Abstract
The MAPK (mitogen-activated protein kinase) pathway is a major intracellular signalling pathway involved in EGF (epithelial growth factor) receptor-mediated cell growth and differentiation. A novel function of MAPK activity in the mechanism of EGF-mediated protection of TJs (tight junctions) from H2O2 was examined in Caco-2 cell monolayers. EGF-mediated prevention of H2O2-induced increase in paracellular permeability was associated with the prevention of H2O2-induced Tyr-phosphorylation, Thr-dephosphorylation and cellular redistribution of occludin and ZO-1 (zonula occludin-1). EGF also prevented H2O2-induced disruption of the actin cytoskeleton and the dissociation of occludin and ZO-1 from the actin-rich detergent-insoluble fractions. MEK (MAPK/ERK kinase, where ERK stands for extracellular signal related kinase) inhibitors, PD98059 and U0126, completely blocked these protective effects of EGF on TJs. EGF rapidly increased the levels of phosphorylated MEK (p-MEK) in detergent-soluble fractions and phosphorylated ERK (p-ERK) in detergent-insoluble fractions. p-ERK was colocalized and co-immunoprecipitated with occludin. GST (glutathione S-transferase) pull-down assay showed that the C-terminal tail of occludin binds to p-ERK in Caco-2 cell extracts. Pair-wise binding studies using recombinant proteins demonstrated that ERK1 directly interacts with the C-terminal tail of occludin. Therefore the present study shows that ERK interacts with the C-terminal region of occludin and mediates the prevention of H2O2-induced disruption of TJs by EGF.
Keywords: barrier function, cell–cell adhesion, epithelium, ERK, occludin, mitogen-activated protein kinase (MAPK), zonula occludins-1 (ZO-1)
Abbreviations: BSA, bovine serum albumin; EGF, epidermal growth factor; ERK, extracellular-signal-related kinase; GSH, glutathione; GST, glutathione S-transferase; HRP, horseradish peroxidase; MAPK, mitogen-activated protein kinase; MDCK, Madin–Darby canine kidney; MEK, MAPK/ERK kinase; PI3K, phosphatidylinositol 3-kinase; TER, transepithelial electrical resistance; TJ, tight junction; ZO, zonula occludin
INTRODUCTION
The TJ (tight junction), located at the apical end of the lateral membrane of epithelial cells, forms a barrier to the diffusion of macromolecules such as allergens, toxins and pathogens. Disruption of TJs and the compromised barrier function play a crucial role in the pathogenesis of a number of gastrointestinal and pulmonary diseases [1–4]. The TJ is organized by the interactions between the transmembrane proteins, such as occludin, claudins and junction adhesion molecule [4–6], the intracellular plaque proteins such as ZO (zonula occludin)-1, ZO-2, ZO-3, cingulin, 7H6 and others [4,7–10], and the actin cytoskeleton [4].
A significant body of evidence indicates that TJs are associated with numerous intracellular signalling molecules, and are regulated by the activity of signal transduction pathways [4]. The integrity of the TJ in different epithelia is regulated by G-proteins [11–13], protein kinase C [14], c-Src [15], PI3K (phosphatidylinositol 3-kinase) [16], phospholipase Cγ [17] and protein phosphatase 2A [18]. Evidence suggests that these signalling activities may affect TJs by inducing phosphorylation and regulation of protein–protein interactions. TJ-proteins such as occludin and ZO-1 have been shown to undergo reversible phosphorylation on Ser, Thr and Tyr residues. Occludin is hyper phosphorylated on Ser/Thr residues [19,20] and undergoes dephosphorylation during the disruption of TJs by calcium depletion [19–21], phorbol esters [21,22] or bacterial infection [23]. On the other hand, occludin undergoes phosphorylation on Tyr residues during the disruption of TJ by oxidative stress and acetaldehyde [24–26]. Tyrosine-mediated phosphorylation of occludin decreases its interaction with ZO-1, ZO-2 and ZO-3 [27].
Oxidative stress disrupts TJs and increases paracellular permeability in a number of epithelial monolayers [28,29] including the Caco-2 cell monolayer, an intestinal epithelium [24,25,30]. Oxidative stress-induced increase in permeability is associated with Tyr-phosphorylation, redistribution from the intercellular junctions of occludin and ZO-1, and dissociation of these proteins from the actin cytoskeleton [25]. Disruption of TJs by oxidative stress is mediated by the activities of PI3K [16] and c-Src [15].
EGF (epidermal growth factor), a gastrointestinal mucosal protective factor, prevents oxidative stress-induced disruption of TJs [31]. EGF plays an important role in promoting cell growth and differentiation [32]. It is secreted in saliva and other gastrointestinal secretions at high concentrations [33] and is considered an important mucosal protective factor. In addition to its growth promoting effect, it prevents gastric ulceration induced by aspirin, stress, ethanol and acid [34–37], and attenuates the intestinal mucosal damage induced by a variety of insults [38–40]. EGF receptor activation turns on the Ras/MAPK (mitogen-activated protein kinase) pathway and mediates the promotion of cell proliferation, growth and differentiation [41]. Previous studies showed that activation of MAPK by active Raf-1 in salivary gland epithelium [42] and by active Ras in MDCK (Madin–Darby canine kidney) cells [43] disrupt the TJ. Disruption of TJs by Ras is associated with a decrease in the levels of occludin [43], whereas Raf-1 decreases the expression of claudin-1 [42]. MAPK activity was also found to be involved in the disruption of TJ by H2O2 in the endothelial cell monolayer [44] and by phorbol ester in corneal epithelium [45]. However, there is no evidence for the interaction of MAPKs with the TJ protein complex. In the present study we show that MAPK is involved in the protection of TJs in an intestinal epithelium.
The role of MAPK in the EGF-mediated prevention of oxidative stress-induced disruption of TJ was examined in Caco-2 cell monolayers. Results show that: i) EGF prevents H2O2-induced Tyr-phosphorylation, Thr-dephosphorylation and redistribution of occludin and ZO-1 from the junctions by a MAPK-dependent mechanism; ii) EGF prevents H2O2-induced disruption of actin cytoskeletal architecture and the dissociation of TJ-proteins from the actin cytoskeleton by a MAPK-dependent mechanism; iii) the active ERK (extracellular-signal-related kinase) in EGF-treated cells is localized in the actin-rich, detergent-insoluble fractions and active ERK is co-localized and co-immunoprecipitated with occludin; iv) ERK directly binds to the C-terminal region of occludin. This study for the first time shows that ERK interacts with occludin in Caco-2 cell monolayers, and plays an important role in the regulation of epithelial TJ by EGF.
MATERIALS AND METHODS
Chemicals
Cell culture reagents and supplies were purchased from GIBCO-BRL (Grand Island, NY, U.S.A.). FITC-inulin, vanadate, SDS, H2O2, EGF, GSH (glutathione)–agarose, protease inhibitors, streptavidin agarose, Protein A–Sepharose and Protein G–Sepharose were purchased from Sigma Chemical Company (St. Louis, MO, U.S.A.). PD98059 and U0126 were from Calbiochem (San Diego, CA, U.S.A.). All other chemicals were of analytical grade purchased either from Sigma Chemical Company or Fisher Scientific (Tustin, CA, U.S.A.).
Antibodies
Mouse monoclonal anti-ERK, anti-MEK (MAPK/ERK kinase), recombinant biotin-conjugated anti-phosphorylated tyrosine (p-Tyr), HRP (horseradish peroxidase)-conjugated anti-(mouse IgG), anti-(rabbit IgG) and HRP-conjugated mouse anti-GST (glutathione S-transferase) antibodies were purchased from BD Biosciences (San Jose, CA, U.S.A.). Rabbit polyclonal anti-ERK (p-Thr-202/p-Tyr-204) or anti-phosphorylated ERK (p-ERK) and anti-MEK (p-Ser-222) or anti-phosphorylated MEK (p-MEK) antibodies were purchased from Cell Signaling Technology, Inc. (Beverly, MA, U.S.A.). Goat polyclonal anti-p-ERK antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, U.S.A.). Mouse monoclonal anti-occludin, rabbit polyclonal anti-ZO-1, rabbit polyclonal antiphosphorylated threonine (p-Thr) and HRP-conjugated anti-occludin antibodies were purchased from Zymed Laboratories (South San Francisco, CA, U.S.A.). Cy3-conjugated anti-(rabbit IgG), Alexa Fluor™ 488-conjugated anti-(mouse IgG) and Alexa Fluor™ 488-conjugated phalloidin were from Molecular Probes (Eugene, OR, U.S.A.).
