Abstract
Cholecystokinin (CCK), one of the most abundant neuropeptides in the brain, plays an important role in anxiogenesis through the activation of CCK receptor-2 (CCKR-2). Accumulating evidence, however, has suggested this role depends on endogenous CCKergic “tone,” which is largely determined by the expression level of the CCKR-2. Using the tTA/tetO-inducible transgenic (tg) approach, we show here that overexpression of the CCKR-2 in neurons of the forebrain significantly increases CCKR-2 binding capacity in tg mice compared with their littermate controls. Interestingly, these tg mice consistently exhibit increased fear responses, which are generally interpreted as anxiety-like behaviors in the rodent, in a battery of behavioral tests, which represented conflict situations or delivered stress to the subjects. The inhibition of transgene expression with doxycycline treatment completely diminished both increased receptor-binding activity and all behavioral phenotypes. Furthermore, treatment of tg mice with diazepam significantly attenuated these anxiety-like behaviors. Our results directly demonstrate that the elevated CCKergic tone via overexpression of the CCKR-2 in the brain may constitute an underlying molecular/neuronal mechanism for the expression of anxiety. In addition, our study has validated a robust genetic anxiety model in the mouse in terms of their face, constructive, and predictive validity.
Keywords: behaviors, transgenic mouse
Anxiety disorders are among the most common psychiatric disorders and affect ≈20 million American adults. Although effective treatments are available, we are still meeting very modest success in curing these disorders, largely due to unknown molecular and neuronal mechanisms (1, 2). In addition, most available medications have certain side effects or problems with tolerance or dependency (3, 4). Therefore, it is of significant interest to further explore mechanisms to develop a novel therapeutic strategy.
Recently, accumulating evidence indicates an important role of the cholecystokinin (CCK) system in the pathogenesis of anxiety (5, 6). CCK, originally found in the gastrointestinal track (7), is one of the most abundant neuropeptides in the brain, with the highest level in the cortex, hippocampus, and amygdala (8, 9). CCK binds to two types of receptors, CCK receptor-1 (CCKR-1) and CCK receptor-2 (CCKR-2) (10, 11). CCKR-1 is abundant in peripheral organs and in few discrete brain regions, and CCKR-2 is predominantly found in the brain, with the highest density in the limbic system (10, 12, 13). The first evidence to show the role of the CCKergic system in anxiogenesis came from a satiety study, in which a CCK agonist was found to have an anxiogenic effect in sheep (14). Injection of CCK-8 into the amygdala reproduced a similar effect in rats (15). The finding that CCK-induced activity in hippocampal neurons can be blocked by very low doses of benzodiazepines, the most commonly used class of anxiolytic drugs, suggested that CCKergic system might be a potential target for pharmacological treatment of anxiety (16). After the development and use of various CCK receptor agonists and antagonists, it is now generally believed that agonism or antagonism of CCKR-2 in the brain can produce anxiogenic or anxiolytic effects, respectively. This notion is supported by the following findings (1). Selective CCKR-2, but not -1, agonists produce anxiogenic effects (2, 17). Selective CCKR-2 agonists can provoke or facilitate panic-like anxiety, and this effect can be blocked by CCKR-2, but not -1, antagonists (3, 5, 18). Selective CCKR-2, but not -1, antagonists produce anxiolytic effects (19). In addition, the evidence that the polymorphisms in CCKR-2 are associated with panic disorder (18, 20) further suggests a primary role of the CCKR-2 in the pathogenesis of anxiety.
Despite these findings, however, conflicting results have been reported. For example, in a well designed experiment, neither CCK-8 nor -4 was found to have anxiogenic effects (21). Similar negative effects were reported in the use of CCKR-2 antagonists in animals (22). Indeed, a number of studies could not reproduce the expected results after treatment of subjects with CCKR-2 agonists or antagonists (23–28). The reasons that led to these controversial findings are not clear (29, 30). Accumulating evidence has indicated that the basal CCKergic tone in the brain, which is largely determined by receptor number and activity, may play a leading role in shaping these pharmacological effects. To establish the roles of elevated CCKergic tone in anxiogenesis, we used a genetic gain-of-function approach in this study and found that overexpression of CCKR-2 in neurons of the forebrain indeed leads to enhanced receptor-binding activity and robust anxiety-like behaviors (ALBs). In addition, these ALBs could be attenuated with diazepam.
Results
Generation of Inducible Forebrain-Specific CCKR-2 tg (IF-CCK-2) Transgenic (tg) Mice.
As described in Fig. 9, which is published as supporting information on the PNAS web site, we have successfully generated IF-CCKR-2 tg mice.
Histological Characterization.
As described in Supporting Text, which is published as supporting information on the PNAS web site, our results indicate that neither transgene overexpression nor random genomic incorporation events result in any neuronal developmental deficits or morphological changes in IF-CCKR-2 tg mice.
Molecular Characterization.
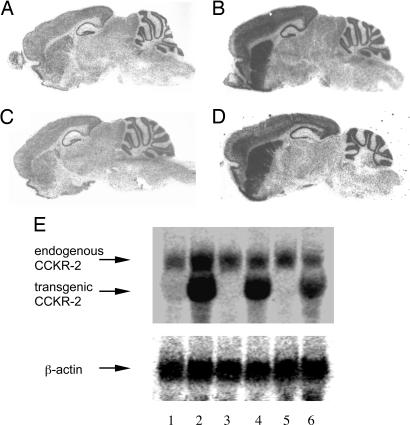
First, we examined whether the expression of the transgene is forebrain-specific and can be inhibited by doxycycline (doxy) treatment by using in situ hybridization. As shown in Fig. 1A, wild-type (wt) mice exhibit a moderate expression level of endogenous CCKR-2 mRNA, which distributes all over the brain with a stronger signal in the forebrain regions. As expected, a much higher expression level is observed in the forebrain regions of IF-CCKR-2 tg mice treated with vehicle (-vehicle mice) (Fig. 1B). After doxy treatment, CCKR-2 expression levels in IF-CCKR-2 tg mice return to the wt level (Fig. 1C). Furthermore, withdrawal of doxy restores transgene expression to the overexpression level (Fig. 1D). Second, we used Northern blot analyses to further detect expression level and inducible/reversible features. It should be noted that the mRNA size for endogenous CCKR-2 is 2.5 kb and for tg CCKR-2 is 1.4 kb. As shown in Fig. 1E, we observed one band in wt mice (lane 1) and two bands in IF-CCKR-2 tg mice (lanes 2 and 4; lane 4 represents another IF-CCKR-2 tg mouse strain not used in this study). Similar to results observed with in situ hybridization, after doxy treatment, transgene expression disappeared in both IF-CCKR-2 tg mouse strains (lanes 3 and 5, respectively). Withdrawal of doxy for 5 days could partially restore transgene expression (Fig. 1E, lane 6). Our further time-course experiment indicated that withdrawal of doxy treatment for 7 days could almost completely restore transgene expression (data not shown). Normalized with the internal control signal (detected by β-actin probe with the same membrane), the transgene expression level in IF-CCKR-2 tg mice used in this study is 5- to 6-fold higher than in wt mice. These data indicate that expression of the transgene is both forebrain-specific and inducible/reversible.
