confocal micrographs (data not shown).
Insights regarding the roles of DC neurons are few and therefore current ideas about their functions are speculative. Ablation of the ato-expressing neurons through ectopic expression of cell-death genes by ato-GAL4 leads to failed or delayed eclosion of the flies (Hassan et al, 2000). This indicates a potential role for these neurons in eclosion but it is currently impossible to distinguish between the requirements for DC neurons versus other ato-expressing neurons. The insights regarding connectivity provided in this paper are consistent with the model that DC neurons handle simultaneous processing of information from both optic lobes.
Global TGF-β/Activin prepares fly brains for temporal metamorphosis
Given that Babo/dSmad2 is required around the mid-3rd instar stage for high-level expression of EcR-B1 in the larval functional MB γ neurons, (Zheng et al, 2003) we wondered if stage-specific TGF-β signaling is also required for timely differentiation of these neurons prior to metamorphosis. We re-examined the expression of EcR-B1 in dSmad2 mutant MB Nb clones at various late larval stages and observed a 12-h delay in the onset of EcR-B1 expression in dSmad2 mutant MB γ neurons (data not shown). As reported previously, 100% of dSmad2 mutant clones of γ neurons fail to remodel their neurites during early metamorphosis, thus the delayed EcR-B1 expression may block the MB γ neurons' responses to the prepupal ecdysone peak. These phenomena imply that TGF-β and ecdysone signals act sequentially in the postembryonic development of the Drosophila brain.
Loss of Babo/dSamd2-mediated TGF-β/Activin signaling leads to delayed neuronal morphogenesis and delayed expressions of genes. We wondered if advanced neuronal development or expression of certain genes would occur if we activated the Babo/dSmad2 pathway early. To test this hypothesis, we ectopically expressed a transgene encoding a constitutively active (CA) form of Babo-a (Brummel et al, 1999) in the MB or DC neurons with GAL4-OK107 and ato-GAL4, respectively. We did not detect early or increased expression of either EcR-B1 or Atonal, nor did we observe precocious morphological changes in MB or DC neurons (data not shown). Of course, it is possible that the activity of the ato-GAL4/(CA)Babo—a combination is too late to affect the morphogenesis of DC neurons, but the onset of GAL4-OK107 is more than 2 days ahead of the requirement of endogenous Babo/dSmad2 signaling. Thus, the Drosophila TGF-β/Activin pathway may play a permissive role in promoting the timely differentiation of postmitotic neurons or additional temporally controlled signaling may be involved.
Nevertheless, our analysis of Babo/dSmad2's functions in various neurons reveals the potential role of global TGF-β signaling in preparing fly brains for metamorphosis. In the larval functional neurons that remodel during early metamorphosis, Babo/dSmad2 acts to upregulate expression of a certain ecdysone receptor isoform before the prepupal ecdysone peak. At the same developmental stage, TGF-β signaling promotes morphological differentiation of larval immature neurons. Interestingly, these distinct developmental processes may both be controlled by transcriptional regulation. Apparent involvement of transcriptional controls in Babo/dSmad2-dependent neuronal morphogenesis is evidenced by a significant delay in the expression of the subtype-specific postmitotic markers, ato-GAL4 (Figure 5), GAL4-EB1 (Figure 7) and EcR-B1 (data not shown). We speculate that such stage-specific global TGF-β signaling may temporally coordinate diverse developmental programs and may help to synchronize development of neurons that are born sequentially over a broad window within individual lineages. Nevertheless, better understanding of the physiological significance awaits characterization of the involved TGF-β(s) and their modes of secretion/action.
Diversity of TGF-β/Activin signaling
Although transcriptional regulation of distinct target genes may underlie different Babo/dSmad2 functions (model 1 in Figure 9B), much remains to be investigated regarding how activation of the same molecules can elicit distinct nuclear responses. TGF-β signaling leads to translocation of phosphorylated R-Smad proteins, which might complex with co-Smad. Because Smad proteins alone confer little DNA-binding specificity (Shi et al, 1998), their induction of specific genes possibly depends on transcription factors that form complexes with nuclear Smads. Some of these factors may be ubiquitous and available in diverse cells, while others may be differentially restricted to activate gene expression in various cell type-specific manners (Shi and Massague, 2003). One also wonders if differential involvement of Punt (Figures 3D and 8F) versus Wit (Figure 8G and K) (Zheng et al, 2003), Babo-a (Figure 8H) versus Babo-b (Figure 8I), Medea (Figure 8E and J) and/or dActivin versus the Activin-like protein (MB O'Connor, personal communication) results in qualitatively and/or quantitatively distinct patterns of TGF-β signaling leading to different cellular responses.
