Abstract
Engagement of the T-cell antigen receptor leads to recruitment of phospholipase Cγ1 (PLCγ1) to the LAT-nucleated signaling complex and to PLCγ1 activation in a tyrosine phosphorylation-dependent manner. The mechanism of PLCγ1 recruitment and the role of PLCγ1 Src homology (SH) domains in this process remain incompletely understood. Using a combination of biochemical methods and real-time fluorescent imaging, we show here that the N-terminal SH2 domain of PLCγ1 is necessary but not sufficient for its recruitment. Either the SH3 or C-terminal SH2 domain of PLCγ1, with the participation of Vav1, c-Cbl and Slp76, are required to stabilize PLCγ1 recruitment. All three PLCγ1 SH domains are required for phosphorylation of PLCγ1 Y783, which is critical for enzyme activation. These novel findings entailed revision of the currently accepted model of PLCγ1 recruitment and activation in T lymphocytes.
Keywords: calcium, confocal microscopy, FRET, protein–protein interaction, TCR
Introduction
Ligation of the T-cell antigen receptor (TCR) initiates a cascade of molecular events leading to T-cell activation, cellular proliferation and cytokine production. Proximal events that immediately follow TCR engagement include activation of protein kinases and phosphorylation of multiple enzymes and adaptor molecules (Burack et al, 2002; Samelson, 2002). The phosphorylation of LAT, a lipid raft-associated adaptor protein, creates docking sites for Src-homology 2 (SH2) domain-containing proteins (Zhang et al, 1998). Slp76, another adaptor protein phosphorylated following TCR engagement (Wu and Koretzky, 2004), is recruited to LAT through its constitutive interaction with a small adaptor protein Gads (Liu et al, 2001). The LAT–Gads–Slp76 complex creates a platform for the recruitment of multiple signaling molecules, including phospholipase Cγ1 (PLCγ1), the adaptors Grb2 and Nck, the Rho-family GTPase exchange factor Vav, the adaptor with ubiquitin-ligase activity c-Cbl, and the Tec-family kinase Itk (Zhang et al, 1998, 2000; Bunnell et al, 2000; Lewis et al, 2001; Tybulewicz et al, 2003; Wu and Koretzky, 2004).
Recruitment and activation of PLCγ1 is a key step in the T-cell activation process triggered by the TCR (Desai et al, 1990; Bonvini et al, 2003). The activated enzyme hydrolyzes phosphatidylinositol-4,5-bisphosphate to inositol-1,4,5-trisphosphate (IP3), which stimulates the release of Ca2+ from intracellular stores, and diacylglycerol, which activates protein kinase C and RasGRP-dependent signaling pathways (Katan, 1998; Ebinu et al, 2000; Rhee, 2001). The increase in intracellular free Ca2+ concentration triggered by IP3 plays a crucial role in the induction of numerous T-cell activation-associated responses (Desai et al, 1990).
Two forms of PLCγ have been identified. PLCγ1 is ubiquitously expressed, whereas PLCγ2 is limited to certain cell types primarily of hematopoietic lineage. T cells express predominantly the PLCγ1 form (Katan, 1998). A common structural feature of all PLCs is a split catalytic domain comprised of two conserved subdomains. In PLCγ, these subdomains are separated by a regulatory region, which includes two SH2 domains followed by a single Src-homology 3 (SH3) domain (Katan, 1998; Rhee, 2001). PLCγ1 contains at least five potential sites of tyrosine phosphorylation. Among them only Y775 and Y783, located between the C-terminal SH2 (SH2C) domain and the SH3 domain, are essential for enzyme activation in vivo (Irvin et al, 2000; Rhee, 2001; Serrano et al, 2005).
Despite the vital function of PLCγ1 in T cells and the ubiquitously pivotal role of PLCs in signal transduction in general, the mechanism of PLCγ1 activation following TCR engagement is incompletely understood. Several proteins participating in formation of the TCR-proximal signaling complex have been implicated in the regulation of PLCγ1 activity. Impaired Ca2+ mobilization has been reported in Jurkat cell lines deficient in LAT (Finco et al, 1998; Zhang et al, 2000), Slp76 (Yablonski et al, 1998), Vav1 (Cao et al, 2002), as well as in c-Cbl-deficient (Naramura et al, 1998) or Vav-deficient thymocytes (Reynolds et al, 2002) and Vav-deficient (Costello et al, 1999) or Itk-deficient T cells (Liu et al, 1998; Schaeffer et al, 1999). LAT has been identified as a primary docking site for PLCγ1 following TCR engagement (Zhang et al, 2000; Zhu et al, 2003). The recruitment of PLCγ1 to LAT occurs through specific binding of the N-terminal SH2 (SH2N) domain of PLCγ1 to the phosphorylated Y132 (pY132) of LAT (Stoica et al, 1998; Zhang et al, 2000). Mutations of either SH2N of PLCγ1 or Y132 of LAT abrogated PLCγ1–LAT association and inhibited PLCγ1 phosphorylation and activation (Stoica et al, 1998; Irvin et al, 2000; Zhang et al, 2000; Lin and Weiss, 2001; Paz et al, 2001; Zhu et al, 2003). No other in vivo binding partners for either LAT-pY132 or the SH2N domain of PLCγ1 have been identified in T cells to date.
On the other hand, the SH2C domain of PLCγ1 is substantially less selective than the SH2N domain. A chimeric protein consisting of GST fused to the SH2C domain is able to precipitate a range of phosphorylated proteins from activated Jurkat T cells (Stoica et al, 1998; Irvin et al, 2000; Bonvini et al, 2003). However, it is still unclear which of these proteins interact with PLCγ1 via its SH2C domain in vivo. The loss-of-function mutation of the SH2C domain (R694K) did not affect PLCγ1 interaction with LAT and was reported to have a mild to negligible effect on the PLCγ1 phosphorylation in Jurkat T cells (Stoica et al, 1998; Irvin et al, 2000). Yet, expression of SH2C-mutated PLCγ1 failed to reverse the impaired IL-2 transcriptional activation in Jurkats expressing low levels of PLCγ1 (Irvin et al, 2000) or rescue IP3 production in PLCγ-deficient B cells (DeBell et al, 1999). The active conformation of PLCγ1 involves intramolecular association of the SH2C domain with phosphorylated Y783 (pY783) (Poulin et al, 2005). Without this association the enzyme is only moderately active. Thus, it has been suggested that, although required for the PLCγ1 activity, the SH2C domain is dispensable for PLCγ1 recruitment and phosphorylation (Irvin et al, 2000; Bonvini et al, 2003).
The SH3 domain of PLCγ1 has been shown to interact constitutively with c-Cbl in T cells (Rellahan et al, 2003). Several studies have demonstrated that the SH3 domain of PLCγ1 can also bind other proline-rich domain-containing proteins, including Slp76 (Yablonski et al, 2001), Sos (Kim et al, 2000), Itk (Perez-Villar and Kanner, 1999) and PIKE (Ye et al, 2002). The significance of these interactions and their occurrence in vivo following TCR engagement remains unclear. Mutation of the SH3 domain has been reported to have no effect on PLCγ1 phosphorylation (DeBell et al, 1999). Moreover, in Jurkat cells expressing low levels of endogenous PLCγ1, the SH3-mutated PLCγ1 was able to recover impaired IL-2 transcriptional activation more efficiently than wild-type (WT) PLCγ1 (Irvin et al, 2000). It has been hypothesized that the SH3 domain is dispensable for PLCγ1 phosphorylation and may negatively regulate PLCγ1 activity in T cells (Irvin et al, 2000; Bonvini et al, 2003; Rellahan et al, 2003).
