Abstract
sPLA2 (secretory phospholipase A2) enzymes have been implicated in various biological events, yet their precise physiological functions remain largely unresolved. In the present study we show that group V and X sPLA2s, which are two potent plasma membrane-acting sPLA2s, are capable of preventing host cells from being infected with an adenovirus. Bronchial epithelial cells and lung fibroblasts pre-expressing group V and X sPLA2s showed marked resistance to adenovirus-mediated gene delivery in a manner dependent on their catalytic activity. Although adenovirus particles were insensitive to recombinant group V and X sPLA2s, direct addition of these enzymes to 293A cells suppressed both number and size of adenovirus plaque formation. Group V and X sPLA2s retarded the entry of adenovirus into endosomes. Moreover, adenoviral infection was suppressed by LPC (lysophosphatidylcholine), a membrane-hydrolytic product of these sPLA2s. Thus hydrolysis of the plasma membrane by these sPLA2s may eventually lead to the protection of host cells from adenovirus entry. Given that group V and X sPLA2s are expressed in human airway epithelium and macrophages and that the expression of endogenous group V sPLA2 is upregulated by virus-related stimuli in these cells, our present results raise the possibility that group V and X sPLA2s may play a role in innate immunity against adenoviral infection in the respiratory tract.
Keywords: adenovirus, bronchial epithelial cell, infection, lysophosphatidylcholine (LPC), plasma membrane, secretory phospholipase A2 (sPLA2)
Abbreviations: AA, arachidonic acid; Ad, adenovirus; CAR, coxsackie virus B adenovirus receptor; cPLA2, cytosolic PLA2; COX, cyclo-oxygenase; EAA1, early endosomal antigen 1; ESI, electrospray ionization; G3PDH, glyceraldehyde-3-phosphate dehydrogenase; HSPG, heparan sulphate proteoglycan; iPLA2, Ca2+-independent PLA2; IFN, interferon; LPA, lysophosphatidic acid; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; LPS, lysophosphatidylserine; mPGES-1, microsomal PGE synthase-1; MOI, multiplicity of infection; NHPF, normal human pulmonary fibroblasts; PC, phosphatidylcholine; PGE2, prostaglandin E2; PLA2, phospholipase A2; RT, reverse transcription; sPLA2, secretory PLA2; TNF, tumour necrosis factor
INTRODUCTION
PLA2 (phospholipase A2) catalyses the hydrolysis of the sn-2 position of membrane glycerophospholipids to yield free fatty acids, including AA (arachidonic acid) and lysophospholipids. Mammalian PLA2s have been classified into four major families including sPLA2 (secretory PLA2), cPLA2 (cytosolic PLA2), iPLA2 (Ca2+-independent PLA2) and platelet-activating factor acetylhydrolases, each of which occur as multiple isoforms [1,2]. Of these, the sPLA2 family represents a group of structurally related, disulphide-rich, Ca2+-dependent, low molecular mass enzymes with a His-Asp catalytic dyad [1,2]. Individual sPLA2s exhibit unique tissue and cellular localization and enzymatic properties, suggesting distinct roles in various physiological events.
Several sPLA2s have the ability to release AA from mammalian cell membranes through multiple mechanisms, an event that can be linked to eicosanoid biosynthesis [3–9]. Among these enzymes, group V and X sPLA2s (sPLA2-V and -X respectively) are particularly potent in eliciting AA release from intact cellular membranes, most likely because of their high capacity to bind PC (phosphatidylcholine), a phospholipid that is enriched in the outer plasma membrane [4–6]. sPLA2-IIA and related enzymes often release AA from activated cells in a manner dependent upon their association with HSPGs (heparan sulphate proteoglycans), although the occurrence of this route appears to be cell-type-specific [4,7,8]. It has also been suggested that sPLA2s can elicit AA release before secretion [9]. sPLA2s also promote various cellular responses, such as cPLA2α-dependent AA release, through binding to sPLA2 receptors in a manner independent of their catalytic activity [10].
