Abstract
Visual motion is sensed by low-level (energy-based) and high-level (feature-based) mechanisms. Our interest is in the motion detectors underlying the initial ocular following responses (OFR) that are elicited at ultrashort latencies by sudden motions of large images. OFR were elicited in humans by applying horizontal motion to vertical square-wave gratings lacking the fundamental. In the frequency domain, a pure square wave is composed of the odd harmonics—first, third, fifth, seventh, etc.—such that the third, fifth, seventh, etc., have amplitudes that are one-third, one-fifth, one-seventh, etc., that of the first, and the missing fundamental stimulus lacks the first harmonic. Motion consisted of successive quarter-wavelength steps, so the features and 4n+1 harmonics (where n = integer) shifted forward, whereas the 4n-1 harmonics—including the strongest Fourier component (the third harmonic)—shifted backward (spatial aliasing). Thus, the net Fourier energy and the non-Fourier features moved in opposite directions. Initial OFR, recorded with the search coil technique, had minimum latencies of 60 to 70 ms and were always in the direction of the third harmonic, for example, leftward steps resulted in right-ward OFR. Thus, the earliest OFR were strongly dependent on the motion of the major Fourier component, consistent with mediation by oriented spatiotemporal visual filters as in the well-known energy model of motion detection. Introducing interstimulus intervals of 10 to 100 ms (during which the screen was uniform gray) reversed the initial direction of tracking, consistent with extensive neurophysiological and psychophysical data suggesting that the visual input to the motion detectors has a biphasic temporal impulse response.
Keywords: visual motion, energy-based mechanisms, biphasic temporal impulse response, missing fundamental
TWO KINDS OF MOTION
There is clear evidence that there are at least two distinct neural subsystems by which we analyze visual motion (for recent reviews, see Lu and Sperling,1 and Derrington et al.2). One of these subsystems underlies our direct sense of motion and gives rise to the well-known waterfall illusion in which the observer experiences a sensation of motion without displacement. (In fact, there is some perceived displacement, but much less than the apparent motion.3) This system is low-level, utilizing dedicated local motion sensors, and functions without regard to form or perceptual features. Many computational models of this process have been suggested and the so-called motion-energy model of Adelson and Bergen4 has been particularly influential. These authors developed the idea that motion is sensed by spatiotemporal filters that are oriented in x-t space and tuned for spatial frequency.5,6 (One study7 reported motion between dissimilar spatial frequencies, but used images of high contrast—0.48—leaving open the possibility that the motion was mediated by distortion products.) Such models are critically sensitive to the Fourier components of the motion stimulus, which must be defined by luminance. However, it is possible to design moving stimuli that lack motion energy and are invisible to these low-level motion sensors—being defined not by luminance but by contrast, disparity, or flicker, for example—and yet we have no problem seeing the motion of such stimuli. This indicates that there must be additional, higher-level systems by which we can sense motion, and these are thought to track the movement of particular features of the image, such as edges, bright areas, shapes, and so forth.
The distinguishing characteristics of these two motion-sensing systems are sometimes controversial, and various descriptors have been used: “short-range” versus “long-range,”8 “first-order” versus “second-order,”9 “Fourier” versus “non-Fourier,”10 “passive” versus “active,”11 and “energy-based” versus “feature-based” or “correspondence-based.”12 Lu and Sperling1,13,14 contend that there are three separate motion systems and invoke a “third-order” (“figure-based”) system that, they argue, is slower than the others and heavily dependent on attention.
Our interest here is in the motion detectors underlying the initial ocular following responses (OFR) that can be elicited at ultrashort latencies by sudden motion of a large textured pattern.15,16 On the one hand, initial OFR have the spatiotemporal properties expected of low-level motion detectors and show clear reversal with “reverse-phi motion,”17 one of the hallmarks of an energy- or Fourier-based mechanism. On the other hand, a study of the initial OFR of one monkey reported that the responses to moving images that were defined by contrast-modulated dynamic noise (pure second-order motion) were virtually identical to those defined by sinusoidal luminance gratings (first-order motion) except for a slightly longer latency (average difference, 10.8 ms).18 This was the case even at low contrast (<5%), suggesting that the sensitivity to second-order motion was not simply due to distortion products that rendered the contrast-defined motion visible to the same mechanism that senses first-order luminance-defined motion. (Note that contrast-modulated noise contains spatial Fourier components in the luminance domain, but along any given axis these cancel, and hence would be invisible to the low-level energy-based mechanism. Such stimuli are said to be “drift-balanced.”10 However, the visual system has a compressive nonlinearity so that with high contrast the locally averaged luminance will now modulate and hence appear in the Fourier spectrum. For discussion of these technical issues, see Derrington,19 and Smith and Ledgeway.20) Also, higher-order stimuli in the form of moving stereograms (generated with dynamic random dots to eliminate monocular motion cues) elicit vigorous optokinetic nystagmus (OKN),21,22 