To summarize, the currently accepted model of PLCγ1 regulation in T cells postulates that the SH2N domain of PLCγ1 is both necessary and sufficient for its recruitment and phosphorylation following TCR engagement, whereas the SH2C and SH3 domains of PLCγ1 are dispensable for this purpose. Our current study contradicts both these propositions. Using a combination of biochemical methods and real-time fluorescent imaging, we show here that the SH2N domain of PLCγ1 is necessary but not sufficient for its recruitment to the LAT-nucleated complex. Furthermore, all three SH domains of PLCγ1 are required for the efficient phosphorylation and activation of PLCγ1 in T cells. In addition, the results of this study contribute new information on the role of other signaling proteins in the process of PLCγ1 activation. Thus, we propose a different model of PLCγ1 regulation in T cells that can account for both our new findings and previously reported data.
Results
To visualize the recruitment of PLCγ1 following TCR stimulation, we have expressed full-length PLCγ1 fused to the monomeric version of yellow fluorescent protein (Zacharias et al, 2002) (PLCwt-YFP) in WT Jurkat E6 T cells. Our imaging analysis was based on a previously published technique (Bunnell et al, 2002). In this protocol, T cells were dropped onto a surface coated with a stimulatory monoclonal antibody binding the TCR. This resulted in TCR clustering and recruitment of multiple signaling molecules to the points of contact with the stimulatory surface (Bunnell et al, 2002; Barda-Saad et al, 2005). Following the initial engagement, the cells spread out on the planar surface over a period of 2–3 min for the Jurkat cells and up to 30 min for human peripheral blood lymphocytes (Bunnell et al, 2002; Barda-Saad et al, 2005). Similar clusters of signaling molecules have recently been observed upon T-cell contact with lipid bilayers containing antigen–MHC complexes (Campi et al, 2005).
Stimulation of E6 cells stably expressing PLCwt-YFP resulted in formation of PLCγ1 clusters within seconds of the initial contact. The cluster formation continued during the cell spreading, then gradually diminished with eventual dissipation within 4–6 min after the beginning of the process (Figure 1A; Supplementary video 1). To evaluate whether the clustering of PLCwt-YFP represented its recruitment to signaling complexes, we have created a Jurkat E6 cell line expressing both PLCwt-YFP with either LAT or Slp76 fused with the Cerulean variant of cyan fluorescent protein (Rizzo et al, 2004) (LAT-CFP and Slp76-CFP, respectively). It has been shown previously that upon cell stimulation, LAT and Slp76 form clusters that colocalize with the sites of tyrosine phosphorylation, with the TCR and with other molecules recruited to the TCR-proximal signaling complex (Bunnell et al, 2002; Barda-Saad et al, 2005; Campi et al, 2005). As is evident from Figure 1B and C, the PLCwt-YFP clusters colocalize completely with LAT-CFP and Slp76-CFP, indicating that the PLCwt-YFP clustering represents recruitment of PLCγ1 to the LAT-nucleated signaling complex.
Figure 1.
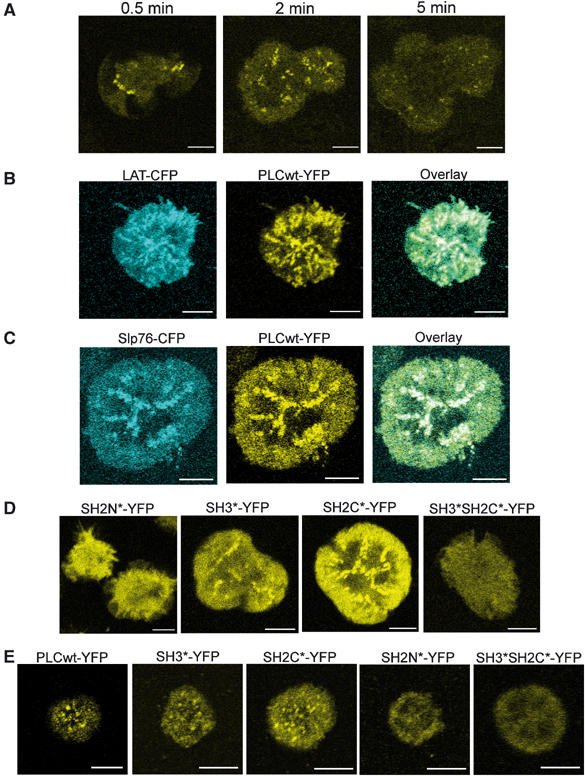
Visualization of PLCγ1 recruitment to signaling clusters in Jurkat E6 cells. (A) Jurkat E6 cells expressing PLCwt-YFP were seeded on stimulatory coverslips. Images obtained 0.5, 2 and 5 min into the spreading process are shown. The size bar corresponds to 5 μm. (B) Jurkat E6 cells expressing LAT-CFP and PLCwt-YFP were seeded on stimulatory coverslips. Images obtained 1.5 min into the spreading process are shown. The size bar corresponds to 5 μm. (C) Same as (B) for cells expressing Slp76-CFP and PLCwt-YFP. (D) Jurkat E6 cells expressing mutant PLCγ1-YFP conjugates as indicated were seeded on stimulatory coverslips. Images obtained 1–1.5 min into the spreading process are shown. The size bar corresponds to 5 μm. (E) Murine primary CD4+ T cells expressing PLCγ1-YFP conjugates as indicated were seeded on stimulatory coverslips and fixed 5 min into the spreading process. The size bar corresponds to 5 μm.
To assess the role of its SH domains in the process of PLCγ1 recruitment, we have created Jurkat E6 cell lines expressing PLCγ1-YFP conjugates bearing the previously described loss-of-function point mutations in the SH3 domain (SH3*-YFP), the SH2C domain (SH2C*-YFP) or the SH2N domain (SH2N*-YFP) (Stoica et al, 1998; Irvin et al, 2000). The mutation of the SH2N domain abrogated the TCR-induced clustering of PLCγ1 (Figure 1D), consistent with the notion that the SH2N domain serves as a binding site for LAT and, as such, is necessary for PLCγ1 recruitment to the LAT-nucleated complex.
The mutations of either the SH3 or the SH2C domain did not affect the clustering of PLCγ1 (Figure 1D). It has been suggested that these domains may negatively regulate PLCγ1 activity, possibly through binding of c-Cbl, and as such may be responsible for the dissipation of the clusters (Stoica et al, 1998; Bonvini et al, 2003; Rellahan et al, 2003). Therefore, disruption of both these domains might enhance or prolong the clustering of PLCγ1. We have created an additional PLCγ1-YFP variant bearing mutations in both the SH3 and the SH2C domains (SH3*SH2C*-YFP). Surprisingly, however, SH3*SH2C*-YFP completely failed to cluster, similar to SH2N*-YFP (Figure 1D). The clusters were undetectable at all stages of cell spreading, suggesting that the inhibition of clustering was absolute and not relative to the duration of TCR stimulation. Similar results were obtained using primary murine CD4+ T cells transiently expressing the PLCγ1-YFP forms mentioned above (Figure 1E).