In addition to their action on mammalian cells, several sPLA2s are capable of killing bacteria by degrading bacterial membranes, thereby potentially providing a first line of anti-microbial host defence [11–14]. The bactericidal activity of sPLA2-IIA, a prototypic anti-bacterial sPLA2, depends on its catalytic activity, as well as on the overall negative charge of the enzyme [11–14]. The rank order of potency among human sPLA2s against Gram-positive bacteria has been reported to be IIA>X>V>XII>IIE>IB, IIF [12]. The ability of sPLA2-X, an acidic sPLA2 to kill some Gram-positive bacteria, and the observation that another acidic sPLA2 sPLA2-XIIA, in which catalytic activity towards phospholipid vesicles is rather low, can also destroy Gram-positive bacteria [12] suggests that the bactericidal activity of sPLA2s cannot be explained simply by their catalytic activity toward pure phospholipid vesicles and their surface charges but may involve additional unresolved parameter(s).
Although the potential host defence role of sPLA2s against bacterial infection has thus been emerging, their effect on viral infection has remained largely obscure. The capsid of parvoviruses contains sPLA2 (group XIII), the activity of which is dispensable for viral attachment to host cell surfaces and subsequent endocytosis but crucial for efficient transfer of the viral genome from endosomes into the nucleus [15,16]. Conversely, exogenous bee venom sPLA2 (group III) inhibits the entry and thereby replication of HIV in host cells through a mechanism linked to sPLA2 binding to cells [17,18]. So far, little is known about the effects of mammalian sPLA2s on viral infection into host cells.
Adenoviruses are non-enveloped icosahedral DNA viruses about 90 nm in diameter that cause infections of the respiratory tract [19,20]. The adenovirus particle is composed of an outer capsid consisting mainly of hexon and an inner DNA-associated core with a 36 kbp linear DNA, two terminal proteins and condensing proteins. The vertices are made up of a penton base containing Arg-Gly-Asp motifs and the protruding fibre protein, which binds to CAR (Coxsackie virus B adenovirus receptor) on host cell surfaces and activates integrins locally. Adenoviruses are then internalized via clathrin-mediated endocytosis into the endosomes, a slightly acidic intracellular compartment, and escape into the cytosol after cell signalling. Viruses then move towards the nucleus, dock with the nuclear pore complex and disassemble to release the viral genome into the nucleus.
During our ongoing studies on the functional aspects of mammalian sPLA2s by taking advantage of the adenovirus-mediated gene transfer strategy [21–24], we unexpectedly found that sPLA2-V and -X have the capacity to prevent host cells from being infected with adenoviruses. Unlike the bactericidal action of sPLA2s, the anti-viral effect of these two sPLA2s depends essentially on their ability to hydrolyse phospholipids in host cell membranes. Considering that both sPLA2-V and -X are expressed in the human respiratory tract [23], our present results open new insights into the potential functional role of these enzymes in innate immunity against viral infection.
MATERIALS AND METHODS
Materials
Human sPLA2 cDNAs, pure recombinant human sPLA2 proteins produced in Escherichia coli and rabbit antisera specific for individual human sPLA2s were described previously [21–25]. Enzyme immunoassay kit for PGE2 (prostaglandin E2) was purchased from Cayman Chemical. cDNAs for human cPLA2α, cPLA2γ, iPLA2β, COX (cyclo-oxygenase)-2 and mPGES-1 (microsomal PGE synthase-1) were described previously [3,26–28]. AA, LPC with various sn-1 fatty acids (C12:0, C14:0, C16:0 and C18:0), 1-palmitoyl-LPE (lysophosphatidylethanolamine), LPS (lysophosphatidylserine), and LPA (lysophosphatidic acid) and human IFN (interferon)-α were purchased from Sigma. Human TNF (tumour necrosis factor)-α was from Genzyme. Poly-I:C was obtained from Amersham Biosciences.
Cell culture
Human bronchial epithelial BEAS-2B cells were cultured in RPMI 1640 medium (Nissui Pharmaceutical) containing 10% (v/v) foetal calf serum as described previously [28]. BEAS-2B cells stably expressing various PLA2s or COX-2 were established as described previously [23,28]. NHPF (normal human pulmonary fibroblasts) and culture media with supplements for these cells were purchased from BioWhittaker [23].
