Abstract
OBJECTIVE
Macrophage migration inhibitory factor (MIF) has emerged as an important mediator of septic shock. The administration of MIF increases lethality during endotoxemia, whereas neutralization of this cytokine prevents endotoxic shock and death associated with bacterial infection. The objective of this study was to determine whether there is a change in the amniotic fluid concentration of MIF in intra-amniotic infection and human parturition.
STUDY DESIGN
A cross-sectional study was conducted in women in the following categories: 1) midtrimester (n=84); 2) preterm labor and intact membranes who delivered at term (n=33), who delivered preterm (n=53), and preterm labor with intra-amniotic infection (n=23); 3) preterm premature rupture of membranes (PROM) with (n=25) and without intra-amniotic infection (n=26); and 4) term with intact membranes, in labor (n=52), and not in labor (n=31). MIF concentrations in amniotic fluid were determined using a sensitive and specific immunoassay. MIF concentrations in maternal plasma were also determined in patients with preterm labor and intact membranes. Immunohistochemistry was conducted in chorioamniotic membranes obtained from a different set of patients presenting with preterm labor with (n=18) and without (n=20) histologic chorioamnionitis. Quantitative RT-PCR was used to measure MIF mRNA expression in chorioamniotic membranes of patients with preterm labor with (n=13) and without (n=13) histologic chorioamnionitis. Parametric and non-parametric, receiver-operating characteristic (ROC) curve, survival analysis, and Cox regression model were used for analysis.
RESULTS
Immunoreactive MIF was detectable in 96% (313/327) of amniotic fluid samples. The concentration of amniotic fluid MIF at term was higher than that in the midtrimester (p=0.004). Intra-amniotic infection in women with preterm labor and preterm PROM was associated with a significant increase in median amniotic fluid MIF concentration (p<0.001 and 0.004, respectively). Patients with preterm labor with sterile amniotic fluid who delivered preterm had a significantly higher median amniotic fluid MIF concentration than those who delivered at term (p=0.007). Among patients with preterm labor with intact membranes, survival analysis indicated that the median amniocentesis-to-delivery interval was significantly shorter in patients whose amniotic fluid concentrations of MIF were above 302 ng/ml than those below this cutoff value (p<0.001). Human parturition at term was not associated with changes in the amniotic fluid MIF concentrations (p>0.05). There was no significant difference in median maternal plasma MIF concentrations among patients with preterm labor and intact membranes who delivered at term, those who delivered preterm, and those who had intra-amniotic infection (p>0.05 for all comparisons). Immunohistochemistry demonstrated that MIF protein was present in amniotic epithelial cells, and the mean percentage of immunoreactive MIF-staining cells was higher in patients with histologic chorioamnionitis than in those without this lesion (p=0.03). Similarly, the mean MIF mRNA expression was higher in chorioamniotic membranes obtained from patients with histologic chorioamnionitis than in those without this lesion (p=0.03).
CONCLUSIONS
Intra-amniotic infection and preterm parturition, but not term parturition, are associated with a significant increase in amniotic fluid MIF concentrations. Among patients with preterm labor with intact membranes, elevated amniotic fluid concentrations of MIF are associated with intra-amniotic inflammation, histologic chorioamnionitis, and shorter amniocentesis-to-delivery interval. These changes in amniotic fluid were not reflected in maternal plasma. An increased expression of MIF protein and mRNA in chorioamniotic membranes was observed in patients with histologic chorioamnionitis.
Keywords: Amniotic fluid, immunohistochemistry, intrauterine infection, macrophage migration inhibitory factor, MIF, MIF gene expression, parturition, placenta, preterm labor
INTRODUCTION
A growing body of evidence suggests an association between intra-amniotic infection and preterm labor.[1,2] The proposed pathophysiology of infection-induced preterm delivery parallels that of septic shock. In both conditions, microbial products are thought to stimulate the production of proinflammatory cytokines, which in turn lead to hemodynamic disturbances of septic shock or the activation of the common pathway of parturition. However, efforts to block or attenuate the action of proinflammatory cytokines such as interleukin (IL)-1 and tumor necrosis factor (TNF)-α have failed to improve the outcome of septic shock[3] or prevent preterm delivery.[4] It is possible that these strategies do not block the effect of other important cytokines, which may be the main mediators of septic shock or preterm delivery.
A cytokine that recently has emerged as a pivotal mediator of innate immunity and septic shock is macrophage migration inhibitory factor (MIF). This cytokine is released by monocytes and corticotropic anterior pituitary cells in response to bacterial products, TNF-α, interferon (IFN)-γ, or corticotropin-releasing hormones (CRH).[5] MIF exerts its biological function to inhibit the migration of macrophages, and stimulates TNF-α and nitric oxide from macrophages, as well as and IL-2 production from T lymphocytes. However, Onodera et al proposed that MIF could induce the migration of inflammatory cell into synovium of rheumatoid joint indirectly by the stimulation of IL-8 through the protein C kinase, activator protein-1 and NF-kB dependent pathways[6]. Other biological functions of MIF[7] include regulation of the adaptive immune response, induction of cell proliferation, and angiogenesis. Thus, MIF has been implicated in a wide variety of inflammatory and non-inflammatory diseases in humans, including rheumatoid arthritis, acute glomerulonephritis, adult respiratory distress syndrome, atherosclerosis, psoriasis vulgaris, diabetes, and renal allograft rejection.
Recent studies suggest that MIF plays an important role as a mediator of sepsis.[8] Evidence in support of this is an increased MIF concentration in blood obtained from patients with septic shock, compared to normal healthy individuals.[9] In addition, administration of recombinant MIF to animals increases lethality during endotoxemia,[10] while a neutralizing MIF antibody prevents septic shock and death from bacterial peritonitis.[11]
The purpose of this study was to determine if MIF is involved in infection/inflammation-associated preterm labor, and whether the concentration changes with intra-amniotic infection, parturition, and preterm PROM.
MATERIALS AND METHODS
A cross-sectional study was conducted by searching our clinical database and bank of biologic samples. This study included women divided into four groups. Group 1 consisted of women in the mid-trimester (14-18 weeks) of pregnancy who underwent amniocentesis for genetic indications and delivered normal infants at term (n=84). Group 2 included women with preterm labor and intact membranes. These women were subdivided into the following categories: (a) preterm labor who delivered at term with a negative amniotic fluid culture for microorganisms (n=33), (b) preterm labor who delivered preterm (<37 weeks) with a negative amniotic fluid culture for microorganisms (n=53), and (c) preterm delivery with intra-amniotic infection (n=23). Preterm labor was defined by the presence of regular uterine contractions occurring at a frequency of at least 2 every 10 minutes and cervical changes before 37 completed weeks of gestation. Intra-amniotic infection was defined as a positive amniotic fluid culture for microorganisms and intra-amniotic inflammation as an amniotic fluid white blood cell (WBC) count of 50 cells/ml or more.[12] Group 3 consisted of women with preterm PROM with (n=25) and without (n=26) intra-amniotic infection. PROM was diagnosed as amniorrhexis before the onset of spontaneous labor. Membrane rupture was diagnosed with the use of vaginal pooling, by ferning, or by a positive nitrazine test. The indications for amniocentesis in patients of both groups 2 and 3 were for the detection of intra-amniotic infection and fetal lung maturity. Group 4 was composed of women with term gestations (≥37 weeks of gestation) with intact membranes without intra-amniotic infection. This group was subdivided into: (a) not in labor (n=31) and (b) in labor (n=52). Women at term not in labor underwent amniocentesis for the assessment of lung maturity prior to cesarean section, whereas those in labor underwent amniocentesis because of labor at an uncertain gestational age or for the diagnosis of intra-amniotic infection. Amniotic fluid not required for clinical purposes was centrifuged at 4°C for 10 minutes and stored at −70°C. A sample of amniotic fluid was transported to the laboratory for aerobic, anaerobic, and genital Mycoplasma cultures. Amniotic fluid WBC count and glucose concentrations were not performed in some cases. The results of these tests were used for subsequent clinical management.