Fig. 1.
CCKR-2 expression in the brain. (A–D) In situ hybridization shows expression of total CCKR-2 in the brain. (A) wt mice-doxy. (B) IF-CCKR-2 tg (tg) mice-vehicle. (C) tg mice-doxy. (D) tg mice-doxy-withdrawal. (E) Northern blot detects both the endogenous and tg CCKR-2 expression. (Upper) Lane 1, wt-doxy; lane 2, tg-vehicle; lane 3, tg-doxy; lane 4, tg-vehicle (another tg line); lane 5, tg-doxy; lane 6, tg-doxy-withdrawal. (Lower) The same membrane was rehybridized with a β-actin cDNA probe.
Real-time RT-PCR analysis did not reveal any significant difference in CCK mRNA expression between wt and IF-CCKR-2 tg mice (data not shown).
Receptor-Binding Properties in IF-CCKR-2 tg Mice.
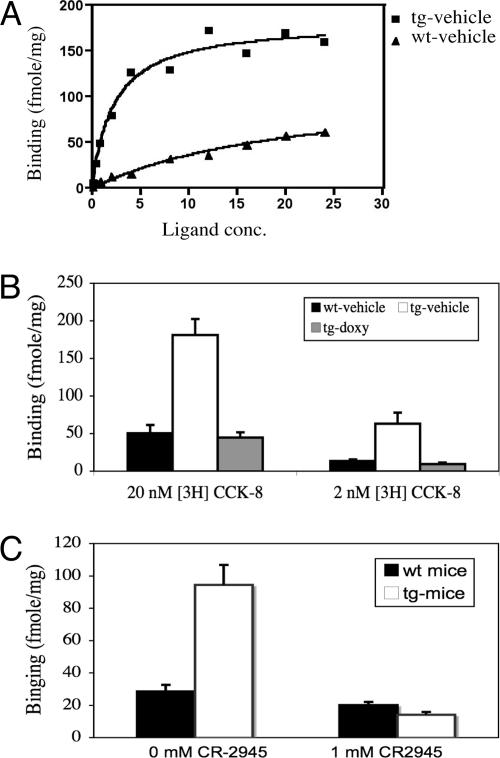
As shown in Fig. 2A, a typical saturated binding curve is observed in both IF-CCKR-2 tg and wt mice. As expected, Bmax in IF-CCKR-2 tg mice (182.70) is ≈7.5-fold higher than in wt mice (24.35). As shown in Fig. 2B, the ratio for binding capacity in wt mice over IF-CCKR-2 tg mice is 1:4.2 in the presence of 20 nM 3H-CCK-8 and 1:5 in the presence of 2 nM 3H-CCK-8. However, after doxy treatment, binding activity in IF-CCKR-2 tg mice returned to the wt level. As shown in Fig. 3C, the use of a CCKR-2 antagonist, CR-2945, is able to completely inhibit increased binding activity. These data indicate that: (i) the difference in receptor-binding capacity between wt and IF-CCKR-2 tg mice is ≈5-fold, which approximates the differences in mRNA expression levels; (ii) the overexpressed receptors are functional; and (iii) the increased CCKergic tone caused by transgene expression can be completely inhibited by either doxy treatment or CCKR-2 antagonism.
Fig. 2.
CCK receptor-binding properties in tg mice. (A) The saturated binding curve for wt mice-vehicle and tg mice-vehicle. (B) Binding capacity in the presence of a high (20 nM) and low (2 nM) dose of ligand and the effects of doxy treatment on binding activity. (C) Binding capacity in the presence of CR-2945 (1 μM). The data are expressed as mean ± SD.
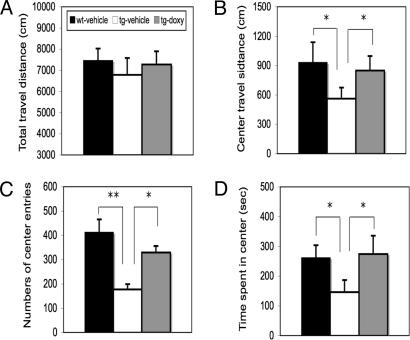
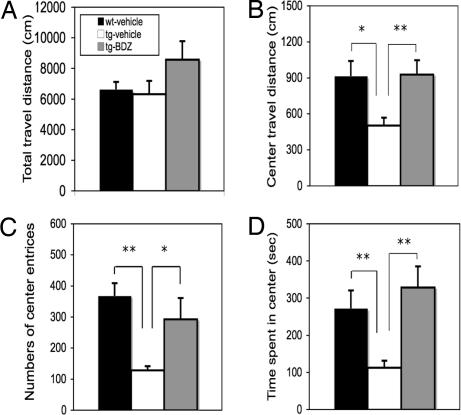
Fig. 3.
Effects of CCKR-2 transgene on ALBs in open-field test. (A) Total travel distance. (B) Travel distance in the center area. (C) Number of entries into the center area. (D) Time spent in the center area. ∗, P < 0.05; ∗∗, P < 0.01, Student's t test. Data are expressed as mean ± SE.