Preparation and activation of peritoneal macrophages
C57BL/6 mice (Saitama Animal Centre) were housed in microisolator cages in a pathogen-free barrier facility and all experiments were performed under approved institutional guidance. Peritoneal cells were recovered from mice that had received thioglycollate medium (Difco) (1 ml per 20 g of body weight) 4 days previously [29]. The peritoneal cells were seeded into 12 well plates (Iwaki Glass) at a cell density of 2×106 cells/ml in 1 ml of RPMI medium supplemented with 10% (v/v) foetal calf serum. After incubation for 2 h in a CO2 incubator, the supernatants and non-adherent cells were removed. More than 90% of adherent cells were macrophages.
Northern-blotting
Equal amounts (approx. 10 μg) of total RNA obtained from cells by use of TRIzol® reagent (Invitrogen) were applied to separate lanes of 1.2% (w/v) formaldehyde-agarose gels, electrophoresed and transferred to Immobilon-N membranes (Millipore). The resulting blots were then probed with appropriate cDNA probes that had been labelled with [32P]dCTP (Amersham Bioscience) by random priming (Takara Biomedicals). Hybridization and subsequent membrane washing were carried out as described previously [3].
Immunoblotting
Lysates from 2×105 cells were subjected to SDS/PAGE on 15% gels under reducing conditions with 2-mercaptoethanol. The separated proteins were electroblotted on to nitrocellulose membranes (Schleicher and Schuell) with a semi-dry blotter (MilliBlot-SDE system, Millipore). After blocking with 5% (w/v) skimmed milk in TBS containing 0.05% (v/v) Tween-20 (TBS-T), the membranes were probed with rabbit antibody against human sPLA2-V or -X at 1:5000 dilution in TBS-T for 2 h, followed by incubation with horseradish peroxidase-conjugated anti-(rabbit IgG) (Zymed) at 1:5000 dilution in TBS-T for 2 h and were visualized using the ECL (enhanced chemiluminesence) Western blot system (PerkinElmer) as described previously [22,23].
RT (reverse transcription)-PCR
Synthesis of cDNA was performed using 0.5 μg of total RNA from cells and tissues, and AMV (avian myeloblastosis virus) reverse transcriptase, according to the manufacturer's instructions supplied with the RNA PCR kit (Takara Biomedicals). Subsequent amplification of the cDNA fragments was performed using 0.5 μl of the reverse-transcribed mixture as a template with specific primers for each sPLA2 (Greiner, Japan). For amplification of human and mouse sPLA2-V and -X, a set of 23 bp oligonucleotide primers corresponding to 5′- and 3′-nt sequences of their open reading frames were used as primers, as described previously [22,23]. The PCR conditions were 94 °C for 30 s and then 30 cycles of amplification at 94 °C for 5 s and 68 °C for 4 min, using the Advantage cDNA polymerase mix (Clontech). The PCR conditions for G3PDH (glyceraldehyde-3-phosphate dehydrogenase) was described previously. The PCR products were analysed by 1% agarose gel electrophoresis with ethidium bromide.
Adenovirus expression system
Adenoviruses bearing cDNAs for human sPLA2s and their mutants; cPLA2s, iPLA2β, COX-2 and mPGES-1 were prepared using the ViraPower Adenovirus Expression System (Invitrogen) as described previously [21–24]. Briefly, the full-length cDNAs were subcloned into the pENTER/D-TOPO vector using a pENTER Directional TOPO Cloning kit (Invitrogen). After purification of the plasmids from the transformed Top10 competent cells (Invitrogen) the cDNA inserts were transferred to the pAd (adenovirus)/CMV/V5-DEST vector (Invitrogen) by means of the Gateway® system using LR Clonase™ (Invitrogen). The plasmids were purified and digested with Pac I (New England BioLabs). The linearized plasmids (1–2 μg) were then mixed with 4 μl of Lipofectamine™ 2000 (Invitrogen) in 200 μl of Opti-MEM® medium (Invirogen) and transfected into subconfluent 293A cells (Invitrogen) in 1 ml of Opti-MEM® in 6 well plates (Iwaki Glass). Then 293A cells were cultured for 1–2 weeks in RPMI 1640 medium containing 10% foetal calf serum, with replacement of the medium every 2 days. When most cells became detached from the plates the cells and culture medium were harvested together, freeze-thawed twice and centrifuged to obtain the adenovirus-enriched supernatants. Then aliquots of the supernatants were added to fresh 293A cells and cultured for 2–3 days to amplify adenoviruses. After 2–4 amplifications, the resulting adenovirus-containing media were used as virus stock. Viral titres were determined by the plaque-forming assay using 293A cells. As a control, PacI-digested pAd/CMV/V5-GW/lacZ vector (Invitrogen) was transfected into 293A cells to produce a lacZ-bearing adenovirus. Aliquots of the adenovirus-containing medium were added to cells and cultured for appropriate periods to allow the expression of adenovirus-delivered genes.