To confirm the results obtained using the imaging technique, we evaluated the interaction of the various PLCγ1-YFP forms with LAT by precipitating the YFP conjugates with an anti-GFP serum and blotting with an antibody recognizing the phosphorylated Y132 residue (pY132) of LAT. Because maximal PLCγ1 phosphorylation is observed by 1 min after stimulation under similar conditions (Houtman et al, 2005), 1-min stimulation was chosen for this and other biochemical experiments in this study. In agreement with the results described above, phospho-LAT co-precipitated with the clustering conjugates—PLCwt-YFP, SH3*-YFP and SH2C*-YFP (Figure 2A and B). However, no co-precipitation of LAT was observed with the nonclustering SH2N*-YFP and SH3*SH2C*-YFP (Figure 2A and B). This result further supports the conclusion that the observed clusters of PLCγ1-YFP (Figure 1) represent recruitment of PLCγ1 to LAT and that the concomitant mutation of both the SH3 and SH2C domains block this recruitment. These results indicate that contrary to the currently accepted model, the SH2N domain alone is not sufficient for the PLCγ1 recruitment to the LAT-nucleated cluster. Although the SH3 and the SH2C domains, taken separately, are dispensable for the PLCγ1 recruitment, at least one of them is required in combination with the SH2N domain to maintain the association of PLCγ1 with activated LAT.
Figure 2.

Effect of SH domain mutations on PLCγ1 recruitment and phosphorylation. (A) Jurkat E6 cells (1 × 107) expressing the indicated PLCγ1-YFP variants were either stimulated with C305 for 1 min or left unstimulated. The cells were lysed and PLCγ1-YFP conjugates were precipitated with anti-GFP. The precipitates were resolved on SDS–PAGE and probed with anti-phospho-LAT-pY132 (upper blot). The same membrane was stripped and probed with anti-GFP (lower blot). The data are representative of three independent experiments. (B) Quantitative analysis of blots obtained in experiments represented by (A). The data represent densitometry of LAT-pY132 bands as a percentage of band intensity obtained in cells expressing PLCwt-YFP. Each bar represents an average over four independent experiments. Error bars indicate s.e. (C) Jurkat E6 cells expressing the indicated PLCγ1-YFP variants were either stimulated with OKT3 for 1 min or left unstimulated and lysed. The lysates were resolved on SDS–PAGE and probed with either anti-phospho-PLCγ1-pY783 (upper blot) or anti-PLCγ1 (lower blot). The data are representative of five independent experiments. (D) Quantitative analysis of blots obtained in experiments in (C). The data represent densitometry of PLCγ1-YFP-pY783 bands as a percentage of endogenous PLCγ1-pY783 band intensity in corresponding lanes. The results were normalized to account for the relative amounts of PLCγ1-YFP and endogenous PLCγ1 in each cell line. Each bar represents an average over five independent experiments. Error bars indicate s.e.
It has been reported previously that mutations of either the SH3 or SH2C domains had little or no effect on the general tyrosine phosphorylation of PLCγ1 (Stoica et al, 1998; DeBell et al, 1999; Irvin et al, 2000). Yet, these findings did not correlate well with the functional impact of the mutations on downstream signaling. PLCγ1 contains several tyrosine residues that can be phosphorylated upon stimulation. However, only two of them, Y783 and the recently discovered Y775, are necessary for enzyme activation (Irvin et al, 2000; Rhee, 2001; Serrano et al, 2005). Therefore, we have assessed the effect of the SH domain mutations on phosphorylation of these critical tyrosine residues using specific antibodies binding these phosphorylated sites—anti-pY783 (Figure 2C and D) and anti-pY775 (not shown). The ability to distinguish the endogenous PLCγ1 and the PLCγ1-YFP conjugates as separate bands on Western blots provided a clear advantage for analyzing these results. Comparison between the level of phosphorylation of the YFP conjugates with that of the endogenous PLCγ1 in the same cells allowed more accurate assessment of the effect produced by the mutants and prevented skewed results because of a possible general effect of the mutants on the stimulation. The results clearly demonstrate (Figure 2C and D) that upon stimulation PLCwt-YFP is phosphorylated to the same extent as endogenous PLCγ1, ruling out the possibility of YFP interfering with phosphorylation. However, the loss-of-function mutation in either of the three SH domains strongly inhibits phosphorylation of Y783. Moreover, despite the fact that SH3*-YFP and SH2C*-YFP are recruited to the LAT-nucleated signaling complex, Y783 phosphorylation in these mutants is inhibited more severely than that in SH2N*-YFP, which is not recruited (Figure 2C and D). Virtually identical phosphorylation pattern was obtained using anti-pY775 (not shown). Note that the mutations did not interfere with the ability of the proteins to act as a substrate for tyrosine kinases, as pervanadate stimulation induced Y783 phosphorylation in all PLC-YFP conjugates at levels comparable with those obtained in endogenous PLCγ1 (Supplementary Figure S1). These results suggest that both the SH3 and SH2C domains are indispensable for the efficient phosphorylation and activation of PLCγ1 and that PLCγ1 recruitment to the signaling complex alone is not sufficient for this purpose.
The deleterious effect of the SH domain mutations on recruitment and activating phosphorylation of PLCγ1 might be explained by disruption of PLCγ1 interactions with other signaling proteins normally mediated by these domains. Precipitation of PLCwt-YFP from the stimulated E6 cells reveals four clear bands of tyrosine-phosphorylated proteins co-precipitating with PLCγ1 (Figure 3A, second lane). The 36–38 kDa band has already been identified as LAT (Figure 2A). The other three bands correspond in sizes to Slp76, Vav1 and c-Cbl, all of which have been implicated in the PLCγ1 regulation in T cells (Naramura et al, 1998; Yablonski et al, 1998; Costello et al, 1999; Cao et al, 2002; Reynolds et al, 2002; Rellahan et al, 2003). Application of antibodies specific to Slp76, phosphorylated Vav1 (pVav1) or phosphorylated c-Cbl (pc-Cbl) confirmed the identity of these bands (Figure 3B). Mutation of any of the SH domains abrogated co-precipitation of PLCγ1 with Slp-76 (Figure 3B). Consistent with the previously reported constitutive interaction between PLCγ1 and c-Cbl mediated via the SH3 domain of PLCγ1 (Rellahan et al, 2003), co-precipitation of these two proteins was completely blocked in the PLCγ1 mutants lacking a functional SH3 domain (Figure 3B and C). Precipitation of Vav1 was substantially inhibited with either SH3*-YFP or SH2C*-YFP and was undetectable with either SH2N*-YFP or SH3*SH2C*-YFP (Figure 3B and D). These results suggest that the SH domains of PLCγ1 mediate its interactions with Slp76, Vav1 and c-Cbl. The loss of these interactions might account for the impaired recruitment and phosphorylation of the SH domain mutants.
Figure 3.

Co-precipitation of PLCγ1-YFP variants with various signaling proteins. (A) Jurkat E6 cells (1 × 107) expressing PLCwt-YFP were either stimulated for 1 min with C305 or left unstimulated and lysed. PLCwt-YFP was precipitated with anti-GFP. The precipitates (three left lanes) and a sample from stimulated whole-cell lysate WCL (right lane) were resolved on SDS–PAGE and probed with anti-phosphotyrosine 4G10 antibody. NC lane represents a control experiment performed without cell lysate. All lanes were from the same gel. The three left lanes were obtained at equal exposure times. WCL lane was obtained at a shorter exposure time. HC, heavy chain of Ig; LC, light chain of Ig. The data are representative of three independent experiments. (B) Jurkat E6 cells (1 × 107) expressing the indicated PLCγ1-YFP variants were either stimulated with C305 for 1 min or left unstimulated. The cells were lysed and PLCγ1-YFP conjugates were precipitated with anti-GFP. The precipitates were resolved on SDS–PAGE and probed with anti-phospho-c-Cbl-pY774 (top blot). The same membrane was stripped and probed with anti-GFP (second from top blot). In a different experiment, the precipitates were resolved on SDS–PAGE and probed consecutively with anti-phospho-Vav1-pY160, anti-Slp76 and anti-GFP (lower blots from top to bottom). NS, nonspecific band. The data are representative of at least four independent experiments. (C) The data represent densitometry of phospho-c-Cbl bands from experiments in (B) as a percentage of band intensity obtained in cells expressing PLCwt-YFP. Each bar represents an average of five independent experiments. Error bars indicate s.e. (D) Same as (C) for phospho-Vav1 bands. Each bar represents an average of four independent experiments.