Behavioral Characterization of IF-CCKR-2 tg Mice.
Four tests were used, each with a separate set of animals. Because there were no significant differences in any measures between wt mice-vehicle and wt mice-doxy groups: we show only data from either group.
ALBs in the open-field test.
As shown in Fig. 3A, there is no significant difference in total distance traveled between any two groups, indicating that the mice in each group have no deficit in general motor activity. However, as shown in Fig. 3B, the travel distance in the center area in IF-CCKR-2 tg mice-vehicle (n = 9) is significantly lower than in wt mice-vehicle (n = 11; P < 0.05), and this difference disappears in IF-CCKR-2 tg mice treated with doxy (-doxy) (n = 13). Furthermore, IF-CCKR-2 tg mice-vehicle show significantly fewer entries into the center area (Fig. 3C, P < 0.01) and spend significantly less time in the center area (Fig. 3D, P < 0.05) compared with wt mice-vehicle or IF-CCKR-2 tg mice-doxy, respectively. These data strongly suggest that IF-CCKR-2 tg mice show a more anxious response in this test.
Behaviors in the forced-swimming test.
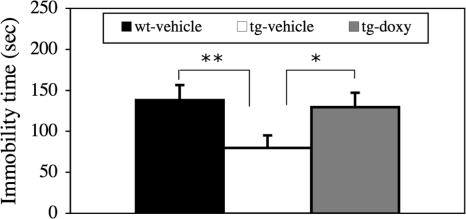
This test consisted of two stages: a pretest on the first day to produce a despairing status and a test on the second day to observe “giving-up” behaviors. There are no significant differences in swimming behaviors during the pretest stage between any two groups (data not shown). As shown in Fig. 4, however, IF-CCKR-2 tg mice-vehicle (n = 13) spent much less time in immobility (P < 0.01) compared with wt mice-vehicle (n = 12) during the test day. In addition, this phenotype is reversed in IF-CCKR-2 tg mice-doxy (n = 13). These results indicate that overexpression of the CCKR-2 leads to a stronger escaping behavior or less giving-up behavior in mice.
Fig. 4.
Effects of CCKR-2 transgene on ALBs in forced-swimming test. Significantly less immobility was observed in tg mice-vehicle, compared with that in either wt mice-vehicle or tg mice-doxy. ∗, P < 0.05; ∗∗, P < 0.01, Student's t test. Data are expressed as mean ± SE.
ALBs in the social interaction test.
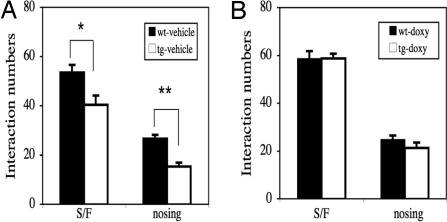
In this test, the amount of sniffing, following, nosing, grooming, and fighting between two naïve mice in a novel environment represents a robust index for social interaction. As shown in Fig. 5A, numbers of both sniffing/following and nosing are significantly lower in IF-CCKR-2 tg mice-vehicle (n = 11) when compared with wt mice-vehicle (n = 8) (P < 0.05 and P < 0.01, respectively), indicating that tg mice are significantly impaired in social interaction. However, in IF-CCKR-2 tg mice-doxy (n = 8), the deficit in either sniffing/following or nosing is reversed (Fig. 5B) when compared with wt mice-doxy (n = 10), indicating that the social deficits are associated with transgene expression. The numbers of grooming, biting, and fighting are very low in all tested mice, and there is no significant difference between any two groups (data not shown). These data indicate that overexpression of the CCKR-2 indeed leads to deficient social interactions, which are among the most problematic symptoms for anxiety patients.
Fig. 5.
Effects of CCKR-2 transgene on ALBs in social-interaction test. (A) Mice were treated with vehicle. (B) Mice were treated with doxy. ∗, P < 0.05; ∗∗, P < 0.01, Student's t test. Data were expressed as mean ± SE. S/F, sniff and following.
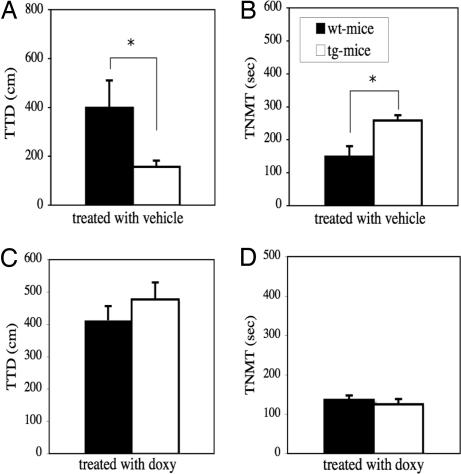
ALBs in the conditioned fear stress test.
As shown in Fig. 6, significantly less total distance traveled (Fig. 6A) and significantly higher total time for nonmovement (Fig. 6B) are observed in IF-CCKR-2 tg mice-vehicle (n = 9) compared with wt mice-vehicle (n = 8) (P < 0.05, respectively), indicating that tg mice show an enhanced fearful response to conditioned shock. Both decreased total traveled distance (Fig. 6C) and increased total nonmovement time (Fig. 6D) are reversed in IF-CCKR-2 tg mice-doxy (n = 10). There is no significant difference compared with wt mice-doxy (n = 13), further confirming that the enhanced fear responses are indeed due to transgene expression.
Fig. 6.
Effects of CCKR-2 transgene on ALBs in conditioned-fear stress test. (A) Total travel distance in mice treated with vehicle. (B) Total time of nonmovement in mice treated with vehicle. (C) Total travel distance in mice treated with doxy. (D) Total time of nonmovement in mice treated with doxy. ∗, P < 0.05, Student's t test. Data were expressed as mean ± SE. TTD, total travel distance; TNMT, total nonmovement time.
Effects of Diazepam (DZP) on ALBs in IF-CCKR-2 tg Mice.
Two tests were used in this experiment.