Construction of sPLA2-V and -X mutants
cDNAs for the catalytic centre mutants of human sPLA2-V and -X (H48Q; numbering in alignment with sPLA2-IB) were prepared by the mismatched PCR method as described previously [4]. The primers used were as follows: V-HQ-S primer, 5′-TGTTGGGCGCAGGACCACTGCTATG-3′; V-HQ-AS primer, 5′-CATAGCAGTGGTCCTGCGCCCAACA-3′; X-HQ-S primer, 5′-TGCCATGGCCAGGACTGTTGTTAC-3′; X-HQ-AS primer, 5′-GTAACAACAGTCCTGGCCATGGCA-3′; V-5′ primer, 5′-ATGAAAGGCCTCCTCCCACTGGC-3′; V-3′ primer, 5′-GGCCTAGGAGCAGAGGATGTTGG-3′; X-5′ primer, 5′-ATGGGGCCGCTACCTGTGTGCC-3′ and X-3′ primer, 5′-TCAGTCACACTTGGGCGAGTCCG-3′. To construct the mutants, PCR was conducted with a set of V- or X-5′ primers and V- or X-HQ-AS primers (for the forward strand), and with a set of V- or X-HQ-S primers and V- or X-3′ primers (for the reverse strand) using Pyrobest polymerase (Takara Biomedicals) and sPLA2-V or -X cDNA as a template with 25 cycles of 95 °C for 30 s, 55 °C for 30 s and 72 °C for 30s. The resulting forward and reverse strands were mixed, heated, annealed and subjected to a second PCR with V- or X-5′ primers and V- or X-3′ primers under the same thermal conditions. The PCR products were subcloned into the pENTER/D-TOPO vector for adenoviral expression as described above. The plasmids were sequenced with a Taq cycle sequencing kit (Takara Biomedicals) and an autofluorometric DNA sequencer 310 Genetic Analyser (Applied Biosystems) to confirm the sequences.
Assays for AA release and PGE2 generation
Cells grown to near confluency in 24-well plates (Iwaki Glass) were incubated with [3H]AA (Amersham Bioscience) (0.1 μCi/ml) overnight. After 3 washes with fresh medium, 250 μl of culture medium with or without 100 units/ml TNFα was added to each well and the radioactivities, released into the supernatants after incubation for appropriate periods, were measured. The percentage release was calculated using the formula [S/(S+P)]×100, where S and P are the radioactivities measured in the supernatant and cell pellet respectively, as described previously [3,4]. Aliquots of the supernatants from replicate positive cells (without preincubation with [3H]AA) were subjected to PGE2 enzyme immunoassay.
Plaque-forming assay
Adenovirus solution in serial dilutions was added to subconfluent 293A cells cultured in 1 ml of culture medium in 24 well plates. After 5 days of culture, the number of plaques formed on the 293A cell monolayer was counted. Photographs were taken using an ECLIPSE TE300 microscope (Nikon).
Measurement of sPLA2 activity
sPLA2 activities in cell lysates and culture supernatants were assayed by measuring the amount of radiolabelled linoleic acid released from the substrate 1-palmitoyl-2-[14C]linoleoyl-phosphatidylethanolamine (Amersham Bioscience). The substrate in ethanol was dried under N2 stream and was dispersed in water by sonication. Each reaction mixture (total volume 250 μl) consisted of appropriate amounts of the required samples, 100 mM Tris/HCl (pH 7.4), 4 mM CaCl2 and 2 μM substrate. After incubation for 30 min at 37 °C, [14C]linoleic acid was extracted and the radioactivity was quantified by liquid-scintillation counter as described previously [3,4].