Co-precipitation does not necessarily indicate direct interaction between the protein molecules. Therefore, we have applied the fluorescence resonance energy transfer (FRET) technique to address this issue. As FRET can occur between fluorescent species separated no further than ∼10 nm, detection of FRET between two protein molecules strongly suggests their direct interaction. Unfortunately, we have not been able to detect FRET between full-length PLCγ1-YFP and either LAT-CFP or Slp76-CFP, although direct interaction between PLCγ1 and LAT is well documented (Stoica et al, 1998; Zhang et al, 2000). This may be due to the size and structure of PLCγ1, which places YFP at a greater distance from the interacting proteins than is allowed for FRET. Therefore, we have created a truncated version of PLCγ1-YFP (PLCs-YFP) by removing the C-terminal part of the PLCγ1 catalytic domain, thereby placing YFP closer to the SH domains of PLCγ1.
Figure 4 demonstrates that PLCs-YFP is recruited to the signaling clusters similarly to PLCwt-YFP. Furthermore, a FRET signal has been detected between PLCs-YFP and either LAT-CFP, c-Cbl-CFP, Vav1-CFP or Slp76-CFP (Figure 4A–D) suggesting direct interaction between these proteins. Note that FRET between PLCs-YFP and c-Cbl-CFP, in contrast to the other proteins, is not limited to the clusters, which is likely due to the constitutive nature of the PLCγ1 and c-Cbl interaction. Interestingly, the C-terminus labeled Slp76 (Slp76-CFP) exhibited substantially lower FRET efficiency with PLCs-YFP than its N-terminus labeled counterpart (CFP-Slp76) (Figure 4D and E). This sensitivity of the observed FRET efficiency to the structural variations within the protein complex suggests that the obtained FRET signal reflects actual protein interactions and is not merely due to high protein density within the cluster. Predictably, no significant FRET was observed between LAT-CFP and PLCs-YFP mutated at the SH2N domain of PLCγ1 (Figure 4F).
Figure 4.

FRET analysis between PLCγ1-YFP and various CFP-conjugated proteins. (A) Jurkat E6 cells expressing PLCs-YFP and LAT-CFP were seeded on a stimulatory coverslip and fixed 2 min into the spreading process. FRET efficiency values are presented in a pseudo-colored scale, with black color representing saturated pixels. The average value of FRET efficiency±s.e. obtained for a given pair is shown. The size bar corresponds to 5 μm. (B) Same as (A) for cells expressing PLCs-YFP and c-Cbl-CFP. (C) Same as (A) for cells expressing PLCs-YFP and Vav1-CFP. (D) Same as (A) for cells expressing PLCs-YFP and CFP-Slp76. (E) Same as (A) for cells expressing PLCs-YFP and Slp76-CFP. (F) Same as (A) for cells expressing PLCs-SH2N*-YFP and LAT-CFP.
To further evaluate the role of Slp76 and Vav1 in PLCγ1 recruitment, we expressed PLCγ1-YFP conjugates in cells deficient in either of these proteins. Expression of PLCwt-YFP in the Slp76-deficient Jurkat J14 cells revealed strong inhibition of PLCγ1 recruitment to the signaling clusters (Figure 5A). This result was unexpected, as normal co-precipitation of PLCγ1 with LAT in the J14 cells has been previously shown (Yablonski et al, 1998). In the course of an independent study, we have discovered that the expression of c-Cbl in the J14 cells used in our experiment is substantially reduced compared to those observed in E6 cells. To assess whether the reduced level of c-Cbl can account for the impaired PLCγ1 recruitment, we have created a J14 cell line expressing c-Cbl-CFP (J14-Cbl) at a level similar to the level of c-Cbl in E6 cells (Supplementary Figure S2). Reconstitution of J14 cells with c-Cbl restored normal clustering of PLC-YFP and SH2C*-YFP (Figure 5B). Colocalization of PLCγ1 and c-Cbl clusters is worth noting. However, c-Cbl-CFP was unable to rescue the clustering of SH3*-YFP (Figure 5B) observed in the WT Jurkats (Figure 1D). These results suggest that the SH3-mediated interaction of PLCγ1 with c-Cbl (Rellahan et al, 2003) stabilizes PLCγ1 recruitment to the signaling complex. Although reconstitution of the J14 cells with c-Cbl restored recruitment of PLCγ1, it was unable to rescue PLCγ1 activity (Figure 5C). Yet, reconstitution of J14 cells with Slp76 (J14-Slp) restored calcium mobilization (Figure 5C) and PLCwt-YFP clustering (not shown) in these cells. Therefore, in the presence of Slp76, c-Cbl is either redundant for PLCγ1 activation or the low levels of c-Cbl present in J14 cells are sufficient to maintain its functionality under these conditions.
Figure 5.
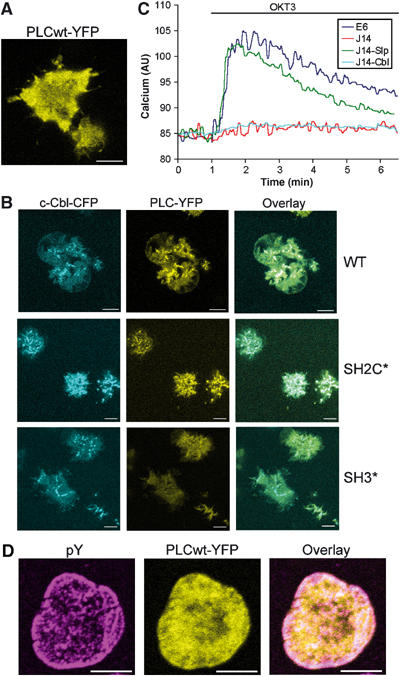
Effect of Slp76 and c-Cbl deficiencies on PLCγ1 recruitment and activation. (A) Jurkat J14 cells expressing PLCwt-YFP were seeded on a stimulatory coverslip. An image obtained 1 min into the spreading process is shown. The size bar corresponds to 5 μm. (B) Jurkat J14 cells expressing c-Cbl-CFP and an indicated PLCγ1-YFP conjugate were seeded on stimulatory coverslips. Images obtained 1–1.5 min into the spreading process are shown. The size bar corresponds to 5 μm. (C) Time course of the response in cytosolic calcium concentration obtained from the indicated cell lines after stimulation with OKT3. AU, arbitrary units. (D) CD4+ T cells from c-Cbl KO mice expressing PLCwt-YFP were seeded on a stimulatory coverslip, fixed 5 min into the spreading process and stained with anti-pY antibody. The size bar corresponds to 5 μm.