though the latency of these responses is not known, hence it is unclear whether such stimuli will generate OFR at the usual short latency for this response. Given this evidence indicating that moving second-order motion can elicit tracking eye movements, it is puzzling that Harris and Smith,23 working on humans, reported only very poor OKN in response to sustained, high-contrast, second-order motion stimuli (again defined by contrast-modulated dynamic noise), though these same authors later showed that low-contrast second-order motion stimuli in the form of flicker frequency-modulated noise were a little more effective and, when combined with first-order motion stimuli, modulated the OKN elicited by the latter.24 There is also clear evidence that OKN25,26 and the very earliest OFR27,28 are very sensitive to binocular disparity, with a preference for images moving in the plane of fixation, findings that led to the suggestion that the underlying motion detectors are disparity-selective and hence not as primitive as previously supposed. However, the motion stimuli in the OFR experiments were luminance-defined and hence first-order. The same is true of the Type I moving-plaid stimuli constructed from two sinusoidal gratings differing in orientation by 90°, which Masson and Castet have shown generate short-latency OFR in the direction of the vector average of their local Fourier motion.29 However, unikinetic plaids consisting of two sine-wave gratings, one that is horizontal and moves vertically while the other is oblique (45°) and remains stationary, generate OFR with two components: the initial response with ultrashort latency is purely vertical (i.e., in the direction of the first-order Fourier motion), and after about 20 ms an additional horizontal component (i.e., in the direction of second-order pattern motion) begins to emerge.29
In all of these studies, the motion stimuli were either brief (duration <200 ms) or, when prolonged, measures were often taken to prevent subjects from actively pursuing elements in the moving patterns (e.g., by defining the moving stimuli with dynamic random dots, and/or excluding moving images from the foveal region). This is necessary because it is clear that subjects can use attentive pursuit to track a variety of discrete second-order motion stimuli.30-32
THE MISSING FUNDAMENTAL STIMULUS: EVIDENCE FOR A MOTION-ENERGY MECHANISM
We have recorded the initial OFR elicited by an apparent-motion stimulus whose features and principal Fourier component move in opposite directions. This stimulus, variously termed a fluted square wave or a square wave with a missing fundamental (and referred to here as “the missing fundamental stimulus”), is constructed by subtracting the fundamental sine-wave component from a square wave. Figure 1 shows the luminance spatial profiles for one-dimensional grating patterns consisting of a square wave, a sine wave with the spatial frequency of the fundamental, and the missing fundamental stimulus. These patterns are seen through a fixed window and when any of them moves smoothly, say rightwards, then it is perceived to move to the right. Clearly, there is nothing surprising about that. However, if this movement is in discrete, quarter-wavelength steps, then the square wave and sine wave are perceived to move veridically (i.e., with apparent motion to the right), but the missing fundamental stimulus is generally seen to move to the left.4,7,33-36 Thus, with quarter-wavelength steps the missing fundamental stimulus is perceived to move in the reverse direction of its actual motion. The usual explanation is that the first-order motion detectors responsible for the perception here do not sense the motion of the raw images (or their features), but rather a spatially filtered version of the images, so that the perceived motion depends critically on the Fourier composition of the spatial stimulus. In the frequency domain, a pure square wave is composed entirely of the odd harmonics—the first, third, fifth, seventh, etc.—with progressively decreasing amplitudes such that the third, fifth, seventh, etc., have amplitudes that are one-third, one-fifth, one-seventh, etc., that of the first. Accordingly, the missing fundamental stimulus lacks the first harmonic and so is composed entirely of the higher odd harmonics, with the third having the lowest spatial frequency and the largest amplitude (see Fig. 1). This means that when the missing fundamental stimulus steps one-quarter of its wavelength, the largest Fourier component, the third harmonic steps three-quarters of its own wavelength in the forward direction. However, a three-quarter wavelength forward step of a sine wave is exactly equivalent to a quarter-wavelength backward step, and, because the brain gives greatest weight to the nearest image matches (spatial aliasing), the perceived motion is invariably in the backward direction (see Fig. 2). (A similar explanation underlies the wagon wheels that seem to rotate backwards in old western movies.) In fact, with quarter-wavelength steps of the missing fundamental stimulus, all of the 4n-1 harmonics (where n is an integer), such as the third, seventh, eleventh, etc., shift in the backward direction, whereas all of the 4n+1 harmonics, such as the fifth, ninth, thirteenth, etc., shift in the forward direction, and it seems that the most prominent component—the third harmonic—, generally wins. (It has been suggested34 that this is a form of motion capture, where-by the largest and/or lowest spatial frequency component somehow suppresses the influence of all other components.37) For our present purposes, the important point here is that the direction of apparent motion is generally determined by the principal Fourier component, consistent with the idea that the underlying detection mechanism is energy-based rather than feature-based.
FIGURE 1.