Among patients with preterm labor and intact membranes who had blood sampling performed within 24 hours of amniocentesis, MIF concentrations in maternal plasma were also determined. Briefly, blood was collected into an EDTA-containing tube and centrifuged, and the supernatant was stored at −70°C. Furthermore, among patients with preterm labor with intact membranes and preterm PROM who delivered within 72 hours of amniocentesis, the presence or absence of acute inflammatory lesions in the extra-placental membranes (histologic chorioamnionitis) was assessed as previously described.[13] This period of time was selected to preserve a meaningful temporal relationship between amniotic fluid MIF concentrations and placental pathologic findings.
All women provided informed consent prior to the collection of amniotic fluid, blood, and placental tissues. The collection of samples was approved by the Human Investigation Committees of the participating institutions and its utilization for research purposes by the IRB of the National Institute of Child Health and Human Development. Many of these samples have been used previously in studies of cytokines and arachidonic acid metabolites.
Assays for MIF in amniotic fluid and plasma
MIF concentrations in amniotic fluid and plasma were determined by using a commercially available enzyme-linked immunosorbent assay (Chemicon International, Temecula, CA). The MIF assay system was validated in our laboratory for amniotic fluid prior to determination (i.e. spike and recovery experiments). Briefly, standard and test specimens were incubated in duplicate wells of the microtiter plates coated with monoclonal antibodies against MIF. During this incubation, the immobilized antibody in the microtiter plate bound the MIF present in the standards and samples. Affinity purified monoclonal antibody to human MIF conjugated to horseradish peroxidase (HRP) was added to the wells. After an incubation period, the plate was washed to remove unbound antibody-enzyme reagents. A substrate solution (TMB: Tetramethyl Benzidine) was added and color developed in proportion to the amount of MIF bound in the initial step. Color development was stopped after a defined period and the microtiter plates were read utilizing a programmable spectrophotometer (Ceres 900 Microplate Workstation, Bio-Tek Instruments, Winooski, VT). The concentration of MIF was determined by interpolation from the respective standard curves. Inter- and intra-assay coefficients of variation (CVs) were 6.4% and 4.8%, respectively. The sensitivity was 1.4 ng/ml.
Immunohistochemistry
Chorioamniotic membranes were obtained from different sets of patients presenting with preterm labor and intact membranes with (n=18) and without histologic chorioamnionitis (n=20). Both groups were matched for gestational age at delivery. The membranes were dissected and washed in sterile phosphate buffered saline (PBS) (Accugene, Rockland, ME), then fixed in 10% buffered formaldehyde and embedded in paraffin. Sections of each specimen were stained with hematoxylin-eosin and examined by a pathologist (YMK) who was blinded to the study population. Five micron-thick sections of paraffin-embedded tissues were mounted on poly-L-lysine-coated microscopic glass slides (Fisher Scientific, Pittsburgh, PA). Deparaffinization, hydration, antigen retrieval, and immunostaining were performed with an automatic immunostainer (Ventana BenchMark, Ventana Medical Systems, Inc., Tucson, AZ). In brief, tissue sections were incubated at 42°C for 8 minutes with a goat polyclonal anti-human MIF antibody (R&D Systems, Minneapolis, MN) (1:100 in PBS), then 30 minutes with a biotin-conjugated rabbit anti-goat antibody (Dako, Carpinteria, CA) as a secondary antibody (1:500 in PBS), followed by the iVIEW DAB Detection kit (Ventana Medical Systems Inc.). Sections were counterstained with hematoxylin. Negative controls were obtained by replacing the specific antibody with non-immune goat immunoglobulins (R&D Systems) at the same concentration as the primary antibody. Immuno-absorption with blocking peptides of MIF (R&D Systems) were also used for negative controls.
Control absorptions were carried out under similar conditions with bovine serum albumin. At least ten microscopic fields (X400) were analyzed using a Nikon microscope with SPOT advanced software. The number of immunoreactive MIF-staining cells was computed by image analysis software (Image-ProPlus, MediaCybernetics, Silver Spring, MD). Immunoreactivity of amnion cells was semi-quantitatively analyzed by the fraction (%) of immuno-positive cells.
Real-time quantitative RT-PCR
Chorioamniotic membranes were obtained from patients with preterm labor and intact membranes with (n=13) and without histologic chorioamnionitis (n=13). Both groups were matched for gestational age at delivery. The membranes were dissected from the placentas, rinsed thoroughly with sterile ice-cold PBS (Sigma, St. Louis, MO), cut into small pieces, placed in RNAlater solution (Ambion, Austin, TX), and stored at 4°C for no longer than two weeks. Total RNA was isolated using guanidinium isothiocyanate/cesium chloride method.[14] MIF sequences were obtained using Accession number NM_002415. Primers and a probe for real-time quantitative RT-PCR assays were designed using Primer Express software (Applied Biosystems, Foster City, CA). The primer sequences were GCAGAACCGCTCCTACAGC (forward) and TAATAGTTGATGTAGACCCTGTCCG (reverse). The probe CAGCAGGCCGCACAGCAGCT contained the fluorescent dye 6FAM at the 5′-end and TAMRA at the 3′-end. In addition, two control genes, namely the 18S ribosomal RNA and glyceraldehyde-3 phosphate dehydrogenase (GAPDH) (J04038, Applied Biosystems, Foster City, CA), were assayed with each RNA sample. The total RNA samples were treated first with RNase-free DNase (Invitrogen, Carlsbad, CA) and then used as templates to synthesize the first strand cDNA in a reaction containing Superscript II reverse transcriptase (Invitrogen, Carldbad, CA), random hexamers, and oligo-d(T) primers. Negative controls without reverse transcriptase were included in the assays. Real-time quantitative PCRs were performed using TaqMan Universal PCR Master Mix reagents and protocols in Sequence Detection System 7700 (Applied Biosystems). After PCR, amplification plots were inspected, and baselines with threshold values were set for FAM and VIC dye layers using the Sequence Detection System (SDS) software according to the manufacturer’s recommendations (Applied Biosystems). The threshold cycle numbers were computed for each well for every dye. The analysis of relative gene expression data was performed according to the method of Livak and Schmittgen.[15]
Statistical analysis
Kruskal Wallis and Mann-Whitney U tests were used to determine the differences of the median amniotic fluid and plasma concentration of MIF among and between groups. Spearman rank correlation was utilized to assess the correlations. Among patients with preterm labor and intact membranes, receiver operating characteristic (ROC) curve analysis was employed for the identification of patients who had intra-amniotic infection or those who had intra-amniotic inflammation. Survival analysis and Cox proportional hazard model were applied to examine the interval from amniocentesis-to-delivery according to MIF amniotic fluid concentrations, while adjusting for confounding factors. Student’s t tests were used to examine the difference of the mean percentage of immunoreactive MIF-staining cells and the mean MIF mRNA expression in chorioamniotic membranes. A p value of <0.05 was considered statistically significant (SPSS 10.0, SPSS Inc, Chicago, Illinois).