Interestingly, Babo is apparently needed for prompt cell differentiation/growth in both neural and non-neural tissues during late larval development (Brummel et al, 1999). However, loss of Babo does not appear to affect the ultimate cell fates. We suggest an alternative model for TGF-β′s mechanism of action in promoting cell differentiation/growth (model 2 in Figure 9B). We propose that independent genetic programs control cell fate determination and the rate of differentiation, and that there is a common molecular network that determines the rate of cell differentiation/growth. It is possible that activation of Babo/dSmad2 may simply intensify a similar genetic program in different cells to facilitate distinct, already ongoing, but otherwise slowly-progressing cell type-specific development. However, it is also possible that cell fate and the rate of differentiation are extensively intertwined. Future identification of Babo/dSmad2 downstream signaling targets in various tissues should provide some fundamental insights into both organism development and brain function.
Materials and methods
Fly strains
Creation of babo MARCM clones involves (1) hs-FLP, FRTG13, tubP-GAL80/CyO,y; (2) FRTG13,babo9,UAS-mCD8-GFP/CyO,y;ato-GAL4,GAL4-EB1; (3) FRTG13, babo9, UAS-mCD8-GFP, GAL4-201Y/CyO,y; (4) UAS-babo-a (X chromosome); and (5) UAS-babo-b (X chromosome). Creation of dSmad2 mutant clones involves (6) FRT19A, tubP-GAL80, hs-FLP; ato-GAL4; (7) FRT19A, tubP-GAL80, hs-FLP; GAL4-OK107; and (8) FRT19A, dSmad21, UAS-mCD8-GFP/Fm7C. (9) FRTG13, UAS-mCD8-GFP; ato-GAL4, GAL4-EB1 and (10) FRTG13, UAS-mCD8-GFP/CyO,y are used for creating wild-type MARCM clones. Creation of punt clones involves (11) ato-GAL4, UAS-mCD8-GFP; FRT82B, punt[Δ61]/TM6B; (12) ato-GAL4, UAS-mCD8-GFP;FRT82B,punt[135-22(ts)]; (13) hs-FLP,UAS-mCD8-GFP;FRT82B, tubP-GAL80; and (14) hs-FLP,UAS-mCD8-GFP;FRT82B,tubP-GAL80;GAL4-OK107. (15) hs-FLP,UAS-mCD8-GFP; tubP-GAL80, FRT[2A]; GAL4-OK107; (16) hs-FLP,UAS-mCD8-GFP; tubP-GAL80, FRT[2A]; and (17) ato-GAL4, UAS-mCD8-GFP; wit[G15], FRT[2A]/TM6B are used for creating wit MARCM clones. Creation of medea clones involves (18) ato-GAL4, UAS-mCD8-GFP; FRT82B, medea13/TM6B. Creation of wild-type MARCM clone with the axonal marker UAS-synaptobrevin-GFP involves (19) FRTG13;UAS-n-syb-GFP/TM3, (20) Pin/CyO;ato-GAL4,GAL4-EB1. Creation of wild-type MARCM clone with the dendritic marker UAS-Dscam[exon17.1]-GFP involves (21) FRTG13 UAS-Dscam[exon17.1]-GFP.
Generation of MARCM clones
Generation of Nb MARCM clones of MB, DC and EB neurons: 0–2 h old larvae were collected and placed on standard fly food at a density of 80 larvae per vial. A 60-min heat shock at 37°C was applied immediately to induce the expression of the hs-FLP transgene, and hence mitotic recombination. After clone induction, the animals were either kept at 25°C, 16°C or shifted back and forth (see the temperature shifting experiments). Brains were dissected at different larval, pupal or adult stages. Generation of single-cell or two-cell MARCM clones of DC and EB neurons: 0–2 h old larvae were collected and placed on standard fly food at a density of 80 larvae per vial. Animals were kept at 25°C before one 15-min heat shock at 37°C around one of the following time points: 0 day after larval hatching (ALH), 0.5 day ALH, 1 day ALH, 1.5 days ALH, 2 days ALH, or eclosion of the flies. Brains were all dissected at the adult stage.