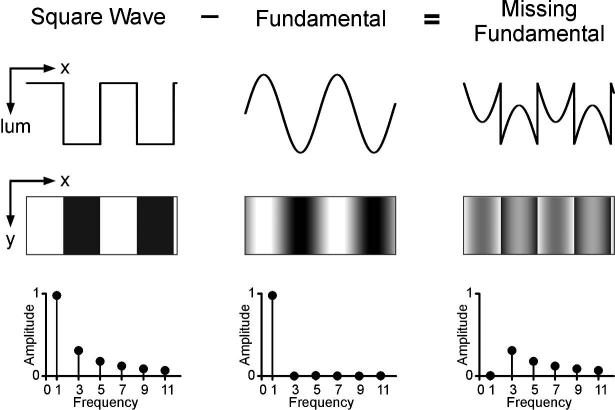
The missing fundamental stimulus is produced by subtracting the fundamental sine wave from a square wave. For the experiments described, visual patterns were all vertical one-dimensional gratings. Upper Row (x-lum plot): luminance plotted as a function of horizontal spatial position for the square-wave, fundamental, and missing fundamental stimuli. Middle Row (x-y plot): luminance as a function of horizontal and vertical position, indicating the visual appearance of the three different grating patterns on the screen. Bottom Row (Fourier spectra): harmonic content of the spatial stimuli.
FIGURE 2.
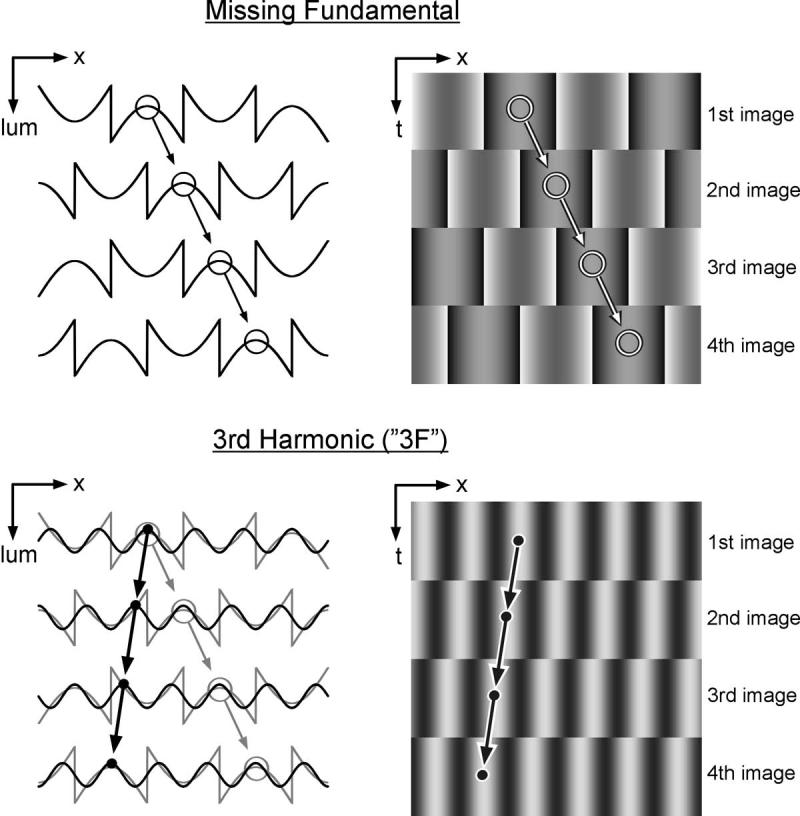
Successive images of the missing fundamental motion stimulus used in the present study. Top: In the example shown, the luminance profiles of successive images shifted rightward by one-quarter wavelength, as indicated by the circles and arrows; plots at left show luminance as a function of horizontal spatial position (x-lum plot) and plots at right show a single horizontal slice through the stimulus at successive points in time, as though the stimulus was viewed through a stationary horizontal slit (x-t plot). Bottom: The luminance profiles for the third harmonic component of the missing fundamental stimulus, which steps three-quarters of its wavelength rightward with the appearance of each new image (see successive circles linked by arrows); these steps cannot be distinguished from quarter-wavelength steps leftward (see successive black dots linked by arrows). In fact, when a pure sinusoid with the wave-length of the third harmonic undergoes such steps it is invariably perceived to move leftwards, indicating that the brain gives greatest weight to the nearest matching images.
In view of the controversy surrounding the motion detectors underlying the initiation of OFR, we used the missing fundamental stimulus to determine whether the earliest OFR are energy-based and/or feature-based. Accordingly, OFR were elicited in human subjects by applying horizontal motion in discrete steps to vertical square-wave grating patterns lacking the fundamental. Successive steps, each one-quarter of the pattern’s wavelength, were applied every 10 ms for 200 ms. Initial OFR, recorded from three human subjects with the search coil technique, had minimum latencies of 60 to 70 ms and were invariably in the direction of the third harmonic over a wide range of wavelengths (0.4 to 7.5 deg/cycle) and contrasts (2.4% to 75.4%). A sample response profile from one subject is shown in Figure 3: successive 1.65-degree rightward steps were applied to a grating of wavelength 6.6 deg/cycle and resulted in leftward OFR (downward deflection in the figure; see the trace labeled “MF,” indicating the “Missing Fundamental” stimulus). The same rightward steps applied to a pure sine-wave grating whose wavelength was the same as that of the missing fundamental resulted in clear rightward OFR, exactly as expected because both the motion energy and the features undergo rightward steps (see the trace labeled “F,” for “Fundamental,” in Fig. 3). If the response generated by the missing fundamental stimulus is due primarily to the shift in the third harmonic, then we would expect to see a very similar response when the same rightward steps were applied to a pure sine-wave grating whose spatial frequency was three times that of the missing fundamental stimulus and, indeed, this was the case (see the dotted trace labeled “3F,” for “third harmonic,” in Fig. 3). Note that the contrast of the MF was arranged to be 18.8%, so that its third harmonic had a contrast that was the same as that of the 3F sine wave (8%). Thus, the earliest OFR were strongly dependent on the motion of the major Fourier component, consistent with mediation by oriented spatiotemporal visual filters, as in the well-known energy model of motion detection.
