Abstract
Leptin is a versatile 16 kDa peptide hormone, with a tertiary structure resembling that of members of the long-chain helical cytokine family. It is mainly produced by adipocytes in proportion to fat size stores, and was originally thought to act only as a satiety factor. However, the ubiquitous distribution of OB-R leptin receptors in almost all tissues underlies the pleiotropism of leptin. OB-Rs belong to the class I cytokine receptor family, which is known to act through JAKs (Janus kinases) and STATs (signal transducers and activators of transcription). The OB-R gene is alternatively spliced to produce at least five isoforms. The full-length isoform, OB-Rb, contains intracellular motifs required for activation of the JAK/STAT signal transduction pathway, and is considered to be the functional receptor. Considerable evidence for systemic effects of leptin on body mass control, reproduction, angiogenesis, immunity, wound healing, bone remodelling and cardiovascular function, as well as on specific metabolic pathways, indicates that leptin operates both directly and indirectly to orchestrate complex pathophysiological processes. Consistent with leptin's pleiotropic role, its participation in and crosstalk with some of the main signalling pathways, including those involving insulin receptor substrates, phosphoinositide 3-kinase, protein kinase B, protein kinase C, extracellular-signal-regulated kinase, mitogen-activated protein kinases, phosphodiesterase, phospholipase C and nitric oxide, has been observed. The impact of leptin on several equally relevant signalling pathways extends also to Rho family GTPases in relation to the actin cytoskeleton, production of reactive oxygen species, stimulation of prostaglandins, binding to diacylglycerol kinase and catecholamine secretion, among others.
Keywords: adipocyte, cytokine, Janus kinase/signal transducer and activator of transcription pathway (JAK/STAT pathway), leptin receptor, obesity, signalling cascade
Abbreviations: ACC, acetyl-CoA carboxylase; AMPK, 5′-AMP-activated protein kinase; CNTF, ciliary neurotrophic factor; CT-1, cardiotrophin-1; ERK, extracellular-signal-regulated kinase; HIF-1α, hypoxia-inducible factor 1α; IL, interleukin; IRS, insulin receptor substrate; JAK, Janus kinase; JNK, c-Jun N-terminal kinase; LIF, leukaemia inhibitory factor; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor κB; NPY, neuropeptide Y; OSM, oncostatin-M; PDE, phosphodiesterase; PI3K, phosphoinositide 3-kinase; PKA, protein kinase A; PKC, protein kinase C; PTP1B, protein tyrosine phosphatase 1B; SH2, Src-like homology 2; SHP-2, SH2 domain-containing protein tyrosine phosphatase; SOCS, suppressor of cytokine signalling; STAT, signal transducer and activator of transcription; TNFα, tumour necrosis factor α; TYK2, tyrosine kinase 2; VEGF, vascular endothelial growth factor
INTRODUCTION
The discovery of leptin at the end of 1994 [1] opened up a whole new perspective to study the role of adipocyte-derived factors in energy balance homoeostasis [2–7]. The 16 kDa non-glycosylated polypeptide product of the ob gene is mainly produced and secreted by fat cells in proportion to fat mass to signal the repletion of body energy stores to the hypothalamus [7–11]. Leptin presents striking structural similarities to members of the long-chain helical cytokine family, including LIF (leukaemia inhibitory factor), CNTF (ciliary neurotrophic factor), OSM (oncostatin-M) and CT-1 (cardiotrophin-1), as well as IL-6 (interleukin-6), IL-11 and IL-12 [8,12–15]. Both the crystal structure and NMR studies of leptin have revealed that the protein adopts a cytokine fold similar to that exhibited by the short-helix subfamily of cytokine folds [13,15]. The three-dimensional structure of the 167-amino-acid leptin molecule is based on four antiparallel α-helices, connected by two long crossover links and one short loop arranged in a left-handed helical bundle, which forms a two-layer packing. A disulphide bond between two cysteine residues (Cys96 and Cys146) of the C-terminus of leptin and the beginning of one of the loops has been shown to be important for structure folding and receptor binding, as mutation of either of the cysteine residues renders the protein biologically inactive [8,12–15].
Circulating leptin concentrations have been reported to correlate closely with both the BMI (body mass index) and the total amount of body fat [7–9]. Although leptin is mainly produced and secreted to the bloodstream by white adipocytes, this is not the only potential source of the hormone. Placenta, gastric mucosa, bone marrow, mammary epithelium, skeletal muscle, pituitary, hypothalamus and bone have also been shown to be able to produce small amounts of leptin in certain circumstances [16–19]. Initially, the effects of leptin were thought to be only centrally mediated. However, leptin shares with other members of the long-chain helical cytokine family an extreme functional pleiotropy. Although originally isolated in relation to a particular biological action, many cytokines have subsequently been shown to be capable of stimulating a variety of biological responses in a wide spectrum of cell types. Based on an almost ubiquitous distribution of receptors, leptin has been reported to play a role in a quite diverse range of physiological functions both in the central nervous system and at the periphery [9,20–24]. Therefore, since its discovery, leptin has caused upheavals not only in the fields of appetite and body mass control, but also in the more broad spheres of general endocrinology, metabolism, reproduction, immunology, cardiovascular pathophysiology, respiratory function and wound healing, as well as in growth and development [25–48]. In this sense, completely disentangling the biochemical and molecular pathways activated by leptin represents a fascinating challenge.
BINDING PROTEINS AND CLEARANCE
In humans, the majority of leptin circulates bound to serum macromolecules, which may modulate ligand bioactivity and bioavailability to target tissues [49]. In lean subjects with a relatively small adipose tissue mass, the majority of leptin is in the bound form, while the proportion of free leptin is increased in the serum of obese patients [49,50]. Free leptin may have a more rapid turnover because of proteolytic cleavage or increased clearance. During fasting, a decrease in free leptin concentrations has been observed, which is more pronounced in lean volunteers compared with obese subjects, whereas no change was observed in bound leptin in either group [50]. It may be speculated that the ratio of free/total leptin is not constant, but rather that, depending on the metabolic and nutritional state, a dynamic balance between circulating binding proteins and free leptin exists. A precedent for the key role played by binding proteins in the transport or uptake of ligands has been shown for other members of the cytokine family. Moreover, for some cytokines and haematopoietic growth factors, association with binding proteins potentiates ligand activity because of biochemical modifications [51]. These phenomena provide a potential explanation for apparent leptin resistance in the setting of increased free leptin concentrations. The short half life of leptin in the circulation is determined mainly by efficient renal clearance by a highcapacity non-saturable process, consistent with glomerular filtration, followed by metabolic degradation in the renal tubules [52,53].
LEPTIN RECEPTORS
The pleiotropic nature of leptin is supported by the universal distribution of OB-R leptin receptors. Leptin acts via transmembrane receptors, which show structural similarity to the class I cytokine receptor family [54–58], which includes the receptors of IL-2, IL-3, IL-4, IL-6, IL-7, LIF, granulocyte colony-stimulating factor, growth hormone, prolactin and erythropoietin [59,60]. Members of this family have characteristic extracellular motifs of four cysteine residues and WSXWS (Trp-Ser-Xaa-Trp-Ser) [61] containing a different number of fibronectin type III domains [62,63]. The OB-R is produced in several alternatively spliced forms, designated OB-Ra, OB-Rb, OB-Rc, OB-Rd, OB-Re and OB-Rf [55,64], that have in common an extracellular domain of over 800 amino acids, a transmembrane domain of 34 amino acids and a variable intracellular domain, characteristic for each of the isoforms. Thus the isoforms can be classified into three classes: short, long and secreted. In addition to containing identical extracellular and transmembrane domains, the short and long isoforms share the same first 29 intracellular amino acids, diverging in sequence secondary to alternative splicing of 3′ exons. The extracellular domain of OB-R has two cytokine-like receptor motifs and four fibronectin type III domains [54,57,58,65]. Mutant receptor constructs have shown that only the second putative binding domain mediates leptin binding and receptor activation, with an affinity which lies in the nanomolar range [66]. The short forms of the receptor, i.e. OB-Ra, OB-Rc, OB-Rd and OB-Rf, consist of 30–40 cytoplasmic residues. However, only the long full-length isoform, OB-Rb, was initially considered to be the functional receptor, based on the finding that it has an extended intracellular domain of approx. 300 cytoplasmic residues (longer in humans than in mouse), containing various motifs required for the interaction with other proteins and subsequent signalling pathway activation [54].
The lack of the full-length OB-R has been shown to be responsible for the obesity phenotypes of the db/db mouse and the fa/fa rat [67]. Selective deletion of all OB-R isoforms in neurons has been shown to lead to obesity in mice, providing evidence for the relevance of neuronal leptin action in body mass regulation [68]. OB-Rb has been found to be expressed at high levels in the hypothalamus. OB-Ra and OB-Rc are highly expressed in choroid plexus and microvessels, where they may play a role in leptin uptake or efflux from the cerebrospinal fluid as well as in receptor-mediated transport of leptin across the blood–brain barrier [24,69]. OB-Re, which lacks the intracellular domain, may encode a soluble receptor [55]. The secreted isoform, OB-Re, represents an alternative splice product or proteolytic cleavage products of membrane-bound OB-R. Secreted extracellular domains of cytokine receptors have been shown to function as specific binding proteins [51]. In mice, it has been reported that the putative soluble isoform, OB-Re, is produced at a sufficiently high level to act as a buffering system for free circulating leptin [56]. It has been shown that the soluble OB-R represents the main leptin-binding activity in human blood [70] and that it is determined by sex, adiposity and leptin administration [71]. Consistent with leptin's role in controlling appetite and energy metabolism, OB-Rs have been found in the hypothalamus and adjacent brain regions [54,57]. Initially, direct actions of leptin were thought to be circumscribed only to the central nervous system. However, the almost universal distribution of OB-Ra and OB-Rb reflects the multiplicity of biological effects in extraneural tissues, providing evidence for the extreme functional pleiotropy of leptin.
RECEPTOR INTERNALIZATION
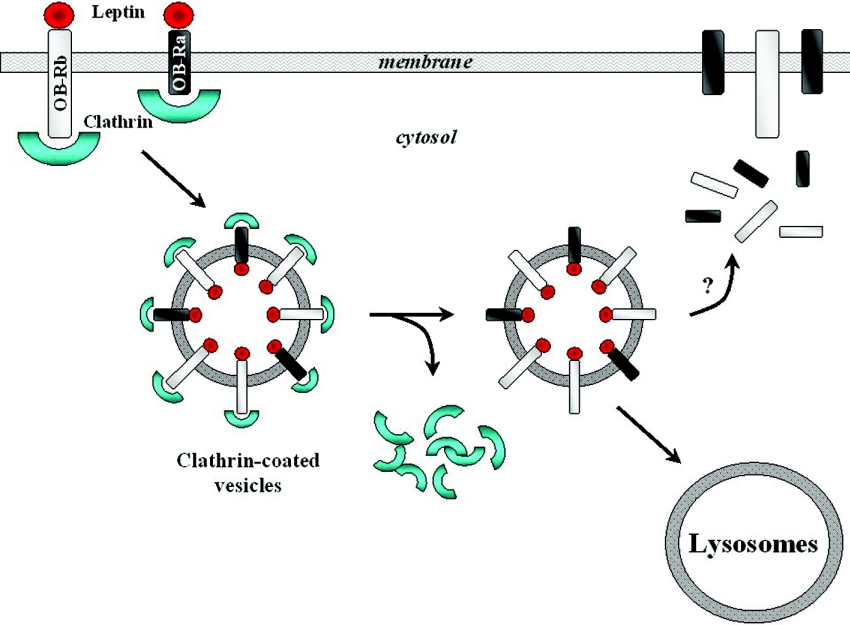
Cytokine receptor family members are known to be internalized upon ligand binding via clathrin-coated vesicles into early endosomes, with the receptor being processed for degradation or efficiently recycled back to the cell surface (Figure 1) [72]. It has been estimated that, under normal conditions, only 5–25% of the total OB-R isoforms are located at the cell surface, with the majority contained in intracellular pools [73]. Both OB-Ra and OB-Rb mediate lysosomal internalization and degradation, with amino acids 8–29 of the intracellular domain determining the process [72,74]. It has been reported by some authors that leptin internalization and down-regulation of surface receptors is greater for OB-Rb [73]. Additionally, the short receptor isoform has been shown to recycle to the cell surface faster [74]. Taken together, these observations provide a plausible explanation for selective leptin resistance linked to hyperleptinaemia in human obesity based on a preferential down-regulation of OB-Rb signalling. Although in vitro studies with transfected OB-R have shown the existence of a large intracellular receptor pool [72–74], the functional meaning of this distribution and the exact in vivo trafficking dynamics have not been fully established. Determination of the cellular mechanisms underlying these processes represents a critical step towards a better understanding of the intracellular traffic, the control of the residence time of receptors at the cell surface, and hence the potential relationship to leptin sensitivity. Both OB-R isoforms have been reported to be short-lived membrane proteins and to follow similar intracellular routes, despite showing structural and functional differences in their cytoplasmic domains [75]. Based on indirect pieces of evidence regarding high turnover rates, levels of recycling pathway markers and stability of endocytosed receptors in response to chloroquine, this study did not support the recycling of OB-R to the cell surface. Furthermore, it suggested that a fraction of neosynthesized OB-R is transported to the plasma membrane, before being constitutively endocytosed and degraded in lysosomes, while another fraction is retained inside the cell, and could possibly follow an alternative pathway leading to lysosomes without prior access to the cell surface [75]. Whether the relative levels of the intracellular and cell-surface pools of OB-R might be modulated in response to physiological stimuli, as well as the potential contribution of regulatory defects in this process to leptin sensitivity, remain to be established.