RESULTS
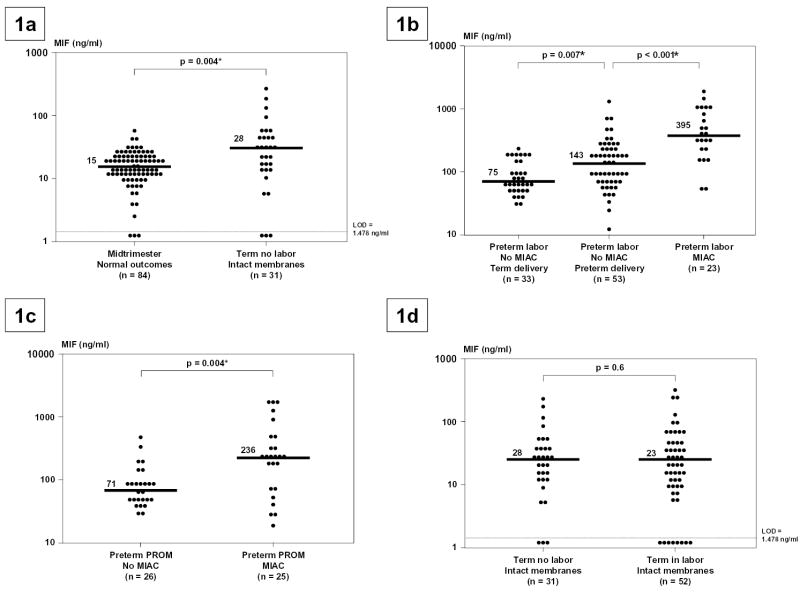
Immunoreactive MIF was detectable in 96% (313/327) of amniotic fluid samples. The median concentration of MIF at term was higher than that in the midtrimester (p=0.004; Figure 1a). There was no significant difference in the median gestational age at amniocentesis among the three subgroups of patients with preterm labor and intact membranes, and between the two subgroups of patients with preterm PROM (p>0.05 for each comparison; Table 1). Patients with intra-amniotic infection (with preterm labor and preterm PROM) had a significantly higher median amniotic fluid MIF concentration than those with sterile amniotic fluid (p<0.001 and 0.004, respectively; Figures 1b and 1c). Patients with preterm labor with sterile amniotic fluid who delivered preterm had a significantly higher median amniotic fluid MIF concentration than those who delivered at term (p=0.007; Figure 1b). There was no significant difference in the median amniotic fluid MIF concentration between patients with preterm labor with intact membranes who subsequently delivered at term and patients with preterm PROM with sterile amniotic fluid (p=0.5). Human parturition at term was not associated with changes in the amniotic fluid MIF concentrations (p=0.6; Figure 1d).
Figure 1.
Amniotic fluid concentrations of Macrophage Migration Inhibitory Factor (MIF). Figure 1a. The median amniotic fluid concentration of MIF in women at term without labor was significantly higher than in those in the mid-trimester (median 28.3 ng/ml; range 0-261.9 vs. median 15.3 ng/ml; range 0-51.6, respectively; p=0.004). Figure 1b. Amniotic fluid concentration of MIF in women with preterm labor and intact membranes. The median amniotic fluid concentration of MIF was significantly higher in women with preterm labor who delivered preterm than in those who delivered at term (median 143 ng/ml, range 11.6-1341.8 vs. median 75 ng/ml, range 30-232.7, respectively; p=0.007). Similarly, the median amniotic fluid concentration of MIF in women with microbial invasion of the amniotic cavity (MIAC) was significantly higher than in those with preterm labor without MIAC who delivered preterm (median 395 ng/ml, range 55.6-1939.9 vs. median 143 ng/ml, range 11.6-1341.8, respectively; p<0.001). Figure 1c. Amniotic fluid concentration of MIF in women with preterm PROM with and without MIAC. The median amniotic fluid concentration of MIF was significantly higher in women with preterm PROM with MIAC than in those without MIAC (median 236 ng/ml, range 20-1929.4 vs. median 71.4 ng/ml, range 30-498.2, respectively; p=0.004). Figure 1d. Amniotic fluid concentration of MIF in women at term with and without labor. The median amniotic fluid concentration of MIF was not significantly different in women at term with and without labor (median 23 ng/ml, range 0-325.4 vs. median 28 ng/ml, range 0-261.9, respectively; p=0.6).
Table I.
Gestational age (median, range) at amniocentesis in each group
| GA at amniocentesis (weeks) median (range) | |
|---|---|
| Midtrimester and normal outcomes | 16 (14-18) |
| Preterm labor who delivered at term | 28.4 (21.2-34.0) |
| Preterm labor who delivered preterm | 27.4 (19.3-34.6) |
| Preterm labor with MIAC | 27.0 (20-34.1) |
| Preterm PROM no MIAC | 30.7 (22-33.7) |
| Preterm PROM with MIAC | 29.0 (19-33.4) |
| Term no Labor | 39.3 (38-42) |
| Term in Labor | 39.3 (37-41.5) |
GA, Gestational age
MIAC, microbial invasion of the amniotic cavity
PROM, prelabor rupture of membranes
The diagnostic efficacy of MIF concentration in amniotic fluid was assessed among patients with preterm labor and intact membranes. Table 2 displays two cutoff values (derived from ROC curves) of amniotic fluid concentration of MIF for the identification of patients who had intra-amniotic infection and those who had intra-amniotic inflammation. To assess the relationship between intra-amniotic inflammation and the duration of the amniocentesis-to-delivery interval, survival analysis was employed. Spontaneous labor and delivery was entered in the analysis as the event of interest, and the patients who delivered by fetal or maternal indications were treated as censored observations with a censoring time equal to the amniocentesis-to-delivery interval. The median amniocentesis-to-delivery interval was significantly shorter in patients whose amniotic fluid concentrations of MIF were above the cutoff values derived from ROC curves than those below the cutoff values (p<0.001; Figure 2). Cox proportional hazard modeling was used to examine the relationship between the duration of the amniocentesis-to-delivery interval and MIF concentrations in amniotic fluid, while adjusting for the status of amniotic fluid culture, gestational age, and cervical dilatation at amniocentesis. The hazard ratio for amniotic fluid MIF concentration of 302 ng/ml or greater in relation to amniocentesis-to-delivery interval was high (7.8; 95% CI 3.3-18.5; see Table 3).
Table II.
