The development of new reactions employing imidozirconium complexes or their derivatives as catalysts is an elusive goal in organometallic chemistry. To date, the imidozirconocene-catalyzed hydroamination of alkynes and allenes to yield new enamines (and, on tautomerization, imines) is the lone example of such a process.[1,2] We recently reported that aldehydes and electron-deficient imines insert into the carbon–zirconium bond of azazirconacyclobutene 1 to afford new six-membered ring metallacycles 2 and 3 (see Scheme 1). On heating, these expanded zirconacycles undergo a retro-[4+2] cycloaddition to afford α,β-unsaturated imine 4 and oxozirconium complex 5 or electron-poor imidozirconocene dimer 6, both of which are unreactive (Scheme 1; EWG =electron-withdrawing group, Cp = C5H5).[3,4] We reasoned that insertion of an aldimine 7 with N-substitution identical to that of the nitrogen group in the metallacyclobutene, followed by subsequent retro-cycloaddition, would not only afford α,β-unsaturated imine 4 but would also generate imidozirconocene complex 8 previously used to prepare the starting azazirconacyclobutene (see Scheme 2).[5] Carrying out the reaction in the presence of the necessary alkyne would regenerate the starting metallacycle and close the catalytic cycle (Scheme 2).[6] This reaction is deemed a carboamination, because it results in the overall cleavage of an imine C=N bond and addition of the resulting C and N fragments across an alkyne, forming a new carbon–carbon double bond and a new ketimine carbon–nitrogen double bond.[7–9] Herein, we present the development of a novel, high-yielding imidozirconocene-catalyzed carboamination reaction that also represents the best method for preparing the highly arylated α,β-unsaturated imine co-products.
Scheme 1.
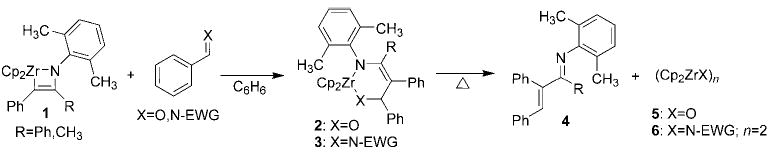
Scheme 2.
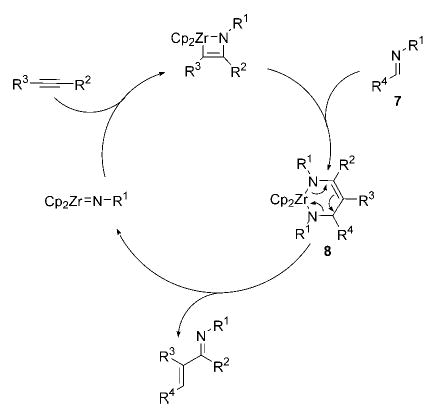
The most frequently studied imidozirconium complexes bear sterically bulky groups on the nitrogen atom to prevent competitive dimerization of the imido compound.[5] As such, N-2,6-dimethylphenyl- and N-tert-butyl-substituted azazirconacyclobutenes 1 and 9 (see Scheme 3) were explored in the desired insertion chemistry. Unfortunately, even at high concentrations of substrate and temperatures up to 165°C, neither of these zirconium compounds was an effective catalyst as no imine insertion products were observed. In the case of metallacycle 1 and imine 10, it appears that the combined steric bulk of the zirconacycle and imine N-substituents prohibits insertion; with metallacycle 9 and N-tert-butyl imine 11, we began to observe C–D bond activation of the [D6]benzene solvent. Presumably, this pathway arises from [2+2]-cycloreversion to afford the free imidozirconocene complex 12, which had been shown to activate benzene C–H bonds at a minimum temperature of 75°C (Scheme 3).[10]
Scheme 3.