To differentiate between these two possibilities, we have transiently expressed PLCwt-YFP in CD4+ T cells derived from c-Cbl knockout (KO) mice. Contrary to the results obtained in WT CD4+ T cells (Figure 1E), PLCwt-YFP clustering was abolished in c-Cbl-deficient T cells, despite normal TCR stimulation as indicated by multiple phospho-tyrosine clusters (Figure 5D). In addition, it has been shown previously that calcium elevation in T cells from c-Cbl KO mice is strongly inhibited (Chiang et al, 2004). These results together indicate that c-Cbl at some level is required for PLCγ1 recruitment and activation.
Impaired calcium mobilization has also been demonstrated in Vav1-deficient T cells (Costello et al, 1999; Cao et al, 2002; Reynolds et al, 2002). However, the general tyrosine phosphorylation of PLCγ1 was not affected by Vav1 deficiency in Jurkat T cells (Reynolds et al, 2002). Because the total tyrosine phosphorylation does not necessarily reflect phosphorylation of the critical Y783 residue, we have evaluated phosphorylation of PLCγ1 in the Vav1-deficient Jurkat J.vav cells using an anti-pY783 antibody. As can be seen in Figure 6A, phosphorylation of Y783 in J.vav cells is greatly reduced compared to E6.1 cells. Predictably, the calcium response in J.vav cells is strongly inhibited (Figure 6B).
Figure 6.

Effect of Vav1 deficiency on PLCγ1 recruitment and activation. (A) Jurkat E6 cells (two left lanes) and Jurkat J.vav cells (two right lanes) were either stimulated with OKT3 for 1 min or left unstimulated and lysed. The lysates were resolved on SDS–PAGE and probed with either anti-phospho-PLCγ1-pY783 (upper blot) or anti-PLCγ1 (lower blot). The data are representative of two independent experiments. (B) Time course of the response in cytosolic calcium concentration obtained from the indicated cell lines after stimulation with OKT3. AU, arbitrary units. (C) Jurkat E6 or J.vav cells (1 × 107) expressing PLCwt-YFP were either stimulated for 1 min with C305 or left unstimulated and lysed. PLCwt-YFP was precipitated with anti-GFP. The precipitates were resolved on SDS–PAGE and probed with anti-phosphotyrosine 4G10 antibody. The data are representative at least two independent experiments. (D) Jurkat J.vav cells expressing an indicated PLCγ1-YFP conjugate were seeded on stimulatory coverslips. Images obtained 1–1.5 min into the spreading process are shown. The size bar corresponds to 5 μm.
Comparison between tyrosine-phosphorylated proteins that co-precipitate with PLCγ1 in J.vav versus E6 cells reveals a conspicuous absence of a band corresponding to Slp76 in precipitate, obtained from the former (Figure 6C). Interestingly, the amount of pc-Cbl co-precipitated with PLCγ1 in J.vav is substantially greater than that obtained in E6 cells. These results suggest that Vav1, and possibly Slp76, may compete with c-Cbl for PLCγ1 binding.
Expression of PLCwt-YFP in J.vav cells demonstrated that in the absence of Vav1, PLCγ1 was still recruited to the signaling clusters (Figure 6D). However, unlike the result we observed in WT Jurkats (Figure 1D), both the SH3*-YFP and SH2C*-YFP failed to cluster in J.vav cells (Figure 6D). Taken together, these data suggest that Vav1 plays an important role in both recruitment and activation of PLCγ1.
Discussion
Stimulation of the TCR leads to recruitment of PLCγ1 to the TCR-proximal signaling complex, followed by PLCγ1 phosphorylation and activation (Zhang et al, 1998, 2000; Bonvini et al, 2003). The hallmark of PLCγ1 recruitment is its association with LAT through binding of the PLCγ1 SH2N domain to pY132 of LAT (Stoica et al, 1998; Zhang et al, 2000). In this study, we show that the PLCγ1 SH2N domain is not sufficient for the recruitment of the enzyme to the LAT-nucleated signaling complex. Although mutation of either the SH2C or SH3 domain of PLCγ1 separately does not interfere with its binding to LAT, mutation of both of these domains abolishes PLCγ1 recruitment and activation (Figures 1D and E and 2C and D).
These results suggest that although the SH3 and SH2C domains of PLCγ1 do not bind directly to LAT, they can participate in the stabilization of the PLCγ1–LAT association via other proteins in the LAT-nucleated signaling complex. These proteins can bind the PLCγ1 SH3 and SH2C domains and bridge between PLCγ1 and LAT. Either of the SH3 or SH2C domains can sufficiently stabilize the PLCγ1–LAT association without participation of the other. However, functional elimination of both of them or, alternatively, mutation of two or all three distal pYs of LAT (Zhang et al, 2000; Lin and Weiss, 2001; Paz et al, 2001; Zhu et al, 2003), destroys the ‘bridge' and abolishes PLCγ1 association with LAT.
We also show that recruitment of PLCγ1 to the LAT-nucleated complex does not necessarily result in PLCγ1 activation. It has been proposed earlier that the SH3 and SH2C domains are dispensable for PLCγ1 phosphorylation, because an application of a general anti-pY serum did not reveal a substantial decrease in the tyrosine phosphorylation of the SH3* and SH2C* mutants (Stoica et al, 1998; DeBell et al, 1999; Irvin et al, 2000; Bonvini et al, 2003). However, the data presented here provide evidence to the contrary. Using site-specific anti-pY antibodies, we show that phosphorylation of Y783, which is critical for PLCγ1 activation, is strongly inhibited in the SH3* and SH2C* mutants (Figure 2C and D). Moreover, the Y783 phosphorylation of the SH2N* mutant, which is not recruited to LAT, appears to be more efficient than that of the SH3* or the SH2C* mutants despite seemingly normal recruitment of these two proteins to the signaling complex. Similar results were obtained for the Y775 site. Thus, in addition to their role in stabilization of the PLCγ1–LAT association, the SH3 and SH2C domains are essential for efficient phosphorylation of Y783 and Y755. It is possible that the collaborative engagement of the SH3 and SH2C domains confers a conformational change exposing Y783 and Y755, otherwise not readily accessible for phosphorylation. Mutation in either the SH3 or SH2C domain results in a nonproductive recruitment of PLCγ1. Yet, this recruitment may still be sufficient to induce phosphorylation of PLCγ1 tyrosine residues other than Y783 or Y755, which may explain seemingly unaffected phosphorylation of the SH3* and SH2C* mutants observed with a general anti-pY serum (Stoica et al, 1998; DeBell et al, 1999; Irvin et al, 2000; Bonvini et al, 2003).
We confirm here that, in addition to LAT, at least three tyrosine-phosphorylated proteins—Slp76, c-Cbl and Vav1—co-precipitate with PLCγ1 from stimulated Jurkat T cells (Figure 3). This co-precipitation is affected by mutations in the PLCγ1 SH domains (Figure 3B). The FRET data indicate that PLCγ1 bind these proteins directly (Figure 4). Together with previously reported evidence (Finco et al, 1998; Liu et al, 1998; Naramura et al, 1998; Yablonski et al, 1998; Costello et al, 1999; Schaeffer et al, 1999; Zhang et al, 2000; Cao et al, 2002; Reynolds et al, 2002), these results suggest that Slp76, c-Cbl and Vav1 are the likely candidates for the proteins participating in PLCγ1 activation within the LAT-nucleated signaling complex. It has been demonstrated that c-Cbl binds constitutively to the PLCγ1 SH3 domain (Rellahan et al, 2003). In agreement with these findings, we show here that co-precipitation of PLCγ1 with c-Cbl critically depends on the functional integrity of the PLCγ1 SH3 domain (Figure 3B and C). Moreover, recruitment (Figure 5) and activation (Chiang et al, 2004) of PLCγ1 is strongly inhibited in c-Cbl-deficient T cells. These data suggest that c-Cbl may facilitate and stabilize recruitment of PLCγ1 through their constitutive SH3-dependent interaction.