Figure 1. Leptin receptor internalization.
Schematic representation of lysosomal internalization and degradation of leptin receptors via clathrin-coated vesicles.
MAIN SIGNALLING PATHWAYS
During the last few years, the study of the signalling events derived from leptin binding to its receptor has promoted a better understanding of the biochemical and molecular mechanisms of leptin function. The homology of OB-R with other class I cytokine receptors, such as the gp130 (glycoprotein 130) subunit of the IL-6 receptor family, suggested the possibility that leptin binding might mediate cytokine receptor-like signals, including the activation of JAKs (Janus kinases) and STATs (signal transducers and activators of transcription) [76–78]. Therefore, although leptin and its receptors were discovered relatively recently, a great deal was already known about the molecular details of class I cytokine receptor-mediated signalling and physiological regulation. Thus early recognition of OB-R as a member of this cytokine receptor superfamily resulted in the prompt identification of the JAK/STAT pathway as one of the main signalling cascades activated by leptin [45,60,72,79]. Subsequent studies showed that only the full-length isoform, OB-Rb, contains intracellular motifs required for activation of the JAK/STAT signal transduction pathway [24,58,80]. Chimaeric receptor heterodimers of OB-Ra and OB-Rb failed to activate the JAK/STAT pathway, whereas dimers of OB-Rb gave rise to the expected ligand-dependent activation of JAK [81]. Furthermore, deletion and substitution mutagenesis experiments of the intracellular domain of OB-Rb have shown that ligand-independent homo-oligomerization by the long isoform is sensitive to reduction in JAK recruitment capability, suggesting that JAK interaction and signalling competency may provide means for specific OB-R sorting [82].
JAK/STAT signal transduction cascade
The JAK/STAT pathway comprises a family of four non-receptor tyrosine kinases (JAKs) and seven 85–95 kDa transcription factors (STATs) that are regulated by phosphorylation on specific serine and tyrosine residues. Typically, the JAK/STAT signal transduction cascade is activated by interferons, interleukins or other cytokines whose receptors lack intrinsic kinase activity. Functional cytokine receptors contain a proline-rich ‘box1’ motif that is required for JAK interaction and activation [83]. Furthermore, less-well-conserved sequences, termed ‘box2’ also play a role in JAK interactions and isoform selectivity. Of the four known members of the JAK family, JAK1, JAK2 and TYK2 (tyrosine kinase 2) are widely expressed, while JAK3 is found only in cells of the haematopoietic immune systems [84]. The OB-R does not have an intrinsic tyrosine kinase domain, and therefore binds cytoplasmic kinases, mainly JAK2 [85]. Box1 and box2 motifs are known to recruit and bind JAKs [86,87]. However, for leptin signalling, it was reported that only box1 and the immediate surrounding amino acids are essential for JAK activation [81,88]. Box1 and amino acids 31–36 of the intracellular domain have been proved to be indispensable for this interaction, while amino acids 37–48 seem to be involved in increasing the signal, but can be replaced by other elements [88]. The intracellular domain of all OB-R isoforms contains in the juxtamembrane region the box1 JAK-binding domain, whereas OB-Rb also includes the box2 motif and STAT-binding sites. Although only OB-Rb was initially viewed as the isoform with signalling capacity, the short isoforms have also demonstrated divergent signalling capacities [89–93]. Both long and short receptor isoforms have the ability of homodimer formation in the absence of a ligand, with the extent of this association not being significantly changed by ligand stimulation, suggesting that dimerization does not play a key regulatory role in receptor activation [94–96]. However, dimer formation appears to be actively involved in post-receptor signalling [66,97]. A 1:1 stoichiometric relationship between OB-R and leptin results in a tetrameric receptor–ligand complex [96]. The conformational change in the structure of the receptor elicited by the complex formation has been reported to be critical for leptin signalling activation [60]. In the absence of ligand, no OB-R heterodimers have been observed; these become readily detectable in the presence of leptin [82,96]. Contrary to what takes place with other class I cytokine receptors, leptin does not undergo heterodimerization with structurally similar cytokine receptors, as is the case with IL-6, IL-11, LIF, CT-1, CNTF or OSM [72,95].
The expression of chimaeric receptors containing the extracellular ligand-binding domain of the erythropoeitin receptor fused to the transmembrane and intracellular domains of the long OB-R isoform allowed the demonstration that the intracellular domain activated JAK2, but not JAK1 or TYK2 [57,58,88,96]. In addition to box1 sequences, intracellular residues 31–36 of OB-Rb were shown to be required for JAK2 activation [88]. However, high-level overexpression of JAKs by transient transfection has been observed to be able to decrease the stringency of the requirement for residues 31–36 [58,88]. This finding is consistent with the observation of weak OB-Rb–JAK1- and OB-Ra–JAK2-induced signalling under transient transfection conditions [80]. It can be concluded that box1 is absolutely necessary for all cytokine receptor–JAK interactions, with residues homologous with intracellular amino acids 31–36 of OB-Rb determining the specificity of JAKs interacting with a particular receptor [58].
Since OB-Rb does not have intrinsic enzymatic activity, it signals by activating non-covalently associated JAK2, which autophosphorylates numerous tyrosine residues at the same time as it phosphorylates tyrosine residues on the functional leptin receptor. JAK2 proteins are associated with membrane-proximal sequences of the receptor intracellular domain, which is phosphorylated upon ligand binding. The phosphorylated intracellular domain then provides a binding site for STAT proteins, which are activated, translocate to the nucleus and stimulate transcription (Figures 2 and 3). Ligand–receptor binding activation of STAT3, STAT5 and STAT6, but not STAT1, STAT2 or STAT4, has been described in relation to OB-R [72]. Furthermore, the SHP-2 [SH2 (Src-like homology 2) domain-containing protein tyrosine phosphatase] has been demonstrated to bind to a phosphotyrosine of the intracellular domain of OB-R with the ability to down-regulate tyrosine phosphorylation-dependent leptin signalling such as STAT3 activation.
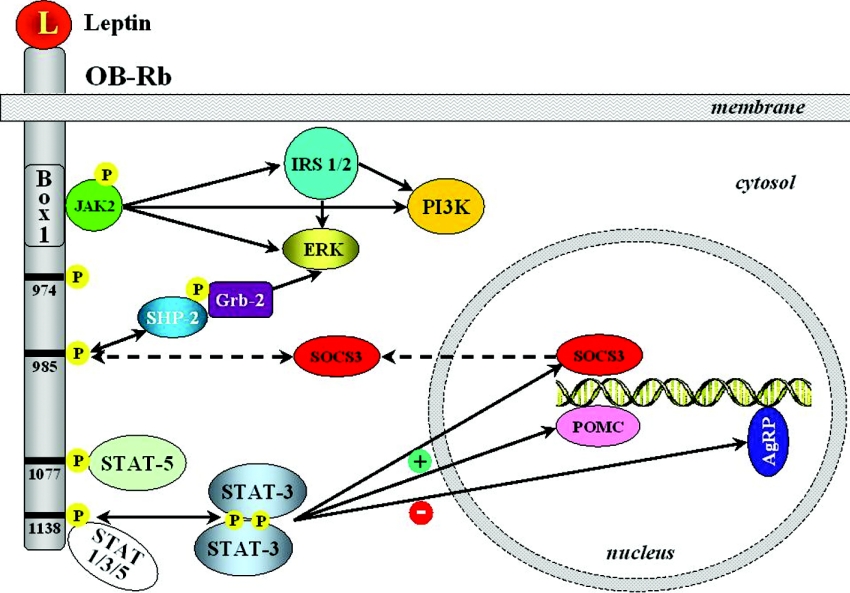
Figure 2. Role of phosphotyrosines of OB-Rb in leptin signalling.
JAK2 associates with the receptor via the box1 motif. The long isoform leptin (L) receptor (OB-Rb) contains four important tyrosine residues (Tyr974, Tyr985, Tyr1077 and Tyr1138). These phosphorylated tyrosine residues provide docking sites for signalling proteins with SH2 domains. Most importantly, Tyr1138 recruits the transcription factor STAT3, which is subsequently phosphorylated by JAK2, dimerizes and translocates to the nucleus, where it induces SOCS3 and POMC (pro-opiomelanocortin) expression, while repressing AgRP (agouti-related peptide). SOCS proteins inhibit signalling by binding to phosphorylated JAK proteins or interacting directly with tyrosine-phosphorylated receptors. The ability of SOCS3 to inhibit leptin-stimulated phosphorylation of JAK2 and ERK provides a negative-feedback mechanism on the leptin signalling system. Grb-2, growth factor receptor binding-2.
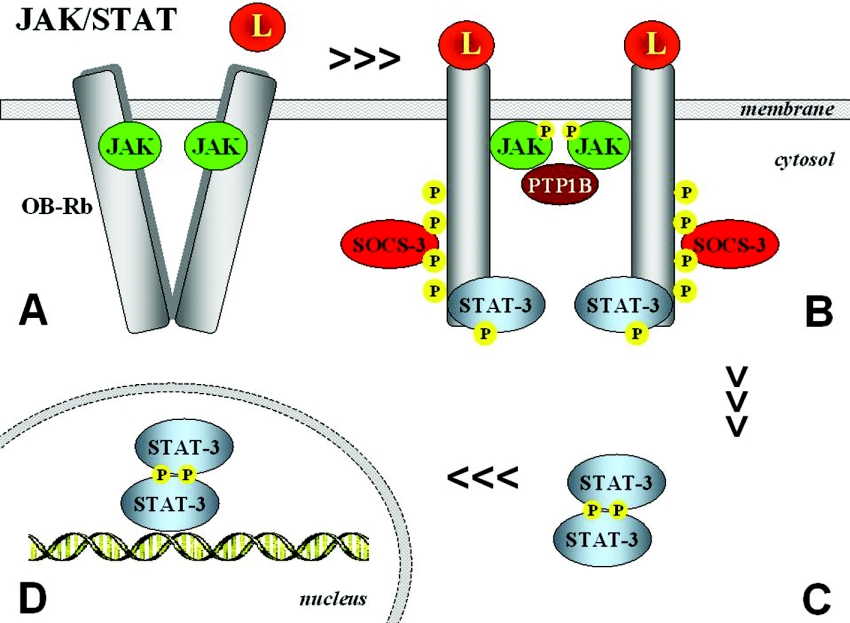
Figure 3. Mechanisms of JAK/STAT activation through OB-Rb.
Upon leptin (L) binding, a conformational change takes place (A) that allows juxtaposition of JAKs, which then become activated and are able to tyrosine-phosphorylate other JAKs and tyrosine residues on the receptor (B). Activation of JAK2 occurs by transphosphorylation and subsequent phosphorylation of tyrosine residues in the cytoplasmic region of the receptor. Phosphorylation of Tyr1138 allows association of STATs, which then become substrates of receptor-associated JAKs. Phosphorylation of STATs leads to their dissociation from the receptor and the formation of active dimers (C), which translocate to the nucleus to regulate gene expression, binding to the promoter regions of target genes (D).
To determine the role of the three intracellular tyrosine residues (Tyr985, Tyr1077 and Tyr1138), researchers created constructs replacing either one of the three tyrosine residues or combinations of them [98,99]. It has been shown that all three intracellular tyrosine residues of OB-Rb exhibit different capabilities for downstream activation signalling. Tyr985 is required for the activation of the Ras/Raf/ERK (extracellular-signal-regulated kinase) pathway. The phosphorylation of Tyr985 creates a binding site for the C-terminal SH2 domain of the tyrosine phosphatase SHP-2, leading to the activation of the canonical p21Rasρ/ERK signalling cascade. The canonical mechanism by which tyrosine kinases activate ERK is through the SH2-domain-containing adapter protein, Grb-2 (growth factor receptor binding-2) [58]. Although Tyr985 mediates the majority of ERK stimulation, leptin-stimulated ERK activation is also regulated, in part, independently of OB-Rb phosphorylation, most probably via tyrosine phosphorylation sites on JAK2 [24,100–102]. Tyrosine phosphorylation of SHP-2 before STAT3 dimerization has been clearly shown to play a pivotal role in leptin-induced stimulation of ERKs [101,102]. However, in macrophages, it has been also reported that STAT3 can be phosphorylated at Ser727 [103]. Furthermore, in these cells, ERK-dependent serine phosphorylation was required for maximal activation of STAT3 DNA binding, providing evidence that this intracellular signalling mechanism is likely to be a relevant pathway in exerting the effects of leptin in relation to immune function.