Effects of DZP on ALBs in the open-field test.
As shown in Fig. 7A, no significant difference in total distance traveled is found between wt mice-vehicle (n = 11) and any group of tg mice, although a slightly increased travel distance is observed in IF-CCKR-2 tg mice treated with DZP (-DZP) (n = 9). However, the distance traveled in the center area of IF-CCKR-2 tg mice-DZP was significantly higher than that of IF-CCKR-2 tg mice-vehicle (n = 10, Fig. 7B, P < 0.01). As shown in Fig. 7 C and D, DZP treatment significantly increases the decreased numbers of entries into and the decreased time spent in the center area, compared with IF-CCKR-2 tg mice-vehicle (P < 0.05 and P < 0.01, respectively). These data indicate that the ALBs in our tg mice are reversible with DZP treatment.
Fig. 7.
Effects of DZP on ALBs in open-field test. (A) Total travel distance. (B) Travel distance in the center area. (C) Number of entries into the center area. (D) Time spent in the center area. ∗, P < 0.05; ∗∗, P < 0.01, Student's t test. Data are expressed as mean ± SE.
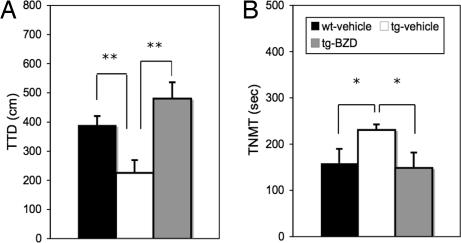
Effects of DZP on ALBs in the conditioned fear stress test.
As shown in Fig. 8A, a significant decrease in total distance traveled is observed in IF-CCKR-2 tg mice-vehicle (n = 11) compared with wt mice-vehicle (n = 14; P < 0.01). However, the total distance traveled in IF-CCKR-2 tg mice-DZP (n = 13) is significantly increased compared with IF-CCKR-2 tg mice-vehicle (P < 0.01). Similarly, the time for nonmovement in IF-CCKR-2 tg mice-DZP is significantly decreased compared with IF-CCKR-2 tg mice-vehicle (P < 0.01). These data further confirm the effects of DZP on CCKR-2 overexpression-triggered ALBs in the mouse.
Fig. 8.
Effects of DZP on ALBs in conditioned fear stress test. (A) Total travel distance. (B) Total time of nonmovement. ∗, P < 0.05, Student's t test. Data were expressed as mean ± SE. TTD, total travel distance; TNMT, total nonmovement time.
Discussion
In this study, we generated IF-CCKR-2 tg mice to determine the role of the CCKergic system in anxiogenesis. We found that the overexpression of the CCKR-2 in the forebrain significantly elevates the CCKergic tone with no observable effect on CCK expression, and that tg mice consistently show enhanced anxious behavioral responses in a battery of behavior tests. Furthermore, these ALBs are sensitive to DZP, a commonly used antianxiety drug in the clinic. Our results indicate that elevated CCKergic tone in the forebrain may constitute a novel molecular/neuronal mechanism for the involvement of the CCKergic system in the expression of anxiety. In addition, our study has validated a robust genetic model for anxiety in the mouse.
There are several unique features in our tg model. First, the transgene expression is limited only to neurons of the forebrain. The restriction of transgene expression to the forebrain and neurons avoids the possible involvement of other factors, such as glial cell function (31), or other brain regions (32) in the interpretation of the phenotypes. The best strategy would be to use a promoter-driving transgene expression in the whole limbic system. Unfortunately, this kind of promoter is currently unavailable. It should be noted, however, that the forebrain region in rodents encompasses most parts of the limbic system in human brains. Most importantly, although the thalamus is defined as a part of the limbic system in human brains, the expression of CCK mRNA was not found in the human thalamus/hypothalamus (33), despite the expression of CCKR-2 in these areas (as shown in Fig. 1A). Therefore, the forebrain-specific expression strategy is a reasonable choice. Second, the transgene expression is inducible/reversible, which not only allows one to exclude the possibility that the observed phenotypes may be due to a genomic locus effect by random incorporation but also provides a very powerful tool for the dynamic analyses of the role of CCKergic tone in anxiogenesis. For example, real-time coupling of elevated CCKergic tone with a particular stressor or anxiogenic process is a potentially powerful approach to address the interaction between the stressor and the CCKergic tone in anxiogenesis. Third, the genetic background in our tg mice is from B6/CBA hybrid mice, one of the best mouse strains for behavioral characterization (34). It should be mentioned that the DBA mouse strain is frequently used in anxiety modeling due to its high sensitivity to anxiogenic factors (35, 36). However, this mouse strain is not optimal for our purpose, because the observed effects of CCKergic tone on ALBs must be compounded by the DBA genetic background.
The most successful achievement in this study is to validate that overexpression of the CCKR-2 transgene significantly increases CCKergic tone, which was evidenced by a significantly increased CCKR-binding capacity in the brain of tg mice (Fig. 2A). Furthermore, either inhibition of the transgene expression by doxy or antagonism of the CCKR-2 with a CCKR-2 antagonist was able to completely block the increased CCKergic tone (Fig. 2 B and C). All these results indicate that our transgene is functional, which constitutes the most important component in determining the CCKergic tone in the brain.