It has been shown that Slp76 is essential for PLCγ1 Y783 phosphorylation and activation (Yablonski et al, 1998; Gonen et al, 2005). We show here that mutation in any of the three SH domains of PLCγ1 results in a complete loss of PLCγ1-Slp76 co-precipitation (Figure 3B). Interestingly, the loss of PLCγ1-Slp76 co-precipitation remarkably coincides with inhibition of the PLCγ1 Y783 phosphorylation (Figures 2C and 3B and elsewhere: Yablonski et al, 1998; Reynolds et al, 2002; Gonen et al, 2005). The N-terminal pY residues of Slp76 have been identified as a binding site for the SH2 domain of the Tec-family kinase Itk, which can phosphorylate PLCγ1 (Bunnell et al, 2000; Lewis et al, 2001). Thus, the key function of Slp76 in PLCγ1 activation may lie in the recruitment of Itk to the signaling complex, whereas the SH3 and SH2C domains of PLCγ1 jointly participate in the formation of a productive interaction between PLCγ1 and the Slp76-Itk pair.
The N-terminal pY residues of Slp76 also provide a binding site for Vav1 (Fang and Koretzky, 1999) with a possible formation of a Slp76-Itk–Vav1 complex (Dombroski et al, 2005). Our results suggest that Vav1 facilitates association between PLCγ1 and Slp76, corroborating similar observations reported previously (Reynolds et al, 2002). Both the SH2C and SH3 domains of PLCγ1 mediate PLCγ1–Vav1 association. This function of Vav1 may underlie its indispensability for PLCγ1 phosphorylation and activation (Figure 6A and B and elsewhere; Costello et al, 1999; Cao et al, 2002; Reynolds et al, 2002).
To summarize the data shown and cited above, we propose the following model for the recruitment and activation of PLCγ1 in T cells (Figure 7). TCR engagement leads to recruitment of PLCγ1 to the LAT-nucleated complex through binding of the PLCγ1 SH2N domain to the pY132 residue of LAT. However, this binding is not sufficient to maintain the PLCγ1–LAT association. Either the SH3 or SH2C domain of PLCγ1 must stabilize this association through binding to other proteins in the LAT-nucleated signaling complex. Vav1, c-Cbl and Slp76 bridge between the PLCγ1 SH3 and SH2C domains and LAT. Although only one of these domains is required for stabilization of the PLCγ1–LAT association, both the SH3 and SH2C domains together are necessary for an efficient presentation of PLCγ1 Y783 and Y755 to the activating kinase, probably Itk. Elimination of either of these domains or, alternatively, elimination of either Slp76 or Vav1 partially destabilizes the PLCγ1–LAT complex and results in a nonproductive recruitment of PLCγ1.
Figure 7.

Schematic model of PLCγ1 recruitment and activation in the LAT-nucleated signaling complex.
Materials and methods
Antibodies
Mouse anti-CD3ɛ, anti-CD28, anti-CD4, human anti-CD3 HIT3a and anti-CD3 PE-Cy5 were purchased from Pharmingen. Anti-phosphotyrosine 4G10, anti-c-Cbl 7G10, goat anti-mouse IgG-HRP, goat anti-rabbit IgG-HRP were from Upstate Biotechnology. Anti-LAT-pY132, anti-PLCγ1-pY783, anti-Vav1-pY160 were from Biosource. Anti-Slp76 was from Antibody Solutions. Anti-c-Cbl-pY774 was from Cell Signaling Technology. Anti-PLCγ1 was from Santa Cruz Biotechnology. Anti-GFP was from Roche. Anti-GAPDH was from Biodesign. Anti-PLCγ1-pY775 was provided by Dr BL Rellahan (Division of Monoclonal Antibodies, US Food and Drug Administration, Bethesda, MD).
Expression vectors and plasmids
The bovine PLCγ1 cDNA was provided by Dr E Bonvini (Division of Monoclonal Antibodies, US Food and Drug Administration, Bethesda, MD). The rat Vav1 cDNA was provided by Dr J Rivera (Section on Chemical Immunology, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda, MD). LAT-CFP, Slp76-CFP and c-Cbl-CFP were generated from previously described constructs (Donovan et al, 1996; Bunnell et al, 2002; Barda-Saad et al, 2005). The monomeric YFP plasmid was created from pEYFP-N1 (Clontech) by A206K substitution (Zacharias et al, 2002). The Cerulean CFP plasmid was created from pECFP-N1 (Clontech) by A206, S72A, Y145A and H148D substitutions (Zacharias et al, 2002; Rizzo et al, 2004). The resultant YFP and CFP variants were used to create all fluorescent conjugates by standard methods. Truncated PLCγ1-YFP vector (PLCs-YFP) encodes for PLCγ1(1–933) fragment followed by YFP. Point mutations were introduced using a QuikChange II XL site-directed mutagenesis kit (Stratagene). The constructs were cloned into either pMSCVhyg or pMSCVneo (Clontech) to create retroviral expression vectors. All constructs were verified using DNA sequencing.
Vector expression and generation of stable cell lines
Jurkat cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum. The recombinant DNA was introduced into the cells using retroviral infection. The retroviral expression vectors and pVSV-G (Clontech) were cotransfected into GP2-293 packaging cells (Clontech) using Lipofectamine 2000 (Invitrogen). After 48 h, the virus-containing medium was removed from the packaging cells and mixed with Jurkat cells. The medium was replaced with the regular growth medium after 24 h and an appropriate selection medium was added 72 h post-infection. The cells were sorted and those expressing fluorescent conjugates at the levels comparable with the corresponding endogenous proteins in the WT Jurkats were collected and expanded. Protein expression was monitored at multiple time points using flow cytometry and Western blotting. TCR expression level was monitored using immunostaining with anti-CD3 PE-Cy5 followed by flow cytometry.
Transfection of murine T cells
Mice used in this study were on a C57BL/6 background. C-Cbl KO (Cbl−/−) mice were described previously (Chiang et al, 2004). CD4+ T cells from lymph nodes were purified by magnetic bead separation as previously described (Sommers et al, 2005). Cells were transfected with PLCγ1-YFP using AMAXA electroporator and AMAXA kit for primary murine T cells and used 48 h post-transfection.
Confocal microscopy
The spreading assays were performed as described previously (Bunnell et al, 2002; Barda-Saad et al, 2005). Briefly, chambered coverslips (LabTek) were coated overnight at 4°C with either the stimulatory antibody anti-CD3ɛ HIT3a (10 μg/ml) or mouse anti-CD3ɛ, anti-CD28 and anti-CD4 (10 μg/ml each). The cells were seeded onto coated coverslips containing imaging buffer (RPMI 1640 without phenol red, 10% fetal calf serum, 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid). Some cells were fixed at different time points with 2–4% paraformaldehyde in phosphate-buffered saline. Immunostaining was performed as described previously (Barda-Saad et al, 2005). Fluorescent images were acquired on a LSM510 confocal system (Carl Zeiss) using a × 63 Plan-Apochromat objective (Carl Zeiss). A hot air blower (Nevetec) was used to maintain live samples at 37°C, and fine adjustments were made with a digital probe monitoring the buffer temperature in the chamber. The acquired images were extracted with the LSM browser (Carl Zeiss), cropped and composed into figures with Adobe Photoshop. The images were pseudo-colored as following, CFP—cyan, YFP—yellow, pY—magenta. No image enhancement procedures have been performed.