While either Tyr1077 or Tyr1138 is required for leptin-induced tyrosine phosphorylation of STAT5, Tyr1138 is essential for activation of STAT1 and STAT3 [99,100]. The presence of Tyr1077 as the only intracellular tyrosine residue was sufficient to induce tyrosine phosphorylation of STAT5 and to stimulate STAT5-driven reporter gene activity in vitro [99]. The critical role of Tyr1138 was elegantly shown in mice by replacing this residue with a serine residue [104]. Interestingly, these mice (LeprS1138) are unable to activate STAT3, thus enabling the disentanglement of the divergent contributions of the individual STATs to the different biological roles. LeprS1138 homozygous mutants were hyperphagic and obese, like db/db mice [104]. Nonetheless, LeprS1138 homozygotes were less hyperglycaemic than db/db mice, reached normal sexual development and fertility, and attained a normal body length. Hypothalamic melanocortin expression was present in both mutants. However, the increased NPY (neuropeptide Y) expression of db/db rodents was not observed in LeprS1138 homozygotes [104]. These observations, together with other findings, suggest that STAT3 signalling participates in energy homoeostasis through the melanocortic/melanocortinergic pathway, while the control of hypothalamic NPY expression, linear growth, glycaemia and reproduction are attained via STAT3-independent pathways [104–107].
Receptor mutants have shown further that lack of Tyr1138 abrogates STAT3 signalling by OB-Rb [57,58,76,97]. Cumulative evidence shows that the JAK/STAT pathway of cytokine signalling is under the negative-feedback control of SOCS (suppressors of cytokine signalling) proteins [45,108]. Members of the SOCS family, which contain an SH2 domain, are induced by a variety of cytokines, acting as a negative regulator of their signalling. Leptin has been reported to induce SOCS3 expression [45,98,109–112]. Initial work showed that Tyr985 of OB-Rb and homologous sites on related receptors represents a high-affinity binding site for SOCS3 during signalling by IL-6 receptor family members and that this site is critical for signalling inhibition by SOCS3 [98,101,102,111,113]. However, a more recent study has provided evidence that Tyr985 is not required for the attenuation of JAK2 and ERK phosphorylation during prolonged signalling by the intracellular domain of OB-Rb [114]. Thus Tyr985 is not necessary for the SOCS3-mediated blockade of JAK2 tyrosine phosphorylation, suggesting the existence of multiple binding sites for SOCS3 within activated cytokine receptor complexes [115]. Brief leptin treatment (30 min) has been shown to stimulate the phosphorylation of STAT3, which peaked after 30–60 min of stimulation, declined to approx. 80% of these levels after 4 h, and remained at approx. 60% of peak levels after 24 h of stimulation [114]. In contrast, JAK2 tyrosine phosphorylation is more transient, declining rapidly to 40–50% of peak levels at 1–4 h of stimulation and to below 30% after 8–24 h. Similarly, ERK phosphorylation declined to 20–30% after peaking following 2–4 h of treatment and decreased further to 0–5% of peak values when 4–8 h had elapsed [114]. The biphasic activation of ERK exhibits an initial peak of activity between 5 and 10 min of activation, followed by a maintained activation from 10 min onwards. SOCS3 protein was undetectable before stimulation, whereas Western blotting was able to detect the induction of SOCS3 protein in cell lysates after 8 h of treatment [114].
Endogenous SOCS3 expression inhibits tyrosine phosphorylation of OB-R, thus providing an important feedback mechanism for receptor signalling at the transcriptional level [111]. Moreover, changes in SOCS3 expression have been postulated to underlie the phenomenon of leptin resistance [112]. Although it is now clear that SOCS3 overexpression is able to inhibit multiple aspects of signalling by the intracellular domain of OB-Rb and other receptors, it had not been shown that SOCS3 itself does actually mediate feedback inhibition of OB-Rb signalling. RNAi (RNA interference)-mediated knockdown of SOCS3 (but not SHP-2) expression increased the tyrosine phosphorylation of JAK2 and STAT3 [114]. The researchers provided evidence, for the first time, that knockdown of SOCS3 not only acutely enhanced ERK phosphorylation, but also blocked the attenuation of this signal after prolonged receptor stimulation. Therefore it is plausible that the Tyr985-independent, Tyr1138-dependent feedback inhibition of ERK signalling, and probably JAK2, relies on accumulation of SOCS3 following extended stimulation of the intracellular domain of OB-Rb [112,114]. Based on these findings, a model is put forward whereby STAT3 signalling by OB-Rb mediates critical effects of leptin on food intake and body mass control, at the same time as mediating a feedback inhibition of the signalling of the receptor during prolonged stimulation via induction of the expression of SOCS3 (Figure 4). The participation of SOCS3 in the negative-feedback mechanism of leptin signalling has been proposed to underlie the development of leptin resistance in relation to the hyperleptinaemia observed in the context of the majority of obesity cases [101]. The relevance of OB-Rb-induced SOCS3 accumulation in the sensitivity to the biological effects of the hormone has been shown further by the fact that SOCS3 deficiency elevates leptin sensitivity and confers resistance to diet-induced obesity [116,117].
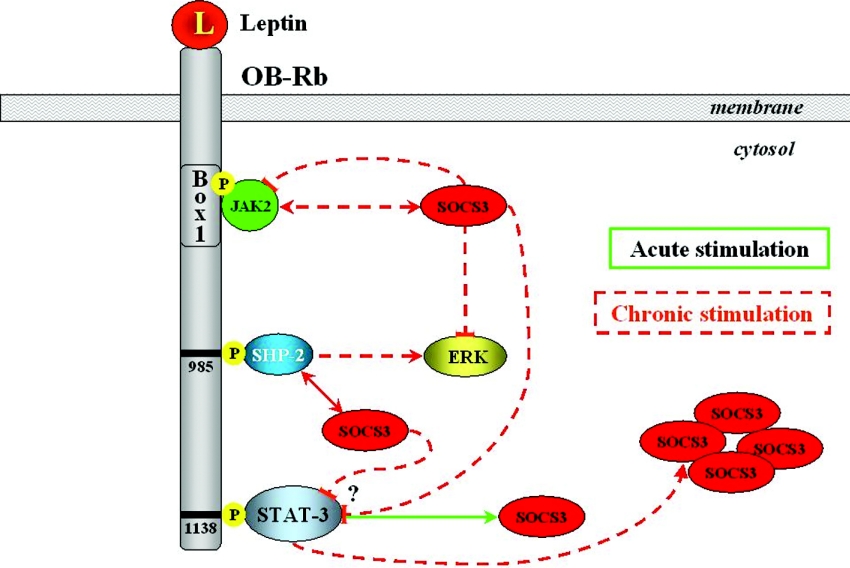
Figure 4. Proposed model for the participation of SOCS3 in leptin resistance.
During prolonged receptor stimulation by leptin (L), the inhibition of JAK2 and ERK phosphorylation is mediated by SOCS3 independently of Tyr985 of OB-Rb.
Another negative regulator of leptin signalling is represented by PTP1B (protein tyrosine phosphatase 1B), which has been shown to regulate leptin signal transduction both in vivo and in vitro, primarily via dephosphorylation of JAK2 [24,45,118–120]. PTP1B has been reported to be a physiologically important negative regulator of insulin signalling [24,121,122]. Mice lacking PTP1B were resistant to developing diet-induced obesity and did not exhibit hyperphagia despite a clear hypoleptinaemia. Under physiological circumstances, the effects of PTP1B are likely to be exerted via central and peripheral actions. Consistent with the involvement of PTP1B in leptin-dependent signalling pathways, administration of leptin to PTP1B-knockout mice was followed by a hypersensitivity to the physiological effects of the hormone in body mass control [24,118].
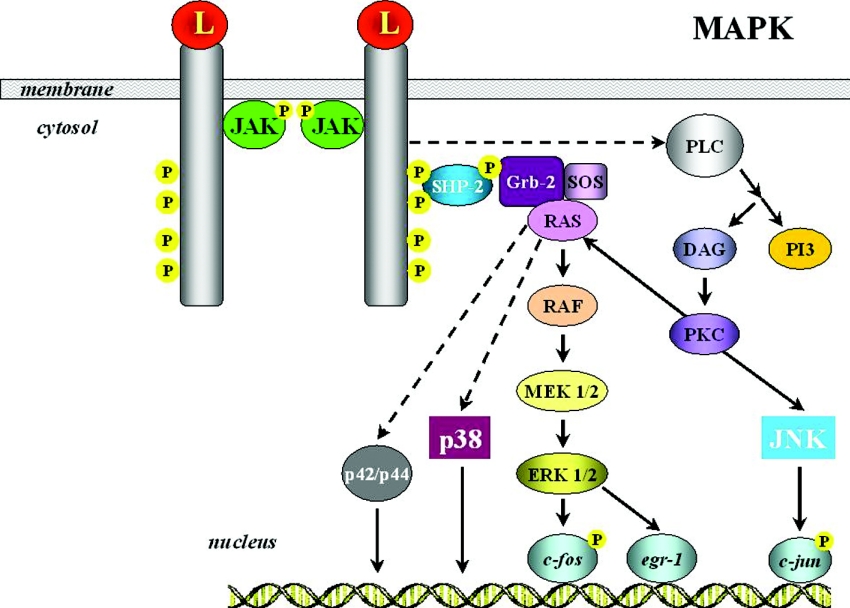
MAPK (mitogen-activated protein kinase) cascade
The ERK members of the MAPK family are components of the well-defined Ras/Raf/MAPK signalling cascade and become activated by a wide range of stimuli, including leptin (Figure 5). The MAPK pathway can be stimulated by either OB-Ra or OB-Rb, although to a lesser extent by the former [80,101]. Although the distal portion of OB-R is not essential for MAPK signalling, the intact intracellular part of the long receptor is needed to achieve maximal activation. This is based on the fact that leptin is able to trigger the MAPK cascade in two different ways, i.e. via tyrosine phosphorylation of JAK2 receptor-associated activation, or independently of receptor phosphorylation [60,80]. Nonetheless, in both pathways, downstream signalling requires an intact catalytic domain of SHP-2. It has been reported that a lack of phosphatase activity causes a failure of ERK phosphorylation [102]. Although it has not been completely elucidated which molecules are involved in transmitting the leptin signalling, activated MEKs (MAPK/ERK kinases) phosphorylate ERKs, leading finally to the expression of specific target genes, such as c-fos and egr-1, that participate in cell proliferation and differentiation.
Figure 5. The MAPK pathway in leptin signalling.
The ERK members of the MAPK family are components of the well-defined Ras/Raf/MAPK signalling cascade and have become activated by leptin (L). For more detailed information, see the text. DAG, diacylglycerol; Grb-2, growth factor receptor binding-2; PI3, PtdIns(3,4,5)P3; PLC, phospholipase C; SOS, son of sevenless.
Activation of the MAPK signalling pathway has been observed both in vivo and in vitro, as well as centrally and peripherally. In precursor cells of the osteoblastic lineage, leptin has been demonstrated to induce apoptosis through the MAPK cascade via ERK1/2 activation of cytosolic phospholipase A, which in turn leads to cytochrome c release and finally to caspase 3 and caspase 9 induction [123]. In monocytes, leptin activates the promoter of IL-1Ra (IL-1 receptor antagonist) through p42/44 MAPK and a composite NF-κB (nuclear factor κB)/PU.1 binding site [124]. A further study has revealed that leptin stimulates nitric oxide synthase activity in white adipose tissue through a complex mechanism that involves PKA (protein kinase A) and p42/44 MAPK [125].
A wide range of stimuli, including osmotic stress, heat shock and cytokines, among others, activate another member of the MAPK family, namely p38 MAPK [72]. Leptin has been shown further to increase the phosphorylation of p38 MAPK in mononuclear cells, as well as in L6 muscle cells, most probably not as a direct effect, but by reducing insulin-stimulated p38 MAPK phosphorylation [126]. Leptin shares with other cytokines, growth factors and stressors the ability to activate the stress-activated protein kinase, JNK (c-Jun N-terminal kinase). In this respect, leptin reportedly enhances TNFα (tumour necrosis factor α) production via p38 and JNK MAPK in LPS (lipopolysaccharide)-stimulated Kupffer cells [127]. In vascular smooth muscle cells, leptin induces hypertrophy via p38 MAPK [128], indicating the potential relevant role of this hormone in cardiovascular physiology and an impact on vascular remodelling [129]. Overall, a definitive picture of leptin signal transduction, including upstream activators and downstream targets of the p38 and JNK MAPK pathways, remains to be completely disentangled. With regard to the downstream targets, the regulation of NF-κB appears as a clear candidate since this essential transcription factor is known to play a pivotal role in the transcriptional regulation of pro-inflammatory cytokines such as TNFα and IL-1β.