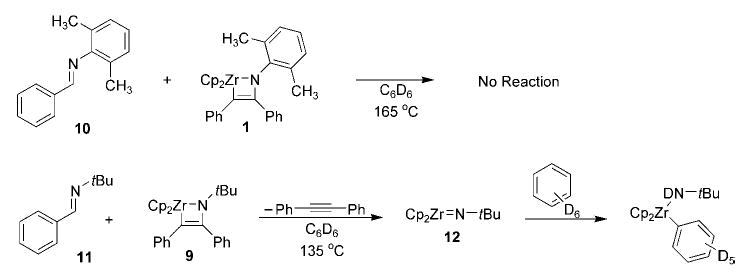
While few obvious modifications can be made to the N-tert-butyl framework, we reasoned that a smaller N-aryl substituent would decrease the steric bulk of both the azazirconacyclobutene and the reacting imine to better facilitate insertion into the metallacycle Zr–C bond. [Cp2Zr=N(p-C6H4CH3)] (13a) undergoes irreversible dimerization in the absence of a trapping agent; however, imido complex 13a will react preferentially with an alkyne to generate the N-tolyl metallacyclobutene 14a (see Table 1).[5] This observation is particularly relevant to a potential catalytic system, since alkyne will be in substantial excess relative to 13a for the majority of the reaction, further decreasing the likelihood of competitive dimerization. We were pleased to find that one equivalent of azazirconacyclobutene 14a reacted with four equivalents of both N-p-tolyl p-tolualdimine (15a) and diphenylacetylene (16a) at 135°C (reactions carried out in sealed tubes, for full experimental details see the Supporting Information) to effect complete consumption of 15a and generation of new α,β-unsaturated imine 17a. No intermediate six-membered-ring metallacycle was observed during the course of the reaction, consistent with rate-determining imine insertion into the azametallacyclobutene Zr–C bond. Optimization of temperature, catalyst concentration, and concentration of reagents led to the development of a system that employs 10 mol% of 14a and one equivalent each of imine and alkyne in benzene at 145°C to afford a 71% yield of α,β-unsaturated imine 17a. This catalytic carboamination reaction generates the (E)-imine and (E)-olefin stereoisomers exclusively and provides the only known synthesis of this product.
Table 1.
Carboamination reactions catalyzed by imidozirconocenes 14. [a]
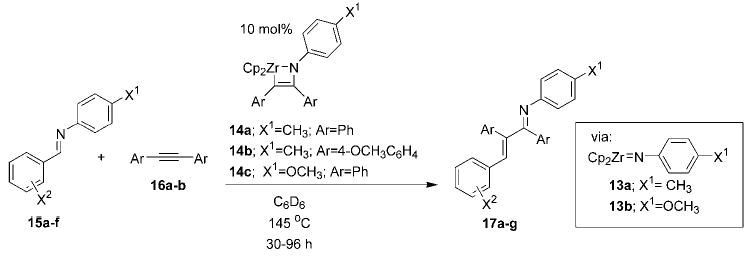
| Entry | Starting Imine | Alkyne | Catalyst | Product | Yield [%][b] |
|---|---|---|---|---|---|
| 1 | 15 a; X1 =CH3; X2 =4-CH3 | 16 a; Ar =Ph | 14 a | 17 a | 71 |
| 2 | 15 b; X1 =CH3; X2 =4-OCH3 | 16 a; Ar =Ph | 14 a | 17 b | 71 |
| 3 | 15 c; X1 =CH3; X2 =4-N(CH3)2 | 16 a; Ar =Ph | 14 a | 17 c | 82 |
| 4 | 15 d; X1 =OCH3; X2 =H | 16 a; Ar =Ph | 14 c | 17 d | 80 |
| 5 | 15 e; X1 =OCH3; X2 =4-CH3 | 16 a; Ar =Ph | 14 c | 17 e | 80 |
| 6 | 15 f; X1 =OCH3; X2 =3-CH3 | 16 a; Ar =Ph | 14 c | 17 f | 85 |
| 7[c] | 15 a; X1 =CH3; X2 =4-CH3 | 16 b; Ar =4-OCH3C6H4 | 14 b | 17 g | 58 |
Reaction is conducted with 10 mol% azazirconacyclobutene 14 (with appropriate X1 and Ar substitution) as catalyst and imine 15 and alkyne 16 at 0.5m in C6D6.