FIGURE 3.
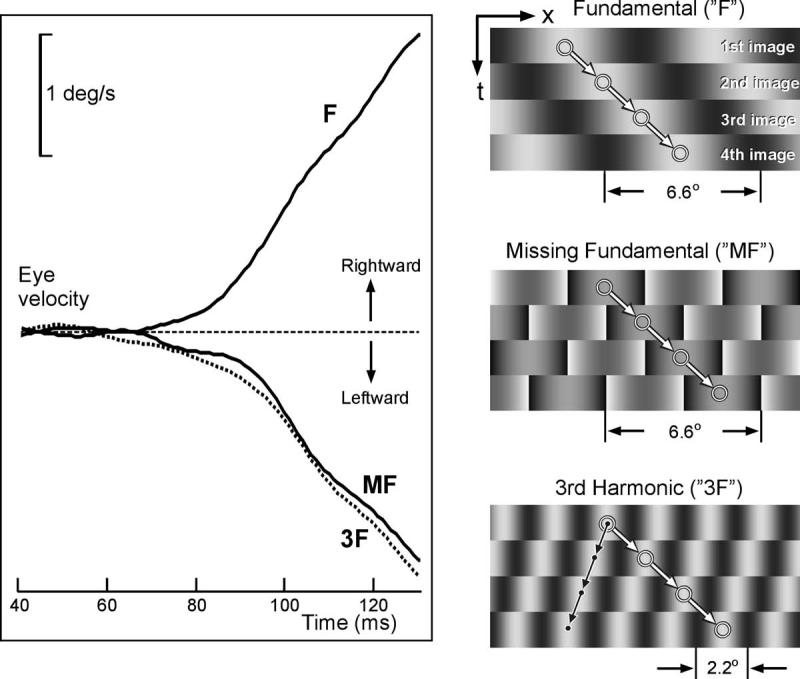
The initial horizontal OFR resulting from successive rightward steps applied to various types of vertical grating pattern. Trace MF: the OFR generated when the missing fundamental stimulus (wavelength, 6.6 deg) underwent quarter-wavelength steps (1.65 deg). Trace F: the OFR when steps of the same magnitude (1.65 deg) were applied to pure sine-wave gratings that had the same spatial frequency as the fundamental. Trace 3F: the OFR when steps of the same magnitude (1.65 deg) were applied to pure sine-wave gratings that had three times the spatial frequency of the fundamental. Note that the responses to the missing fundamental stimulus strongly resemble those to the 3F grating (and that the contrast of the 3F component of the missing fundamental stimulus was the same as that of the pure 3F sine-wave stimulus—8%). The cartoons at the right show x-t plots of the stimuli.
THE EFFECT OF AN INTERSTIMULUS INTERVAL: EVIDENCE FOR A BIPHASIC TEMPORAL IMPULSE RESPONSE
Georgeson and Harris35 examined the direction of perceived motion associated with quarter-wavelength steps applied to missing fundamental gratings and found that it could be reversed by introducing interstimulus intervals (ISI) of more than 40 ms, so that the motion was now perceived to be in the direction of the pattern or feature. In this situation, the display goes blank (i.e., the pattern contrast is reduced to zero and the luminance is maintained at the same mean level) between each quarter-wavelength step of the missing fundamental stimulus for a period of at least 40 ms. The authors suggested that the ISI disabled the energy-based mechanism and uncovered a feature-based one. A number of other authors have also advanced this same explanation for the reversal of perceived motion by an ISI.2,36,38,39 Consistent with these observations we have found that the initial OFR to quarter-wavelength steps applied to missing fundamental stimuli are reversed by an ISI of 10 to 100 ms. However, we have a rather different interpretation, because we found that such ISI also reversed the initial OFR when quarter-wavelength steps were applied to pure sine waves. Sample response profiles for one subject are shown in Figure 4: the responses to quarter-wavelength rightward single steps of a sine-wave grating (“two-image movie”) resulted in the expected rightward initial OFR, albeit very transient (note the initial upward deflection of the continuous trace labeled “0 ms” in Fig. 4), and interposing a blank period of 30 ms (ISI) between the two missing-fundamental images resulted in a clear reversal of the direction of the initial OFR (note the initial downward deflection of the dotted trace labeled “30 ms” in Fig. 4). The reversal of tracking here cannot be explained by a feature-based mechanism because the features of a pure sine wave shift in the same direction as its Fourier energy. Instead, we attribute the reversal in the direction of the initial tracking to the temporal characteristics of the neural input reaching the motion detectors. Thus, neurophysiological data40-42 from early in the visual pathway (e.g., retina, lateral geniculate nucleus, striate cortex) and psychophysical data43-47 indicate that, with the low spatial frequencies and photopic conditions used in our experiments, many of the visual responses reaching the motion detectors have a biphasic temporal impulse response (see Fig. 5A). A critical feature of this temporal-filter hypothesis, which is illustrated in Figure 5B, is that the neural representation of the first image undergoes reversal during the ISI. Because of this, the neural representation of the second image— whose appearance marks the onset of motion—is matched to a representation of the first image that is positive at short ISI and negative (i.e., inverted) at longer ones (see Fig. 5B). Thus, at short ISI, the second image is matched to a direct replica of the first image, whereas at long ISI the second image is matched to an inverted replica of the first image, which is equivalent to a 180° phase shift. As a consequence, the quarter-wavelength difference between the first and second images is seen as a 90° phase shift in the forward direction at short ISI and a 90° phase shift in the backward direction at long ISI (see Fig. 5C). The net result is that initial OFR are in the forward direction with short ISI and in the reverse direction with longer ISI. Actually, the reversed responses reach a maximum with an ISI of about 40 ms and return to baseline when the ISI reaches about 100 ms (not shown), presumably because this is the maximum time over which the low-level motion detectors can integrate motion.