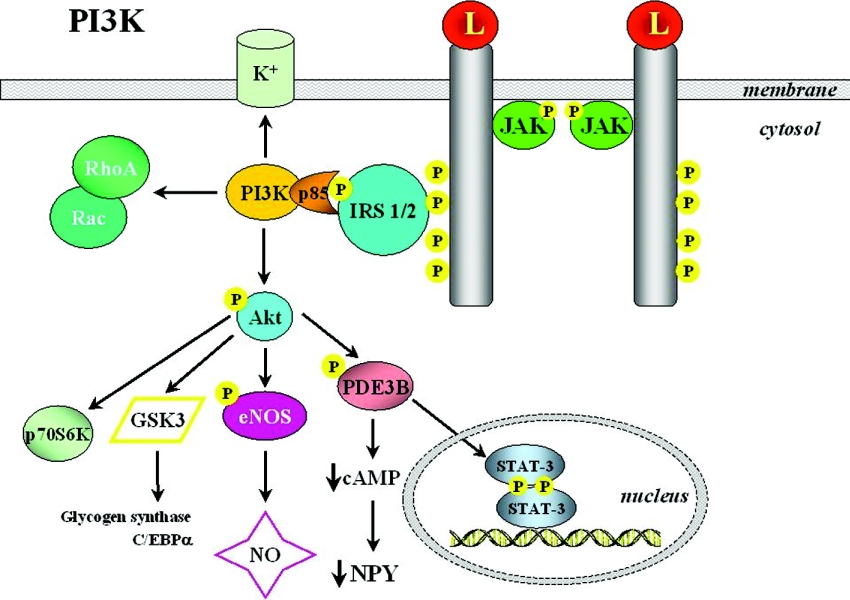
The PI3K (phosphoinositide 3-kinase)/PDE3B (phosphodiesterase 3B)/cAMP pathway
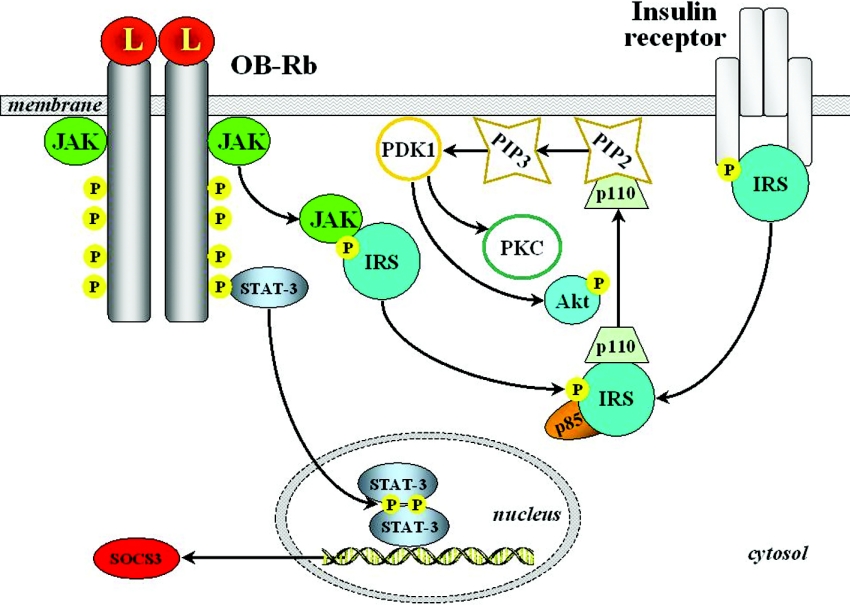
PI3K activity represents a key target regulated by a broad spectrum of ligands, with insulin requiring special mention. In fact, most insulin-dependent actions involve PI3K activation, making this a relevant point of cross-talk between the insulin and leptin signalling pathways [32,60,130,131]. PI3K products typically stimulate protein kinases such as Akt (protein kinase B) and PKC (protein kinase C) isoforms [72]. Leptin reportedly acts through some of the components of the insulin signalling cascade (Figures 6 and 7). The binding of insulin to its receptor recruits several IRSs (insulin receptor substrates) that are tyrosine-phosphorylated by the intrinsic kinase activity of the receptor. In turn, phosphorylation of IRSs increases their affinity for binding other signalling molecules, thus initiating further steps of the pathway. IRS proteins exert PI3K activation via association with its regulatory subunit, p85, and increasing the activity of the catalytic domain. The stimulation of PI3K leads to activation of PtdIns(3,4,5)P3-dependent serine/threonine kinases such as PDK1 (phosphoinositide-dependent kinase 1), which is able to activate Akt, a further serine/threonine kinase representing a key step in subsequent downstream signalling. In the central nervous system, as well as in, for instance, adipose tissue, pancreas and liver, leptin has been shown to induce an insulin-like signalling pathway involving PI3K-dependent activation of PDE3B and eventual cAMP reduction [132–134]. Indirect evidence for a plausible participation of the IRS/PI3K cascade in leptin action came from the phenotype of IRS2-null mice, which exhibited hyperphagia and a hypometabolic state in the presence of increased adiposity and hyperleptinaemia, but not as marked as for db/db animals [58]. Leptin has been shown to stimulate IRS2-mediated hypothalamic PI3K activity, while pharmacological blockade of PI3K abrogated the leptin-induced hyperpolarization of NPY/AgRP (agouti-related peptide) neurons, thus blocking the anorectic effect of leptin [58,135]. Interestingly, inhibition of PI3K is not able to influence the anorectic effect of melanocortin agonists operating downstream of OB-Rb. Moreover, PI3K activity is also required for leptin-mediated sympathoactivation [136]. Although the precise contribution of insulin-versus leptin-induced PI3K stimulation to actual signalling remains difficult to dissect, overall findings indicate that the PI3K/PDE3B/cAMP pathway interacting with the JAK2/STAT3 cascade constitutes a critical component of leptin signalling in the hypothalamus [45].
Figure 6. The PI3K/PDE3B/cAMP cascade.
Stimulation of the PI3K pathway by leptin (L) represents a key cascade to exert several different effects of the hormone at multiple sites. For more detailed information, see the text. C/EBP, CCAAT/enhancer-binding protein; eNOS, endothelial nitric oxide synthase; GSK3, glycogen synthase kinase 3.
Figure 7. Cross-talk of leptin signalling with insulin-induced pathways.
Leptin (L) receptor (OB-Rb) activation acts through some of the components of the insulin signalling cascade, recruiting several IRSs. PIP2, PtdIns(4,5)P2, PIP3, PtdIns(3,4,5)P3.
However, results are inconsistent in different cell lines and the nature of the leptin–insulin cross-talk mechanisms operating in each cell type may exhibit certain particularities. Thus leptin itself has no direct effect on the insulin pathway in a well-differentiated hepatoma cell line, while pre-treatment with leptin transiently enhances insulin-induced IRS1 phosphorylation and its association with p85. Leptin administration results further in Akt phosphorylation, without affecting insulin-induced phosphorylation. Furthermore, leptin alone exerts a less pronounced effect on GSK3 (glycogen synthase kinase 3) serine-phosphorylation than insulin [137]. In C2C12 muscle cells, leptin reportedly activates PI3K via JAK2- and IRS2-dependent pathways [89]. In these myotubes, leptin also recruits GLUT4 to the cell surface and stimulates glucose transport. This effect can be blocked by wortmannin, which can inhibit both PI3K and MAPK [60]. Leptin-induced stimulation of PI3K has been observed to affect hormone-sensitive lipase activity in macrophages, which can be blocked using PI3K inhibitors [138]. In pancreatic β-cells, leptin participates in the regulation of PDE3B, decreasing cAMP levels and inhibiting the insulin secretion stimulated by glucagon-like peptide-1 [133].
A signalling pathway divergent from activated PI3K results in the induction of K+/ATP channels, which trigger a hyperpolarization of the cell membrane. This intracellular mechanism has been observed to take place in insulinoma cells, isolated pancreatic islets and glucose-sensitive hypothalamic neurons [60,72]. Apparently, Akt, p70S6K or MAPK downstream cascades stimulated by insulin are not used by leptin in this alternative pathway. The most probable candidate is PtdIns(3,4,5)P3, which leads to disruption of actin filaments, the last known step for K+/ATP channel enhancement by leptin [60,139].
Analogous to other dual actions of leptin, the hormone is able to trigger both stimulatory and inhibitory effects on PKC. The release of insulin from pancreatic islets of ob/ob mice in response to PKC stimulation has been shown to be blunted by leptin [72]. It has been observed further that the ability of leptin to lower glucose-mediated insulin secretion was correlated with its capacity to decrease the activity of Ca2+-dependent PKC. There is also evidence suggesting that leptin action in pancreatic islets may block the PKC-regulated component of the phospholipase C–PKC signalling system that is involved physiologically in insulin secretion [72].
AMPK (5′-AMP-activated protein kinase)
It has been clearly established that leptin stimulates fatty acid oxidation, thereby exerting a protective effect against lipotoxicity in non-adipose tissues [11]. However, the signalling elements counteracting lipotoxicity were not fully understood until some years ago when a novel pathway participating in leptin's action on metabolism was identified [140]. Leptin was observed to selectively activate the α2 catalytic subunit of AMPK in skeletal muscle, which stimulates fatty-acid oxidation by blocking the effect of ACC (acetyl-CoA carboxylase). AMPK represents a heterotrimeric enzyme that functions as a ‘fuel gauge’ to monitor cellular energy status [141,142]. AMPK regulates food intake by responding to hormonal and nutrient signals in the hypothalamus [141]. Activation of AMPK represents a signal to shut down anabolic pathways and to promote catabolic processes in response to a decrease in the ATP/AMP ratio by phosphorylating key enzymes of intermediary metabolism. In parallel with AMPK activation, leptin suppresses ACC activity, thereby stimulating β-oxidation in muscle by disinhibiting CTP1 (carnitine palmitoyltransferase 1). After leptin treatment, the increased AMP levels activate AMPK after only 15 min. This rapid response relies on a direct effect following leptin binding to OB-Rb in skeletal muscle, although the exact post-receptor activation needs to be elucidated fully. It is not yet clear how leptin increases AMP levels and activates AMPK when targeting muscle cells directly. Leptin is also able to cause a similar effect, although with a time-lag, by acting through the α-adrenergic system as a result of hypothalamic stimulation [60,142–144]. The direct activation of AMPK by leptin also explains, at least in part, the findings that leptin increases both in vitro and in vivo glucose uptake and metabolism.
OTHER SIGNALLING CASCADES ACTIVATED BY LEPTIN
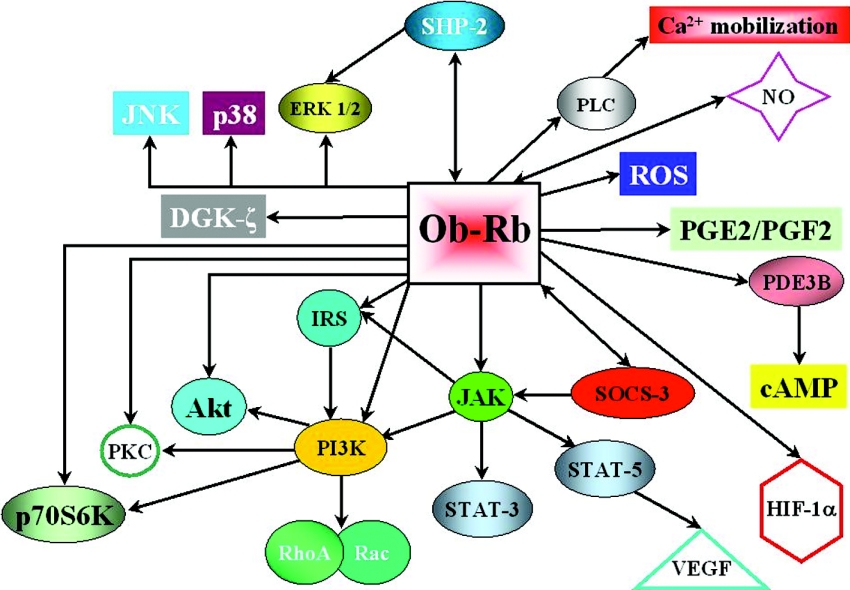
Leptin has been discovered to act as a multifunctional cytokine in all tissues, being involved in many cellular functions throughout the whole body. To develop its widespread effects, leptin interacts with many signalling factors, cross-talking with different signal transduction pathways through its ubiquitous receptors (Figure 8). The main cascades activated by leptin have been described above. However, there are several other signalling events in which leptin has been observed to be involved. Some of them have been clearly established, while others are only beginning to unfold.
Figure 8. Signalling pathways reported to be regulated by leptin.
The schematic diagram summarizes the numerous and diverse pathways in which leptin has been shown to be involved. DGK, diacylglycerol kinase; PG, prostaglandin; PLC, phospholipase C; ROS, reactive oxygen species. Modified from Cellular Signalling, vol. 14, Sweeney, G., ‘Leptin signalling’, pp. 655–663, © 2002, with permission from Elsevier.
The relevance of leptin in the pathogenesis of cardiovascular complications associated with obesity is being extensively studied [41,145]. Taking into consideration the morphological and physiological resemblance between NO (nitric oxide) and leptin, the potential functional relationship between them has been established [2]. Leptin administration has been shown to increase serum NO concentrations [29]. In addition, NO has been observed to facilitate leptin-induced lipolysis [146]. As regards blood pressure homoeostasis, leptin has been shown to be involved in vascular tone control by simultaneously producing a neurogenic pressor action and an opposing NO-mediated depressor effect [29]. Subsequent studies have disentangled further the participation of leptin in blood pressure regulation, showing that the inhibition of angiotensin II-induced intracellular calcium increase and vasoconstriction elicited by leptin is via an NO-dependent mechanism [147]. Moreover, it has been shown recently that the leptin-induced NO production in white adipocytes is mediated through PKA and MAPK activation [125].