Diagnostic efficacy of amniotic fluid concentration of macrophage migration inhibitory factor (MIF) in patients with preterm labor and intact membranes
| Outcome of interest | Cut-off value (ng/ml) | Sensitivity (%) | Specificity (%) | Area under ROC curve (%) | p |
|---|---|---|---|---|---|
| Intra-amniotic infection | ≥220 | 78 | 81 | 84 | <0.001* |
| Intra-amniotic inflammation | ≥302 | 95 | 95 | 96 | <0.001* |
ROC = Receiver operating characteristic
Figure 2.
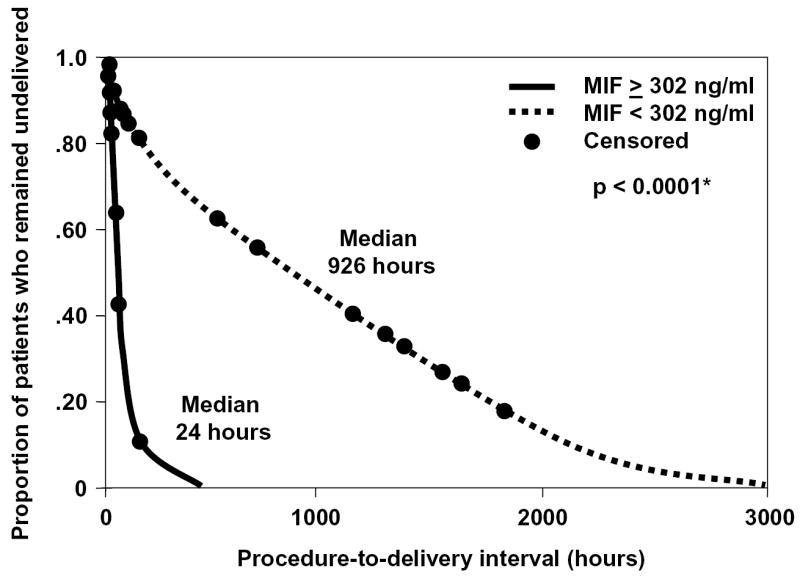
Survival curves for amniocentesis-to-delivery interval according to amniotic fluid concentrations of MIF above or below the cutoff value for the identification of intra-amniotic inflammation. Spontaneous labor and delivery was entered in the analysis as the event of interest, and the patients who delivered by fetal or maternal indications were excluded from the analysis (censored). Patients with amniotic fluid concentrations of MIF ≥ 302 ng/ml had a significantly shorter amniocentesis-to-delivery interval than those with amniotic fluid MIF concentrations below this cutoff value (MIF 302 ng/ml or greater: events 18, censored 6; median 24 hours; 95% CI 10-37 hours vs. MIF lower than 302 ng/ml: events 70, censored 15; median 926 hours; 95% CI 627-1224 hours; p<0.0001).
Table III.
Hazard ratio of amniotic fluid concentration of macrophage migration inhibitory factor (MIF) above the cutoff value used for the identification of intra-amniotic inflammation (≥50 cells/ml) in relation to interval from amniocentesis to delivery (hours)
| Variables | Hazard ratio | 95% CI | p |
|---|---|---|---|
| MIF ≥ 302 ng/ml | 7.8 | 3.3-18.5 | <0.001* |
| MIAC | 2.6 | 1.1-6.6 | 0.03* |
| Cervical dilation (cm.) | 1.4 | 1.2-1.7 | <0.001* |
| Gestational age (weeks) | 1.1 | 1.02-1.2 | 0.01* |
MIAC, microbial invasion of the amniotic cavity.
Statistically significant.
There was a positive correlation between the concentration of MIF and the amniotic fluid WBC count (Spearman’s rho = 0.5; p<0.001), and a negative correlation between the concentrations of MIF and glucose (Spearman’s rho = −0.5; p<0.001) in amniotic fluid. Among patients with preterm labor with intact membranes and preterm PROM, the median amniotic fluid MIF concentration was significantly higher in those with Gram-negative infections than in those with intra-amniotic infection in whom isolation was either Gram-positive microorganisms (p=0.004) or genital Mycoplasmas (p=0.03; Figure 3).
Figure 3.
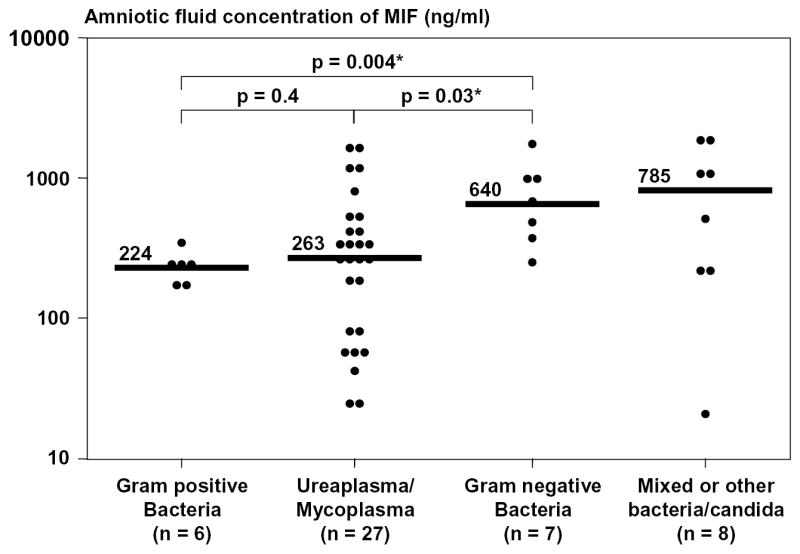
Amniotic fluid concentration of Macrophage Migration Inhibitory Factor (MIF) according to the types of microorganisms. The median amniotic fluid MIF concentration was significantly higher in patients with Gram-negative intra-amniotic infections than in those with microbial invasion of the amniotic cavity caused by either Gram-positive microorganisms or genital Mycoplasmas (Gram-negative bacteria: median 640 ng/ml; range 243-1732 vs. Gram-positive bacteria: median 224 ng/ml range 157-323; p=0.004 and Gram-negative bacteria: median 640 ng/ml; range 243-1732 vs. genital Mycoplasmas: median 263 ng/ml range 27-1678; p=0.03). The median amniotic fluid concentration of MIF in patients with intra-amniotic infection caused by mixed organisms was 785 ng/ml and ranged from 20 to 1939 ng/ml.