FRET was measured by the donor-sensitized acceptor fluorescence technique as described previously (Barda-Saad et al, 2005). Briefly, three images were acquired for each set of measurements: YFP excitation/YFP emission image (YFP channel); CFP excitation/CFP emission image (CFP channel) and CFP excitation/YFP emission image (FRET channel). A set of reference images was acquired from single-labeled CFP or YFP-expressing cells for each set of acquisition parameters and a calibration curve was derived to allow elimination of the non-FRET components from the FRET channel. The FRET efficiency was calculated on a pixel-by-pixel basis using the following equation:
![]()
where FRETcorr is the pixel intensity in the corrected FRET image and CFP is the intensity of the corresponding pixel in the CFP channel image. To estimate the significance of the obtained FRET efficiency values and to exclude the possibility of getting false-positive FRET, three negative control systems were prepared: cells expressing LAT-CFP chimera and free YFP; cells expressing SLP-76 YFP chimera and free CFP; and cells expressing free CFP and free YFP. FRET efficiency values obtained from the negative control samples were subtracted from the ones obtained in the main experiments and the resulted efficiency values were reported.
Measurement of intracellular calcium concentration
Cells were incubated with 5 μM Indo-1-AM (Molecular Probes) and 0.5 mM probenecid (Sigma) in RPMI 1640 medium at 37°C for 45 min. The cells were washed with RPMI 1640, resuspended in imaging buffer containing 0.5 mM probenecid, and kept at room temperature for 30–45 min. The cells were incubated at 37°C for 5 min before measurements and analyzed using the LSR II (BD Biosciences). The data were processed using Tree Star FlowJo software.
Immunoblotting and immunoprecipitation
Jurkat cells were either stimulated with anti-CD3 C305 or OKT3 for 1 min or left untreated. The optimal concentration of the stimulatory antibody was determined by titration. Murine cells were stimulated by incubating with biotinylated anti-CD3ɛ and anti-CD28 (10 μg each) for 15 min on ice, crosslinking with streptavidin (25 μg) and incubating at 37°C for 4 min. Cells were lysed in ice-cold lysis buffer containing 1% Brij, 1% n-Octyl-β-D-glucoside, 50 mM Tris–HCl, pH 7.6, 150 mM NaCl, 5 mM ethylenediaminetetraacetic acid, 1 mM Na3VO4 and complete protease inhibitor tablets (Roche). Protein A/G Plus-Agarose beads (Santa Cruz Biotechnology) were used for immunoprecipitation. Protein samples were resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE), transferred to nitrocellulose membrane, and immunoblotted with appropriate primary antibodies. Immunoreactive proteins were detected with either anti-mouse or anti-rabbit horseradish peroxidase-coupled secondary antibody (Upstate Biotechnology) followed by detection with enhanced chemiluminescence (Amersham Biosciences).
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Video 1
Acknowledgments
The authors thank B Taylor for cell sorting, P Schwartzberg and B Rellahan for providing reagents and critical reading of the manuscript, and RJ Hodes for providing KO mice. This research was supported, in part, by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- Barda-Saad M, Braiman A, Titerence R, Bunnell SC, Barr VA, Samelson LE (2005) Dynamic molecular interactions linking the T cell antigen receptor to the actin cytoskeleton. Nat Immunol 6: 80–89 [DOI] [PubMed] [Google Scholar]
- Bonvini E, DeBell KE, Veri MC, Graham L, Stoica B, Laborda J, Aman MJ, DiBaldassarre A, Miscia S, Rellahan BL (2003) On the mechanism coupling phospholipase Cgamma1 to the B- and T-cell antigen receptors. Adv Enzyme Regul 43: 245–269 [DOI] [PubMed] [Google Scholar]
- Bunnell SC, Diehn M, Yaffe MB, Findell PR, Cantley LC, Berg LJ (2000) Biochemical interactions integrating Itk with the T cell receptor-initiated signaling cascade. J Biol Chem 275: 2219–2230 [DOI] [PubMed] [Google Scholar]
- Bunnell SC, Hong DI, Kardon JR, Yamazaki T, McGlade CJ, Barr VA, Samelson LE (2002) T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J Cell Biol 158: 1263–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burack WR, Cheng AM, Shaw AS (2002) Scaffolds, adaptors and linkers of TCR signaling: theory and practice. Curr Opin Immunol 14: 312–316 [DOI] [PubMed] [Google Scholar]
- Campi G, Varma R, Dustin ML (2005) Actin and agonist MHC–peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med 202: 1031–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Janssen EM, Duncan AW, Altman A, Billadeau DD, Abraham RT (2002) Pleiotropic defects in TCR signaling in a Vav-1-null Jurkat T-cell line. EMBO J 21: 4809–4819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang YJ, Sommers CL, Jordan MS, Gu H, Samelson LE, Koretzky GA, Hodes RJ (2004) Inactivation of c-Cbl reverses neonatal lethality and T cell developmental arrest of SLP-76-deficient mice. J Exp Med 200: 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello PS, Walters AE, Mee PJ, Turner M, Reynolds LF, Prisco A, Sarner N, Zamoyska R, Tybulewicz VLJ (1999) The Rho-family GTP exchange factor Vav is a critical transducer of T cell receptor signals to the calcium, ERK, and NF-kappa B pathways. Proc Natl Acad Sci USA 96: 3035–3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBell KE, Stoica BA, Veri M-C, Di Baldassarre A, Miscia S, Graham LJ, Rellahan BL, Ishiai M, Kurosaki T, Bonvini E (1999) Functional independence and interdependence of the Src homology domains of phospholipase C-gamma 1 in B-cell receptor signal transduction. Mol Cell Biol 19: 7388–7398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai DM, Newton ME, Kadlecek T, Weiss A (1990) Stimulation of the phosphatidylinositol pathway can induce T-cell activation. Nature 348: 66–69 [DOI] [PubMed] [Google Scholar]
- Dombroski D, Houghtling RA, Labno CM, Precht P, Takesono A, Caplen NJ, Billadeau DD, Wange RL, Burkhardt JK, Schwartzberg PL (2005) Kinase-independent functions for Itk in TCR-induced regulation of Vav and the actin cytoskeleton. J Immunol 174: 1385–1392 [DOI] [PubMed] [Google Scholar]
- Donovan J, Ota Y, Langdon W, Samelson L (1996) Regulation of the association of p120cbl with Grb2 in Jurkat T cells. J Biol Chem 271: 26369–26374 [DOI] [PubMed] [Google Scholar]
- Ebinu JO, Stang SL, Teixeira C, Bottorff DA, Hooton J, Blumberg PM, Barry M, Bleakley RC, Ostergaard HL, Stone JC (2000) RasGRP links T-cell receptor signaling to Ras. Blood 95: 3199–3203 [PubMed] [Google Scholar]
- Fang N, Koretzky GA (1999) SLP-76 and Vav function in separate, but overlapping pathways to augment interleukin-2 promoter activity. J Biol Chem 274: 16206–16212 [DOI] [PubMed] [Google Scholar]
- Finco TS, Kadlecek T, Zhang W, Samelson LE, Weiss A (1998) LAT is required for TCR-mediated activation of PLCgamma1 and the Ras pathway. Immunity 9: 617–626 [DOI] [PubMed] [Google Scholar]