Yield of isolated product after chromatography.
Reaction was conducted at 160°C using a 3:1 mixture of 14 c:16 b.
With optimized conditions in hand, we next sought to expand the scope of all components of the reaction. The more electron-rich substrates, N-p-tolyl p-anisaldimine (15b) and N-p-tolyl p-N,N-dimethylaminobenzaldimine (15c) were also competent in this chemistry (Table 1), whereas electron-poor N-p-tolyl p-trifluoromethylbenzaldimine failed to insert. These observations were somewhat surprising given the ease with which electron-deficient imines were shown to insert previously.[11] We have also been able to employ bis(p-methoxyphenyl)acetylene (16b) by preparing the requisite azazirconacyclobutene 14b. This metallacycle catalyzed the carboamination of alkyne 16b with imine 15a in good yield at 160°C.[12,13] The azazirconacyclobutene formed by reaction of imidozirconocene complex 13a with 1-phenyl-1-propyne was unstable at the high temperatures required for insertion.[14] Finally, N-p-methoxyphenyl (PMP) azametallacyclobutene 14c catalyzed the carboamination reaction between N-p-methoxyphenyl p-benzaldimine (15d) or N-p-methoxyphenyl p-tolualdimine (15e) and diphenylacetylene (16a).[15] N-p-Methoxy-phenyl m-tolualdimine (15 f) was also a competent substrate in this chemistry, while only approximately 30% of N-p-methoxyphenyl o-tolualdimine was converted into product. Use of the PMP group as a substituent on the nitrogen atom is particularly attractive, since nucleophilic addition to or reduction of the product PMP-imine provides a p-methoxyphenyl-protected primary amine, which may be liberated under mild oxidative conditions.[16]
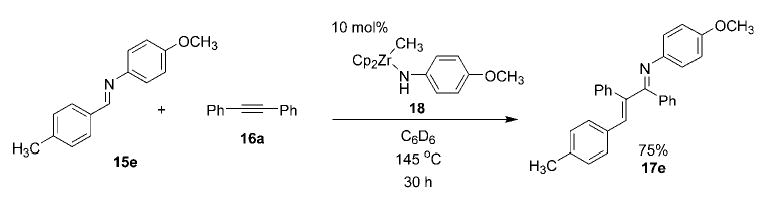
We have employed methylzirconocene (p-methoxyphenyl)amide (18) as the catalyst in the carboamination reaction between imine 15e and alkyne 16a to generate α,β-unsaturated imine product 17e in 75% yield (Scheme 4).[17] This yield is comparable to that obtained with azazirconacyclobutene 14c as catalyst (Table 1, entry 5). From a practical standpoint, this development allows the chemist to by-pass the additional step of azazirconacyclobutene formation, while also eliminating the necessity of preparing a different azazirconacyclobutene catalyst for each new alkyne considered. From a mechanistic standpoint, this result supports the intermediacy of imidozirconium compound 13b (generated in situ on elimination of methane from 18) along the proposed catalytic cycle.
Scheme 4.
In summary, we have succeeded in developing a novel imidozirconocene-catalyzed carboamination reaction that adds an imine C=N bond across an alkyne to generate synthetically interesting α,β-unsaturated imine products. While the substrate scope of the transformation is, at present, limited to all-aryl substitution, the products generated have not been accessed by alternative means. We will continue to develop this chemistry with the goal of expanding the substrate scope to include new imines and alkynes. Full experimental details can be found in the Supporting Information. A related paper that reports the insertion of N-acylamines into azazirconacyclobutenes also appears in this issue.[18]
Supplementary Material
Footnotes
This work was supported by the National Institutes of Health (GM-25459) and by an NIH post-doctoral fellowship to R.T.R.