Cell culture
Caco-2bbe cells, obtained from Dr Jerold Turner (University of Chicago, Chicago, IL, U.S.A.), were grown under standard cell culture conditions as described previously [15,16]. Caco-2bbe is a sub-clone of the Caco-2 cell line selected on the basis of a well-developed brush border and greater expression of alkaline phosphatase. These cells are considered to differentiate better than the original Caco-2 cell line. Cells were grown on polycarbonate membranes in Transwells (12 or 24 mm; Costar, Cambridge, MA, U.S.A.) and experiments conducted 12–14 days (12 mm Transwells) or 17–19 days (24 mm Transwell) after seeding.
Treatment with oxidative stress
H2O2 (20 μM) in PBS [Dulbecco's saline containing 1.2 mM CaCl2, 1 mM MgCl2 and 0.6% (v/v) BSA] was administered to both the apical and the basal medium as previously described [15,16]. Human EGF (3–30 nM) was administered to both apical and basal medium 10 min before H2O2 administration. U0126 (10 μM) or PD98059 (30 μM) was administered to both the apical and basal media 50 min prior to EGF administration. Control cell monolayers were incubated in PBS without H2O2 and with or without inhibitors.
Measurement of TER (transepithelial electrical resistance)
TER was measured as described previously [24] using a Millicell-ERS (electrical resistance system) (Millipore, Bedford, MA, U.S.A.). TER was calculated as Ω/cm2 by multiplying it with the surface area of the monolayer. The resistance of the polycarbonate membrane in Transwells (approximately 30 Ω/cm2) was subtracted from all readings. Basal TER varied from 350–400 Ω/cm2. Changes in TER during experimental conditions were calculated as a percentage of corresponding basal values. Duplicate cell monolayers were used for each group in each experiment, and the experiment was repeated at least four times.
Unidirectional flux of inulin
Transwells with the cell monolayers were incubated under different experimental conditions in the presence of FITC-inulin (0.5 mg/ml) in the basal well. At 3 h after H2O2 treatment, 100 μl each of apical and basal media were withdrawn and the fluorescence was measured at 528 nm using a fluorescence plate reader (BioTEK Instruments, Winooski, Vermont, U.S.A.). The flux into the apical well was calculated as the percent of total fluorescence administered into the basal well h−1 per cm2 surface area. Duplicate cell monolayers were used for each group in each experiment and the experiment was repeated at least four times. Analysis of cell viability was performed at the end of experiments by lactate dehydrogenase release assay, Trypan Blue exclusion and DNA damage by propidium iodide demonstrated the absence of cell damage during these experimental conditions.
Immunofluorescence microscopy
After experimental treatments, Caco-2 cell monolayers (12 mm) were washed in PBS and fixed in acetone/methanol (1:1, v/v) at 0 °C for 5 min or in 3% paraformaldehyde for 15 min at room temperature for staining of actin. Cell monolayers were blocked in 3% non-fat milk or 1% BSA in TBS-T [20 mM Tris (pH 7.2), 0.1% (v/v) Triton X-100 and 150 mM NaCl] and incubated for 1 h with primary antibodies, rabbit polyclonal anti-ZO-1, mouse monoclonal anti-occludin, mouse monoclonal anti-ERK and rabbit polyclonal anti-p-ERK antibodies, followed by incubation for 1 h with secondary antibodies, Alexa Fluor™ 488-conjugated anti-(mouse IgG) and Cy3-conjugated anti-(rabbit IgG) antibodies. For actin staining, paraformaldehyde-fixed cells were incubated with Alexa Fluor™-conjugated phalloidin. The fluorescence was examined by a Zeiss LSM 510 laser-scanning microscope (Carl Zeiss MicroImaging, Inc., Thornnwood, NY, U.S.A.) and images from x-axes, y-axes and z-axes (sections thickness 1 μm) were collected by using LSM PASCAL software. Images were stacked by using the software, Image J and processed by using the software, Adobe Photoshop (Adobe Systems Inc., San Jose, CA, U.S.A.). Fluorescence for actin appeared in 14–16 sections. There was no significant difference in the number of sections with fluorescence among different groups. Fluorescence in the top 4–5 sections, middle 4–5 sections and bottom 4–5 sections were stacked separately for apical, middle and basal images, respectively. In some images, fluorescence intensity at the intercellular junctions was quantified using the software, Image J and the values were presented as arbitrary units.
Preparation of detergent-insoluble fractions
Cell monolayers in Transwells (24 mm) were washed twice with ice-cold PBS and incubated for 15 min with lysis buffer-CS (cytoskeleton) (Tris buffer containing 1.0% Triton X-100, 2 μg/ml leupeptin, 10 μg/ml aprotinin, 10 μg/ml bestatin, 10 μg/ml pepstatin-A, 1 mM vanadate and 1 mM PMSF) at 4 °C. Cell lysates were scraped from the substratum and passed through a pipette tip five times. It was then centrifuged at 15600 g for 4 min at 4 °C to sediment the high-density actin cytoskeleton. The pellet was resuspended in 200 μl of lysis buffer-CS. Protein contents in different fractions were measured by BCA (bicinchoninic acid) Pierce Biotechnology method (Rockford, IL, U.S.A.). Triton-insoluble and Triton-soluble fractions were mixed with equal volumes of Laemmli's sample buffer (2× concentrated) and heated at 100 °C for 5 min and stored until immunoblot analysis. Triton-insoluble and -soluble fractions were immunoblotted for occludin and ZO-1. Samples were also immunoblotted for actin as a housekeeping protein. The experiment was repeated at least twice.
Immunoprecipitation of phosphoproteins
Tyrosine phosphorylation of occludin and ZO-1 in H2O2-treated cells was previously demonstrated by immunoprecipitation of p-Tyr followed by immunoblot analysis for occludin and ZO-1, and immunoprecipitation of occludin and ZO-1 followed by immunoblot analysis for p-Tyr [25]. Immunoprecipitation of phospho-occludin with anti-p-Tyr antibody was found to be much cleaner with less non-specific binding than precipitation with anti-occludin antibody. Therefore in the present study, we chose to immunoblot anti-p-Tyr immunoprecipitates for occludin and ZO-1. After the experimental treatment, Caco-2 cell monolayers (24 mm) were washed with ice-cold 20 mM Tris (pH 7.4) and denatured protein extract was prepared in lysis buffer-D [0.3% SDS in 10 mM Tris buffer (pH 7.4), containing 1 mM vanadate and 0.33 mM PMSF] by heating at 100 °C for 10 min. Insoluble particles were centrifuged down and the supernatant was used for immunoprecipitation. Protein extracts (1.0 mg protein/ml) were incubated with 2 μg of anti-p-Thr antibodies or biotin-conjugated anti-p-Tyr antibodies at 4 °C for 16 h. Immune complexes were isolated by precipitation using Protein A–Sepharose or streptavidin–agarose (for 1 h at 4 °C). Washed beads were suspended in 20 μl of Laemmli's sample buffer for immunoblot analysis. Control binding was determined by conducting the assay using preimmune IgG or streptavidin–agarose.
Co-immunoprecipitation of occludin and p-ERK
Detergent-insoluble cell fractions were extracted in lysis buffer N [20 mM Tris (pH 7.4), containing 150 mM NaCl, 0.5% NP40, 2 μg/ml leupeptin, 10 μg/ml aprotinin, 10 μg/ml bestatin, 10 μg/ml pepstatin-A, 1 mM vanadate and 1 mM PMSF] by sonication for 10 s in an ice bath, to fragment the F-actin filaments and release the bound protein complexes as described previously [18]. F-actin fragments were pelleted down at 15600 g for 30 min and the supernatant was used for immunoprecipitation experiments. Extracts were incubated overnight at 4 °C with 2 μg of rabbit polyclonal anti-occludin or anti-p-ERK antibodies. Immunoprecipitation was carried out overnight as described above. Immune complexes were precipitated by incubation for 1 h with Protein A–Sepharose at 4 °C. Anti-occludin immunocomplexes were immunoblotted for p-ERK, and anti-p-ERK immunocomplexes were immunoblotted for occludin. Control binding was determined by performing the assay using preimmune IgG.
Immunoblot analysis
Proteins were separated by SDS/PAGE (7% or 4–12% gradient) and transferred to PVDF membranes. Membranes were blotted for occludin, ZO-1, ERK, p-ERK, MEK and p-MEK by using specific antibodies in combination with HRP-conjugated anti-(mouse IgG) or HRP-conjugated anti-(rabbit IgG) antibodies. For GST, blots were incubated with HRP-conjugated anti-GST antibody. The blot was developed using ECL™ (enhanced chemiluminescence) (Amersham, Arlington Heights, IL, U.S.A.).