Likewise, the most important finding in this study is the demonstration that IF-CCKR-2 tg mice consistently show robust ALBs in all tests used. It should be noted that, so far, we are still lacking a specific test that can directly measure “anxiety” in rodents. However, certain behavioral responses in some behavior tests represent behavioral analogues of human anxiety. An integrative analysis of behavioral responses in a multiple testing system may provide reliable indices to show ALBs in rodents. Therefore, the use of a battery of tests, rather than any single test, as well as a consistent finding in multiple tests, is most convincing in demonstrating ALBs in animals. In the present study, we used four different tests. The open-field test is based on rodents' natural tendency to stay near the perimeters of a novel environment (thigmotaxis), which may serve to protect the animals from an avian predator (37). Therefore, this test is a very essential measurement for ALBs in rodents. Forced swimming is a well used test to evaluate depressive behaviors, which express as more time in immobility during testing (38). However, in our study, IF-CCKR-2 tg mice spent much less time in immobility than did wt mice (Fig. 4). These results were similar to those reported by other groups, in which they also found that mice with anxiety had less immobility (39). Together with these results, our results indicate it might be possible that the behavioral response between anxiety and depression in this test is different. Unlike depressive animals, which show more giving-up behaviors in a conflicting environment, more anxious mice may spend more attention or efforts on escaping from the conflicting environment and thus show less immobility. Social interaction, either in human or animal models, is another well established behavioral paradigm to evaluate behavioral traits associated with many abnormal mental statuses, including anxiety. Our results, as shown in Fig. 5, indicate that IF-CCKR-2 tg mice show behavior analogous to social withdrawal. Fearful behaviors are significantly enhanced in response to stressors in patients with anxiety. In rodents, the conditioned-fear stress test is a robust paradigm to evaluate fearful behaviors. As shown in Fig. 6, we found that IF-CCKR-2 tg mice show an enhanced fear response, consistent with many other reports in anxiety modeling (40). Of importance also is that behavioral responses in our tg mice in the elevated-plus maze are different from wt mice, but it is difficult to use the current index to describe the difference (data not shown). Obviously, both the molecular and behavioral characterizations have validated our tg mice as a robust genetic anxiety model in terms of both constructive (CCKergic mechanisms) and face validity (behavioral homologous). Another important requirement to validate the anxiety model is termed predictive validity, which indicates that ALBs in animal models should be sensitive to clinical antianxiety treatment. As shown in Figs. 7 and 8, DZP, one of the most commonly used drugs to treat human anxiety, is most effective in reversing ALBs associated with elevated CCKergic tone, indicating that our animal model also has predictive validity.
Taken together, these results directly demonstrate that elevated CCKergic tone constitutes an important molecular/neuronal mechanism underlying the expression of ALBs. Several lines of evidence have indirectly indicated that a basal CCKergic tone in the brain may play a determinative role in shaping effects associated with anxiogenic factors. For example, many studies have found that CCKR-2 agonists can produce more pronounced anxiogenic effects only in stressed but not unstressed animals (41, 42), and that patients with generalized anxiety or panic disorder are more sensitive to CCKR-2 agonists (43–45). In addition, after stressors, both CCK-like immunoreactivity and CCK receptor density in the brain are increased, indicating that elevated CCKergic tone is associated with these stressors (46, 47). Moreover, expression of anxiety is correlated with increased CCKergic tone, as indicated by a higher CCKR-binding activity in the brain in anxious than in nonanxious animals (48, 49). These findings indicate that a CCKergic tone may dynamically associate with stressors to cause anxiety, and that the expression of anxiety may be dynamically regulated by CCKergic tone. Other evidence suggesting a role of CCKergic tone in the pathogenesis of anxiety includes: (i) knockout of the CCKR-2 or the use of the CCKR-2 antisense oligo in animals produces anxiolytic effects (50, 51), and (ii) varying fear responses among different animal strains can be attributed to different expression levels of CCKR-2 (52, 53). Our results further support these findings to indicate an important role of the CCKergic tone in anxiogenesis. However, the neuronal pathways involved in this CCKergic tone-triggered anxiogenesis need further study.
In conclusion, the demonstration of the role of CCKergic tone in anxiogenesis in this study not only validates a robust genetic anxiety model in the mouse but also reveals a molecular/neuronal mechanism for the expression of anxiety. This robust genetic model may provide a powerful tool for the pharmacological screening of antianxiety compounds. Another important implication from this study is that it might be worthwhile to establish a diagnostic device such as isotope-labeled ligand-receptor imaging (54, 55), which can be used to monitor the CCKergic tone in the brain of anxiety patients resistant to currently available therapy or in patients who have developed a dependency on drugs such as DZP.
Materials and Methods
Generation of IF-CCKR-2 tg Mice.
As described in Supporting Text, two expression vectors were constructed. One is for the tTA transgene, which is downstream of a α-Ca2+ calmodulin kinase II promoter, and the other one is for the CCKR-2 transgene, which is downstream of the tetO promoter. After being linearized with suitable enzymes, the constructs were respectively injected into the pronucleoli of B6/CBA F1 zygotes, as described (56). Southern blot was used to screen tg founder mice. Genotypes were determined with PCR analyses of tail DNA.
Characterization of IF-CCKR-2 tg Mice at the Histological Level.
As described in Supporting Text, we examined the possible morphological changes at both the gross and microscopic levels.
Characterization of IF-CCKR-2 tg Mice at the Molecular Level.
Treatment with or withdrawal of doxy from IF-CCKR-2 tg mice is able to turn off or restore transgene expression (“on”), respectively. In this study, doxy treatment indicates that mice were given doxy for at least 5 days up to the completion of the experiment, and doxy withdrawal indicates that the withdrawal of doxy lasted for at least 7 days, up to the completion of the experiment, unless stated otherwise. Doxy was administered in drinking water at a concentration of 2 mg per 100 ml. We first performed in situ hybridization to confirm the expression pattern of the CCKR-2 transgene and to test the effects of doxy on transgene expression. A cRNA probe, which could recognize both the endogenous and tg CCKR-2 genes, was labeled with 35S UTP [>1,000 Ci/mmol (1 Ci = 37 GBq); Amersham Pharmacia]. A commercial in vitro transcription kit (Invitrogen) was used for cRNA synthesis. Hybridization was performed overnight at 55°C. After washing, the slides were exposed to Kodak BioMax film for 3 days. To quantitatively analyze transgene expression, Northern blot was used, as described (57). A cDNA probe recognizing both the endogenous and tg CCKR-2 genes was labeled by a 32P dATP random labeling kit (New England Biolabs). To normalize total RNA quantity, we used the same membrane to rehybridize with a β-actin probe. The density of each band was analyzed with a computer-assisted imaging system (NorthenMax; Ambion, Austin, TX). In addition, we used a real-time RT-PCR (Applied Biosystems 7900THFast Real-Time PCR System) to detect possible changes in CCK expression in the forebrain between wt mice-vehicle and IF-CCKR-2 tg mice-vehicle (each contained three mice).