Among the extraneural effects of leptin, one of the first to be identified was the participation of the hormone in angiogenesis [27]. It was observed that endothelial cells express functionally competent OB-R. Leptin has been shown to cause cultured endothelial cells to aggregate, form tubes and display a reticular array reminiscent of tissue vasculature. The effects, tested both in vitro and in vivo, indicate that leptin contributes to the promotion of angiogenic processes [27,148].
The angiogenic effect of leptin suggests several intriguing possibilities. One is that leptin contributes to the formation of the new blood vessels needed when the fat mass increases in volume, thus driving the blood vessels to match the amount of fat. The hormone produced by adipocytes not only is secreted into the bloodstream, but also may act locally upon endothelial cells in a paracrine fashion, assuring an appropriate balance between blood supply and fat depot size [9]. However, it does not appear to be essential for this function, because the enormous fat depots in mutant mice that completely lack leptin manage to recruit an adequate blood supply. Cell growth, cell migration and angiogenesis are normal biological processes hijacked by tumour cells to promote tumour proliferation and invasion. Being an angiogenic factor, leptin may be deployed by some cancers to recruit blood vessels. Both primary tumour growth and the formation of metastasis depend on the establishment of new blood vessels. It is interesting to note that rat insulinoma-derived pancreatic β-cells express a functional leptin receptor that mediates a proliferative response [149]. Analogously, OB-Rs have been shown to be expressed in human colon cancer cell lines as well as in human colonic tissue [150]. In addition, stimulation of colonic epithelial cells with leptin has been reported to increase proliferation both in vitro and in vivo.
To maintain oxygen homoeostasis, the mammalian microvasculature undergoes dramatic reorganization in order to supply oxygen and nutrients to hypoxic tissues. Several transcription factors operate to promote angiogenesis, sensing the environmental cues that drive the process. One of the factors that stands out is HIF-1α (hypoxia-inducible factor 1α), which acts as a master transcription switch for the regulation of oxygen homoeostasis [151]. In physiological circumstances, HIF-1α is targeted for ubiquitination and rapid degradation. Under hypoxic conditions, HIF-1α is activated through PI3K/Akt and MAPK/ERK pathways. HIF-1α then translocates to the nucleus and binds to the promoters of genes involved in angiogenesis, such as the gene encoding VEGF (vascular endothelial growth factor) [151]. Interestingly, the JAK2/STAT5 pathway has been shown to be involved in mediating processes related to angiogenesis [152]. A key molecule in this axis is VEGF, whose tyrosine kinase receptor has been demonstrated to exhibit specific STAT activation capabilities. Consistent with their complementary role in angiogenesis, VEGF and STATs are both sensitive to diverse cellular stressors, including hypoxia, which is a well-known stimulator of leptin synthesis and secretion [9]. Hypoxia markedly enhances the expression of leptin and VEGF as well as stimulating HIF-1α [153]. In fact, HIF-1α has been shown to transactivate the leptin gene promoter and to act synergistically with insulin, regulating, via different transcriptional elements, the human leptin promoter [154,155]. The relevance of the VEGF/leptin/HIF-1α axis merits detailed consideration as regards processes involving neovascularization, such as tumour growth, diabetic retinopathy, adipose mass enlargement, atherosclerosis and liver regeneration.
The impact of leptin on several equally relevant signalling pathways extends also to Rho family GTPases, which are implicated in numerous cellular processes, such as apoptosis and regulation of the actin–myosin cytoskeleton [72]. Remodelling of the actin cytoskeleton represents a plausible key element in the promotion of invasiveness of colon epithelial cells in response to leptin, since leptin's effect was potentiated by constitutively active RhoA and decreased by dominant-negative RhoA, Rac1 or the p110α catalytic subunit of PI3K [72]. There are many other signalling molecules that interact with leptin at different levels that might deserve more careful consideration, e.g. leptin's participation in the production of reactive oxygen species, stimulation of prostaglandins, binding to diacylglycerol kinase-ς, activation of the p90 ribosomal protein S6 and p70S6K, as well as catecholamine secretion [72,126,156–159].
PERSPECTIVES
Leptin has contributed significantly to broadening our understanding of the intracellular signalling cascades involved in the development of a myriad of neuroendocrine functions. The almost ubiquitous distribution of leptin receptors in peripheral tissues has provided a fertile area for investigation, and a more dynamic view of leptin has started to unfold. The notion of a mere lipostatic factor has been surpassed by that of a pleiotropic leptin system. The ability of leptin to activate several different pathways, apart from its central role in energy homoeostasis, has been uncovered. However, many keys remain to be deciphered with regard to leptin production, rhythmicity, transport, intracellular pathways and their teleological meaning. The relative contribution of the complex network of signalling pathways that participate in the periphery remains to be determined fully. It will be interesting to gain more insight into how the different pathways downstream of leptin are integrated in the diverse peripheral tissues [160,161]. It will be also worthwhile to focus on how leptin signalling integrates with the intracellular cascades activated by other more recently discovered hormones, adipokines, receptors, channels and peptides both in the central nervous system as well as in the periphery, such as resistin [162,163], ghrelin [164], adiponectin [165], peptide YY3-36 [166], visfatin [167], vaspin (visceral adipose tissue-derived serine protease inhibitor) [168], endocannabinoids [169,170], aquaporin-7 [171] and FAT-ATTAC (fat apoptosis through targeted activation of caspase 8) [172]. In addition, major advances in the molecular mechanisms underlying leptin resistance and its consequences are to be expected. The diverse mechanisms that link leptin signalling in the brain, as well as in peripheral tissues, will clarify the pathogenesis not only of obesity but also of other associated diseases. To close the gaps or to complete the signalling network map for leptin's actions will provide valuable information with regard to the knowledge of the complex signalling patterns characteristic of cell biology, as well as opening up potentially effective ways for therapeutic manipulation. Undoubtedly, given leptin's versatile and ever-expanding list of activities and involvement in signalling cascades, additional and unexpected consequences of leptin action are sure to emerge. The intense investigations under way on many different frontiers of leptin research will add more information to the already large body of knowledge. Disentangling the biochemical and molecular mechanisms in which leptin is involved represents an exciting challenge ahead.
Acknowledgments
This work was supported by a grant from the Spanish Ministerio de Ciencia y Tecnología from the Plan Nacional de I+D+I (SAF2003-09225). The PIUNA (Plan Investigación Universidad de Navarra) Foundation is also gratefully acknowledged.
References
- 1.Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J. M. Positional cloning of the mouse obese gene and its human homologue. Nature (London) 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Frühbeck G., Gómez-Ambrosi J., Muruzábal F. J., Burrell M. A. The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am. J. Physiol. Endocrinol. Metab. 2001;280:E827–E847. doi: 10.1152/ajpendo.2001.280.6.E827. [DOI] [PubMed] [Google Scholar]
- 3.Frühbeck G., Gómez-Ambrosi J. Rationale for the existence of additional adipostatic hormones. FASEB J. 2001;15:1996–2006. doi: 10.1096/fj.00-0829hyp. [DOI] [PubMed] [Google Scholar]
- 4.Mora S., Pessin J. E. An adipocentric view of signaling and intracellular trafficking. Diabetes Metab. Res. Rev. 2002;18:345–356. doi: 10.1002/dmrr.321. [DOI] [PubMed] [Google Scholar]
- 5.Seeley R. J., Woods S. C. Monitoring of stored and available fuel by the CNS: implications for obesity. Nat. Rev. Neurosci. 2003;4:901–909. doi: 10.1038/nrn1245. [DOI] [PubMed] [Google Scholar]
- 6.Flier J. S. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–350. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- 7.Banks W. A. The many lives of leptin. Peptides. 2004;25:331–338. doi: 10.1016/j.peptides.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Frühbeck G., Jebb S. A., Prentice A. M. Leptin: physiology and pathophysiology. Clin. Physiol. 1998;18:399–419. doi: 10.1046/j.1365-2281.1998.00129.x. [DOI] [PubMed] [Google Scholar]
- 9.Frühbeck G. A heliocentric view of leptin. Proc. Nutr. Soc. 2001;60:301–318. doi: 10.1079/pns200196. [DOI] [PubMed] [Google Scholar]
- 10.Ravussin E. Cellular sensors of feast and famine. J. Clin. Invest. 2002;109:1537–1540. doi: 10.1172/JCI16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unger R. H. The hyperleptinemia of obesity – regulator of caloric surpluses. Cell. 2004;117:145–151. doi: 10.1016/s0092-8674(04)00339-3. [DOI] [PubMed] [Google Scholar]
- 12.Madej T., Boguski M. S., Bryant S. H. Threading analysis suggests that the obese gene product may be a helical cytokine. FEBS Lett. 1995;373:13–18. doi: 10.1016/0014-5793(95)00977-h. [DOI] [PubMed] [Google Scholar]
- 13.Zhang F., Basinski M. B., Beals J. M., Briggs S. L., Churgay L. M., Clawson D. K., DiMarchi R. D., Furman T. C., Hale J. E., Hsiung H. M., et al. Crystal structure of the obese protein leptin-E100. Nature (London) 1997;387:206–209. doi: 10.1038/387206a0. [DOI] [PubMed] [Google Scholar]
- 14.Kline A. D., Becker G. W., Churgay L. M., Landen B. E., Martin D. K., Muth W. L., Rathnachalam R., Richardson J. M., Schoner B., Ulmer M., Hale J. E. Leptin is a four-helix bundle: secondary structure by NMR. FEBS Lett. 1997;407:239–242. doi: 10.1016/s0014-5793(97)00353-0. [DOI] [PubMed] [Google Scholar]
- 15.Prolo P., Wong M., Licinio J. Leptin. Int. J. Biochem. Cell Biol. 1998;30:1285–1290. doi: 10.1016/s1357-2725(98)00094-6. [DOI] [PubMed] [Google Scholar]
- 16.Masuzaki H., Ogawa Y., Sagawa N., Hosoda K., Matsumoto T., Mise H., Nishimura H., Yoshimasa Y., Tanaka I., Mori T., Nakao K. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat. Med. 1997;3:1029–1033. doi: 10.1038/nm0997-1029. [DOI] [PubMed] [Google Scholar]
- 17.Bado A., Levasseur S., Attoub S., Kermorgant S., Laigneau J. P., Bortoluzzi M. N., Moizo L., Lehy T., Guerre-Millo M., Le Marchand-Brustel Y., Lewin M. J. The stomach is a source of leptin. Nature (London) 1998;394:790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- 18.Morash B., Li A., Murphy P. R., Wilkinson M., Ur E. Leptin gene expression in the brain and pituitary gland. Endocrinology. 1999;140:5995–5998. doi: 10.1210/endo.140.12.7288. [DOI] [PubMed] [Google Scholar]
- 19.Ahima R. S., Flier J. S. Leptin. Annu. Rev. Physiol. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 20.Frühbeck G. Peripheral actions of leptin and its involvement in disease. Nutr. Rev. 2002;60:S47–S55. doi: 10.1301/002966402320634931. [DOI] [PubMed] [Google Scholar]
- 21.Baratta M. Leptin – from a signal of adiposity to a hormonal mediator in peripheral tissues. Med. Sci. Monit. 2002;8:RA282–RA292. [PubMed] [Google Scholar]
- 22.Muoio D. M., Dohm G. L. Peripheral metabolic actions of leptin. Best Pract. Res. Clin. Endocrinol. Metab. 2002;16:653–666. doi: 10.1053/beem.2002.0223. [DOI] [PubMed] [Google Scholar]
- 23.Harvey J., Ashford M. L. J. Leptin in the CNS: much more than a satiety signal. Neuropharmacology. 2003;44:845–854. doi: 10.1016/s0028-3908(03)00076-5. [DOI] [PubMed] [Google Scholar]