Recombinant GST–occludin-C and ERK1
cDNA for C-terminal tail of chicken occludin (amino acids 354–503) in the pGEX vector was a gift from Dr J. M. Anderson and A. Fanning (University of North Carolina, Chapel Hill, NC, U.S.A.). C-terminal tail, as a GST fusion protein (GST–Occludin-C), was prepared in E. coli DH5α cells and purified using GSH–agarose as described previously [27]. Recombinant human ERK1 as a GST fusion protein was purchased from Upstate Biotech Inc. (Lake Placid, NY, U.S.A.) and incubated with thrombin in 50 mM Tris buffer containing 10 mM CaCl2 to cleave GST. GST was removed by binding to GSH-agarose.
GST pull-down assay
Protein extracts were prepared from Caco-2 cells. Confluent cell monolayers were lysed in 0.2% Triton X-100 in PBS containing 1 mM sodium orthovanadate, 10 mM sodium fluoride and 10 mM sodium pyrophosphate (3 ml/100 mm plate). Cell lysates were centrifuged at 15000 g for 15 min and the supernatant was used for pull-down assay. Cell lysate (0.6 ml) was incubated with 10 μg of GST–occludin-C and 20 μl of GSH–agarose at 4 °C for 3 h on an inverter. Agarose beads were washed 3 times with PBS and proteins extracted by heating at 100 °C for 5 min in 20 μl of Laemmli's sample buffer. The amounts of p-ERK present in GSH–agarose pull-down were determined by immunoblot analysis. Control-binding was determined by using GST instead of GST–occludin-C. At the end of the experiment, the blots were stained with Ponceau-S to confirm the use of equal amount of GST–occludin-C in different samples. Interaction between ERK1 and occludin was also confirmed by incubating GST–ERK1 with the protein extracts from Caco-2 cells. GSH–agarose pull-down was immunoblotted for occludin.
Pair-wise binding assay
To determine the direct interaction between occludin and ERK1, GST–occludin-C (10 μg) was incubated with thrombin-cleaved recombinant ERK1 (0.1–0.3 μg) in PBS containing 0.2% Triton X-100, 1 mM vanadate and 10 mM sodium fluoride for 16 h at 4 °C on an inverter. GST–occludin-C was pulled down by binding to 20 μl of GSH–agarose at 4 °C for 1 h. The amount of ERK1 bound to GSH–agarose pull-down was determined by immunoblot analysis. Non-specific binding was determined by using GST instead of GST–occludin-C. Density of ERK1 bands were quantified by densitometric analysis using the software, Image J and the density is presented as arbitrary units.
Blot overlay assay
Direct binding of ERK1 to occludin was also confirmed by slot blotting different amounts (10–30 ng) of recombinant thrombin-cleaved ERK1 to a PVDF membrane. Membrane was overlayed with 1 μg/ml of GST or GST–occludin-C for 3 h at 4 °C. Washed blots were probed for GST using HRP-conjugated anti-GST antibody.
RESULTS
EGF prevents H2O2-induced disruption of TJ and increase in paracellular permeability by a MAPK-dependent mechanism
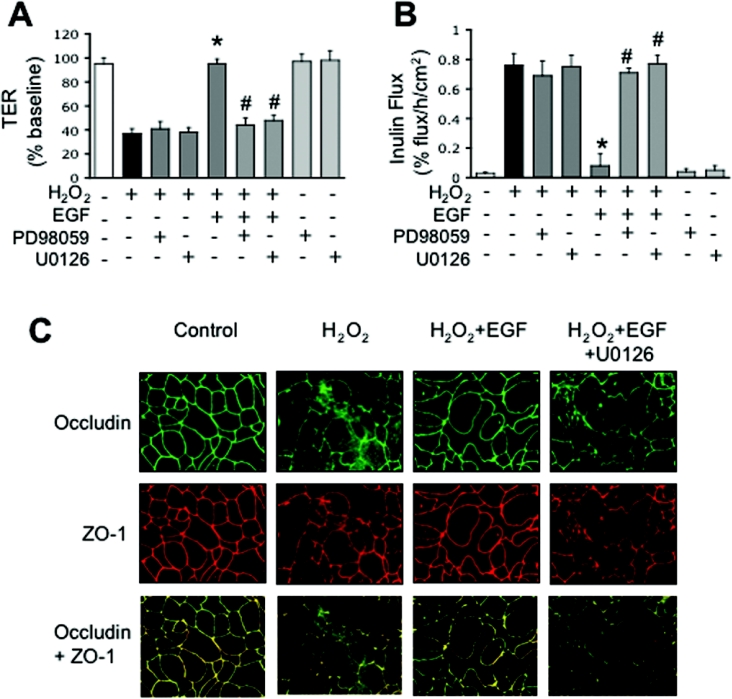
Previous studies showed that EGF prevents H2O2-induced increase in paracellular permeability in Caco-2 cell monolayers [31]. The present study confirms this effect of EGF on TER (Figure 1A) and inulin permeability (Figure 1B). One of the major intracellular signalling pathways that is activated by EGF receptor is the MAPK pathway [41]. Therefore we evaluated the effect of MEK inhibitors (PD98059 and U0126) on EGF-mediated prevention of H2O2-induced permeability. PD98059 and U0126 attenuated the EGF-mediated prevention of H2O2-induced decrease in TER (Figure 1A) and increase in inulin flux (Figure 1B). PD98059 and U0126 produced no significant effect on basal or H2O2-induced changes in TER or inulin flux. Confocal microscopy showed that treatment of Caco-2 cells with EGF dramatically prevents H2O2-induced disruption of junctional organization of occludin and ZO-1, and attenuates the redistribution of occludin and ZO-1 from the intercellular junctions (Figure 1C). EGF-mediated prevention of H2O2-induced redistribution of occludin and ZO-1 was abrogated by the treatment of cell monolayers with U0126 (Figure 1C).
Figure 1. EGF prevents H2O2-induced increases in paracellular permeability and redistribution of occludin and ZO-1 by a MAPK-dependent mechanism.
Caco-2 cell monolayers were incubated with or without 20 μM H2O2 in the presence or absence of 30 nM EGF for 3 h. In some groups cell monolayers were pretreated with PD98059 (30 μM) or U0126 (10 μM) for 1 h before administration of EGF and H2O2. TER (A) was recorded at various times and values at 3 h were calculated as percentage of basal values. Unidirectional flux of FITC-inulin (B) measured during 3 h of treatment with H2O2. Values are mean±S.E.M. (n=8). *, indicates the values that are significantly different (P<0.05) from values for H2O2; #, indicates the values that are significantly different from values for the EGF+H2O2 group. (C) Caco-2 cell monolayers were incubated with H2O2 in the presence or absence of EGF and in the presence or absence of U0126. After 3 h treatment, cell monolayers were fixed and double-labelled for occludin and ZO-1 by immunofluorescence methods. Fluorescence images were collected by confocal microscopy.
EGF prevents H2O2-induced phosphorylation of occludin by a MAPK-dependent mechanism
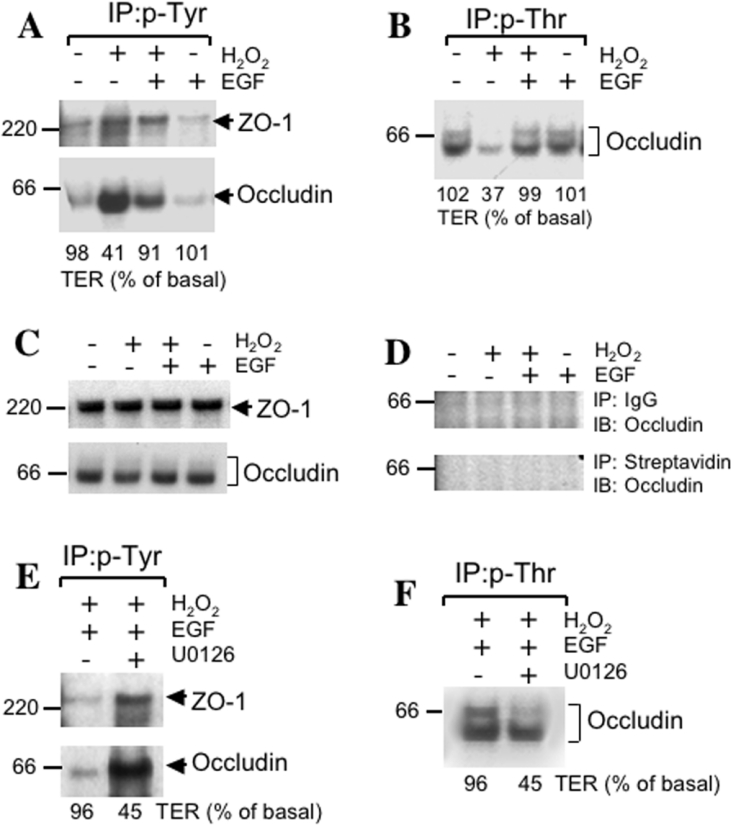
Our previous study showed that a H2O2-induced increase in paracellular permeability was associated with phosphorylation of occludin and ZO-1 on tyrosine residues [25]. Therefore the effect of EGF on Tyr-phosphorylation of occludin and ZO-1 was examined. The results show that EGF prevents H2O2-induced Tyr-phosphorylation of occludin and ZO-1 (Figure 2A). In the normal epithelium, occludin was shown to be hyperphosphorylated on Ser and Thr residues [19,20], and it is dephosphorylated during the disruption of TJ [19–23]. Therefore the effect of H2O2 and EGF on Thr-phosphorylation of occludin was examined. The treatment of cells with H2O2 resulted in dramatic decrease in the phosphorylation of occludin on threonine residues and the treatment of cells with EGF prevented this effect of H2O2 on phosphorylation of occludin on threonine residues (Figure 2B). The total amount of ZO-1 and occludin used for immunoprecipitation was similar in different groups of cells (Figure 2C). Control immunoprecipitation using preimmune IgG and Protein A–Sepharose or streptavidin–agarose showed no non-specific binding of occludin (Figure 2D). The preventive effect of EGF on H2O2-induced Tyr-phosphorylation (Figure 2E) and Thr-dephosphorylation (Figure 2F) of occludin was completely attenuated by U0126. EGF by itself produced no considerable influence on the Tyr-phosphorylation or Thr-phosphorylation of occludin (Figures 2A and 2B).