FIGURE 4.
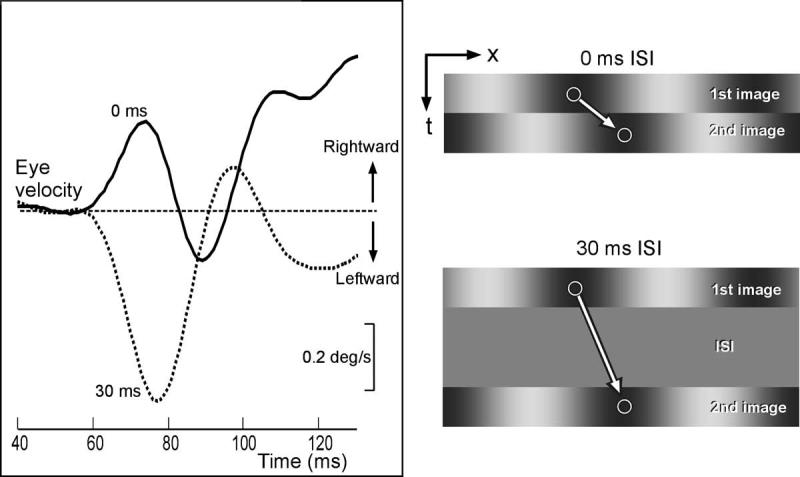
Interposing an ISI (of 30 ms) reversed the OFR resulting from quarter-wavelength steps applied to a pure sine-wave grating. Rightward steps with no ISI generated rightward OFR (continuous trace) and the same steps with a 30-ms ISI generated leftward OFR (dotted trace).
FIGURE 5.
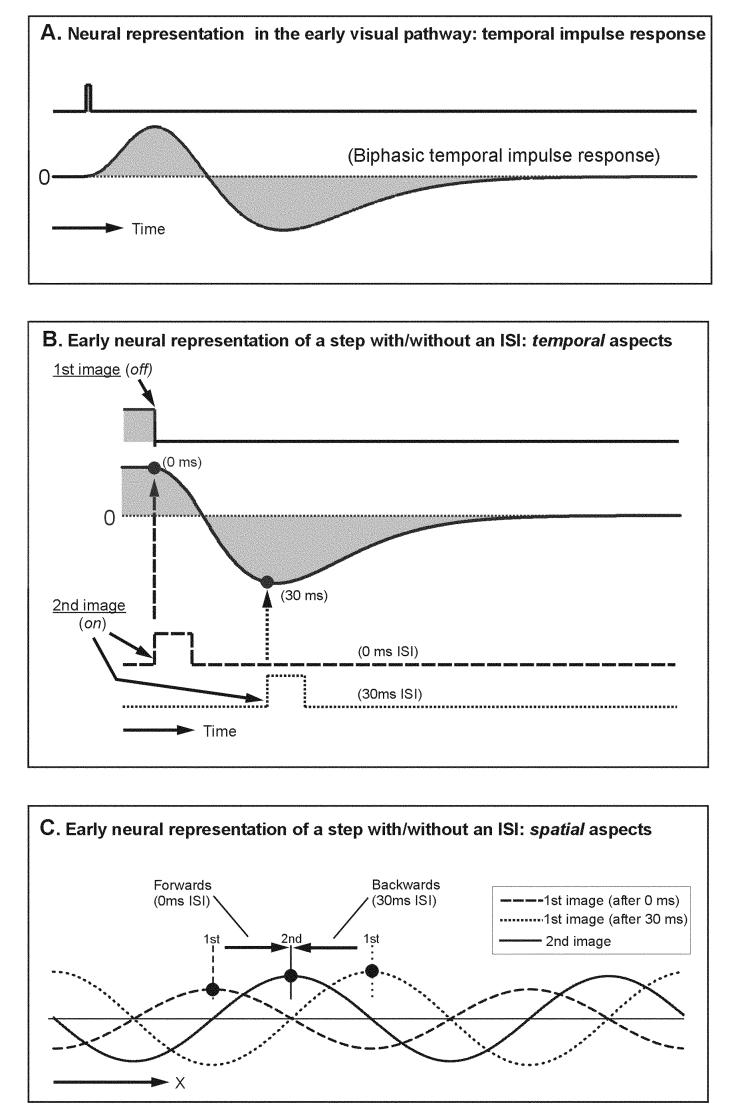
The reversal of OFR seen in Figure 4 can be explained by the biphasic temporal impulse response of the visual responses reaching the motion detectors. (A) The neural responses to a light flash in the early stages of the visual pathway (retina, lateral geniculate, striate cortex) show an initial transient wave of excitation followed by inhibition. (B) This means that when two gratings that differ in phase by one-quarter wavelength are presented in sequence with an intervening interval (ISI) the neural representation of the first-image gradually undergoes reversal. Because of this, the neural representation of the second image—whose appearance marks the onset of motion—is matched to a representation of the first image that is positive at short ISI (0 ms in the figure) and negative, that is, inverted, at longer ones (30 ms in the figure). (C) The net result is that, at short ISI (here, 0 ms), the representation of the second image (continuous trace) is matched to a direct replica of the first image (dashed trace), whereas at long ISI (here, 30 ms) the second image is matched to an inverted replica of the first image (dotted trace), which is equivalent to a 180° phase shift. As a consequence, the quarter-wavelength difference between the first and second images is seen as a 90° phase shift in the forward direction with the 0-ms ISI—leading to initial OFR in the forward direction—and a 90° phase shift in the backward direction with the 30-ms ISI—leading to OFR in the reverse direction.
In sum, the reversal of the initial OFR with ISI greater than 10 ms seems to be readily accounted for by the known biphasic temporal impulse response function of the early visual pathway, especially the so-called magnocellular pathway that has been implicated in the motion responses in cortical areas MT and MST42,48-51 (but see De Valois and colleagues,41,42 who make a case for the parvocellular pathway also being involved), which have in turn been strongly implicated in the genesis of the earliest OFR.52-55 Interestingly, it has been reported that with high-contrast sine-wave gratings, motion can still be perceived with ISI > ms and is always in the forward direction.47 This was attributed to a feature-based mechanism and it is significant that initial OFR showed no such tendency in our study, further reinforcing the idea that the eye movements result entirely from an energy-based detection mechanism.
INITIAL OFR: A MODEL SYSTEM FOR STUDYING ENERGY-BASED VISUAL MOTION?
We have employed complex visual gratings that have long been used by visual psychophysicists to study the mechanisms by which we sense visual motion. These stimuli are relatively novel to oculomotor research and have allowed us to uncover some new aspects of the spatiotemporal characteristics of the motion detectors mediating early OFR. Our preliminary data on OFR point to low-level mechanisms and indicate that initial OFR is a promising model system for studying the neural mechanisms sensing first-order motion energy.
REFERENCES
- Lu ZL, Sperling G. Three-systems theory of human visual motion perception: review and update. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2001;18:2331–2370. doi: 10.1364/josaa.18.002331. [DOI] [PubMed] [Google Scholar]