- 24.Bjørbæck C., Kahn B. B. Leptin signaling in the central nervous system and the periphery. Rec. Prog. Horm. Res. 2004;59:305–331. doi: 10.1210/rp.59.1.305. [DOI] [PubMed] [Google Scholar]
- 25.Cioffi J. A., Shafer A. W., Zupancic T. J., Smith-Gbur J., Mikhail A., Platika D., Snodgrass H. R. Novel B219/OB receptor isoforms: possible role of leptin in hematopoiesis and reproduction. Nat. Med. 1996;2:585–589. doi: 10.1038/nm0596-585. [DOI] [PubMed] [Google Scholar]
- 26.Gainsford T., Willson T. A., Metcalf D., Handman E., McFarlane C., Ng A., Nicola N. A., Alexander W. S., Hilton D. J. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc. Natl. Acad. Sci. U.S.A. 1996;93:14564–14568. doi: 10.1073/pnas.93.25.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sierra-Honigmann M. R., Nath A. K., Murakami C., Garcia-Cardena G., Papapetropoulos A., Sessa W. C., Madge L. A., Schechner J. S., Schwabb M. B., Polverini P. J., Flores-Riveros J. R. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 28.Holness M. J., Munns M. J., Sugden M. C. Current concepts concerning the role of leptin in reproductive function. Mol. Cell. Endocrinol. 1999;157:11–20. doi: 10.1016/s0303-7207(99)00126-4. [DOI] [PubMed] [Google Scholar]
- 29.Frühbeck G. Pivotal role of nitric oxide in the control of blood pressure following leptin administration. Diabetes. 1999;48:903–908. doi: 10.2337/diabetes.48.4.903. [DOI] [PubMed] [Google Scholar]
- 30.Ducy P., Amling M., Takeda S., Priemel M., Schilling A. F., Beil F. T., Shen J., Vinson C., Rueger J. M., Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:195–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 31.Frank S., Stallmeyer B., Kämpfer H., Kolb N., Pfeilschifter J. Leptin enhances wound re-epithelialization and constitutes a direct function of leptin in skin repair. J. Clin. Invest. 2000;106:501–509. doi: 10.1172/JCI9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frühbeck G., Salvador J. Relation between leptin and the regulation of glucose metabolism. Diabetologia. 2000;43:3–12. doi: 10.1007/s001250050002. [DOI] [PubMed] [Google Scholar]
- 33.Shimokawa I., Higami Y. Leptin signaling and aging: insight from caloric restriction. Mech. Ageing Dev. 2001;122:1511–1519. doi: 10.1016/s0047-6374(01)00284-6. [DOI] [PubMed] [Google Scholar]
- 34.Frühbeck G., Gómez-Ambrosi J. Control of body weight: a physiologic and transgenic perspective. Diabetologia. 2003;46:143–172. doi: 10.1007/s00125-003-1053-4. [DOI] [PubMed] [Google Scholar]
- 35.Shalitin S., Phillip M. Role of obesity and leptin in the pubertal process and pubertal growth – a review. Int. J. Obes. 2003;27:869–874. doi: 10.1038/sj.ijo.0802328. [DOI] [PubMed] [Google Scholar]
- 36.Takeda S., Elefteriou F., Karsenty G. Common endocrine control of body weight, reproduction, and bone mass. Annu. Rev. Nutr. 2003;23:403–411. doi: 10.1146/annurev.nutr.23.011702.073312. [DOI] [PubMed] [Google Scholar]
- 37.Chehab F. F., Qiu J., Ogus S. The use of animal models to dissect the biology of leptin. Rec. Prog. Horm. Res. 2004;59:245–266. doi: 10.1210/rp.59.1.245. [DOI] [PubMed] [Google Scholar]
- 38.La Cava A., Matarese G. The weight of leptin in immunity. Nat. Rev. Immunol. 2004;4:371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- 39.La Cava A., Alviggi C., Matarese G. Unraveling the multiple roles of leptin in inflammation and autoimmunity. J. Mol. Med. 2004;82:4–11. doi: 10.1007/s00109-003-0492-1. [DOI] [PubMed] [Google Scholar]
- 40.Hukshorn C. J., Lindeman J. H. N., Toet K. H., Saris W. H. M., Eilers P. H. C., Westerterp-Plantenga M. S., Kooistra T. Leptin and the proinflammatory state associated with human obesity. J. Clin. Endocrinol. Metab. 2004;89:1773–1778. doi: 10.1210/jc.2003-030803. [DOI] [PubMed] [Google Scholar]
- 41.Correia M. L., Haynes W. G. Leptin, obesity and cardiovascular disease. Curr. Opin. Nephrol. Hypertens. 2004;13:215–223. doi: 10.1097/00041552-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Seufert J. Leptin effects on pancreatic β-cell gene expression and function. Diabetes. 2004;53(suppl. 1):S152–S158. doi: 10.2337/diabetes.53.2007.s152. [DOI] [PubMed] [Google Scholar]
- 43.Utsumi H., Iwakiri R., Fujise T., Sakata H., Shimoda R., Amemori S., Tsunada S., Ootani A., Fujimoto K. Intracerebroventricular administration of leptin-induced apoptosis in the rat small intestinal mucosa. Exp. Biol. Med. 2003;228:1239–1244. doi: 10.1177/153537020322801022. [DOI] [PubMed] [Google Scholar]
- 44.VanPatten S., Karkanias G. B., Rossetti L., Cohen D. E. Intracerebroventricular leptin regulates hepatic cholesterol metabolism. Biochem. J. 2004;379:229–233. doi: 10.1042/BJ20040134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sahu A. Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front. Neuroendocrinol. 2004;24:225–253. doi: 10.1016/j.yfrne.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Thomas T. The complex effects of leptin on bone metabolism through multiple pathways. Curr. Opin. Pharmacol. 2004;4:295–300. doi: 10.1016/j.coph.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Peelman F., Waelput W., Iserentant H., Lavens D., Eyckerman S., Zabeau L., Tavernier J. Leptin: linking adipocyte metabolism with cardiovascular and autoimmune diseases. Prog. Lipid Res. 2004;43:283–301. doi: 10.1016/j.plipres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Somasundar P., McFadden D. W., Hileman S. M., Vona-Davis L. Leptin is a growth factor in cancer. J. Surg. Res. 2004;116:337–349. doi: 10.1016/j.jss.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Houseknecht K. L., Mantzoros C. S., Kuliawat R., Hadro E., Flier J. S., Kahn B. B. Evidence for leptin binding proteins in serum of rodents and humans: modulation with obesity. Diabetes. 1996;45:1638–1643. doi: 10.2337/diab.45.11.1638. [DOI] [PubMed] [Google Scholar]
- 50.Sinha M. K., Opentanova I., Ohannesian J. P., Kolaczynski J. W., Heiman M. L., Hale J., Becker G. W., Bowsher R. R., Stephens T. W., Caro J. F. Evidence of free and bound leptin in human circulation. Studies in lean and obese subjects and during short-term fasting. J. Clin. Invest. 1996;98:1277–1282. doi: 10.1172/JCI118913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heaney M. L., Golde D. W. Soluble hormone receptors. Blood. 1993;82:1945–1948. [PubMed] [Google Scholar]
- 52.Cumin F., Baum H.-P., Levens N. Leptin is cleared from the circulation primarily by the kidney. Int. J. Obes. 1996;20:1120–1126. [PubMed] [Google Scholar]
- 53.Cumin F., Baum H.-P., de Gasparo M., Levens N. Removal of endogenous leptin from the circulation by the kidney. Int. J. Obes. 1997;21:495–504. doi: 10.1038/sj.ijo.0800428. [DOI] [PubMed] [Google Scholar]
- 54.Tartaglia L. A., Dembski M., Weng X., Deng N., Culpepper J., Devos R., Richards G. J., Campfield L. A., Clark F. T., Deeds J., et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 55.Lee G.-H., Proenca R., Montez J. M., Carroll K. M., Darvishzadeh J. G., Lee J. I., Friedman J. M. Abnormal splicing of the leptin receptor in diabetic mice. Nature (London) 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 56.Löllmann B., Grüninger S., Stricker-Krongrad A., Chiesi M. Detection and quantification of the leptin receptor splice variants Ob-Ra, b, and e in different mouse tissues. Biochem. Biophys. Res. Commun. 1997;238:648–652. doi: 10.1006/bbrc.1997.7205. [DOI] [PubMed] [Google Scholar]
- 57.Tartaglia L. A. The leptin receptor. J. Biol. Chem. 1997;272:6093–6096. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 58.Myers M. G., Jr Leptin receptor signaling and the regulation of mammalian physiology. Rec. Prog. Horm. Res. 2004;59:287–304. doi: 10.1210/rp.59.1.287. [DOI] [PubMed] [Google Scholar]
- 59.Bazan J. F. A novel family of growth factor receptors: a common binding domain in the growth hormone, prolactin, erythropoietin and IL-6 receptors, and the p75 IL-2 receptor β chain. Biochem. Biophys. Res. Commun. 1989;164:788–795. doi: 10.1016/0006-291x(89)91528-3. [DOI] [PubMed] [Google Scholar]
- 60.Hegyi K., Fülöp K., Kovács K., Tóth S., Falus A. Leptin-induced signal transduction pathways. Cell Biol. Int. 2004;28:159–169. doi: 10.1016/j.cellbi.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 61.Bazan J. F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc. Natl. Acad. Sci. U.S.A. 1990;87:6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kishimoto T., Akira S., Narazaki M., Taga T. Interleukin-6 family of cytokines and gp130. Blood. 1995;86:1243–1254. [PubMed] [Google Scholar]
- 63.Heim M. H. The Jak-STAT pathway: specific signal transduction from the cell membrane to the nucleus. Eur. J. Clin. Invest. 1996;26:1–12. doi: 10.1046/j.1365-2362.1996.103248.x. [DOI] [PubMed] [Google Scholar]
- 64.Wang M.-Y., Zhou Y. T., Newgard C. B., Unger R. H. A novel leptin receptor isoform in rat. FEBS Lett. 1998;392:87–90. doi: 10.1016/0014-5793(96)00790-9. [DOI] [PubMed] [Google Scholar]
- 65.Heshka J. T., Jones P. J. A role for dietary fat in leptin receptor, OB-Rb, function. Life Sci. 2001;69:987–1003. doi: 10.1016/s0024-3205(01)01201-2. [DOI] [PubMed] [Google Scholar]
- 66.Fong T. M., Huang R.-R., Tota M. R., Mao C., Smith T., Varnerin J., Karpitskiy V. V., Krause J. E., Van der Ploeg L. H. T. Localization of leptin binding domain in the leptin receptor. Mol. Pharmacol. 1998;53:234–240. doi: 10.1124/mol.53.2.234. [DOI] [PubMed] [Google Scholar]
- 67.Chua S. C., Jr, Chung W. K., Wu-Peng X. S., Zhang Y., Liu S.-M., Tartaglia L., Leibel R. L. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (Leptin) receptor. Science. 1996;271:994–996. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- 68.Cohen P., Zhao C., Cai X., Montez J. M., Rohani S. C., Feinstein P., Mombaerts P., Friedman J. M. Selective deletion of leptin receptor in neurons leads to obesity. J. Clin. Invest. 2001;108:1113–1121. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hileman S. M., Pierroz D. D., Masuzaki H., Bjørbæck C., El-Haschimi K., Banks W. A., Flier J. S. Characterization of short isoforms of the leptin receptor in rat cerebral microvessels and of brain uptake of leptin in mouse models of obesity. Endocrinology. 2002;143:775–783. doi: 10.1210/endo.143.3.8669. [DOI] [PubMed] [Google Scholar]
- 70.Lammert A., Kiess W., Bottner A., Glasow A., Kratzsch J. Soluble leptin receptor represents the main leptin binding activity in human blood. Biochem. Biophys. Res. Commun. 2001;283:982–988. doi: 10.1006/bbrc.2001.4885. [DOI] [PubMed] [Google Scholar]
- 71.Chan J. L., Bluher S., Yiannakouris N., Suchard M. A., Kratzsch J., Mantzoros C. S. Regulation of circulating soluble leptin receptor levels by gender, adiposity, sex steroids, and leptin: observational and interventional studies in humans. Diabetes. 2002;51:2105–2112. doi: 10.2337/diabetes.51.7.2105. [DOI] [PubMed] [Google Scholar]
- 72.Sweeney G. Leptin signalling. Cell. Signalling. 2002;14:655–663. doi: 10.1016/s0898-6568(02)00006-2. [DOI] [PubMed] [Google Scholar]
- 73.Barr V. A., Lane K., Taylor S. I. Subcellular localization and internalization of the four human leptin receptor isoforms. J. Biol. Chem. 1999;274:21416–21424. doi: 10.1074/jbc.274.30.21416. [DOI] [PubMed] [Google Scholar]
- 74.Lundin A., Rondahl H., Walum E., Wilcke M. Expression and intracellular localization of leptin receptor long isoform-GFP chimera. Biochim. Biophys. Acta. 2000;1499:130–138. doi: 10.1016/s0167-4889(00)00114-2. [DOI] [PubMed] [Google Scholar]
- 75.Belouzard S., Delcroix D., Rouillé Y. Low levels of expression of leptin receptor at the cell surface result from constitutive endocytosis and intracellular retention in the biosynthetic pathway. J. Biol. Chem. 2004;279:28499–28508. doi: 10.1074/jbc.M400508200. [DOI] [PubMed] [Google Scholar]