Figure 2. EGF prevents H2O2-induced alteration in phosphorylation of occludin and ZO-1 by a MAPK-dependent mechanism.
(A) and (B) Caco-2 cell monolayers were incubated with or without H2O2 in the presence or absence of 30 nM EGF for 3 h. Proteins extracted under denatured conditions were subjected to immunoprecipitation of p-Tyr (A) or p-Thr (B) followed by immunoblot analysis for occludin and ZO-1. Values at the bottom of immunoblots represent the TER values for the corresponding experiment. This experiment was repeated twice with similar results. (C) and (D) Caco-2 cell monolayers were incubated as described above for (A) and (B). Tissue extracts were either directly immunoblotted for total occludin and ZO-1 (C) or control-immunoprecipitation was performed using preimmune rabbit-IgG with Protein A–Sepharose or streptavidin–agarose followed by immunoblot analysis for occludin and ZO-1. (E) and (F) Caco-2 cell monolayers were incubated with EGF and H2O2 in the presence or absence of U0126 for 3 h. Extracted proteins were subjected to immunoprecipitation of p-Tyr (E) or p-Thr (F) followed by immunoblot analysis for occludin and ZO-1. This experiment was repeated twice with similar results.
EGF prevents H2O2-induced reorganization of actin cytoskeleton and dissociation of occludin and ZO-1 from the actin cytoskeleton by a MAPK-dependent mechanism
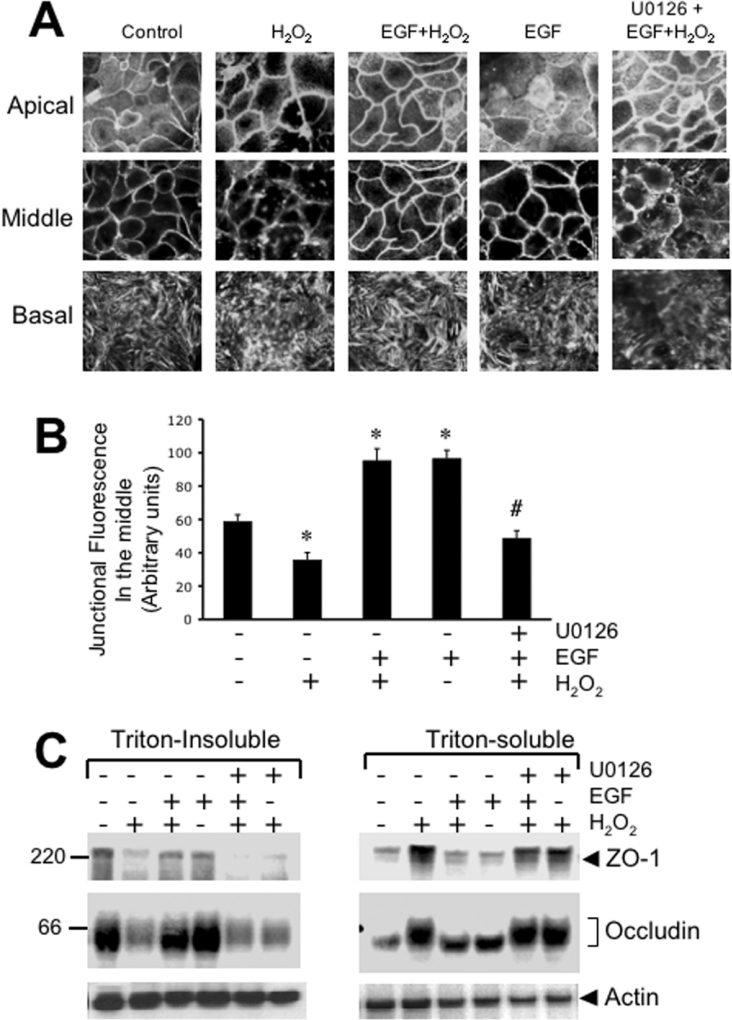
The interaction of TJ proteins with the actin cytoskeleton is essential for the maintenance of the integrity of TJ and the barrier function [4]. Our previous studies showed that the disruption of TJ by H2O2 is associated with the decrease in the levels of occludin and ZO-1 in the actin-rich detergent-insoluble fraction of the cell [25]. Therefore in the present study we examined the organization of actin filaments at the apical, middle and basal parts of the epithelium. At the apical end, actin appeared to be organized into a network, whereas the cortical network of actin was present at the middle part of the cell as a circumferential ring. At the base of the cell, actin filaments were organized into stress fibres. H2O2 treatment disrupted the organization of the actin cytoskeleton at all levels (Figure 3A). The treatment of cells with EGF prevented H2O2-induced alteration of the actin cytoskeletal architecture (Figure 3A). EGF by itself increased the intensity of actin staining in the middle part of the cell (Figure 3B). Treatment of cells with U0126 attenuated the EGF-mediated prevention of H2O2-induced changes in actin cytoskeletal architecture.
Figure 3. EGF prevents H2O2-induced reorganization of actin cytoskeleton and its interaction with TJ proteins by a MAPK-dependent mechanism.
Caco-2 cell monolayers were incubated with or without H2O2 in the presence or absence of EGF and U0126 for 3 h. (A) Cell monolayers were fixed in paraformaldehyde and stained with Alexa Fluor™ 488-conjugated phalloidin. Fluorescence images from different depths in epithelium (apical, middle and basal) were obtained by confocal microscopy. (B) Semi-quantitative analysis of junctional fluorescence in the middle part of the cells. Intensity of fluorescence at the junctions was measured over a constant area of 1 mm2 and the values are presented as arbitrary units. Values are mean±S.E.M. (n=4; each value is the average of 4 different regions from the same image). *, indicate the values that are significantly different (P<0.05) from values for control cells; # indicates the values that are significantly different from values for the EGF+H2O2 group. (C) Triton-insoluble and Triton-soluble fractions were prepared from different cell monolayers and immunoblotted for occludin, ZO-1 and actin.
Our previous study demonstrated that H2O2 rapidly reduces the association of occludin and ZO-1 with the actin-rich Triton-insoluble fractions [25]. The present study shows that EGF prevents the H2O2-induced decrease in the levels of occludin and ZO-1 in the detergent-insoluble fraction (Figure 4C). This effect of EGF was accompanied by the prevention of H2O2-induced increase in the levels of occludin and ZO-1 in Triton-soluble fractions. Treatment of cell monolayers with U0126 abolished the preventive effect of EGF on H2O2-induced changes in the levels of occludin and ZO-1 in Triton-insoluble and Triton-soluble fractions. EGF by itself produced no considerable influence on the levels of occludin or ZO-1 in the Triton-insoluble or Triton-soluble fractions.