Supporting information for this article (full experimental details) is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.a Walsh PJ, Bergman RG. J Am Chem Soc. 1992;114:1708. [Google Scholar]; b Baranger AM, Walsh PJ, Bergman RG. J Am Chem Soc. 1993;115:2753. [Google Scholar]
- 2.Imidotitanium-catalyzed hydroamination reactions are also known: a) Ackermann L, Loy RN, Bergman RG.J Am Chem Soc 200312511956. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Johnson JS, Bergman RG. J Am Chem Soc. 2001;123:2923. doi: 10.1021/ja005685h. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Shi Y, Ciszewski JT, Odom AL. Organometallics. 2001;20:3967. [Google Scholar]; d Haak E, Bytschkov I, Doye S. Angew Chem. 1999;111:3584. Angew. Chem. Int. Ed. 1999, 38, 3389. [PubMed] [Google Scholar]
- 3.Hanna TA, Baranger AM, Bergman RG. J Org Chem. 1995;60:4532. doi: 10.1021/jo9521561. [DOI] [PubMed] [Google Scholar]
- 4.Ruck RT, Bergman RG. Organometallics. 2004;23:2231. doi: 10.1021/om0497994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh PJ, Bergman RG. Organometallics. 1993;12:3705. [Google Scholar]
- 6.Odom and co-workers have developed a titanium-catalyzed three-component coupling of amines, alkynes, and isonitriles to afford α,β-unsaturated β-iminoamines: C. Cao, Y. Shi, A. L. Odom, J. Am. Chem. Soc. 2003, 125, 2880. [DOI] [PubMed]
- 7.Kobayashi and co-workers have reported a Lewis acid-catalyzed reaction between imines and alkynyl sulfides to generate α,β-unsaturated thioimidates. This reaction appears to proceed by [2+2] cycloaddition followed by retro-[2+2] cycloaddition to afford the observed products: H. Ishitani, S. Nagayama, S. Kobayashi, J. Org. Chem. 1996, 61, 1902.
- 8.Qian and Ma have reported a reaction analogous to that described in reference [7] with alkynyl selenides as substrates: Y. Ma, C. Qian, Tetrahedron Lett. 2000, 41, 945.
- 9.Coupling reactions between alkynes and oxyalkynes have been reported to yield α,β-unsaturated carbonyl compounds: a) Curini M, Epitano F, Maltese F, Rosati O.Synlett 2003552 [Google Scholar]; b Shindo M, Oya S, Murakami R, Sato Y, Shishido K. Tetrahedron Lett. 2000;41:5947. [Google Scholar]; c Shindo M, Oya S, Sato Y, Shishido K. Heterocycles. 2000;52:1143. [Google Scholar]
- 10.Walsh PJ, Hollander FJ, Bergman RG. J Am Chem Soc. 1988;110:8729. [Google Scholar]
- 11.See ref. [4]. This observation implicates an alternative effect, speculatively pre-coordination of the electron-withdrawing group oxygen atom to zirconium, in the previously reported chemistry.
- 12.The product of this reaction has been designated 17g to maximize consistency between starting imine and product numbering.
- 13.Attempts to insert imines into the analogous bis(p-bromophenyl)acetylene were unsuccessful.
- 14.For more information on potential suitable alkynes, see: S. Y. Lee, R. G. Bergman, Tetrahedron 1995, 51, 4255.
- 15.This reaction proceeds via p-methoxyphenylimidozirconocene complex 13b.
- 16.a Overman LE, Owen CE, Pavan MM, Richards CJ. Org Lett. 2003;5:1809. doi: 10.1021/ol0271786. [DOI] [PubMed] [Google Scholar]; b Kronenthal DR, Han CY, Taylor MK. J Org Chem. 1982;47:2765. [Google Scholar]
- 17.Efforts to generate imidozirconium compound 13a in situ from an analogous precursor led to multiple decomposition products.
- 18.Ruck RT, Bergman RG. Angew Chem. 2004;116 doi: 10.1002/anie.200461064. Angew. Chem. Int. Ed. 2004, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