- Derrington AM, Allen HA, Delicato LS. Visual mechanisms of motion analysis and motion perception. Annu. Rev. Psychol. 2004;55:181–205. doi: 10.1146/annurev.psych.55.090902.141903. [DOI] [PubMed] [Google Scholar]
- McGraw PV, et al. Motion adaptation distorts perceived visual position. Curr. Biol. 2002;12:2042–2047. doi: 10.1016/s0960-9822(02)01354-4. [DOI] [PubMed] [Google Scholar]
- Adelson EH, Bergen JR. Spatiotemporal energy models for the perception of motion. J. Opt. Soc. Am. A. 1985;2:284–299. doi: 10.1364/josaa.2.000284. [DOI] [PubMed] [Google Scholar]
- Ledgeway T. How similar must the Fourier spectra of the frames of a random-dot kinematogram be to support motion perception? Vision Res. 1996;36:2489–2495. doi: 10.1016/0042-6989(95)00315-0. [DOI] [PubMed] [Google Scholar]
- Watson AB. Apparent motion occurs only between similar spatial frequencies. Vision Res. 1986;26:1727–1730. doi: 10.1016/0042-6989(86)90059-3. [DOI] [PubMed] [Google Scholar]
- Baro JA, Levinson E. Apparent motion can be perceived between patterns with dissimilar spatial frequencies. Vision Res. 1988;28:1311–1313. doi: 10.1016/0042-6989(88)90062-4. [DOI] [PubMed] [Google Scholar]
- Braddick O. A short-range process in apparent motion. Vision Res. 1974;14:519–527. doi: 10.1016/0042-6989(74)90041-8. [DOI] [PubMed] [Google Scholar]
- Cavanagh P, Mather G. Motion: the long and short of it. Spat. Vis. 1989;4:103–129. doi: 10.1163/156856889x00077. [DOI] [PubMed] [Google Scholar]
- Chubb C, Sperling G. Drift-balanced random stimuli: a general basis for studying non-Fourier motion perception. J. Opt. Soc. Am. A. 1988;5:1986–2007. doi: 10.1364/josaa.5.001986. [DOI] [PubMed] [Google Scholar]
- Cavanagh P. Attention-based motion perception. Science. 1992;257:1563–1565. doi: 10.1126/science.1523411. [DOI] [PubMed] [Google Scholar]
- Smith AT. Correspondence-based and energy-based detection of second-order motion in human vision. J. Opt. Soc. Am. A. 1994;11:1940–1948. doi: 10.1364/josaa.11.001940. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Sperling G. Three systems for visual motion perception. Curr. Dir. Psych. Sci. 1996;5:44–53. [Google Scholar]
- Lu ZL, Sperling G. The functional architecture of human visual motion perception. Vision Res. 1995;35:2697–2722. doi: 10.1016/0042-6989(95)00025-u. [DOI] [PubMed] [Google Scholar]
- Miles FA, Kawano K, Optican LM. Short-latency ocular following responses of monkey. I. Dependence on temporospatial properties of visual input. J. Neurophysiol. 1986;56:1321–1354. doi: 10.1152/jn.1986.56.5.1321. [DOI] [PubMed] [Google Scholar]
- Gellman RS, Carl JR, Miles FA. Short latency ocular-following responses in man. Vis. Neurosci. 1990;5:107–122. doi: 10.1017/s0952523800000158. [DOI] [PubMed] [Google Scholar]
- Masson GS, Yang DS, Miles FA. Reversed short-latency ocular following. Vision Res. 2002;42:2081–2087. doi: 10.1016/s0042-6989(02)00082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson PJ, Guo K. Stages in motion processing revealed by the ocular following response. Neuroreport. 1999;10:3803–3807. doi: 10.1097/00001756-199912160-00015. [DOI] [PubMed] [Google Scholar]
- Derrington AM. Distortion products in geniculate X-cells: a physiological basis for masking by spatially modulated gratings? Vision Res. 1987;27:1377–1386. doi: 10.1016/0042-6989(87)90214-8. [DOI] [PubMed] [Google Scholar]
- Smith AT, Ledgeway T. Separate detection of moving luminance and contrast modulations: fact or artifact? Vision Res. 1997;37:45–62. doi: 10.1016/s0042-6989(96)00147-2. [DOI] [PubMed] [Google Scholar]