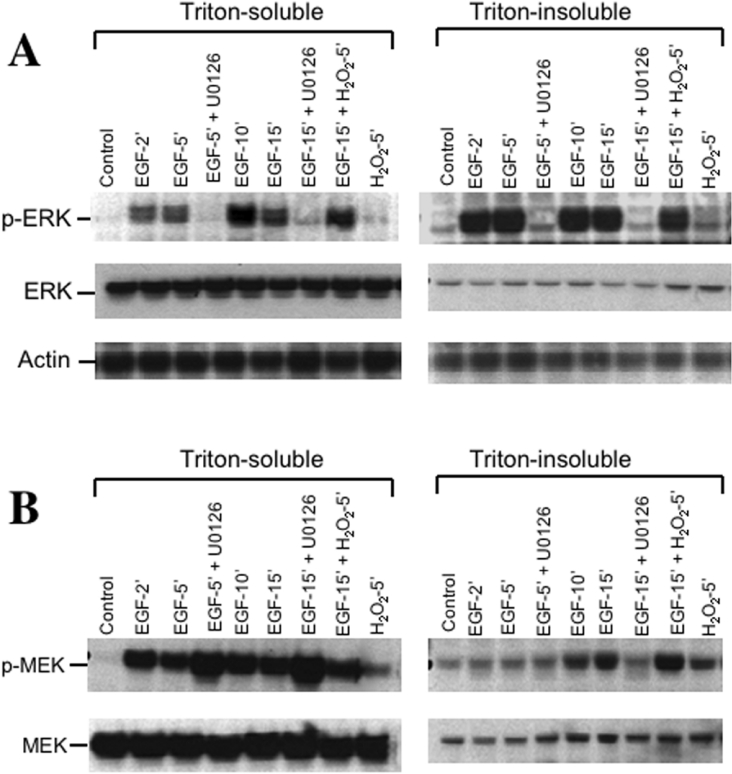
Figure 4. EGF activates ERK in Caco-2 cell monolayers by a MEK-dependent mechanism.
Caco-2 cell monolayers were pretreated with or without U0126 for 1 h followed by incubation with EGF for various times. In additional cell monolayers, H2O2 was added 10 min after EGF and the incubation was continued for a further 5 min. Triton-insoluble and Triton-soluble fractions were prepared and immunoblotted for ERK and p-ERK (A), or MEK and p-MEK (B). Samples were also immunoblotted for actin as a house keeping protein (A).
Activation and localization of ERK at the TJ
Determination of ERK activation by immunoblot analysis for p-ERK showed that EGF rapidly increased the levels of active ERK in both the Triton-soluble and Triton-insoluble fractions (Figure 4A); maximal activation was achieved by 2 min. H2O2 did not influence the activation of ERK by EGF. However, the levels of p-ERK detected were severalfold greater in Triton-insoluble fraction compared with their levels in Triton-soluble fractions. Activation of ERK was completely prevented by U0126, suggesting a role for MEK in EGF-induced activation of ERK. EGF rapidly increased the level of p-MEK (Figure 4B) and p-MEK was localized predominantly to the Triton-soluble fraction. A relatively low level of p-MEK was detected in the Triton-insoluble fraction. The inhibitor U0126 slightly potentiated the effect of EGF on the levels of p-MEK. There were no considerable changes in the levels of total ERK or total MEK under these experimental conditions (Figures 4A and 4B).
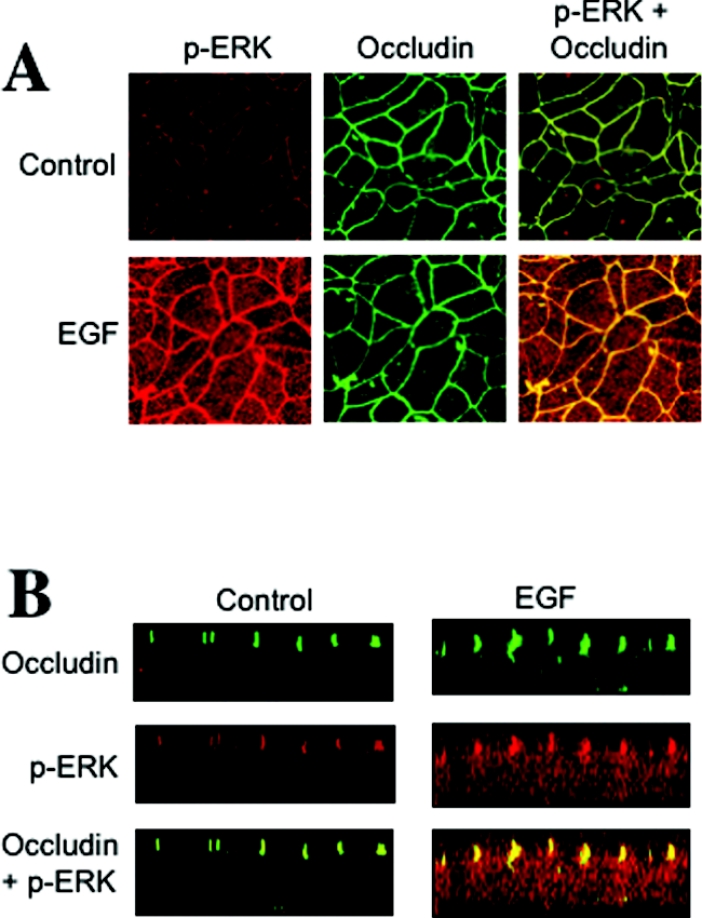
Immunofluorescence confocal microscopy showed that p-ERK is localized predominantly at the intercellular junctions, and co-localized with occludin (Figure 5A). The optical z-sections of confocal images show the co-localization of p-ERK with occludin in control cell-monolayers at the apical end of the lateral membrane (Figure 5B). The amounts of p-ERK localized at the junction were much greater in EGF-treated cell monolayers.
Figure 5. p-ERK is co-localized with occludin at the TJ in Caco-2 cell monolayers.
(A) Caco-2 cell monolayers were incubated in the presence or absence of EGF for 5 min. Cell monolayers were fixed in acetone/methanol and stained for occludin and p-ERK by immunofluorescence methods. (B) Optical z-sections of fluorescence images were collected by confocal microscopy.
Direct interaction of ERK with the C-terminal region of occludin
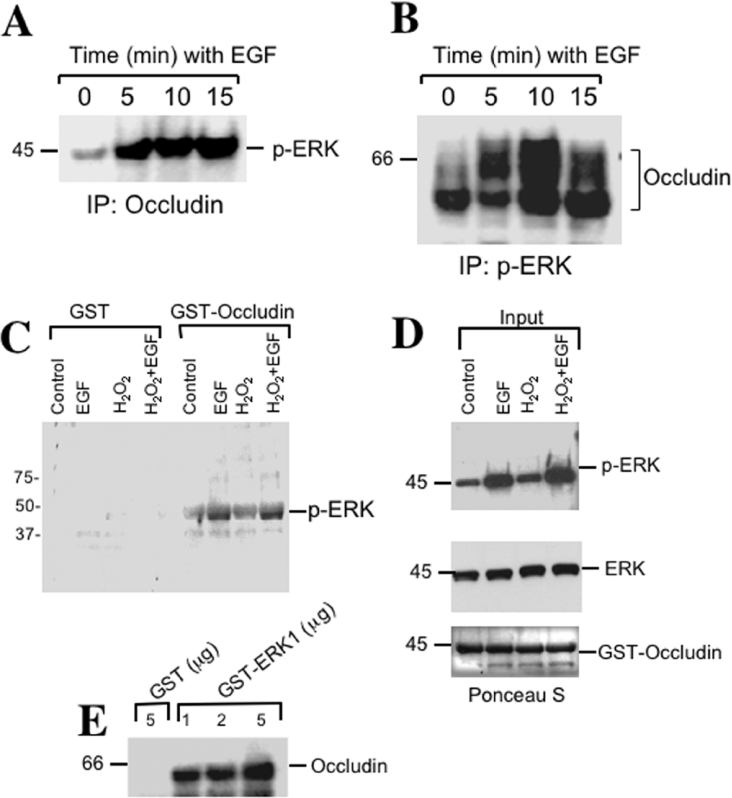
The co-localization of ERK with occludin suggested a possible interaction between occludin and ERK. To determine the association of ERK with the TJ protein complex we investigated the possibility of co-immunoprecipitation of p-ERK with occludin. Considerable levels of p-ERK were co-immunoprecipitated with occludin in control cell-monolayers and the levels of p-ERK detected in anti-occludin immunoprecipitates were much greater in EGF-treated cells (Figure 6A). The association of p-ERK with occludin was further confirmed by the immunoprecipitation of p-ERK, and subsequently immunoblot analysis for occludin (Figure 6B).
Figure 6. Phospho-ERK interacts with TJ protein complex.
(A) Caco-2 cell monolayers were incubated with EGF for various durations. Occludin was immunoprecipitated from proteins extracted from Triton-insoluble fractions under native conditions. Immunocomplexes were then immunoblotted for p-ERK. (B) After EGF treatment, p-ERK was immunoprecipitated from native protein extracts of the Triton-insoluble fractions and immunoblotted for occludin. (C) The C-terminal tail of occludin (150 amino acids) was expressed in E. coli as a GST fusion protein and incubated with Triton-soluble protein extracts from Caco-2 cells, with or without, EGF for 5 min and H2O2 for 30 min. GST–occludin was pulled-down with GSH–agarose and immunoblotted for p-ERK. Non-specific binding was determined by incubation of Triton-soluble protein extracts with GST. (D) Immunoblot analysis of p-ERK and ERK protein extracts used in (C). The blot was stained for total protein by Ponceau S dye to visualize the amount of GST–occludin. (E) GST, or various amounts of recombinant GST–ERK1 incubated with Triton-soluble protein extracts from untreated Caco-2 cells, and GSH–agarose pull-down were immunoblotted for occludin.