Confocal laser microscopy
Cells grown to subconfluency on glass-bottom dishes (Matsunami) pre-coated with 5 μg/ml fibronectin (Sigma) were incubated with adenovirus at 4 °C or 37 °C for adequate periods. Then the cells were washed twice with PBS and fixed with 3% paraformaldehyde in PBS for 1 h. After 3 washes with TBS, the fixed cells were treated with 1% (w/v) BSA and 0.5% (w/v) saponin in TBS (blocking solution) for 1 h, with rabbit antiadenovirus hexon protein (Chemicon) or goat anti-EEA1 (early endosomal antigen 1) antibody (B.D. Biosciences) at 1:200 dilution in blocking solution for 2 h and then with species-matched FITC-conjugated or carbocyanine 3-conjugated second antibody at 1:200 dilution in blocking solution for 2 h, with 3 washes after each interval. After 6 washes with TBS, specific immunofluorescent signals were visualized using a laser scanning confocal microscope (IX70, Olympus).
ESI (electrospray ionization) MS
MS spectra were obtained using a Quattro Micro tandem quadrupole mass spectrometer (Micromass) equipped with an ESI as described previously [24,27]. Lipid extracts obtained from BEAS-2B cells with or without sPLA2 transfection [100 μg protein equivalents; protein concentrations were determined by BCA (bicinchoninic acid) protein assay kit (Pierce)] were reconstituted in chloroform/methanol (2:1) (100–300 μmol phosphorus/l), and 2 μl of the sample was injected per run. As internal standards, 100 pmol of PC (C14:0–C14:1, diacyl), LPC (C12:0) and PE (C14:0–C14:0, diacyl) were added to the samples. The samples were introduced by means of a flow injector into the ESI chamber at a flow rate of 4 μl/min in a solvent system of acetonitrile/methanol/water (2:3:1, by vol.) containing 0.1% (v/v) ammonium formate (pH 6.4). The mass spectrometer was operated in the positive and negative scan modes. The flow rate of the nitrogen drying gas was 12 l/min at 80 °C. The capillary and cone voltages were set at 3.7 kV and 30 V respectively, argon at 3–4×104 Torr was used as the collision gas and a collision energy of 30–40 V was used for obtaining fragment ions for precursor ions.
In situ hybridization
In situ hybridization experiments were performed on the paraffin embedded tissue serial sections with the Ventana HX system (Ventana Medical System) as described previously [30]. Briefly, sPLA2 cDNAs were each subcloned into the pCR-II vector (Invitrogen) and the sense and antisense cRNA probes for sPLA2s were synthesized from the T7 and SP6 promoters respectively, on the Not I-digested, linearized plasmid. Sections were pre-treated and hybridized with a Ventana Ribo Map kit (Ventana Medical Systems) on the automated Ventana HX system. Detection of hybrid was performed using a digoxigenin nucleic kit (Boehringer Mannheim GmbH), according to the manufacturer's instructions. Signal detection with Nitro Blue tetrazolium salt was performed at 20 °C for 30 h in the dark. Sections were then dehydrated through an increasing concentration of ethanol (30, 50, 70, 90 and 100% ethanol, 2 min each) and washed twice for 5 min in xylene before mounting in Malinol mounting medium (Muto Pure Chemicals).