Characterization of IF-CCKR-2 tg Mice at the Receptor Functional Level.
To determine whether overexpressed receptors are functional, we performed three experiments. The first one was to determine the binding saturation curve, and the second one was to determine the effects of doxy on receptor-binding activity. The procedures for the binding assay were as described (58). The binding assay was carried out in the presence of 10 totally different 3H-CCK-8 (propiony-3H-sulfated and propionylated CCK-8, 93.0 Ci/mmol; Amersham Pharmacia Radiochemicals) concentrations ranging from 0.08 to 24 nM for each 0.28 mg of membrane protein at room temperature for 2 h. The same assay was then carried out in the presence of 2 and 20 nM 3H-CCK-8. Nonspecific binding was determined by using 1 μM cold CCK-8 with the same incubation conditions. Lastly, the assay was carried out in the presence of CR-2945 (Sigma), a CCKR-2 antagonist, at 1 μM with 2 nM 3H-CCK-8 with the same conditions. Triplicate measurements were performed in each group. The specific binding was calculated as total minus nonspecific binding cpm. The saturation curve was analyzed by using GraphPad prism (Ver. 4.03).
Characterization of IF-CCKR-2 tg Mice at Behavioral Levels.
We used a battery of behavioral tests to evaluate ALBs. Because there was no significant difference in behavior between wt mice and any single (either α-Ca2+ calmodulin kinase II or tetO-CCKR-2) tg mice, these mice were pooled together as the controls and are simply referred to wt mice. In all behavioral tests, experimenters were blind to genotypes of each individual mouse. The statistical significance between each two groups was determined by Student's t test.
Open-field behaviors.
An automatic-recording open-field working station (MED Associates, Georgia, VT) was used. The field of the box (40 × 40 × 30 cm high) was divided into 16 identical squares by computer-defined grids and illuminated with a 120-W bulb. The area between 4.5 and 11.5 squares on each side was defined as the “center area.” During the test, mice were individually released into the center of the box and allowed to explore the field for 60 min. Exploratory behaviors were recorded by a photobeam-scanning system and a video camera simultaneously. Data were analyzed automatically by the computer-sampling system. Less travel distance in the center area, fewer numbers of center area entries, or/and less time spent in the center area indicated a more anxious status in the mice.
Forced-swimming test.
The procedures were similar to those described (59). The instrument was a 4-liter Pyrexiglas beaker (19 cm diameter) (Corning), containing 25°C water and 20 cm deep, so the mouse could not support itself by touching the bottom with its feet. During the pretest, each mouse was allowed to swim in the beaker for 15 min. The mice were individually tested in the same beaker again for 6 min 24 h after the pretest. All procedures were tape-recorded. An examiner unaware of mouse genotypes collected behavioral indices, including swimming and immobility from the videotape. Immobility was defined as floating in the water without struggling, but movement necessary only to keep the head above the water may be allowed. Swimming was defined as making active swimming motions, more than necessary to merely keep the head above water. Climbing movements with forepaws in and out of the water were also recorded as swimming. More immobility is related to giving-up behaviors.
Social interaction test.
All subjects, including testing and intruder mice, were male. Intruder mice were matched with testing mice in both body weight and age but had never interacted before the test. The open field was illuminated with a 60-W bulb. The experimental procedures were as described (60). Briefly, during the test, one testing and one intruder mouse were introduced into the open field simultaneously and observed for 10 min for their interaction. All procedures were recorded by a video camera, and the indices for social interaction, including sniffing and following, nosing, grooming, biting, and fighting, were collected by persons blind to the genotypes of each mouse. Fewer numbers of social interactions, including sniffing, following, and nosing, indicated a more anxious status in the mice.
Conditioned-fear stress test.
The instrument used was a fear-conditioning system (Coulbourn Instruments, Lehigh Valley, PA). In this test, mice first individually received inescapable scrambled shock (0.4 mA) in the chamber 36 times in a period of 6 min. Each shock lasted for 1 sec, and the interval between two shocks was 10 sec. The mice were then put individually into the same chamber with no shock, and both total distance traveled and total nonmovement time were automatically recorded for 6 min by the photobeam-scanning system 24 h after the shock. Less travel distance and/or more time for nonmovement indicated a more anxious status in the mice.
tg mice were double-hemizygous tg, and all were ≈2–4 months old. Except in social interaction, male and female mice were mixed. Four groups of mice, wt mice-vehicle, wt mice-doxy, IF-CCKR-2 tg mice-vehicle, and IF-CCKR-2 tg mice-doxy, were used.
Effects of DZP on ALBs in IF-CCKR-2 tg Mice.
We examined the effects of DZP on ALBs in two tests, open-field and conditioned-fear stress tests. The procedures were the same as described above. DZP (0.5 mg/kg) was administered i.p. 30 min before the test.
Supplementary Material
Acknowledgments
We thank Drs. Elliot S. Gershon, Sangram S. Sisodia, and Xioaxi Zhuang (all at the University of Chicago) for helpful discussions. We thank Ms. Linda Degenstein at the tg core facility for the pronucleus injection and Ms. Hong Kang for her excellent skills in keeping the mouse colonies. This study was partially supported by grants from Brain Research Foundation (Chicago), the Louis Block Foundation, and the National Institute of Mental Health/National Institutes of Health (MH066243) (all to Y.-P.T.).