Occludin is a transmembrane protein and its C-terminal region extends into the intracellular compartment. The C-terminal region interacts with plaque proteins of the TJ such as ZO-1, ZO-2 and ZO-3 [27]. To determine the interaction of p-ERK with the C-terminal tail of occludin we generated recombinant GST-fused occludin-C (the C-terminal 150 amino acids). GST–occludin-C was incubated with protein extracts prepared from Caco-2 cell monolayers that were pre-incubated with or without EGF or H2O2. GST–occludin-C pulled-down p-ERK from protein extracts prepared from control cells (Figure 6C) and the binding was markedly higher in proteins extracted from EGF-treated cells. On the other hand, GST did not pull-down p-ERK from protein extracts from control or EGF-treated cells. Treatment of cells with H2O2 did not alter the binding of p-ERK to GST–occludin-C in control or EGF-treated cells. The amount of p-ERK present in the protein extracts used for the pull-down assay was much higher in cells treated with EGF (Figure 6D), whereas the amount of total ERK was unaffected. Ponceau-S staining at the end of the experiment indicated that similar amounts of GST–occludin-C were used under different experimental conditions (Figure 6D).
Interaction of ERK1 and occludin was further confirmed by incubation of GST–ERK1 with protein extracts from Caco-2 cells. Immunoblot analysis of GST–ERK1 pull-down showed the presence of occludin and the amount of occludin pulled-down was dependent on the concentration of GST–ERK1 (Figure 6E). Control-binding using GST showed the absence of non-specific binding of occludin.
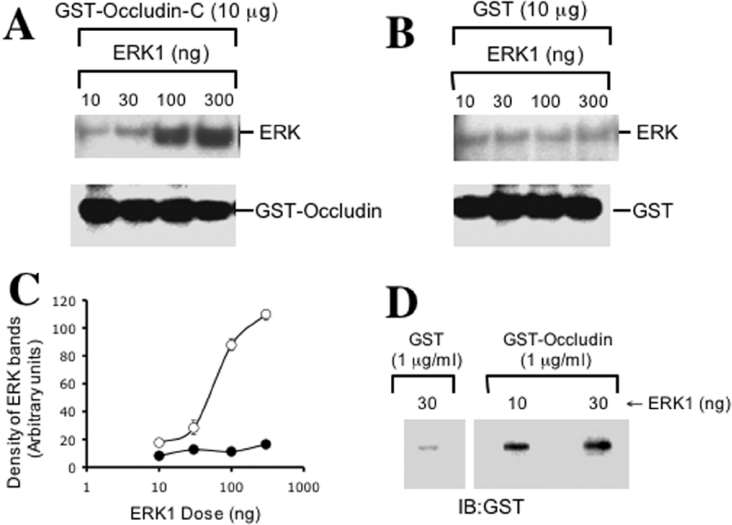
Co-immunoprecipitation studies and GST pull-down assays clearly indicate an interaction between ERK and the TJ-protein complex. However, these results do not demonstrate a direct interaction between ERK and occludin. To determine if there is a direct interaction between ERK and the C-terminal region of occludin, we studied the interaction between these two proteins by a pair-wise binding assay using GST–occludin-C and recombinant thrombin-cleaved ERK1. ERK1 binds to GST–occludin-C in a dose-dependent manner (Figures 7A and 7C), but it did not bind to GST (Figure 7B). The binding of ERK1 to occludin was further confirmed by an overlay of ERK1 slot-blots with GST–occludin-C (Figure 7D). The overlay assay confirmed that GST–occludin-C, but not GST, directly binds to ERK1.
Figure 7. ERK1 directly interacts with the C-terminal region of occludin.
(A) Various amounts of thrombin-cleaved recombinant ERK1 were incubated with GST–occludin-C. GSH–agarose pull-down was immunoblotted for ERK. Blot was also immunoblotted for GST to visualize the amount of GST–occludin used in the assay. (B) Binding assay was conducted as described in (A) using GST instead of GST–occludin to determine the non-specific binding of ERK1. (C) Densitometric analysis of ERK1 bands in panels A and B. Density is presented as arbitrary units. Values are mean±S.E.M. (n=3). (D) Thrombin-cleaved ERK1 was slot blotted on PVDF membrane and overlayed with GST–occludin-C or GST. Washed blots were then probed for GST.
DISCUSSION
Numerous injurious factors, including reactive oxygen species, compromise the epithelial TJ and the barrier function. Evidence indicates that EGF, a gastrointestinal mucosal protective factor, prevents oxidative stress-induced disruption of the intestinal epithelial TJ [31]. The present study demonstrates for the first time that ERK directly interacts with the C-terminal region of occludin and that MAPK activity is required for the EGF-mediated protection of TJ from oxidative stress. Interaction of ERK may mediate the phosphorylation of certain TJ-proteins or signalling molecules associated with the TJ-proteins and regulate the integrity of TJ and the barrier function of epithelium.
Previous studies showed that H2O2, but not superoxide or hydroxyl radical, disrupt the TJ and increase paracellular permeability in Caco-2 cell monolayers by a tyrosine kinase-dependent mechanism [24,25]. The intracellular signalling molecules such as PI3K [16] and c-Src [15] are involved in the H2O2-induced disruption of TJs. The present study confirms that EGF prevents H2O2-induced increases in paracellular permeability and demonstrates for the first time that the protection of TJs by EGF requires MAPK activity. EGFR-induced MAPK activation is the major intracellular signalling pathway that mediates the effect of EGF on cell proliferation and differentiation [41]. The attenuation of EGF-mediated prevention of H2O2-induced permeability by PD98059 and U0126 clearly demonstrate that the activity of MEK is required for EGF-mediated protection of TJ from H2O2. MEK inhibitors also abolished the EGF-mediated prevention of H2O2-induced redistribution of occludin and ZO-1 from the intercellular junctions.
A previous study demonstrated that H2O2 induces rapid increases in the Tyr-phosphorylation of occludin and ZO-1 in Caco-2 cells, and that H2O2-induced Tyr-phosphorylation of occludin and ZO-1, and the disruption of TJ were attenuated by genistein, a tyrosine kinase inhibitor [25]. Furthermore, Tyr-phosphorylation of the C-terminal region of occludin prevents its interaction with ZO-1, ZO-2 and ZO-3 [27]. Therefore Tyr-phosphorylation of occludin is a good indicator of the loss of integrity at the TJ. The present study shows that EGF prevents H2O2-induced Tyr-phosphorylation of occludin and ZO-1. The mechanism of Tyr-phosphorylation of occludin and ZO-1 is not clear. However, the present study shows that EGF receptor activation leads to a modulation of the mechanism involved in Tyr-phosphorylation of occludin and ZO-1. The inhibition of this effect of EGF by MEK inhibitors indicates that EGF prevents H2O2-induced Tyr-phosphorylation of occludin and ZO-1 by a MAPK-dependent mechanism.
Occludin has been shown to be hyperphosphorylated on serine and threonine residues in the normal epithelium [19,20] and it undergoes dephosphorylation during the disruption of TJs by calcium depletion [19–21], phorbol esters [21,22] and Escherichia coli infection [23]. The present study shows that H2O2 induces a dramatic decrease in the level of Thr-phosphorylated occludin and that EGF completely prevents H2O2-induced dephosphorylation of occludin on threonine residues. Therefore EGF-mediated protection of TJ from oxidative stress involves an interruption of the mechanism involved in the H2O2-induced phosphorylation of occludin on threonine residues. Inhibition of this effect of EGF by MEK inhibitors indicates that EGF prevents H2O2-induced Thr-dephosphorylation of occludin by a MAPK-dependent mechanism.