RESULTS
Overexpression of sPLA2-V and -X prevents adenoviral infection in host cells
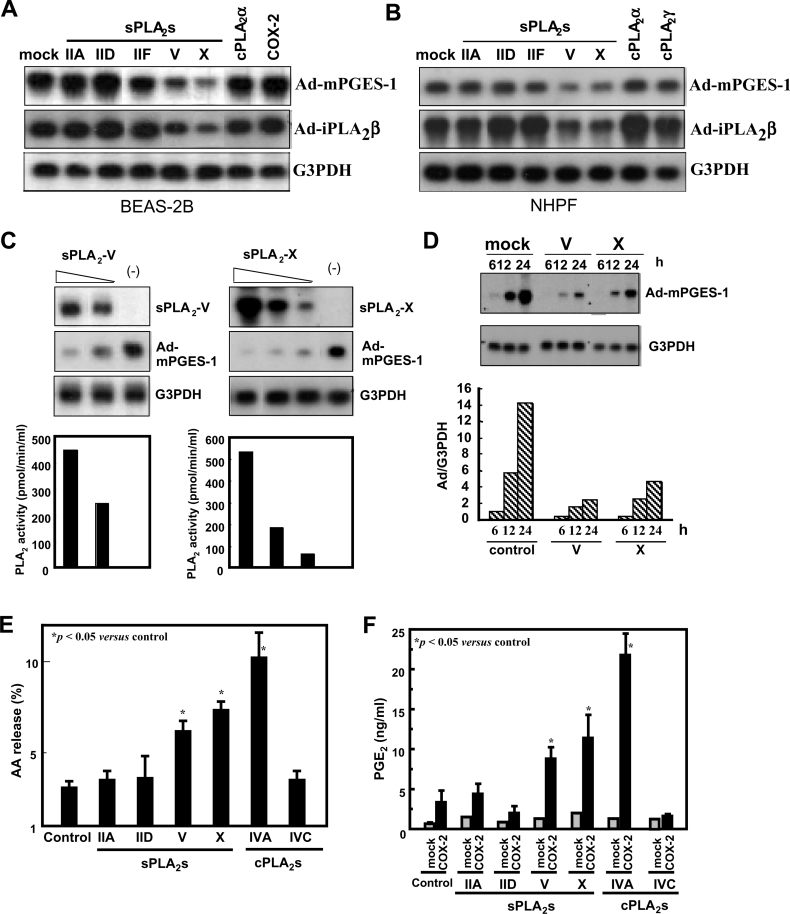
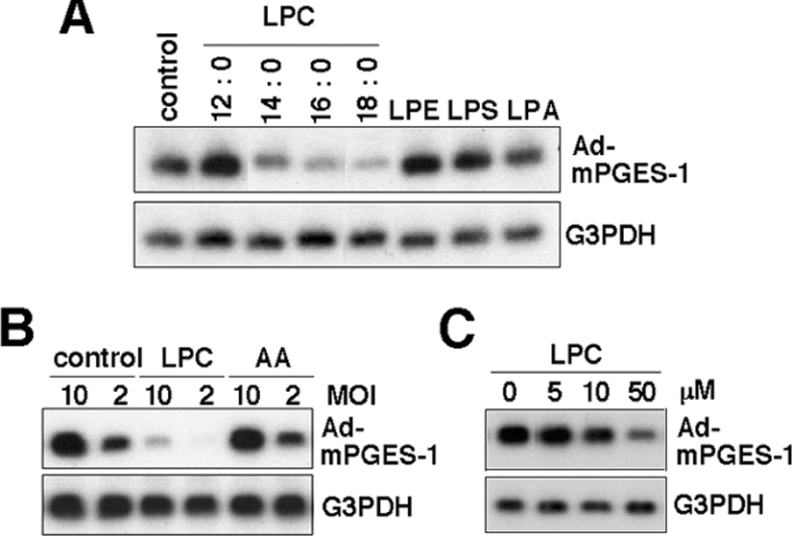
In an initial effort to address the eicosanoid-biosynthetic capacity of various PLA2s in combination with downstream COX and PGES enzymes, human bronchial epithelial cells BEAS-2B, which had been stably transfected with various PLA2 and COX enzymes, were infected with adenovirus expressing mPGES-1 (Ad-mPGES-1), the most downstream enzyme in the PGE2-synthetic pathway. During the course of this study, we unexpectedly found that the expression level of adenovirally delivered mPGES-1 was significantly lower in cells pre-expressing sPLA2-V or -X than that in cells pre-expressing other sPLA2s (sPLA2-IIA, -IID and -IF), cPLA2α, and COX-2 (Figure 1A). It is unlikely that the event observed in cells pre-expressing sPLA2-V or -X was due to overexpression of mPGES-1, since decreased expression of other adenovirus-delivered genes (e.g. iPLA2β) was also evident in cells pre-expressing sPLA2-V or -X, but not in cells pre-expressing other PLA2s and COX-2 (Figure 1A).
Figure 1. sPLA2-V and -X suppress adenovirus-mediated gene delivery into human lung-derived cells.