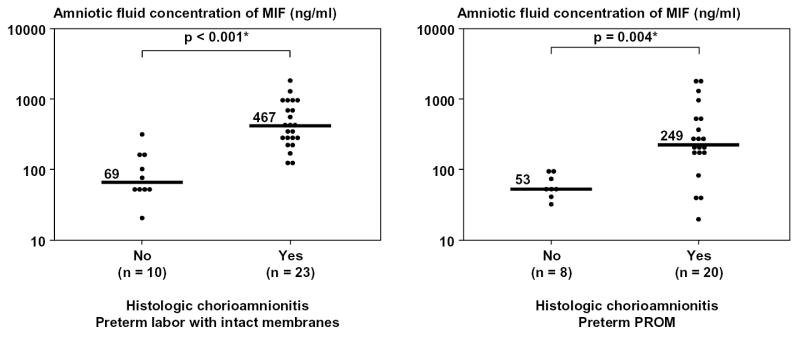
Furthermore, among patients with preterm labor with intact membranes, 53 had blood sampling performed within 24 hours of amniocentesis and had plasma samples available for MIF determination. There was no significant difference in the median plasma MIF concentrations among patients with preterm labor and intact membranes who delivered at term, those who delivered preterm, and those who had intra-amniotic infection [term delivery (n=18), median 52 ng/ml, range 24-152; preterm delivery (n=27), median 55 ng/ml, range 13-160; and intra-amniotic infection (n=8), median 28 ng/ml, range 11-113; p>0.05 for all comparisons]. Nor was there significant correlation between MIF concentrations in amniotic fluid and peripheral blood (Spearman’s rho = −0.2; p=0.1). Among patients who delivered within 72 hours of amniocenteses, placental pathology was available in 92% (33/36) of those with preterm labor with intact membranes, and in all those with preterm PROM (28/28). Patients with evidence of inflammation in the extraplacental membranes (histologic chorioamnionitis) and/or umbilical cord (funisitis) had a significantly higher median amniotic fluid concentration of MIF than those without inflammation (p values <0.05 for each comparison; Figure 4).
Figure 4.
Amniotic fluid concentration of Macrophage Migration Inhibitory Factor (MIF) according to the presence of acute inflammatory lesions in the extraplacental membranes (histologic chorioamnionitis) and/or umbilical cords (funisitis). Among patients who delivered within 72 hours of amniocenteses, those with evidence of inflammation had a significantly higher median amniotic fluid concentration of MIF than those without inflammation (preterm labor, with inflammation: median 467 ng/ml; range 143-1939 vs. without inflammation: median 69 ng/ml; range 24-353; p<0.001 and preterm PROM, with inflammation: median 249 ng/ml; range 20-1929 vs. without inflammation: median 53 ng/ml; range 32-94; p=0.004).
Immunohistochemical analysis of the chorioamniotic membranes demonstrated that MIF protein was present in amniotic epithelial cells and localized mainly in the cytoplasm (see Figures 5a and 5b). Preterm labor patients with acute histologic chorioamnionitis had a higher mean percentage of immunoreactive MIF-staining cells in amnion epithelium than those without chorioamnionitis (p=0.03; Figure 5c). Similarly, the mean MIF mRNA expression was higher in the chorioamniotic membranes obtained from patients with histologic chorioamnionitis than in those without this lesion (p=0.03; Figure 6).
Figure 5.
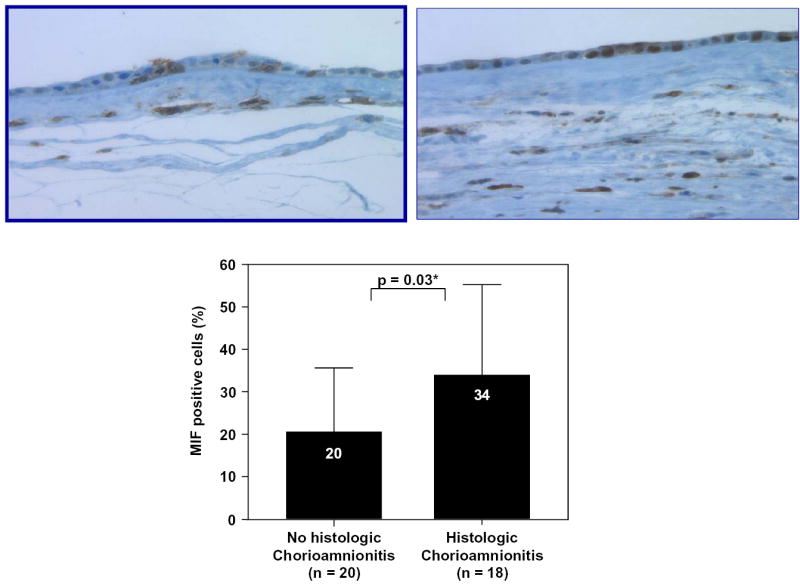
Immunohistochemical analysis of the fetal membranes. Macrophage migration inhibitory factor (MIF) protein was present in amniotic epithelial cells, and the number of MIF-positive cells was increased with histologic chorioamnionitis (5a: preterm labor without chorioamnionitis; 5b: preterm labor with chorioamnionitis). Image analysis system confirmed this result. Patients with preterm labor with acute histologic chorioamnionitis had a higher mean percentage of MIF-positive cells in amnion epithelium than those without chorioamnionitis (chorioamnionitis: mean ± SD = 34 ± 21% vs. without chorioamnionitis: mean ± SD = 20 ± 15%; p=0.03; Figure 5c).
Figure 6.
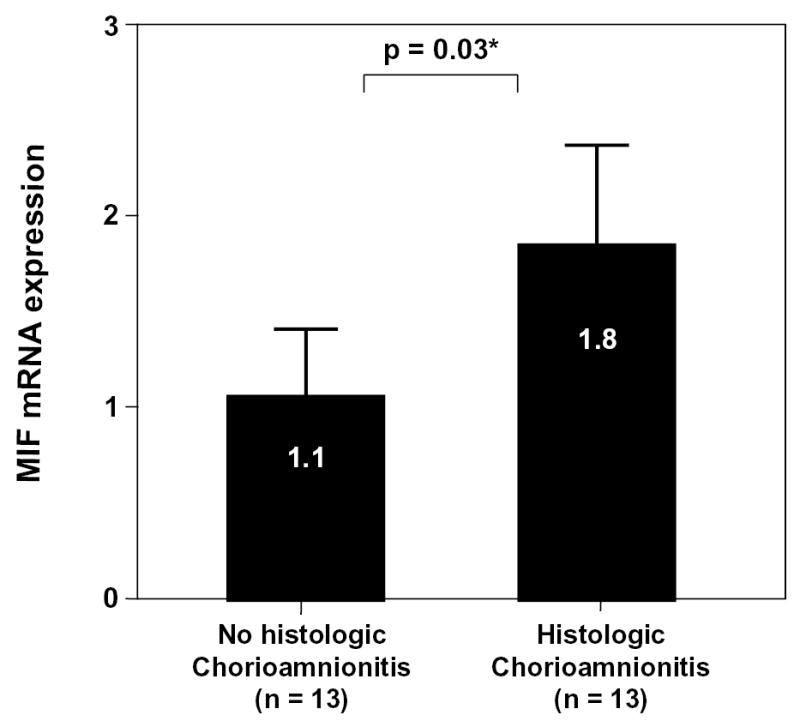
Real-time quantitative RT-PCR of MIF mRNA in chorioamniotic membranes. The mean MIF mRNA expression was higher in the chorioamniotic membranes obtained from patients with histologic chorioamnionitis than those without this lesion (chorioamnionitis: mean ± SD = 1.8 ± 1.0 vs. without chorioamnionitis: mean ± SD = 1.1 ± 0.5; p=0.03).