H2O2 disrupted the actin architecture in the apical, middle and basal parts of the cell; however, the effect was more dramatic in the middle part of cell, suggesting that H2O2 may destabilize the perijunctional actomyosin ring. EGF prevents the H2O2-induced disruption of actin architecture in all three levels of the epithelium. EGF by itself increased the immunostaining intensity of the actin ring in the middle part of cell, suggesting that EGF may stabilize the actomyosin ring by an unknown mechanism. Prevention of a H2O2-induced decrease in the levels of detergent-insoluble pools of occludin and ZO-1 by EGF indicates that it protects the integrity of TJs by preventing the loss of interaction between TJ-proteins and the actin cytoskeleton. TJ-proteins were shown to interact with the actin cytoskeleton and that this interaction was essential for the maintenance of the integrity of the TJ [4]. A previous study showed that increased paracellular permeability through oxidative stress and its prevention by genistein correlated well with changes in the levels of occludin and ZO-1 in actin-rich detergent-insoluble fractions [25]. Changes in the levels of these proteins in the membrane cytoskeleton did not correlate with the permeability changes, indicating that actin-bound pools of TJ-proteins are correlated with the integrity of TJs. The attenuation of an EGF-mediated prevention of the disruption of actin cytoskeleton and the decrease in the levels of detergent-insoluble occludin and ZO-1 by MEK inhibitors demonstrates that EGF-activated MAPK activity interrupts the mechanism involved in H2O2-induced disruption of the actin cytoskeleton and the loss of interaction between TJ-proteins and the actin cytoskeleton.
A rapid increase in the levels of p-MEK and p-ERK mediated by EGF indicates that EGF activates the MAPK pathway in differentiated non-proliferating Caco-2 cells. Attenuation of ERK phosphorylation by MEK inhibitors indicates that EGF activates ERK by a MEK-dependent mechanism. Interestingly, p-ERK was localized predominantly to the detergent-insoluble fractions of Caco-2 cell monolayers, although a major portion of total ERK was present in the detergent-soluble fraction. On the other hand, p-MEK was localized predominantly to the detergent-soluble fraction. EGF did not alter the levels of ERK and MEK, suggesting that EGF induces activation of MEK and ERK without altering their levels or localization within the cell. High levels of p-ERK in the detergent-insoluble fractions raised the question as to whether p-ERK is localized to the TJ. Immunofluorescence confocal microscopy showed that a considerable amount of p-ERK is localized at the apical end of the lateral membrane of the epithelial cell and is co-localized with occludin. Co-localization of p-ERK with occludin was profoundly increased by EGF administration due to increased levels of p-ERK in these cells.
Previous studies showed that expression of active Raf-1 in salivary gland epithelial monolayers [42] and transformation by Ras in MDCK cells [43] activates MAPK and disrupts TJs. Ras-induced transformation of MDCK cells prevents the assembly of TJs by decreasing the levels of occludin [43], whereas Raf-1 in salivary gland epithelial cells decreases the expression of claudin-1 [42]. MAPK activity was also found to be involved in the disruption of TJs by H2O2 in endothelial cell monolayers [44] and by phorbol ester in human corneal epithelial monolayers [45], indicating that the activation of MAPK in these cells results in the disruption of TJs. On the contrary, our present study shows that MAPK activity is involved in the stabilization of TJ integrity in a differentiated, non-proliferating Caco-2 cell monolayer. MAPK activity is required for the EGF-induced protection of TJs from H2O2. The reason for such opposing effects of MAPK in epithelia from different origins is not clear. However, basal activity levels of MAPKs and/or their subcellular localization may determine the type of influence on the TJ. In Ras-transformed MDCK cells active ERK was localized exclusively to the detergent-soluble fractions [43], whereas in the present study we show that the active ERK is localized predominantly to the detergent-insoluble fraction of Caco-2 cells.
Co-localization of p-ERK with occludin suggests a possible interaction between p-ERK and the TJ-protein complex. Co-immunoprecipitation of p-ERK with occludin and co-immunoprecipitation of occludin with p-ERK indicated that p-ERK does interact with the TJ-protein complex and that this interaction is rapidly increased by EGF administration. This was further confirmed by the GST–occludin pull-down assay. Incubation of recombinant GST-fused C-terminal tail of occludin with protein extracts prepared from control and EGF-treated cells showed that p-ERK binds to the C-terminal sequence of occludin. The amount of binding was higher in proteins extracted from EGF-treated cells, as there were higher levels of p-ERK present in these extracts compared with protein extracts prepared from the control cells.
Although the GST pull-down assay showed that p-ERK binds to the C-terminal region of occludin, it is not clear if it binds to occludin directly, or if the binding was indirectly mediated by its interaction with other components of the TJ-protein complex. Therefore, we evaluated the direct interaction between occludin and ERK by a pair-wise binding assay using recombinant GST–occludin-C and ERK. Results demonstrate that ERK1 binds directly to the C-terminal region of occludin. The direct interaction of ERK1 with occludin suggests that ERK1 may play a crucial role in the regulation of the TJ-protein complex. However, the precise function of ERK1 in the assembly or stabilization of TJs is not clear. ERK may play a role in the phosphorylation and the regulation of TJ proteins or the signalling proteins that are associated with the components of TJ-protein complexes.
In summary, this study shows that ERK interacts directly with the C-terminal region of occludin and plays a crucial role in the EGF-mediated protection of TJs from H2O2-induced phosphorylation of occludin, loss of its interaction with the actin cytoskeleton, disruption of TJs and increases in paracellular permeability.
Acknowledgments
This study was supported by grants R01-DK55532 and R01-AA12307 from the National Institute of Health to R.K.R.
References
- 1.Trier J. S. Celiac sprue and refractory sprue. In: Sleisenger M., Fordstran J., editors. Gastrointestinal Disease. Philadelphia: W. B. Saunders; 1978. pp. 1029–1051. [Google Scholar]
- 2.Hollander D. Permeability in Crohn's disease: altered barrier functions in healthy relatives? Gastroenterology. 1993;104:1848–1851. doi: 10.1016/0016-5085(93)90668-3. [DOI] [PubMed] [Google Scholar]
- 3.Mathay M. A., Fukuda N., Frank J., Kallet R., Daniel B., Sakuma T. Alveolar epithelial barrier. Role in lung fluid balance in clinical lung injury. Clin. Chest Med. 2000;21:477–490. doi: 10.1016/s0272-5231(05)70160-x. [DOI] [PubMed] [Google Scholar]
- 4.Anderson J. M., van Italie C. M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. 1995;269:G467–G475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- 5.Tsukita S., Furuse M. Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol. 1999;9:268–273. doi: 10.1016/s0962-8924(99)01578-0. [DOI] [PubMed] [Google Scholar]
- 6.Martin-Padura L., Lostaglio S., Schneemann M., Williams L., Romano M., Feuscella P., Panzeri C., Stoppacciaro A., Ruco L., Villa A., Simmons D., Dejana E. Junctional adhesion molecule, a novel member of the immunoglobulin super family that distributes at intercellular junctions and modulates monocyte transmigration. J. Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wittchen E. S., Haskins J., Stevenson B. R. Protein interactions at the tight junction. Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3. J. Biol. Chem. 1999;49:35179–35185. doi: 10.1074/jbc.274.49.35179. [DOI] [PubMed] [Google Scholar]
- 8.Furuse M., Itoh M., Hirose T., Nagafuchi A., Yonemura S., Tsukita S., Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J. Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodenough D. A. Plugging the leaks. Proc. Natl. Acad. Sci. U.S.A. 1999;96:319–321. doi: 10.1073/pnas.96.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itoh M., Furuse M., Morita K., Kubota K., Saitou M., Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of Claudins. J. Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nusrat A., Giry M., Turner J. R., Colgan S. P., Parkos C. A., Carnes D., Lemichez E., Boquet P., Madara J. L. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc. Natl. Acad. Sci. U.S.A. 1995;92:10629–10633. doi: 10.1073/pnas.92.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh S. W., Hopkins A. M., Chen J., Narumiya S., Parkos C. A., Nusrat A. Rho kinase regulates tight junction function and is necessary for tight junction assembly in polarized intestinal epithelia. Gastroenterology. 2001;121:566–579. doi: 10.1053/gast.2001.27060. [DOI] [PubMed] [Google Scholar]