- Gonen R, Beach D, Ainey C, Yablonski D (2005) T Cell Receptor-induced activation of phospholipase C-gamma1 depends on a sequence-independent function of the P-I region of SLP-76. J Biol Chem 280: 8364–8370 [DOI] [PubMed] [Google Scholar]
- Houtman JCD, Houghtling RA, Barda-Saad M, Toda Y, Samelson LE (2005) Early phosphorylation kinetics of proteins involved in proximal TCR-mediated signaling pathways. J Immunol 175: 2449–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin BJ, Williams BL, Nilson AE, Maynor HO, Abraham RT (2000) Pleiotropic contributions of phospholipase C-gamma 1 (PLC-gamma 1) to T-cell antigen receptor-mediated signaling: reconstitution studies of a PLC-gamma 1-deficient Jurkat T-cell line. Mol Cell Biol 20: 9149–9161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katan M (1998) Families of phosphoinositide-specific phospholipase C: structure and function. Biochim Biophys Acta 1436: 5–17 [DOI] [PubMed] [Google Scholar]
- Kim MJ, Chang JS, Park SK, Hwang JI, Ryu SH, Suh PG (2000) Direct interaction of SOS1 Ras exchange protein with the SH3 domain of phospholipase C-gamma1. Biochemistry 39: 8674–8682 [DOI] [PubMed] [Google Scholar]
- Lewis CM, Broussard C, Czar MJ, Schwartzberg PL (2001) Tec kinases: modulators of lymphocyte signaling and development. Curr Opin Immunol 13: 317–325 [DOI] [PubMed] [Google Scholar]
- Lin J, Weiss A (2001) Identification of the minimal tyrosine residues required for linker for activation of T cell function. J Biol Chem 276: 29588–29595 [DOI] [PubMed] [Google Scholar]
- Liu KQ, Bunnell SC, Gurniak CB, Berg LJ (1998) T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J Exp Med 187: 1721–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SK, Berry DM, McGlade CJ (2001) The role of Gads in hematopoietic cell signalling. Oncogene 20: 6284–6290 [DOI] [PubMed] [Google Scholar]
- Naramura M, Kole HK, Hu R-J, Gu H (1998) Altered thymic positive selection and intracellular signals in Cbl-deficient mice. Proc Natl Acad Sci USA 95: 15547–15552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz PE, Wang S, Clarke H, Lu X, Stokoe D, Abo A (2001) Mapping the Zap-70 phosphorylation sites on LAT (linker for activation of T cells) required for recruitment and activation of signalling proteins in T cells. Biochem J 356: 461–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Villar JJ, Kanner SB (1999) Regulated association between the tyrosine kinase Emt/Itk/Tsk and phospholipase-Cgamma1 in human T lymphocytes. J Immunol 163: 6435–6441 [PubMed] [Google Scholar]
- Poulin B, Sekiya F, Rhee SG (2005) Intramolecular interaction between phosphorylated tyrosine-783 and the C-terminal Src homology 2 domain activates phospholipase C-gamma1. Proc Natl Acad Sci USA 102: 4276–4281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rellahan BL, Graham LJ, Tysgankov AY, DeBell KE, Veri M-C, Noviello C, Bonvini E (2003) A dynamic constitutive and inducible binding of c-Cbl by PLCgamma1 SH3 and SH2 domains (negatively) regulates antigen receptor-induced PLCgamma1 activation in lymphocytes. Exp Cell Res 289: 184–194 [DOI] [PubMed] [Google Scholar]
- Reynolds LF, Smyth LA, Norton T, Freshney N, Downward J, Kioussis D, Tybulewicz VLJ (2002) Vav1 Transduces T cell receptor signals to the activation of phospholipase C-gamma1 via phosphoinositide 3-kinase-dependent and -independent pathways. J Exp Med 195: 1103–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG (2001) Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem 70: 281–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo MA, Springer GH, Granada B, Piston DW (2004) An improved cyan fluorescent protein variant useful for FRET. Nat Biotechnol 22: 445–449 [DOI] [PubMed] [Google Scholar]
- Samelson LE (2002) Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu Rev Immunol 20: 371–394 [DOI] [PubMed] [Google Scholar]
- Schaeffer EM, Debnath J, Yap G, McVicar D, Liao XC, Littman DR, Sher A, Varmus HE, Lenardo MJ, Schwartzberg PL (1999) Requirement for Tec kinases Rlk and Itk in T cell receptor signaling and immunity. Science 284: 638–641 [DOI] [PubMed] [Google Scholar]
- Serrano CJ, Graham L, DeBell K, Rawat R, Veri MC, Bonvini E, Rellahan BL, Reischl IG (2005) A new tyrosine phosphorylation SITE in PLCgamma1: The role of tyrosine 775 in immune receptor signaling. J Immunol 174: 6233–6237 [DOI] [PubMed] [Google Scholar]
- Sommers CL, Lee J, Steiner KL, Gurson JM, DePersis CL, El-Khoury D, Fuller CL, Shores EW, Love PE, Samelson LE (2005) Mutation of the phospholipase C-gamma1-binding site of LAT affects both positive and negative thymocyte selection. J Exp Med 201: 1125–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica B, DeBell KE, Graham L, Rellahan BL, Alava MA, Laborda J, Bonvini E (1998) The amino-terminal Src homology 2 domain of phospholipase cgamma1 is essential for TCR-induced tyrosine phosphorylation of phospholipase Cgamma1. J Immunol 160: 1059–1066 [PubMed] [Google Scholar]
- Tybulewicz VLJ, Ardouin L, Prisco A, Reynolds LF (2003) Vav1: a key signal transducer downstream of the TCR. Immunol Rev 192: 42–52 [DOI] [PubMed] [Google Scholar]
- Wu JN, Koretzky GA (2004) The SLP-76 family of adapter proteins. Semin Immunol 16: 379–393 [DOI] [PubMed] [Google Scholar]
- Yablonski D, Kadlecek T, Weiss A (2001) Identification of a phospholipase C-gamma1 (PLC-gamma1) SH3 domain-binding site in SLP-76 required for T-cell receptor-mediated activation of PLC-gamma1 and NFAT. Mol Cell Biol 21: 4208–4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonski D, Kuhne MR, Kadlecek T, Weiss A (1998) Uncoupling of nonreceptor tyrosine kinases from PLC-gamma1 in an SLP-76-deficient T cell. Science 281: 413–416 [DOI] [PubMed] [Google Scholar]
- Ye K, Aghdasi B, Luo HR, Moriarity JL, Wu FY, Hong JJ, Hurt KJ, Bae SS, Suh PG, Snyder SH (2002) Phospholipase C gamma 1 is a physiological guanine nucleotide exchange factor for the nuclear GTPase PIKE. Nature 415: 541–544 [DOI] [PubMed] [Google Scholar]
- Zacharias DA, Violin JD, Newton AC, Tsien RY (2002) Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296: 913–916 [DOI] [PubMed] [Google Scholar]
- Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE (1998) LAT: The ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 92: 83–92 [DOI] [PubMed] [Google Scholar]
- Zhang W, Trible RP, Zhu M, Liu SK, McGlade CJ, Samelson LE (2000) Association of Grb2, Gads, and phospholipase C-gamma 1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell antigen receptor-mediated signaling. J Biol Chem 275: 23355–23361 [DOI] [PubMed] [Google Scholar]
- Zhu M, Janssen E, Zhang W (2003) Minimal requirement of tyrosine residues of linker for activation of T cells in TCR signaling and thymocyte development. J Immunol 170: 325–333 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Video 1