DISCUSSION
Principal findings of the study
1) Immunoreactive MIF is frequently detectable in amniotic fluid; 2) the concentration of immunoreactive MIF increases as a function of gestational age; 3) patients with microbiologically-proven intra-amniotic infection have a higher median amniotic fluid concentration of MIF than those with sterile amniotic fluid (with intact or ruptured membranes); 4) human spontaneous term parturition is not associated with changes in amniotic fluid concentrations of immunoreactive MIF; 5) immunohistochemistry studies demonstrated that MIF protein is present in amniotic epithelial cells, and that the number of cells staining positive is increased in cases of histologic chorioamnionitis than in those without evidence of histologic chorioamnionitis; 6) the mean MIF mRNA expression is higher in the chorioamniotic membranes obtained from patients with histologic chorioamnionitis than in those without these lesions; and 7) changes in amniotic fluid concentrations of MIF are not reflected in maternal plasma concentrations of this cytokine. Collectively, these observations suggest that MIF plays a role in histologic chorioamnionitis and preterm parturition associated with microbial invasion of the amniotic cavity (MIAC).
What is MIF?
This cytokine was originally identified in 1966[16,17] as a factor released by sensitized lymphocytes that inhibited the random migration of macrophages. Some have proposed that MIF should be called “interleukin-0,” because it was the first cytokine to be characterized.[8] After a gap in the study of this protein, MIF was “rediscovered” as a hormone/cytokine released by cells of the anterior pituitary gland[18] and monocytes/macrophages, which has unique properties. Specifically, MIF production can be induced by glucocorticoid administration, and MIF can regulate the anti-inflammatory properties of glucocorticoids. For example, when monocytes are stimulated with lipopolysaccharide (LPS) in the presence of MIF and dexamethasone, MIF can overcome the glucocorticoid inhibitory effects on monocyte secretion of TNF-α, IL-1β, IL-6 and IL-8.[18] Conversely, in the presence of dexamethasone, an antibody against MIF can inhibit the production of TNF-α.[18] These observations suggest that the balance between glucocorticoid and MIF plays an important role in determining cytokine production by macrophages. MIF and glucocorticoids are proposed to function as “a physiologic counter-regulatory dyad” that controls the balance of the host inflammatory immune response.[19]
MIF and histologic chorioamnionitis
A major observation of this study is that the median amniotic fluid MIF concentration was increased in patients with intra-amniotic infection/inflammation and histologic chorioamnionitis, regardless of the membrane status. This finding suggests that MIF either participates in the host response to intrauterine infection or is involved in the inflammatory response observed in chorioamnionitis.
Given the importance of cytokines in assessing the presence or absence of inflammation in biological fluids, we also examined the ability of MIF amniotic fluid concentrations to identify intra-amniotic inflammation. Our observations indicate that MIF concentrations in amniotic fluid perform better in the identification of intra-amniotic inflammation (an elevated WBC count in the amniotic fluid) than intra-amniotic infection (a positive amniotic fluid culture for microorganisms), as demonstrated by a larger area under the ROC curve (96% vs. 84%; see Table 2). This is consistent with the interpretation that MIF is part of the inflammatory process. Therefore, its concentration will reflect the intensity of the inflammatory response, as gauged by the number of white blood cells in the amniotic cavity, rather than the success of standard microbiological techniques to isolate a microorganism in the amniotic cavity. This observation is consistent with previously published evidence by our group that many patients have intra-amniotic inflammation, but also a negative culture of amniotic fluid for microorganisms.[20] This may represent either inflammation of non-infection-related origin, or MIAC by organisms that have escaped detection with conventional microbiologic techniques. We have previously demonstrated that molecular microbiologic techniques can identify MIAC twice as frequently as standard microbiologic methods. [21]
Maternal plasma and amniotic fluid MIF:
The finding of this study was that there was no correlation between maternal plasma MIF concentration and amniotic fluid concentration of MIF. Indeed, plasma MIF concentrations were not higher in patients with intra-amniotic infection than in those with sterile amniotic fluid. This indicates that maternal plasma MIF concentrations are not representative of intrauterine--specifically intra-amniotic- inflammatory processes.
What type of infection results in an elevation of amniotic fluid MIF concentration?
Microbial products such as LPS from Gram-negative[22] and exotoxins from Gram-positive[23] bacteria are potent inducers of MIF production. However, our results indicate that amniotic fluid MIF concentrations were higher in patients with Gram-negative intra-amniotic infection than in those with MIAC caused by either Gram-positive microorganisms or genital Mycoplasmas. MIF has been implicated in the regulation of the innate immune response to Gram-negative bacteria, as suggested by recent gene knock-out animal experiments.[24] The expression of TLR-4 mRNA and protein, as well as TNF-α production after LPS stimulation, is lower in macrophages obtained from MIF knock-out mice than from wild mice.[24] Further studies are required to understand the mechanisms by which MIF modulates the immune response in cases of Gram-positive bacteria and genital Mycoplasma infection.
MIF and MMPs
MIF has been reported to stimulate mRNA expression of matrix metalloproteinase (MMP)-1 and MMP-3 in synovial fibroblasts of patients with rheumatoid arthritis,[25] as well as MMP-9 in rat osteoblasts.[26] Although several MMPs have been implicated in the mechanism of membrane rupture in preterm PROM[27,28] and MIF could stimulate MMP production, we did not find a significant difference in the amniotic fluid concentration of MIF between patients with preterm PROM without intra-amniotic infection and those with preterm labor and intact membranes who delivered at term. It is possible that MIF in amniotic fluid does not participate in the mechanism of membrane rupture in preterm PROM.
Sources of amniotic fluid MIF
Sources of amniotic fluid MIF include the amniotic epithelial cells, which were positive for MIF with immunohistochemistry, and amniotic fluid leukocytes (there was a positive correlation between the amniotic fluid WBC count and MIF concentration in amniotic fluid). We found that the mRNA expression and protein (as determined by semi-quantitative techniques: immunohistochemistry) were higher in membranes from patients with histologic chorioamnionitis than in those without acute inflammatory lesions. Another potential source of amniotic fluid MIF is the fetus, as MIF could be detected in the umbilical cord serum of women at term.[29] The median concentration of MIF in amniotic fluid in our study was generally higher than that in maternal plasma. This finding is consistent with a previous study of Ietta et al. who reported that the concentration of MIF in amniotic fluid is higher than that in maternal and umbilical cord serum among pregnant women at term.[29]
Amniotic fluid concentrations of MIF as a function of gestational age
Our findings that MIF is a physiologic constituent of midtrimester amniotic fluid and that MIF concentration in amniotic fluid increases at term are also consistent with the previous observation.[29] MIF protein and mRNA have been detected in the ovary[30] (follicular fluid and granulosa cells), endometrium[31] (stroma and glandular epithelium), and cytotrophoblast[32] as early as 6 weeks of gestation.