- 76.Baumann H., Morella K. K., White D. W., Dembski M., Bailon P. S., Kim H., Lai C.-F., Tartaglia L. A. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8374–8378. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ghilardi N., Ziegler S., Wiestner A., Stoffel R., Heim M. H., Skoda R. C. Defective STAT signaling by the leptin receptor in diabetic mice. Proc. Natl. Acad. Sci. U.S.A. 1996;93:6231–6235. doi: 10.1073/pnas.93.13.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heinrich P. C., Behrmann I., Haan S., Hermanns H. M., Müller-Newen G., Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2002;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ahima R. S., Osei S. Y. Leptin signaling. Physiol. Behav. 2004;81:223–241. doi: 10.1016/j.physbeh.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 80.Bjørbæck C., Uotani S., da Silva B., Flier J. S. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J. Biol. Chem. 1997;272:32686–32695. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- 81.Bahrenberg G., Behrmann I., Barthel A., Hekerman P., Heinrich P. C., Joost H. G., Becker W. Identification of the critical sequence elements in the cytoplasmic domain of leptin receptor isoforms required for Janus kinase/signal transducer and activator of transcription activation by receptor heterodimers. Mol. Endocrinol. 2002;16:859–872. doi: 10.1210/mend.16.4.0800. [DOI] [PubMed] [Google Scholar]
- 82.White D. W., Tartaglia L. A. Evidence for ligand-independent homo-oligomerization of leptin receptor (OB-R) isoforms: a proposed mechanism permitting productive long-form signaling in the presence of excess short-form expression. J. Cell. Biochem. 1999;73:278–288. [PubMed] [Google Scholar]
- 83.Ihle I. N., Kerr I. M. Jaks and Stats in signalling by the cytokine receptor superfamily. Trends Genet. 1995;11:69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- 84.Ihle I. N. Cytokine receptor signalling. Nature (London) 1995;377:591–594. doi: 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- 85.Ghilardi N., Skoda R. C. The leptin receptor activates janus kinase 2 and signals for proliferation in a factor-dependent cell line. Mol. Endocrinol. 1997;11:393–399. doi: 10.1210/mend.11.4.9907. [DOI] [PubMed] [Google Scholar]
- 86.Murakami M., Narazaki M., Hibi M., Yawata H., Yasukawa K., Hamaguchi M., Taga T., Kishimoto T. Critical cytoplasmic region of the interleukin 6 signal transducer gp130 is conserved in the cytokine receptor family. Proc. Natl. Acad. Sci. U.S.A. 1991;88:11349–11353. doi: 10.1073/pnas.88.24.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiang N., He T. C., Miyajima A., Wojchowski D. M. The box1 domain of the erythropoietin receptor specifies Janus kinase 2 activation and functions mitogenically within an interleukin 2 β-receptor chimera. J. Biol. Chem. 1996;271:16472–16476. doi: 10.1074/jbc.271.28.16472. [DOI] [PubMed] [Google Scholar]
- 88.Kloek C., Haq A. K., Dunn S. L. Regulation of Jak kinases by intracellular leptin receptor sequences. J. Biol. Chem. 2002;277:41547–41555. doi: 10.1074/jbc.M205148200. [DOI] [PubMed] [Google Scholar]
- 89.Kellerer M., Koch M., Metzinger E., Mushack J., Capp E., Haring H. U. Leptin activates PI-3 kinase in C2C12 myotubes via janus kinase-2 (JAK-2) and insulin receptor substrate-2 (IRS-2) dependent pathways. Diabetologia. 1997;40:1358–1362. doi: 10.1007/s001250050832. [DOI] [PubMed] [Google Scholar]
- 90.Murakami T., Yamashita T., Iida M., Kuwajima M., Shima K. A short form of leptin receptor performs signal transduction. Biochem. Biophys. Res. Commun. 1997;231:26–29. doi: 10.1006/bbrc.1996.6030. [DOI] [PubMed] [Google Scholar]
- 91.Yamashita T., Murakami T., Iida M., Kuwajima M., Shima K. Leptin receptor of Zucker fatty rat performs signal transduction. Diabetes. 1997;46:1077–1080. doi: 10.2337/diab.46.6.1077. [DOI] [PubMed] [Google Scholar]
- 92.Bjørbæck C., Elmquist J. K., Michl P., Ahima R. S., van Bueren A., McCall A. L., Flier J. S. Expression of leptin receptor isoforms in rat brain microvessels. Endocrinology. 1998;139:3485–3491. doi: 10.1210/endo.139.8.6154. [DOI] [PubMed] [Google Scholar]
- 93.Hileman S. M., Tornoe J., Flier J. S., Bjørbaeck C. Transcellular transport of leptin by the short leptin receptor isoform Ob-Ra in Madin–Darby canine kidney cells. Endocrinology. 2000;141:1955–1961. doi: 10.1210/endo.141.6.7450. [DOI] [PubMed] [Google Scholar]
- 94.Nakashima K., Narazaki M., Taga T. Overlapping and distinct signals through leptin receptor (OB-R) and a closely related cytokine signal transducer, gp130. FEBS Lett. 1997;401:49–52. doi: 10.1016/s0014-5793(96)01430-5. [DOI] [PubMed] [Google Scholar]
- 95.Nakashima K., Narazaki M., Taga T. Leptin receptor (OB-R) oligomerizes with itself but not with its closely related cytokine signal transducer gp130. FEBS Lett. 1997;403:79–82. doi: 10.1016/s0014-5793(97)00013-6. [DOI] [PubMed] [Google Scholar]
- 96.Devos R., Guisez Y., Van der Heyden J., White D. W., Kalai M., Fountoulakis M., Plaetinck G. Ligand-independent dimerization of the extracellular domain of the leptin receptor and determination of the stoichiometry of leptin binding. J. Biol. Chem. 1997;272:18304–18310. doi: 10.1074/jbc.272.29.18304. [DOI] [PubMed] [Google Scholar]
- 97.White D. W., Kuropatwinski K. K., Devos R., Baumann H., Tartaglia L. A. Leptin receptor (OB-R) signaling: cytoplasmic domain mutational analysis and evidence for receptor homooligomerization. J. Biol. Chem. 1997;272:4065–4071. doi: 10.1074/jbc.272.7.4065. [DOI] [PubMed] [Google Scholar]
- 98.Eyckerman S., Broekaert D., Verhee A., Vandekerckhove J., Tavernier J. Identification of the Y985 and Y1077 motifs as SOCS3 recruitment sites in the murine leptin receptor. FEBS Lett. 2000;486:33–37. doi: 10.1016/s0014-5793(00)02205-5. [DOI] [PubMed] [Google Scholar]
- 99.Hekerman P., Zeidler J., Bamberg-Lemper S., Knobelspies H., Lavens D., Tavernier J., Joost H.-G., Becker W. Pleiotropy of leptin receptor signalling is defined by distinct roles of the intracellular tyrosines. FEBS J. 2005;272:109–119. doi: 10.1111/j.1742-4658.2004.04391.x. [DOI] [PubMed] [Google Scholar]
- 100.Münzberg H., Björnholm M., Bates S. H., Myers M. G., Jr Leptin receptor action and mechanisms of leptin resistance. Cell. Mol. Life Sci. 2005;62:642–652. doi: 10.1007/s00018-004-4432-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Banks A. S., Davis S. M., Bates S. H., Myers M. G., Jr Activation of downstream signals by the long form of the leptin receptor. J. Biol. Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 102.Bjørbæck C., Buchholz R. M., Davis S. M., Bates S. H., Pierroz D. D., Gu H., Neel B. G., Myers M. G., Jr, Flier J. S. Divergent roles of SHP-2 in ERK activation by leptin receptors. J. Biol. Chem. 2001;276:4747–4755. doi: 10.1074/jbc.M007439200. [DOI] [PubMed] [Google Scholar]
- 103.O'Rourke L., Shepherd P. R. Biphasic regulation of extracellular-signal-regulated protein kinase by leptin in macrophages: role in regulating STAT3 Ser727 phosphorylation and DNA binding. Biochem. J. 2002;364:875–879. doi: 10.1042/BJ20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bates S. H., Stearns W. H., Dundon T. A., Schubert M., Tso A. W. K., Wang Y., Banks A. S., Lavery H. J., Haq A. K., Maratos-Flier E., et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature (London) 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 105.Bates S. H., Myers M. G. The role of leptin-STAT3 signaling in neuroendocrine function: an integrative perspective. J. Mol. Med. 2004;82:12–20. doi: 10.1007/s00109-003-0494-z. [DOI] [PubMed] [Google Scholar]
- 106.Cui Y., Huang L., Elefteriou F., Yang G., Shelton J. M., Giles J. E., Oz O. K., Pourbahrami T., Lu C. Y. H., Richardson J. A., et al. Essential role of STAT3 in body weight and glucose homeostasis. Mol. Cell. Biol. 2004;24:258–269. doi: 10.1128/MCB.24.1.258-269.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gao Q., Wolfgang M. J., Neschen S., Morino K., Horvath T. L., Shulman G. I., Fu X.-Y. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc. Natl. Acad. Sci. U.S.A. 2004;101:4661–4666. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Müller P., Kuttenkeuler D., Gesellchen V., Zeidler M. P., Boutros M. Identification of JAK/STAT signalling components by genome-wide RNA interference. Nature (London) 2005;436:871–875. doi: 10.1038/nature03869. [DOI] [PubMed] [Google Scholar]
- 109.Bjørbæck C., Elmquist J. K., Frantz J. D., Shoelson S. E., Flier J. S. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol. Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 110.Bjørbæck C., El Haschimi K., Frantz J. D., Flier J. S. The role of SOCS-3 in leptin signaling and leptin resistance. J. Biol. Chem. 1999;274:30059–30065. doi: 10.1074/jbc.274.42.30059. [DOI] [PubMed] [Google Scholar]
- 111.Bjørbæck C., Lavery H. J., Bates S. H., Olson R. K., Davis S. M., Flier J. S., Myers M. G., Jr SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J. Biol. Chem. 2000;275:40649–40657. doi: 10.1074/jbc.M007577200. [DOI] [PubMed] [Google Scholar]
- 112.Münzberg H., Myers M. G., Jr Molecular and anatomical determinants of central leptin resistance. Nat. Neurosci. 2005;5:566–570. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- 113.Fairlie W. D., De Souza D., Nicola N. A., Baca M. Negative regulation of gp130 signalling mediated through tyrosine-757 is not dependent on the recruitment of SHP2. Biochem. J. 2003;372:495–502. doi: 10.1042/BJ20030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dunn S. L., Björnholm M., Bates S. H., Chen Z., Seifert M., Myers M. G., Jr Feedback inhibition of leptin receptor/Jak2 signaling via Tyr1138 of the leptin receptor and suppressor of cytokine signaling 3. Mol. Endocrinol. 2005;19:925–938. doi: 10.1210/me.2004-0353. [DOI] [PubMed] [Google Scholar]
- 115.Sasaki A., Yasukawa H., Suzuki A., Kamizono S., Syoda T., Kinjyo I., Sasaki M., Johnston J. A., Yoshimura A. Cytokine-inducible SH2 protein-3 (CIS3/SOCS3) inhibits Janus tyrosine kinase by binding through the N-terminal kinase inhibitory region as well as SH2 domain. Genes Cells. 1999;4:339–351. doi: 10.1046/j.1365-2443.1999.00263.x. [DOI] [PubMed] [Google Scholar]
- 116.Howard J. K., Cave B. J., Oksanen L. J., Tzameli I., Bjørbæk C., Flier J. S. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat. Med. 2004;10:734–738. doi: 10.1038/nm1072. [DOI] [PubMed] [Google Scholar]
- 117.Mori H., Hanada R., Aki D., Mashima R., Nishinakamura H., Torisu T., Chien K. R., Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat. Med. 2004;10:739–743. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- 118.Zabolotny J. M., Bence-Hanulec K. K., Stricker-Kongrad A., Haj F., Wang Y., Minokoshi Y., Kim Y.-B., Elmquist J. K., Tartaglia L. A., Kahn B. B., Neel B. G. PTB1 regulates leptin signal transduction in vivo. Dev. Cell. 2002;2:489–495. doi: 10.1016/s1534-5807(02)00148-x. [DOI] [PubMed] [Google Scholar]
- 119.Kaszubska W., Falls H. D., Schaefer V. G., Haasch D., Frost L., Hessler P., Kroeger P. E., White D. W., Jirousek M. R., Trevillyan J. M. Protein tyrosine phosphatase 1B negatively regulates leptin signaling in a hypothalamic cell line. Mol. Cell. Endocrinol. 2002;195:109–118. doi: 10.1016/s0303-7207(02)00178-8. [DOI] [PubMed] [Google Scholar]
- 120.Cook W. S., Unger R. H. Protein tyrosine phosphatase 1B: a potential leptin resistance factor of obesity. Dev. Cell. 2002;2:385–387. doi: 10.1016/s1534-5807(02)00158-2. [DOI] [PubMed] [Google Scholar]
- 121.Elchebly M., Payette P., Michaliszyn E., Cromlish W., Collins S., Loy A. L., Normandin D., Cheng A., Himms-Hagen J., Chan C. C., et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 122.Klaman L. D., Boss O., Peroni O. D., Kim J. K., Martino J. L., Zabolotny J. M., Moghal N., Lubkin M., Kim Y. B., Sharpe A. H., et al. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein tyrosine phosphatase 1B-deficient mice. Mol. Cell. Biol. 2000;20:5479–5489. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim G. S., Hong J. S., Kim S. W., Koh J. M., An C. S., Choi J. Y., Cheng S. L. Leptin induces apoptosis via ERK/cPLA2/cytochrome c pathway in human bone marrow stromal cells. J. Biol. Chem. 2003;278:21920–21929. doi: 10.1074/jbc.M204598200. [DOI] [PubMed] [Google Scholar]