- Fox R, Lehmkuhle S, Leguire LE. Stereoscopic contours induce optokinetic nystagmus. Vision Res. 1978;18:1189–1192. doi: 10.1016/0042-6989(78)90103-7. [DOI] [PubMed] [Google Scholar]
- Archer SM, Miller KK, Helveston EM. Stereoscopic contours and optokinetic nystagmus in normal and stereoblind subjects. Vision Res. 1987;27:841–844. doi: 10.1016/0042-6989(87)90080-0. [DOI] [PubMed] [Google Scholar]
- Harris LR, Smith AT. Motion defined exclusively by second-order characteristics does not evoke optokinetic nystagmus. Vis. Neurosci. 1992;9:565–570. doi: 10.1017/s0952523800001802. [DOI] [PubMed] [Google Scholar]
- Harris LR, Smith AT. Interactions between first- and second-order motion revealed by optokinetic nystagmus. Exp. Brain Res. 2000;130:67–72. doi: 10.1007/s002219900232. [DOI] [PubMed] [Google Scholar]
- Howard IP, Gonzalez EG. Human optokinetic nystagmus in response to moving binocularly disparate stimuli. Vision Res. 1987;27:1807–1816. doi: 10.1016/0042-6989(87)90109-x. [DOI] [PubMed] [Google Scholar]
- Howard IP, Simpson WA. Human optokinetic nystagmus is linked to the stereoscopic system. Exp. Brain Res. 1989;78:309–314. doi: 10.1007/BF00228902. [DOI] [PubMed] [Google Scholar]
- Masson GS, et al. Short-latency ocular following in humans: sensitivity to binocular disparity. Vision Res. 2001;41:3371–3387. doi: 10.1016/s0042-6989(01)00029-3. [DOI] [PubMed] [Google Scholar]
- Yang DS, Miles FA. Short-latency ocular following in humans is dependent on absolute (rather than relative) binocular disparity. Vision Res. 2003;43:1387–1396. doi: 10.1016/s0042-6989(03)00146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson GS, Castet E. Parallel motion processing for the initiation of short-latency ocular following in humans. J. Neurosci. 2002;22:5149–5163. doi: 10.1523/JNEUROSCI.22-12-05149.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner A, Ilg UJ. Initiation of smooth-pursuit eye movements to first-order and second-order motion stimuli. Exp. Brain Res. 2000;133:450–456. doi: 10.1007/s002210000459. [DOI] [PubMed] [Google Scholar]
- Butzer F, Ilg UJ, Zanker JM. Smooth-pursuit eye movements elicited by first-order and second-order motion. Exp. Brain Res. 1997;115:61–70. doi: 10.1007/pl00005686. [DOI] [PubMed] [Google Scholar]
- Hawken MJ, Gegenfurtner KR. Pursuit eye movements to second-order motion targets. J. Opt. Soc. Am. A Opt. Image. Sci. Vis. 2001;18:2282–2296. doi: 10.1364/josaa.18.002282. [DOI] [PubMed] [Google Scholar]
- Adelson EH. Some new motion illusions, and some old ones, analysed in terms of their Fourier components. Invest. Ophthalmol. Vis. Sci. ARVO. 1982;(Suppl 34):144. [Google Scholar]
- Georgeson MA, Shackleton TM. Monocular motion sensing, binocular motion perception. Vision Res. 1989;29:1511–1523. doi: 10.1016/0042-6989(89)90135-1. [DOI] [PubMed] [Google Scholar]
- Georgeson MA, Harris MG. The temporal range of motion sensing and motion perception. Vision Res. 1990;30:615–619. doi: 10.1016/0042-6989(90)90072-s. [DOI] [PubMed] [Google Scholar]
- Brown RO, He S. Visual motion of missing-fundamental patterns: motion energy versus feature correspondence. Vision Res. 2000;40:2135–2147. doi: 10.1016/s0042-6989(00)00080-8. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Cavanagh P. Motion capture anisotropy. Vision Res. 1987;27:97–106. doi: 10.1016/0042-6989(87)90146-5. [DOI] [PubMed] [Google Scholar]