Detection of MARCM clones by immunohistochemistry
Immunohistochemistry was performed according to Lee et al (1999). MARCM clone was labeled using the rat anti-mCD8 mAb (1:100, Caltag, Burlingame, CA). Synaptobrevin-GFP and Dscam[exon17.1]-GFP were detected by the rabbit anti-GFP polyclonal antibody (1:200, Molecular Probes, Eugene, OR). Florescence signals were captured with Zeiss LSM510 confocal microscope (Carl Zeiss, Oberkochen, Germany) and processed using Adobe Photoshop (Adobe system, San Jose, CA).
Acknowledgments
We thank H Bellen for ato-GAL4 flies; M Ramaswami for UAS-n-syb-GFP flies; MB O'Connor for punt[Δ61] and punt[135-22(ts)] flies. We thank Dr Mike O'Connor and members of the Lee lab for comments on the manuscript. This work was supported by a predoctoral fellowship (0415533Z) from the American Heart Association to XZ, a training grant (HD07333) from National Institutes of Health to CTZ, and a National Institutes of Health grant to TL. TL is a Klingenstein fellow.
References
- Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhaes TR, Goodman CS (2002) Wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron 33: 545–558 [DOI] [PubMed] [Google Scholar]
- Armstrong JD, de Belle JS, Wang Z, Kaiser K (1998) Metamorphosis of the mushroom bodies; large-scale rearrangements of the neural substrates for associative learning and memory in Drosophila. Learn Mem 5: 102–114 [PMC free article] [PubMed] [Google Scholar]
- Augsburger A, Schuchardt A, Hoskins S, Dodd J, Butler S (1999) BMPs as mediators of roof plate repulsion of commissural neurons. Neuron 24: 127–141 [DOI] [PubMed] [Google Scholar]
- Baarends WM, van Helmond MJ, Post M, van der Schoot PJ, Hoogerbrugge JW, de Winter JP, Uilenbroek JT, Karels B, Wilming LG, Meijers JH, Themmen APN, Grootegoed JA (1994) A novel member of the transmembrane serine/threonine kinase receptor family is specifically expressed in the gonads and in mesenchymal cells adjacent to the mullerian duct. Development 120: 189–197 [DOI] [PubMed] [Google Scholar]
- Booker R, Truman JW (1987) Postembryonic neurogenesis in the CNS of the tobacco hornworm, Manduca sexta. I. Neuroblast arrays and the fate of their progeny during metamorphosis. J Comp Neurol 255: 548–559 [DOI] [PubMed] [Google Scholar]
- Brummel T, Abdollah S, Haerry TE, Shimell MJ, Merriam J, Raftery L, Wrana JL, O'Connor MB (1999) The Drosophila activin receptor baboon signals through dSmad2 and controls cell proliferation but not patterning during larval development. Genes Dev 13: 98–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SJ, Dodd J (2003) A role for BMP heterodimers in roof plate-mediated repulsion of commissural axons. Neuron 38: 389–401 [DOI] [PubMed] [Google Scholar]
- Callahan CA, Thomas JB (1994) Tau-β-galactosidase, an axon-targeted fusion protein. Proc Natl Acad Sci USA 91: 5972–5976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Weisberg E, Fridmacher V, Watanabe M, Naco G, Whitman M (1997) Smad4 and FAST-1 in the assembly of activin-responsive factor. Nature 389: 85–89 [DOI] [PubMed] [Google Scholar]
- Childs SR, Wrana JL, Arora K, Attisano L, O'Connor MB, Massague J (1993) Identification of a Drosophila activin receptor. Proc Natl Acad Sci USA 90: 9475–9479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark IE, Jan LY, Jan YN (1997) Reciprocal localization of Nod and kinesin fusion proteins indicates microtubule polarity in the Drosophila oocyte, epithelium, neuron and muscle. Development 124: 461–470 [DOI] [PubMed] [Google Scholar]
- Colavita A, Krishna S, Zheng H, Padgett RW, Culotti JG (1998) Pioneer axon guidance by UNC-129, a C. elegans TGF-β. Science 281: 706–709 [DOI] [PubMed] [Google Scholar]