- 124.Dreyer M. G., Juge-Aubry C. E., Gabay C., Lang U., Rohner-Jeanrenaud F., Dayer J.-M., Meier C. A. Leptin activates the promoter of the interleukin-1 receptor antagonist through p42/44 mitogen-activated protein kinase and a composite nuclear factor κB/PU.1 binding site. Biochem. J. 2003;370:591–599. doi: 10.1042/BJ20021270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mehebik N., Jaubert A.-M., Sabouralt D., Giudicelli Y., Ribière C. Leptin-induced nitric oxide production in white adipocytes is mediated through PKA and MAP kinase activation. Am. J. Physiol. Cell Physiol. 2005;289:C379–C387. doi: 10.1152/ajpcell.00320.2004. [DOI] [PubMed] [Google Scholar]
- 126.van den Brink G. R., O'Toole T., Hardwick J. C., van den Boogaardt D. E., Versteeg H. H., van Deventer H. H., Peppelenbosch M. P. Leptin signaling in human peripheral blood mononuclear cells, activation of p38 and p42/44 mitogen-activated protein (MAP) kinase and p70 S6 kinase. Mol. Cell Biol. Res. Commun. 2000;4:144–150. doi: 10.1006/mcbr.2001.0270. [DOI] [PubMed] [Google Scholar]
- 127.Shen J., Sakaida I., Uchida K., Terai S., Okita K. Leptin enhances TNF-α production via p38 and JNK MAPK in LPS-stimulated Kupffer cells. Life Sci. 2005;77:1502–1515. doi: 10.1016/j.lfs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 128.Shin H.-J., Oh J., Kang S. M., Lee J. H., Shin M.-J., Hwang K.-C., Jang Y., Chung J. H. Leptin induces hypertrophy via p38 mitogen-activated protein kinase in rat vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2005;329:18–24. doi: 10.1016/j.bbrc.2004.12.195. [DOI] [PubMed] [Google Scholar]
- 129.Frühbeck G., Salvador J. Is leptin involved in the signaling cascade after myocardial ischemia and reperfusion? Circulation. 2000;101:e194. doi: 10.1161/01.cir.101.18.e194. [DOI] [PubMed] [Google Scholar]
- 130.Niswender K. D., Schwartz M. W. Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front. Neuroendocrinol. 2003;24:1–10. doi: 10.1016/s0091-3022(02)00105-x. [DOI] [PubMed] [Google Scholar]
- 131.Benomar Y., Roy A.-F., Aubourg A., Djiane J., Taouis M. Cross down-regulation of leptin and insulin receptor expression and signalling in a human neuronal cell line. Biochem. J. 2005;388:929–939. doi: 10.1042/BJ20041621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhao A. Z., Bornfeldt K. E., Beavo J. A. Leptin inhibits insulin secretion by activation of phosphodiesterase 3B. J. Clin. Invest. 1998;102:869–873. doi: 10.1172/JCI3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhao A. Z., Shinohara M. M., Huang D., Shimizu M., Eldar-Finkelman H., Krebs E. G., Beavo J. A., Bornfeldt K. E. Leptin induces insulin-like signaling that antagonizes cAMP elevation by glucagon in hepatocytes. J. Biol. Chem. 2000;275:11348–11354. doi: 10.1074/jbc.275.15.11348. [DOI] [PubMed] [Google Scholar]
- 134.Zhao A. Z., Huan J.-N., Gupta S., Pal R., Sahu A. A phosphatidylinositol 3-kinase-phosphodiesterase 3B-cyclic AMP pathway in hypothalamic action of leptin on feeding. Nat. Neurosci. 2002;5:727–728. doi: 10.1038/nn885. [DOI] [PubMed] [Google Scholar]
- 135.Niswender K. D., Morton G. J., Stearns W. H. Intracellular signaling: key enzyme in leptin-induced anorexia. Nature (London) 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 136.Rahmouni K., Haynes W. G., Morgan D. A. Intracellular mechanisms involved in leptin regulation of sympathetic outflow. Hypertension. 2003;41:763–767. doi: 10.1161/01.HYP.0000048342.54392.40. [DOI] [PubMed] [Google Scholar]
- 137.Szanto I., Kahn C. R. Selective interaction between leptin and insulin signaling pathways in a hepatic cell line. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2355–2360. doi: 10.1073/pnas.050580497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.O'Rourke L., Yeaman S. J., Shepherd P. R. Insulin and leptin acutely regulate cholesterol ester metabolism in macrophages by novel signaling pathways. Diabetes. 2001;50:955–961. doi: 10.2337/diabetes.50.5.955. [DOI] [PubMed] [Google Scholar]
- 139.Harvey J., McKay N. G., Walker K. S., van der Kaay J., Downes C. P., Ashford M. L. Essential role of phosphoinositide 3-kinase in leptin-induced KATP channel activation in the rat CRI-G1 insulinoma cell line. J. Biol. Chem. 2000;275:4660–4669. doi: 10.1074/jbc.275.7.4660. [DOI] [PubMed] [Google Scholar]
- 140.Minokoshi Y., Kim Y.-B., Peroni O. D., Fryer L. G. D., Müller C., Carling D., Kahn B. B. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature (London) 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 141.Minokoshi Y., Alquier T., Furukawa N., Kim Y.-B., Lee A., Xue B., Mu J., Foufelle F., Ferré P., Birnbaum M. J., et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature (London) 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 142.Ceddia R. B. Direct metabolic regulation in skeletal muscle and fat tissue by leptin: implications for glucose and fatty acid homeostasis. Int. J. Obes. 2005;29:1175–1183. doi: 10.1038/sj.ijo.0803025. [DOI] [PubMed] [Google Scholar]
- 143.Minokoshi Y., Kahn B. B. Role of AMP-activated protein kinase in leptin-induced fatty acid oxidation in muscle. Biochem. Soc. Trans. 2003;31:196–201. doi: 10.1042/bst0310196. [DOI] [PubMed] [Google Scholar]
- 144.Steinberg G. R., Rush J. W. E., Dyck D. J. AMPK expression and phosphorylation are increased in rodent muscle after chronic leptin treatment. Am. J. Physiol. Endocrinol. Metab. 2003;284:E648–E654. doi: 10.1152/ajpendo.00318.2002. [DOI] [PubMed] [Google Scholar]
- 145.Frühbeck G. The adipose tissue as a source of vasoactive factors. Curr. Med. Chem. Cardiovasc. Hematol. Agents. 2004;2:197–208. doi: 10.2174/1568016043356255. [DOI] [PubMed] [Google Scholar]
- 146.Frühbeck G., Gómez-Ambrosi J. Modulation of the leptin-induced white adipose tissue lipolysis by nitric oxide. Cell. Signalling. 2001;13:827–833. doi: 10.1016/s0898-6568(01)00211-x. [DOI] [PubMed] [Google Scholar]
- 147.Fortuño A., Rodríguez A., Gómez-Ambrosi J., Muñiz P., Salvador J., Díez J., Frühbeck G. Leptin inhibits angiotensin II-induced intracellular calcium increase and vasoconstriction in the rat aorta. Endocrinology. 2002;143:3555–3560. doi: 10.1210/en.2002-220075. [DOI] [PubMed] [Google Scholar]
- 148.Bouloumie A., Drexler H. C., Lafontan M., Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ. Res. 1998;83:1059–1066. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- 149.Islam M. S., Morton N. M., Hansson A., Emilsson V. Rat insulinoma-derived pancreatic β-cells express a functional leptin receptor that mediates a proliferative response. Biochem. Biophys. Res. Commun. 1997;238:851–855. doi: 10.1006/bbrc.1997.7399. [DOI] [PubMed] [Google Scholar]
- 150.Hardwick J. C., Van Den Brink G. R., Offerhaus G. J., Van Deventer S. J., Peppelenbosch M. P. Leptin is a growth factor for colonic epithelial cells. Gastroenterology. 2001;121:79–90. doi: 10.1053/gast.2001.25490. [DOI] [PubMed] [Google Scholar]
- 151.Strieter R. M. Masters of angiogenesis. Nat. Med. 2005;11:925–927. doi: 10.1038/nm0905-925. [DOI] [PubMed] [Google Scholar]
- 152.Dudley A. C., Thomas D., Best J., Jenkins A. A VEGF/JAK2/STAT5 axis may partially mediate endothelial cell tolerance to hypoxia. Biochem. J. 2005;390:427–436. doi: 10.1042/BJ20050351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lolmède K., Durand de Saint Front V., Galitzky J., Lafontan M., Bouloumié A. Effects of hypoxia on the expression of proangiogenic factors in differentiated 3T3-F442A adipocytes. Int. J. Obes. 2003;27:1187–1195. doi: 10.1038/sj.ijo.0802407. [DOI] [PubMed] [Google Scholar]
- 154.Grosfeld A., André J., Hauguel-de Mouzon S., Berra E., Pouysségur J., Guerre-Millo M. Hypoxia-inducible factor 1 transactivates the human leptin gene promoter. J. Biol. Chem. 2002;277:42953–42957. doi: 10.1074/jbc.M206775200. [DOI] [PubMed] [Google Scholar]
- 155.Meissner U., Östreicher I., Allabauer I., Rascher W., Dötsch J. Synergistic effects of hypoxia and insulin are regulated by different transcriptional elements of the human leptin promoter. Biochem. Biophys. Res. Commun. 2003;303:707–712. doi: 10.1016/s0006-291x(03)00401-7. [DOI] [PubMed] [Google Scholar]
- 156.Attoub S., Noe V., Pirola L., Bruyneel E., Chastre E., Mareel M., Wymann M. P., Gespach C. Leptin promotes invasiveness of kidney and colonic epithelial cells via phosphoinositide 3-kinase-, Rho-, and Rac-dependent signaling pathways. FASEB J. 2000;14:2329–2338. doi: 10.1096/fj.00-0162. [DOI] [PubMed] [Google Scholar]
- 157.Liu Z., Chang G.-Q., Leibowitz S. F. Diacylglycerol kinase ς in hypothalamus interacts with long form leptin receptor. J. Biol. Chem. 2001;276:5900–5907. doi: 10.1074/jbc.M007311200. [DOI] [PubMed] [Google Scholar]
- 158.El-Haschimi K., Dufresne S. D., Hirshman M. F., Flier J. S., Goodyear L. J., Bjørbæck C. Insulin resistance and lipodystrophy in mice lacking ribosomal S6 kinase 2. Diabetes. 2003;52:1340–1346. doi: 10.2337/diabetes.52.6.1340. [DOI] [PubMed] [Google Scholar]
- 159.Lappas M., Permezel M., Rice G. E. Leptin and adiponectin stimulate the release of proinflammatory cytokines and prostaglandins from human placenta and maternal adipose tissue via nuclear factor-κB, peroxisomal proliferator-activated receptor-γ and extracellularly regulated kinase1/2. Endocrinology. 2005;146:3334–3342. doi: 10.1210/en.2005-0406. [DOI] [PubMed] [Google Scholar]
- 160.Frühbeck G., Salvador S. Role of adipocytokines in metabolism and disease. Nutr. Res. 2004;24:803–826. [Google Scholar]
- 161.Zvonic S., Baugh J. E., Arbour-Reily P., Mynatt R. L., Stephens J. M. Cross-talk among gp130 cytokines in adipocytokines. J. Biol. Chem. 2005;280:33856–33863. doi: 10.1074/jbc.M508020200. [DOI] [PubMed] [Google Scholar]
- 162.Steppan C. M., Lazar M. A. The current biology of resistin. J. Int. Med. 2004;255:439–447. doi: 10.1111/j.1365-2796.2004.01306.x. [DOI] [PubMed] [Google Scholar]
- 163.Gómez-Ambrosi J., Frühbeck G. Resistin: a promising therapeutic target for the management of type 2 diabetes mellitus? Drug Des. Rev. Online. 2005;2:1–12. [Google Scholar]
- 164.Cummings D. E., Overduin J., Foster-Schubert K. E. Roles for ghrelin in the regulation of appetite and body weight. Curr. Opin. Endocrinol. Diabetes. 2005;12:72–79. doi: 10.1097/01.med.0000152035.62993.5a. [DOI] [PubMed] [Google Scholar]
- 165.Kadowaki T., Yamauchi T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 166.Hanusch-Enserer U., Roden M. News in gut–brain communication: a role of peptide YY (PYY) in human obesity and following bariatric surgery? Eur. J. Clin. Invest. 2005;35:425–430. doi: 10.1111/j.1365-2362.2005.01514.x. [DOI] [PubMed] [Google Scholar]
- 167.Fukuhara A., Matsuda M., Nishizawa M., Segawa K., Tanaka M., Kishimoto K., Matsuki Y., Murakami M., Ichisaka T., Murakami H., et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 168.Hida K., Wada J., Eguchi J., Zhang H., Baba M., Seida A., Hashimoto I., Okada T., Yasuhara A., Nakatsuka A., et al. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc. Natl. Acad. Sci. U.S.A. 2005;102:10610–10615. doi: 10.1073/pnas.0504703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Di Marzo V., Goparaju S. K., Wang L., Liu J., Batkai S., Jarai Z., Fezza F., Miura G. I., Palmiter R. D., Sugiura T., Kunos G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature (London) 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 170.Horvath T. L. Endocannabinoids and the regulation of body fat: the smoke is clearing. J. Clin. Invest. 2003;112:323–326. doi: 10.1172/JCI19376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hibuse T., Maeda N., Funahashi T., Yamamoto K., Nagasawa A., Mizunoya W., Kishida K., Inoue K., Kuriyama H., Nakamura T., et al. Aquaporin 7 deficiency is associated with development of obesity through activation of adipose glycerol kinase. Proc. Natl. Acad. Sci. U.S.A. 2005;102:10993–10998. doi: 10.1073/pnas.0503291102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pajvani U. B., Trujillo M. E., Combs T. P., Iyengar P., Jelicks L., Roth K. A., Kitsis R. N., Scherer P. E. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat. Med. 2005;11:797–803. doi: 10.1038/nm1262. [DOI] [PubMed] [Google Scholar]