Amniotic fluid MIF and parturition
Parturition has been proposed to be an inflammatory process.[33] The concentrations of several cytokines in amniotic fluid, including IL-6,[34,35] IL-8,[35,36] and TNF-α[37], increase during labor (term and preterm). Amniotic fluid MIF concentration did not increase during parturition at term. In contrast, preterm labor leading to preterm delivery was associated with a higher amniotic fluid MIF concentration than patients with preterm labor who delivered at term. These results hint at differences in the cytokine profile between term and preterm parturition, and support the concept that a fraction of patients with preterm labor may have a different mechanism for parturition from that of spontaneous labor at term.[38] It is important to mention that our study includes solely patients at term without intra-amniotic infection (as identified by negative amniotic fluid culture), a different population from the patients in the study of Ietta et al., who described an increased amniotic fluid concentration of MIF in women at term parturition, and no microbial status of amniotic fluid was reported. [29]
MIF: a mediator of sepsis and preterm labor in the context of infection/inflammation
The role of MIF as a mediator of sepsis has been demonstrated in several studies. Recombinant MIF administration increases the mortality rate induced by the administration of endotoxin[10] or the induction of bacterial peritonitis[11] in animals. MIF knock-out mice are resistant to the lethal effects of LPS[39] or staphylococcal enterotoxin B,[39] and have a lower plasma TNF-α concentration after endotoxin injection than wild-type mice.[39] Notably, these MIF gene-knock-out mice had a normal plasma concentration of IL-6 and IL-10 after an endotoxin challenge,[39] and could efficiently clear Pseudomonas aureuginosa after intratracheal administration.[39] They were, however, more susceptible to infections by intracellular organisms such as Listeria monocytogenes[39] and Salmonella typhimurium.[40] Similarly, the administration of a neutralizing antibody to MIF protects mice from death after the administration of endotoxin,[10] live bacteria,[11] or cecal ligation and bowel puncture.[11] This protective effect is apparent even when the administration occurs late in the course of infection. Moreover, a neutralizing antibody against MIF protects mice from death due to infection even in TNF-α gene knock-out mice,[11] suggesting that the neutralizing antibody may rescue immuno-compromised hosts, and that this protective effect does not depend on the reduction of TNF-α concentration alone. It is noteworthy that neutralization of the MIF activity does not impair the host’s ability to combat a mild infection caused by extracellular Gram-negative bacteria (Escherichia coli).[11] Collectively, this evidence supports the view that MIF plays a crucial role in the pathogenesis of sepsis, and that an anti-MIF strategy may have a potential benefit for the treatment of sepsis.[41] Our finding that a high amniotic fluid concentration of MIF in patients with preterm labor and intact membranes was associated with intrauterine infection/inflammation and a shorter amniocentesis-to-delivery interval suggests that MIF participates in the mechanisms leading to preterm delivery. Indeed, recombinant MIF has been demonstrated to directly up-regulate mRNA of IL-1β, IL-8,[6] cytosolic phospholipase (cPL)-A2, cyclooxygenase (COX)-2 and increase prostaglandin E2 production in cultured synoviocytes from rheumatoid arthritis patients.[42] It is possible that MIF may stimulate the enzymes involved in prostaglandin synthesis in fetal membranes. Finally, the observation that the changes of amniotic fluid MIF concentration were not mirrored in maternal blood underscores the fact that the process of blocking MIF activity to prevent preterm delivery may need to occur inside the amniotic cavity, but not in the peripheral circulation. However, whether or not neutralization of MIF activity can prevent or attenuate infection-induced preterm delivery remains to be determined.
Is MIF required for reproduction?
A recent publication has reported that women with recurrent miscarriage had lower serum concentrations of MIF at 4-6 weeks gestation than normal pregnant women.[43] This observation suggests that MIF may play a role in pregnancy failure. However, the finding that female MIF gene-knockout mice are fertile and their newborns develop normally in size[39] indicates that MIF is not essential for reproduction.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH, DHHS.
Footnotes
Presented at the 23rd Annual Meeting of the Society for Maternal-Fetal Medicine, San Francisco, February 3-8, 2003.
Note from the authors: The work described in this manuscript was submitted for presentation to the 23rd Annual Meeting of the Society for Maternal-Fetal Medicine (Am J Obstet Gynecol 2002; 187: S73). The numbers in the abstract and in this manuscript have changed slightly to accurately reflect the classification of patients and information obtained after the submission of the original abstract. The conclusions of the abstract and the manuscript are the same.
References
- 1.Gomez R, Romero R, Mazor M, Ghezzi F, David C, Yoon BH. The role of infection in preterm labor and delivery. 1997:85–125. [Google Scholar]
- 2.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 3.Cain BS, Meldrum DR, Harken AH, McIntyre RC., Jr The physiologic basis for anticytokine clinical trials in the treatment of sepsis. J Am Coll Surg. 1998:337–350. doi: 10.1016/s1072-7515(98)00036-2. [DOI] [PubMed] [Google Scholar]
- 4.Fidel PL, Jr, Romero R, Cutright J, Wolf N, Gomez R, Araneda H, Ramirez M, Yoon BH. Treatment with the interleukin-I receptor antagonist and soluble tumor necrosis factor receptor Fc fusion protein does not prevent endotoxin-induced preterm parturition in mice. J Soc Gynecol Investig. 1997:22–26. doi: 10.1177/107155769700400104. [DOI] [PubMed] [Google Scholar]
- 5.Baugh JA, Bucala R. Macrophage migration inhibitory factor. Crit Care Med. 2002:S27–S35. [PubMed] [Google Scholar]
- 6.Onodera S, Nishihira J, Koyama Y, Majima T, Aoki Y, Ichiyama H, Ishibashi T, Minami A. Macrophage migration inhibitory factor up-regulates the expression of interleukin-8 messenger RNA in synovial fibroblasts of rheumatoid arthritis patients: common transcriptional regulatory mechanism between interleukin-8 and interleukin-1beta. Arthritis Rheum. 2004:1437–1447. doi: 10.1002/art.20190. [DOI] [PubMed] [Google Scholar]
- 7.Lue H, Kleemann R, Calandra T, Roger T, Bernhagen J. Macrophage migration inhibitory factor (MIF): mechanisms of action and role in disease. Microbes Infect. 2002:449–460. doi: 10.1016/s1286-4579(02)01560-5. [DOI] [PubMed] [Google Scholar]
- 8.Martin TR. MIF mediation of sepsis. Nat Med. 2000:140–141. doi: 10.1038/72230. [DOI] [PubMed] [Google Scholar]
- 9.Beishuizen A, Thijs LG, Haanen C, Vermes I. Macrophage migration inhibitory factor and hypothalamo-pituitary-adrenal function during critical illness. J Clin Endocrinol Metab. 2001:2811–2816. doi: 10.1210/jcem.86.6.7570. [DOI] [PubMed] [Google Scholar]