- Das P, Inoue H, Baker JC, Beppu H, Kawabata M, Harland RM, Miyazono K, Padgett RW (1999) Drosophila dSmad2 and Atr-I transmit activin/TGF-β signals. Genes Cells 4: 123–134 [DOI] [PubMed] [Google Scholar]
- Derynck R, Feng XH (1997) TGF-β receptor signaling. Biochim Biophys Acta 1333: F105–F150 [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang Y, Feng XH (1998) Smads: transcriptional activators of TGF-β responses. Cell 95: 737–740 [DOI] [PubMed] [Google Scholar]
- Ebendal T, Bengtsson H, Soderstrom S (1998) Bone morphogenetic proteins and their receptors: potential functions in the brain. J Neurosci Res 51: 139–146 [DOI] [PubMed] [Google Scholar]
- Hassan BA, Bermingham NA, He Y, Sun Y, Jan YN, Zoghbi HY, Bellen HJ (2000) atonal regulates neurite arborization but does not act as a proneural gene in the Drosophila brain. Neuron 25: 549–561 [DOI] [PubMed] [Google Scholar]
- Heldin CH, Miyazono K, ten Dijke P (1997) TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature 390: 465–471 [DOI] [PubMed] [Google Scholar]
- Ito K, Suzuki K, Estes P, Ramaswami M, Yamamoto D, Strausfeld NJ (1998) The organization of extrinsic neurons and their implications in the functional roles of the mushroom bodies in Drosophila melanogaster Meigen. Learn Mem 5: 52–77 [PMC free article] [PubMed] [Google Scholar]
- Kawabata M, Chytil A, Moses HL (1995) Cloning of a novel type II serine/threonine kinase receptor through interaction with the type I transforming growth factor-β receptor. J Biol Chem 270: 5625–5630 [DOI] [PubMed] [Google Scholar]
- Kingsley DM (1994) The TGF-β superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev 8: 133–146 [DOI] [PubMed] [Google Scholar]
- Labbe E, Silvestri C, Hoodless PA, Wrana JL, Attisano L (1998) Smad2 and Smad3 positively and negatively regulate TGF-β-dependent transcription through the forkhead DNA-binding protein FAST2. Mol Cell 2: 109–120 [DOI] [PubMed] [Google Scholar]
- Lee T, Lee A, Luo L (1999) Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development 126: 4065–4076 [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L (1999) Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22: 451–461 [DOI] [PubMed] [Google Scholar]
- Lee T, Marticke S, Sung C, Robinow S, Luo L (2000) Cell-autonomous requirement of the USP/EcR-B ecdysone receptor for mushroom body neuronal remodeling in Drosophila. Neuron 28: 807–818 [DOI] [PubMed] [Google Scholar]
- Levine RB, Morton DB, Restifo LL (1995) Remodeling of the insect nervous system. Curr Opin Neurobiol 5: 28–35 [DOI] [PubMed] [Google Scholar]
- Marques G, Bao H, Haerry TE, Shimell MJ, Duchek P, Zhang B, O'Connor MB (2002) The Drosophila BMP type II receptor Wishful Thinking regulates neuromuscular synapse morphology and function. Neuron 33: 529–543 [DOI] [PubMed] [Google Scholar]
- Prokop A, Technau GM (1991) The origin of postembryonic neuroblasts in the ventral nerve cord of Drosophila melanogaster. Development 111: 79–88 [DOI] [PubMed] [Google Scholar]
- Raftery LA, Sutherland DJ (1999) TGF-β family signal transduction in Drosophila development: from Mad to Smads. Dev Biol 210: 251–268 [DOI] [PubMed] [Google Scholar]
- Robinow S, Talbot WS, Hogness DS, Truman JW (1993) Programmed cell death in the Drosophila CNS is ecdysone-regulated and coupled with a specific ecdysone receptor isoform. Development 119: 1251–1259 [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J (2003) Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113: 685–700 [DOI] [PubMed] [Google Scholar]
- Shi Y, Wang YF, Jayaraman L, Yang H, Massague J, Pavletich NP (1998) Crystal structure of a Smad MH1 domain bound to DNA: insights on DNA binding in TGF-β signaling. Cell 94: 585–594 [DOI] [PubMed] [Google Scholar]