Abbreviations
- ALB
anxiety-like behavior
- CCK
cholecystokinin
- CCKR-2
CCK receptor-2
- tg
transgenic
- IF-CCKR-2 tg
inducible forebrain-specific CCKR-2 tg
- doxy
doxycycline
- DZP
diazepam
- wt
wild type.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Gorman J. M. J. Clin. Psychiatry. 2003;64(Suppl. 3):28–35. [PubMed] [Google Scholar]
- 2.Shelton R. C., Brown L. L. J. Clin. Psychiatry. 2001;62(Suppl. 12):10–15. [PubMed] [Google Scholar]
- 3.Michelini S., Cassano G. B., Frare F., Perugi G. Pharmacopsychiatry. 1996;29:127–134. doi: 10.1055/s-2007-979558. [DOI] [PubMed] [Google Scholar]
- 4.Asnis G. M., Kohn S. R., Henderson M., Brown N. L. Drugs. 2004;64:383–404. doi: 10.2165/00003495-200464040-00004. [DOI] [PubMed] [Google Scholar]
- 5.Bradwejn J., Koszycki D. Ann. NY Acad. Sci. 1994;713:273–282. doi: 10.1111/j.1749-6632.1994.tb44075.x. [DOI] [PubMed] [Google Scholar]
- 6.Rodgers R. J., Johnson N. J. Crit. Rev. Neurobiol. 1995;9:345–369. [PubMed] [Google Scholar]
- 7.Harper E. A., Raoer H. S. J. Physiol. 1943;102:11–125. [Google Scholar]
- 8.Dockray G. J. Nature. 1976;264:568–570. doi: 10.1038/264568a0. [DOI] [PubMed] [Google Scholar]
- 9.Crawley J. N. Ann. NY Acad. Sci. 1985;448:1–8. doi: 10.1111/j.1749-6632.1985.tb29900.x. [DOI] [PubMed] [Google Scholar]
- 10.Moran T. H., Robinson P. H., Goldrich M. S., McHugh P. R. Brain Res. 1986;362:175–179. doi: 10.1016/0006-8993(86)91413-7. [DOI] [PubMed] [Google Scholar]
- 11.Wank S. A. Am. J. Physiol. 1998;274:G607–G613. doi: 10.1152/ajpgi.1998.274.4.g607. [DOI] [PubMed] [Google Scholar]
- 12.Hill D. R., Campbell N. J., Shaw T. M., Woodruff G. N. J. Neurosci. 1987;7:2967–2976. doi: 10.1523/JNEUROSCI.07-09-02967.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crawley J. N., Corwin R. L. Peptides. 1994;15:731–755. doi: 10.1016/0196-9781(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 14.Della-Fera M. A., Baile C. A. Science. 1979;206:471–473. doi: 10.1126/science.504989. [DOI] [PubMed] [Google Scholar]
- 15.Belcheva I., Belcheva S., Petkov V. V., Petkov V. D. Neuropharmacology. 1994;33:995–1002. doi: 10.1016/0028-3908(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 16.Bradwejn J., de Montigny C. Nature. 1984;312:363–364. doi: 10.1038/312363a0. [DOI] [PubMed] [Google Scholar]
- 17.van Megen H. J., Westenberg H. G., den Boer J. A., Kahn R. S. Eur. Neuropsychopharmacol. 1996;6:263–280. doi: 10.1016/s0924-977x(96)00038-7. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy J. L., Bradwejn J., Koszycki D., King N., Crowe R., Vincent J., Fourie O. Mol. Psychiatry. 1999;4:284–285. doi: 10.1038/sj.mp.4000507. [DOI] [PubMed] [Google Scholar]
- 19.Hughes J., Boden P., Costall B., Domeney A., Kelly E., Horwell D. C., Hunter J. C., Pinnock R. D., Woodruff G. N. Proc. Natl. Acad. Sci. USA. 1990;87:6728–6732. doi: 10.1073/pnas.87.17.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kopin A. S., McBride E. W., Schaffer K., Beinborn M. Trends Pharmacol. Sci. 2000;21:346–353. doi: 10.1016/s0165-6147(00)01526-1. [DOI] [PubMed] [Google Scholar]
- 21.Johnson N. J., Rodgers R. J. Psychopharmacology (Berlin) 1996;124:355–364. doi: 10.1007/BF02247441. [DOI] [PubMed] [Google Scholar]
- 22.Wilson J., Watson W. P., Little H. J. Psychopharmacology. 1998;137:120–131. doi: 10.1007/s002130050601. [DOI] [PubMed] [Google Scholar]
- 23.Charrier D., Dangoumau L., Puech A. J., Hamon M., Thiebot M. H. Psychopharmacology. 1995;121:127–134. doi: 10.1007/BF02245599. [DOI] [PubMed] [Google Scholar]
- 24.Katzman M. A., Koszycki D., Bradwejn J. Depress. Anxiety. 2004;20:51–58. doi: 10.1002/da.20012. [DOI] [PubMed] [Google Scholar]
- 25.Griebel G., Perrault G., Sanger D. J. Behav. Pharmacol. 1997;8:549–560. doi: 10.1097/00008877-199711000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Kramer M. S., Cutler N. R., Ballenger J. C., Patterson W. M., Mendels J., Chenault A., Shrivastava R., Matzura-Wolfe D., Lines C., Reines S. Biol. Psychiatry. 1995;37:462–466. doi: 10.1016/0006-3223(94)00190-E. [DOI] [PubMed] [Google Scholar]
- 27.Adams J. B., Pyke R. E., Costa J., Cutler N. R., Schweizer E., Wilcox C. S., Wisselink P. G., Greiner M., Pierce M. W., Pande A. C. J. Clin. Psychopharmacol. 1995;15:428–434. doi: 10.1097/00004714-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Cowley D. S., Adams J. B., Pyke R. E., Cook J., Zaccharias P., Wingerson D., Roy-Byrne P. P. Biol. Psychiatry. 1996;40:550–552. doi: 10.1016/0006-3223(96)00163-1. [DOI] [PubMed] [Google Scholar]
- 29.Lydiard R. B. Clin. Chem. 1994;40:315–318. [PubMed] [Google Scholar]
- 30.Fink H., Rex A., Voits M., Voigt J. P. Exp. Brain Res. 1998;123:77–83. doi: 10.1007/s002210050546. [DOI] [PubMed] [Google Scholar]