- 10.Bernhagen J, Calandra T, Mitchell RA, Martin SB, Tracey KJ, Voelter W, Manogue KR, Cerami A, Bucala R. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993:756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- 11.Calandra T, Echtenacher B, Roy DL, Pugin J, Metz CN, Hultner L, Heumann D, Mannel D, Bucala R, Glauser MP. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med. 2000:164–170. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- 12.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, Hagay Z, Merchant L, Hobbins JC. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol. 1991:821–830. doi: 10.1016/0002-9378(91)90423-o. [DOI] [PubMed] [Google Scholar]
- 13.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, Ghezzi F, Berry SM, Qureshi F, Jacques SM, Kim JC, Kadar N, Romero R. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 14.Maniatis T, Fritsch EF, Sambrook J. Molecular cloning: A laboratory manual. 1982;
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Bloom BR, Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966:80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- 17.David JR. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci U S A. 1966:72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, Cerami A, Bucala R. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- 19.Calandra T, Bucala R. Macrophage migration inhibitory factor (MIF): a glucocorticoid counter-regulator within the immune system. Crit Rev Immunol. 1997:77–88. doi: 10.1615/critrevimmunol.v17.i1.30. [DOI] [PubMed] [Google Scholar]
- 20.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 21.Yoon BH, Romero R, Kim M, Kim EC, Kim T, Park JS, Jun JK. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol. 2000:1130–1137. doi: 10.1067/mob.2000.109036. [DOI] [PubMed] [Google Scholar]
- 22.Roger T, Glauser MP, Calandra T. Macrophage migration inhibitory factor (MIF) modulates innate immune responses induced by endotoxin and Gram-negative bacteria. J Endotoxin Res. 2001:456–460. [PubMed] [Google Scholar]
- 23.Calandra T, Spiegel LA, Metz CN, Bucala R. Macrophage migration inhibitory factor is a critical mediator of the activation of immune cells by exotoxins of Gram-positive bacteria. Proc Natl Acad Sci U S A. 1998:11383–11388. doi: 10.1073/pnas.95.19.11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roger T, David J, Glauser MP, Calandra T. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature. 2001:920–924. doi: 10.1038/414920a. [DOI] [PubMed] [Google Scholar]
- 25.Onodera S, Kaneda K, Mizue Y, Koyama Y, Fujinaga M, Nishihira J. Macrophage migration inhibitory factor up-regulates expression of matrix metalloproteinases in synovial fibroblasts of rheumatoid arthritis. J Biol Chem. 2000:444–450. doi: 10.1074/jbc.275.1.444. [DOI] [PubMed] [Google Scholar]
- 26.Onodera S, Nishihira J, Iwabuchi K, Koyama Y, Yoshida K, Tanaka S, Minami A. Macrophage migration inhibitory factor up-regulates matrix metalloproteinase-9 and -13 in rat osteoblasts. Relevance to intracellular signaling pathways. J Biol Chem. 2002:7865–7874. doi: 10.1074/jbc.M106020200. [DOI] [PubMed] [Google Scholar]
- 27.Maymon E, Romero R, Pacora P, Gervasi MT, Bianco K, Ghezzi F, Yoon BH. Evidence for the participation of interstitial collagenase (matrix metalloproteinase 1) in preterm premature rupture of membranes. Am J Obstet Gynecol. 2000:914–920. doi: 10.1067/mob.2000.108879. [DOI] [PubMed] [Google Scholar]
- 28.Maymon E, Romero R, Pacora P, Gervasi MT, Gomez R, Edwin SS, Yoon BH. Evidence of in vivo differential bioavailability of the active forms of matrix metalloproteinases 9 and 2 in parturition, spontaneous rupture of membranes, and intra-amniotic infection. Am J Obstet Gynecol. 2000:887–894. doi: 10.1067/mob.2000.108878. [DOI] [PubMed] [Google Scholar]
- 29.Ietta F, Todros T, Ticconi C, Piccoli E, Zicari A, Piccione E, Paulesu L. Macrophage migration inhibitory factor in human pregnancy and labor. Am J Reprod Immunol. 2002:404–409. doi: 10.1034/j.1600-0897.2002.01152.x. [DOI] [PubMed] [Google Scholar]
- 30.Wada S, Fujimoto S, Mizue Y, Nishihira J. Macrophage migration inhibitory factor in the human ovary: presence in the follicular fluids and production by granulosa cells. Biochem Mol Biol Int. 1997:805–814. doi: 10.1080/15216549700201841. [DOI] [PubMed] [Google Scholar]
- 31.Arcuri F, Ricci C, Ietta F, Cintorino M, Tripodi SA, Cetin I, Garzia E, Schatz F, Klemi P, Santopietro R, Paulesu L. Macrophage migration inhibitory factor in the human endometrium: expression and localization during the menstrual cycle and early pregnancy. Biol Reprod. 2001:1200–1205. doi: 10.1095/biolreprod64.4.1200. [DOI] [PubMed] [Google Scholar]
- 32.Arcuri F, Cintorino M, Vatti R, Carducci A, Liberatori S, Paulesu L. Expression of macrophage migration inhibitory factor transcript and protein by first-trimester human trophoblasts. Biol Reprod. 1999:1299–1303. doi: 10.1095/biolreprod60.6.1299. [DOI] [PubMed] [Google Scholar]
- 33.Keelan JA, Marvin KW, Sato TA, Coleman M, McCowan LM, Mitchell MD. Cytokine abundance in placental tissues: evidence of inflammatory activation in gestational membranes with term and preterm parturition. Am J Obstet Gynecol. 1999:1530–1536. doi: 10.1016/s0002-9378(99)70400-x. [DOI] [PubMed] [Google Scholar]
- 34.Romero R, Yoon BH, Kenney JS, Gomez R, Allison AC, Sehgal PB. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol. 1993:167–183. doi: 10.1111/j.1600-0897.1993.tb00618.x. [DOI] [PubMed] [Google Scholar]
- 35.Kemp B, Winkler M, Maas A, Maul H, Ruck P, Reineke T, Rath W. Cytokine concentrations in the amniotic fluid during parturition at term: correlation to lower uterine segment values and to labor. Acta Obstet Gynecol Scand. 2002:938–942. doi: 10.1034/j.1600-0412.2002.811007.x. [DOI] [PubMed] [Google Scholar]
- 36.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991:813–820. doi: 10.1016/0002-9378(91)90422-n. [DOI] [PubMed] [Google Scholar]
- 37.Maymon E, Ghezzi F, Edwin SS, Mazor M, Yoon BH, Gomez R, Romero R. The tumor necrosis factor alpha and its soluble receptor profile in term and preterm parturition. Am J Obstet Gynecol. 1999:1142–1148. doi: 10.1016/s0002-9378(99)70097-9. [DOI] [PubMed] [Google Scholar]
- 38.Romero R, Gomez R, Mazor M, Ghezzi F, Yoon BH. The preterm labor syndrome. 1997:29–49. [Google Scholar]
- 39.Bozza M, Satoskar AR, Lin G, Lu B, Humbles AA, Gerard C, David JR. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J Exp Med. 1999:341–346. doi: 10.1084/jem.189.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koebernick H, Grode L, David JR, Rohde W, Rolph MS, Mittrucker HW, Kaufmann SH. Macrophage migration inhibitory factor (MIF) plays a pivotal role in immunity against Salmonella typhimurium. Proc Natl Acad Sci U S A. 2002:13681–13686. doi: 10.1073/pnas.212488699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 42.Sampey AV, Hall PH, Mitchell RA, Metz CN, Morand EF. Regulation of synoviocyte phospholipase A2 and cyclooxygenase 2 by macrophage migration inhibitory factor. Arthritis Rheum. 2001:1273–1280. doi: 10.1002/1529-0131(200106)44:6<1273::AID-ART219>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 43.Yamada H, Kato EH, Morikawa M, Shimada S, Saito H, Watari M, Minakami H, Nishihira J. Decreased serum levels of macrophage migration inhibition factor in miscarriages with normal chromosome karyotype. Hum Reprod. 2003:616–620. doi: 10.1093/humrep/deg147. [DOI] [PubMed] [Google Scholar]