- Shyamala BV, Bhat KM (2002) A positive role for patched-smoothened signaling in promoting cell proliferation during normal head development in Drosophila. Development 129: 1839–1847 [DOI] [PubMed] [Google Scholar]
- Simin K, Bates EA, Horner MA, Letsou A (1998) Genetic analysis of punt, a type II Dpp receptor that functions throughout the Drosophila melanogaster life cycle. Genetics 148: 801–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausfeld NJ (1976) Atlas of an Insect Brain. Berlin: Springer-Verlag [Google Scholar]
- Talbot WS, Swyryd EA, Hogness DS (1993) Drosophila tissues with different metamorphic responses to ecdysone express different ecdysone receptor isoforms. Cell 73: 1323–1337 [DOI] [PubMed] [Google Scholar]
- Technau G, Heisenberg M (1982) Neural reorganization during metamorphosis of the corpora pedunculata in Drosophila melanogaster. Nature 295: 405–407 [DOI] [PubMed] [Google Scholar]
- Truman JW (1990) Metamorphosis of the central nervous system of Drosophila. J Neurobiol 21: 1072–1084 [DOI] [PubMed] [Google Scholar]
- Truman JW, Bate M (1988) Spatial and temporal patterns of neurogenesis in the central nervous system of Drosophila melanogaster. Dev Biol 125: 145–157 [DOI] [PubMed] [Google Scholar]
- Truman JW, Schuppe H, Shepherd D, Williams DW (2004) Developmental architecture of adult-specific lineages in the ventral CNS of Drosophila. Development 131: 5167–5184 [DOI] [PubMed] [Google Scholar]
- Truman JW, Talbot WS, Fahrbach SE, Hogness DS (1994) Ecdysone receptor expression in the CNS correlates with stage-specific responses to ecdysteroids during Drosophila and Manduca development. Development 120: 219–234 [DOI] [PubMed] [Google Scholar]
- Wang J, Ma X, Yang JS, Zheng X, Zugates CT, Lee CH, Lee T (2004) Transmembrane/juxtamembrane domain-dependent Dscam distribution and function during mushroom body neuronal morphogenesis. Neuron 43: 663–672 [DOI] [PubMed] [Google Scholar]
- Wang J, Zugates CT, Liang IH, Lee CH, Lee T (2002) Drosophila Dscam is required for divergent segregation of sister branches and suppresses ectopic bifurcation of axons. Neuron 33: 559–571 [DOI] [PubMed] [Google Scholar]
- Weeks JC, Levine RB (1990) Postembryonic neuronal plasticity and its hormonal control during insect metamorphosis. Annu Rev Neurosci 13: 183–194 [DOI] [PubMed] [Google Scholar]
- Wrana JL, Tran H, Attisano L, Arora K, Childs SR, Massague J, O'Connor MB (1994) Two distinct transmembrane serine/threonine kinases from Drosophila melanogaster form an activin receptor complex. Mol Cell Biol 14: 944–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Yin Z, Hudson JB, Ferguson EL, Frasch M (1998) Smad proteins act in combination with synergistic and antagonistic regulators to target Dpp responses to the Drosophila mesoderm. Genes Dev 12: 2354–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa J, Yanagi Y, Masuhiro Y, Suzawa M, Watanabe M, Kashiwagi K, Toriyabe T, Kawabata M, Miyazono K, Kato S (1999) Convergence of transforming growth factor-β and vitamin D signaling pathways on SMAD transcriptional coactivators. Science 283: 1317–1321 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Feng XH, Derynck R (1998) Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-β-induced transcription. Nature 394: 909–913 [DOI] [PubMed] [Google Scholar]
- Zheng X, Wang J, Haerry TE, Wu AY, Martin J, O'Connor MB, Lee CH, Lee T (2003) TGF-β signaling activates steroid hormone receptor expression during neuronal remodeling in the Drosophila brain. Cell 112: 303–315 [DOI] [PubMed] [Google Scholar]