- 31.Newman E. A. Trends Neurosci. 2003;26:536–542. doi: 10.1016/S0166-2236(03)00237-6. [DOI] [PubMed] [Google Scholar]
- 32.Beinfeld M. C., Palkovits M. Brain Res. 1982;238:260–265. doi: 10.1016/0006-8993(82)90794-6. [DOI] [PubMed] [Google Scholar]
- 33.Lindefors N., Linden A., Brene S., Sedvall G., Persson H. Prog. Neurobiol. 1993;40:671–690. doi: 10.1016/0301-0082(93)90010-p. [DOI] [PubMed] [Google Scholar]
- 34.Gerlai R. Trends Neurosci. 1996;19:177–181. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- 35.Falls W. A., Carlson S., Turner J. G., Willott J. F. Behav. Neurosci. 1997;111:855–861. [PubMed] [Google Scholar]
- 36.McCaughran J. A., Jr., Bell J., III, Hitzemann R. J. Pharmacol. Biochem. Behav. 2000;65:301–312. doi: 10.1016/s0091-3057(99)00216-6. [DOI] [PubMed] [Google Scholar]
- 37.Henderson N. D. Anim. Behav. 1967;15:364–376. doi: 10.1016/0003-3472(67)90023-1. [DOI] [PubMed] [Google Scholar]
- 38.Lucki I. Behav. Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Ramboz S., Oosting R., Amara D. A., Kung H. F., Blier P., Mendelsohn M., Mann J. J., Brunner D., Hen R. Proc. Natl. Acad. Sci. USA. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Battaglia M., Ogliari A. Neurosci. Biobehav. Rev. 2005;29:169–179. doi: 10.1016/j.neubiorev.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Cohen H., Kaplan Z., Kotler M. Depress. Anxiety. 1999;10:8–17. [PubMed] [Google Scholar]
- 42.Koks S., Mannisto P. T., Bourin M., Shlik J., Vasar V., Vasar E. J. Psychiatry Neurosci. 2000;25:33–42. [PMC free article] [PubMed] [Google Scholar]
- 43.Bradwejn J., Koszycki D., Shriqui C. Arch. Gen. Psychiatry. 1991;48:603–610. doi: 10.1001/archpsyc.1991.01810310021005. [DOI] [PubMed] [Google Scholar]
- 44.McCann U. D., Slate S. O., Geraci M., Roscow-Terrill D., Uhde T. W. Neuropsychopharmacology. 1997;16:229–237. doi: 10.1016/S0893-133X(96)00197-2. [DOI] [PubMed] [Google Scholar]
- 45.van Vliet I. M., Westenberg H. G., Slaap B. R., den Boer J. A., Ho Pian K. L. Biol. Psychiatry. 1997;42:76–78. doi: 10.1016/S0006-3223(97)00185-6. [DOI] [PubMed] [Google Scholar]
- 46.Harro J., Lofberg C., Rehfeld J. F., Oreland L. Naunyn. Schmiedebergs Arch. Pharmacol. 1996;354:59–66. doi: 10.1007/BF00168707. [DOI] [PubMed] [Google Scholar]
- 47.Siegel R. A., Duker E. M., Pahnke U., Wuttke W. Neuroendocrinology. 1987;46:75–81. doi: 10.1159/000124799. [DOI] [PubMed] [Google Scholar]
- 48.Harro J., Marcusson J., Oreland L. Eur. Neuropsychopharmacol. 1992;2:57–63. doi: 10.1016/0924-977x(92)90037-9. [DOI] [PubMed] [Google Scholar]
- 49.MacNeil G., Sela Y., McIntosh J., Zacharko R. M. Pharmacol. Biochem. Behav. 1997;58:737–746. doi: 10.1016/s0091-3057(97)00037-3. [DOI] [PubMed] [Google Scholar]
- 50.Miyasaka K., Kobayashi S., Ohta M., Kanai S., Yoshida Y., Nagata A., Matsui T., Noda T., Takiguchi S., Takata Y., et al. Neurosci. Lett. 2002;335:115–118. doi: 10.1016/s0304-3940(02)01176-x. [DOI] [PubMed] [Google Scholar]
- 51.Tsutsumi T., Akiyoshi J., Hikichi T., Kiyota A., Kohno Y., Katsuragi S., Yamamoto Y., Isogawa K., Nagayama H. Pharmacopsychiatry. 2001;34:232–237. doi: 10.1055/s-2001-18030. [DOI] [PubMed] [Google Scholar]
- 52.Farook J. M., McLachlan C. S., Zhu Y. Z., Lee L., Moochhala S. M., Wong P. T. Neurosci. Lett. 2004;355:205–208. doi: 10.1016/j.neulet.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 53.Wang H., Zhu Y. Z., Farook J. M., Moochhala S., Teo A. L., Lee L. K., Wong P. T. Behav. Neurosci. 2003;117:385–390. doi: 10.1037/0735-7044.117.2.385. [DOI] [PubMed] [Google Scholar]
- 54.Reubi J. C., Macke H. R., Krenning E. P. J. Nucl. Med. 2005;46(Suppl. 1):67S–75S. [PubMed] [Google Scholar]
- 55.Smith G. S., Koppel J., Goldberg S. Psychopharmacol. Bull. 2003;37:26–65. [PubMed] [Google Scholar]
- 56.Tang Y. P., Shimizu E., Dube G. R., Rampon C., Kerchner G. A., Zhuo M., Liu G., Tsien J. Z. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 57.Tang Y. P., Murata Y., Nagaya T., Noda Y., Seo H., Nabeshima T. J. Cereb. Blood Flow. Metab. 1997;17:771–780. doi: 10.1097/00004647-199707000-00007. [DOI] [PubMed] [Google Scholar]
- 58.Koks S., Vasar E., Soosaar A., Lang A., Volke V., Voikar V., Bourin M., Mannisto P. T. Eur. Neuropsychopharmacol. 1997;7:289–294. doi: 10.1016/s0924-977x(97)00034-5. [DOI] [PubMed] [Google Scholar]
- 59.Detke M. J., Lucki I. Behav. Brain Res. 1996;73:43–46. doi: 10.1016/0166-4328(96)00067-8. [DOI] [PubMed] [Google Scholar]
- 60.Yamada K., Wada E., Wada K. Brain Res. 2000;870:20–26. doi: 10.1016/s0006-8993(00)02395-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.