- 13.Denker B. M., Saha C., Khawaja S., Nigam S. K. Involvement of a heterotrimeric G protein α subunit in tight junction biogenesis. J. Biol. Chem. 1996;271:25750–25753. doi: 10.1074/jbc.271.42.25750. [DOI] [PubMed] [Google Scholar]
- 14.Stuart R. O., Nigam S. K. Regulated assembly of tight junctions by protein kinase C. Proc. Natl. Acad. Sci. U.S.A. 1995;92:6072–6076. doi: 10.1073/pnas.92.13.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basuroy S., Sheth P., Kuppuswamy D., Balasubramanian S., Rayr R. M., Rao R. K. Expression of kinase-inactive c-Src delays oxidative stress-induced disassembly and accelerates calcium-mediated reassembly of tight junctions in the Caco-2 cell monolayer. J. Biol. Chem. 2003;278:11916–11924. doi: 10.1074/jbc.M211710200. [DOI] [PubMed] [Google Scholar]
- 16.Sheth P., Basuroy S., Li C., Naren A. P., Rao R. K. Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J. Biol. Chem. 2003;278:49239–49245. doi: 10.1074/jbc.M305654200. [DOI] [PubMed] [Google Scholar]
- 17.Ward P. D., Klein R. R., Troutman M. D., Desai S., Thakker D. R. Phospholipase C-γ modulates epithelial tight junction permeability through hyperphosphorylation of tight junction proteins. J. Biol. Chem. 2002;277:35760–35765. doi: 10.1074/jbc.M203134200. [DOI] [PubMed] [Google Scholar]
- 18.Numbhakdi-Craig V., Machleidt T., Ogris E., Bellotto D., White C. L., Sontag E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J. Cell Biol. 2002;158:967–978. doi: 10.1083/jcb.200206114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakakibara A., Furuse M., Saitou M., Ando-Akatsuka Y., Tsukita S. Possible involvement of phosphorylation of occludin in tight junction formation. J. Cell Biol. 1997;137:1393–1401. doi: 10.1083/jcb.137.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong V. Phosphorylation of occludin correlates with occludin localization and function at the tight junction. Am. J. Physiol. 1997;273:C1859–C1867. doi: 10.1152/ajpcell.1997.273.6.C1859. [DOI] [PubMed] [Google Scholar]
- 21.Farshori P., Kachar B. Redistribution and phosphorylation of occludin during opening and resealing of tight junctions in cultured epithelial cells. J. Membr. Biol. 1999;170:147–156. doi: 10.1007/s002329900544. [DOI] [PubMed] [Google Scholar]
- 22.Clarke H., Soler A. P., Mullin J. M. Protein kinase C activation leads to dephosphorylation of occludin and tight junction permeability increase in LLC-PK1 epithelial cell sheets. J. Cell Sci. 2000;133:3187–3196. doi: 10.1242/jcs.113.18.3187. [DOI] [PubMed] [Google Scholar]
- 23.Simonovic I., Rosenberg J., Koutsouris A., Hecht G. Enteropathogenic Escherichia coli dephosphorylates and dissociates occludin from intestinal epithelial tight junctions. Cell. Microbiol. 2000;2:305–315. doi: 10.1046/j.1462-5822.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- 24.Rao R. K., Baker R. D., Baker S. S., Gupta A., Holycross M. Oxidant-induced disruption of intestinal epithelial barrier function: role of protein tyrosine phosphorylation. Am. J. Physiol. 1997;273:G812–G823. doi: 10.1152/ajpgi.1997.273.4.G812. [DOI] [PubMed] [Google Scholar]
- 25.Rao R. K., Basuroy S., Rao V. U., Karnaky K. J., Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-β-catenin complexes from the cytoskeleton by oxidative stress. Biochem. J. 2002;368:471–481. doi: 10.1042/BJ20011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atkinson K. A., Rao R. K. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am. J. Physiol. 2001;280:G1280–G1288. doi: 10.1152/ajpgi.2001.280.6.G1280. [DOI] [PubMed] [Google Scholar]
- 27.Kale G., Rao R. K. Tyrosine phosphorylation of occludin attenuates its interactions with ZO-1, ZO-2, and ZO-3. Biochem. Biophys. Res. Commun. 2003;302:324–329. doi: 10.1016/s0006-291x(03)00167-0. [DOI] [PubMed] [Google Scholar]
- 28.Chapman K. E., Waters C. M., Miller W. M. Continuous exposure of airway epithelial cells to hydrogen peroxide: protection by KGF. J. Cell Physiol. 2002;192:71–80. doi: 10.1002/jcp.10115. [DOI] [PubMed] [Google Scholar]
- 29.Collares-Buzoto C. B., Jepson M. A., McEwan G. T., Simmones N. L., Hirst B. H. Junctional uvomorulin/E-cadherin and phosphotyrosine-modified protein content are correlated with paracellular permeability in Madin-Darby canine kidney (MDCK) epithelia. Histochemistry. 1994;101:185–194. doi: 10.1007/BF00269543. [DOI] [PubMed] [Google Scholar]
- 30.Rao R. K., Li L., Baker R. D., Baker S. S., Gupta A. Glutathione oxidation and PTPase inhibition by hydrogen peroxide in Caco-2 cell monolayer. Am. J. Physiol. 2000;279:G332–G340. doi: 10.1152/ajpgi.2000.279.2.G332. [DOI] [PubMed] [Google Scholar]
- 31.Rao R., Baker R. D., Baker S. S. Inhibition of oxidant-induced barrier disruption and protein tyrosine phosphorylation in Caco-2 cell monolayers by epidermal growth factor. Biochem. Pharmacol. 1999;57:685–695. doi: 10.1016/s0006-2952(98)00333-5. [DOI] [PubMed] [Google Scholar]
- 32.Carpenter G., Cohen S. Epidermal growth factor. J. Biol. Chem. 1990;265:7709–7712. [PubMed] [Google Scholar]
- 33.Rao R. K. Biologically active peptides in the gastrointestinal lumen. Life Sci. 1991;48:1685–1704. doi: 10.1016/0024-3205(91)90205-p. [DOI] [PubMed] [Google Scholar]
- 34.Brzozowski T., Majka J., Garlicki J., Drozdowicz D., Konturek S. J. Role of polyamines and prostaglandins in gastroprotective action of epidermal growth factor against ethanol injury. J. Clin. Gastroenterol. 1991;13:S98–S102. doi: 10.1097/00004836-199112001-00016. [DOI] [PubMed] [Google Scholar]
- 35.Konturek J. W., Brzozowski T., Konturek S. J. Epidermal growth factor in protection, repair, and healing of gastroduodenal mucosa. J. Clin. Gastroenterol. 1991;13:S88–S97. doi: 10.1097/00004836-199112001-00015. [DOI] [PubMed] [Google Scholar]
- 36.Konturek P. K., Brzozowski T., Konturek S. J., Dembinski A. Role of epidermal growth factor, prostaglandin, and sulfhydryls in stress-induced gastric lesions. Gastroenterology. 1990;99:1607–1615. doi: 10.1016/0016-5085(90)90464-c. [DOI] [PubMed] [Google Scholar]
- 37.Tepperman B. L., Vozzolo B. L., Soper B. D. Effect of maternal sialoadenectomy on ontogenic response of rat gastric mucosa to luminal H+ Am. J. Physiol. 1993;265:G354–G360. doi: 10.1152/ajpgi.1993.265.2.G354. [DOI] [PubMed] [Google Scholar]
- 38.Ishikawa S., Cepinskas G., Specian R. D., Itoh M., Kveitys P. R. Epidermal growth factor attenuates jejunal mucosal injury induced by oleic acid: role of mucus. Am. J. Physiol. 1994;267:G1067–G1077. doi: 10.1152/ajpgi.1994.267.6.G1067. [DOI] [PubMed] [Google Scholar]
- 39.Lawrence J. P., Brevetti L., Obiso R. J. Effects of epidermal growth factor and Clostridium difficile toxin B in a model of mucosal injury. J. Pediatr. Surg. 1997;32:430–433. doi: 10.1016/s0022-3468(97)90598-4. [DOI] [PubMed] [Google Scholar]
- 40.Rao R., Porreca F. Epidermal growth factor protects mouse ileal mucosa from Triton X-100-induced injury. Eur. J. Phamacol. 1996;303:209–212. doi: 10.1016/0014-2999(96)00186-0. [DOI] [PubMed] [Google Scholar]
- 41.Agell N., Bachs O., Rocamora N., Villalonga P. Modulation of the Ras/Raf/MEK/ERK pathway by Ca2+, and calmodulin. Cell Signal. 2002;14:649–654. doi: 10.1016/s0898-6568(02)00007-4. [DOI] [PubMed] [Google Scholar]
- 42.Li D., Mrsny R. J. Oncogenic Raf-1 disrupts epithelial tight junctions via down regulation of occludin. J. Cell Biol. 2000;148:791–800. doi: 10.1083/jcb.148.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y., Lu Q., Schneeberger E. E., Goodenough D. A. Restoration of tight junction structure and barrier function by down-regulation of the mitogen-activated protein kinase pathway in ras-transformed Madin-Darby canine kidney cells. Mol. Cell Biol. 2000;11:849–862. doi: 10.1091/mbc.11.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kevil C. G., Oshima T., Alexander B., Coe L. L., Alexander J. S. H(2)O(2)-mediated permeability: role of MAPK and occludin. Am. J. Physiol. 2000;279:C21–C30. doi: 10.1152/ajpcell.2000.279.1.C21. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y., Zhang J., Yi X. J., Yu F. S. Activation of ERK1/2 MAPK pathway induces tight junction disruption in human corneal epithelial cells. Exp. Eye Res. 2004;78:125–136. doi: 10.1016/j.exer.2003.09.002. [DOI] [PubMed] [Google Scholar]