- Smith AT. Correspondence-based and energy-based detection of second-order motion in human vision. J. Opt. Soc. Am. A. 1994;11:1940–1948. doi: 10.1364/josaa.11.001940. [DOI] [PubMed] [Google Scholar]
- Hammett ST, Ledgeway T, Smith AT. Transparent motion from feature- and luminance-based processes. Vision Res. 1993;33:1119–1122. doi: 10.1016/0042-6989(93)90245-r. [DOI] [PubMed] [Google Scholar]
- Purpura K, et al. Light adaptation in the primate retina: analysis of changes in gain and dynamics of monkey retinal ganglion cells. Vis. Neurosci. 1990;4:75–93. doi: 10.1017/s0952523800002789. [DOI] [PubMed] [Google Scholar]
- De Valois RL, Cottaris NP. Inputs to directionally selective simple cells in macaque striate cortex. Proc. Natl. Acad. Sci.; USA. 1998. pp. 14488–14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Valois RL, et al. Spatial and temporal receptive fields of geniculate and cortical cells and directional selectivity. Vision Res. 2000;40:3685–3702. doi: 10.1016/s0042-6989(00)00210-8. [DOI] [PubMed] [Google Scholar]
- Bex PJ, Baker CL., Jr. Motion perception over long interstimulus intervals. Percept. Psychophys. 1999;61:1066–1074. doi: 10.3758/bf03207614. [DOI] [PubMed] [Google Scholar]
- Shioiri S, Cavanagh P. ISI produces reverse apparent motion. Vision Res. 1990;30:757–768. doi: 10.1016/0042-6989(90)90101-p. [DOI] [PubMed] [Google Scholar]
- Pantle A, Turano K. Visual resolution of motion ambiguity with periodic luminance- and contrast-domain stimuli. Vision Res. 1992;32:2093–2106. doi: 10.1016/0042-6989(92)90071-p. [DOI] [PubMed] [Google Scholar]
- Strout JJ, Pantle A, Mills SL. An energy model of interframe interval effects in single-step apparent motion. Vision Res. 1994;34:3223–3240. doi: 10.1016/0042-6989(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, De Valois KK. Motion-reversal reveals two motion mechanisms functioning in scotopic vision. Vision Res. 1997;37:745–755. doi: 10.1016/s0042-6989(96)00207-6. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Psychophysical evidence for separate channels for the perception of form, color, movement, and depth. J. Neurosci. 1987;7:3416–3468. doi: 10.1523/JNEUROSCI.07-11-03416.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M, Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988;240:740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- Maunsell JH, Nealey TA, DePriest DD. Magnocellular and parvocellular contributions to responses in the middle temporal visual area (MT) of the macaque monkey. J. Neurosci. 1990;10:3323–3334. doi: 10.1523/JNEUROSCI.10-10-03323.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JH. Macaque vision after magnocellular lateral geniculate lesions. Vis. Neurosci. 1990;5:347–352. doi: 10.1017/s0952523800000432. [DOI] [PubMed] [Google Scholar]
- Takemura A, Inoue Y, Kawano K. Visually driven eye movements elicited at ultra-short latency are severely impaired by MST lesions. Ann. N.Y. Acad. Sci. 2002;956:456–459. doi: 10.1111/j.1749-6632.2002.tb02854.x. [DOI] [PubMed] [Google Scholar]
- Kawano K, et al. Neural activity in cortical area MST of alert monkey during ocular following responses. J. Neurophysiol. 1994;71:2305–2324. doi: 10.1152/jn.1994.71.6.2305. [DOI] [PubMed] [Google Scholar]
- Kawano K, et al. The role of MST neurons during ocular tracking in 3D space. Int. Rev. Neurobiol. 2000;44:49–63. doi: 10.1016/s0074-7742(08)60737-0. [DOI] [PubMed] [Google Scholar]
- Miles FA. The neural processing of 3-D visual information: evidence from eye movements. Euro. J. Neurosci. 1998;10:811–822. doi: 10.1046/j.1460-9568.1998.00112.x. [DOI] [PubMed] [Google Scholar]