(A) Human bronchial epithelial BEAS-2B cells, which had been stably transfected with various PLA2s and COX-2 were subjected to infection with Ad-mPGES-1 or Ad-iPLA2β at MOI (multiplicity of infection)=10. After 24 h the cells were harvested and taken for Northern-blotting with probes against mPGES-1, iPLA2β and G3PDH. (B) NHPF were infected with adenoviruses for various PLA2s at MOI=10 for 24 h and were then infected with Ad-mPGES-1 or Ad-iPLA2β at MOI=2 for an additional 24 h. The cells were harvested and taken for Northern-blotting with probes against mPGES-1, iPLA2β and G3PDH. (C) BEAS-2B cells stably expressing different amounts of sPLA2-V (left panel) or -X (right panel) or mock-transfected cells (−) were infected with Ad-mPGES-1 at MOI=10 for 24 h. Then the cells were harvested and taken for Northern-blotting with probes for sPLA2-V or -X, mPGES-1 and G3PDH. sPLA2 activity released into the supernatants from individual clones is shown in the bottom panel. (D) Control BEAS-2B cells and those stably transfected with sPLA2-V or -X were infected for the indicated periods with Ad-mPGES-1 at MOI=10. Then the cells were subjected to Northern-blotting with probes against mPGES-1 and G3PDH. The bottom panel shows the ratio of signal intensities between mPGES-1 and G3PDH. (E) AA release from BEAS-2B cells transfected with various PLA2s. Cells prelabelled with [3H]AA for 24 h were stimulated for 4 h with 100 units/ml TNFα to assess [3H]AA release. (F) BEAS-2B cells stably expressing various PLA2s with or without cotransfection of COX-2 were incubated with TNFα for 4 h to assess generation of PGE2. (A) to (D) representative results of 3–5 experiments and values are mean±S.E.M. for three independent experiments in (E) and (F).
To verify if the above result is particular to BEAS-2B cells or also occurs in other cell types, normal human fibroblasts NHPF were first transfected adenovirally with various sPLA2s and cPLA2s and then subjected to second transfection with Ad-mPGES-1 or Ad-iPLA2β. As shown in Figure 1(B), expression of the genes delivered by Ad-mPGES-1 and Ad-iPLA2β was significantly lower in cells pre-expressing sPLA2-V or -X than in cells pre-expressing other PLA2s or control cells. Moreover, adenoviral expression of sPLA2-IIA-G30S, a mutant that lacks catalytic activity and thus is devoid of the cellular AA-releasing function [3], was significantly decreased in cells pre-expressing sPLA2-V or -X relative to that in control cells (results not shown), indicating that the adenoviral delivery of the gene irrelevant to eicosanoid biosynthesis is also suppressed by sPLA2-V or -X.
To confirm these observations further, we used several stable clones of sPLA2-V- or -X-transfected BEAS-2B cells, in which the expression levels of these enzymes differed among individual clones, as assessed by RNA blotting (Figure 1C, top panel) and by enzymatic activity released into the culture supernatants (Figure 1C, bottom panel). When individual clones were infected with Ad-mPGES-1, the expression of mPGES-1 showed a reciprocal correlation with that of sPLA2-V or -X (Figure 1C). Thus the expression of mPGES-1 was lower in cells expressing higher levels of sPLA2-V or -X. Time-course experiments demonstrated that the adenoviral delivery of mPGES-1 into BEAS-2B (Figure 1D) and NHPF (results not shown) was markedly delayed in sPLA2-V- or -X-expressing cells compared with that in control cells. On the basis of these observations, we hypothesized that sPLA2-V and -X have the unique ability to prevent adenoviral infection into mammalian cells.
BEAS-2B cells expressing sPLA2-V or -X, but not sPLA2-IIA and -IID and cPLA2γ, exhibited increased AA release (Figure 1E) and PGE2 production (when cotransfected with COX-2) (Figure 1F) compared with replicate control cells, indicating that sPLA2-V and -X can hydrolyse BEAS-2B cell membranes to supply AA for eicosanoid production. However, greater AA release (Figure 1E) and PGE2 production (Figure 1F) were evident in cells expressing cPLA2α, which displayed no inhibitory effect on adenoviral infection (Figure 1A) than in cells expressing sPLA2-V or -X. Moreover, cells transfected with COX-2 alone, in which no inhibition of adenoviral infection occurred (Figure 1A) produced more PGE2 than did cells transfected with sPLA2-V or -X alone (Figure 1F). These observations thus argue against a contribution of AA or its metabolites to sPLA2-V- or -X-mediated suppression of adenoviral infection.
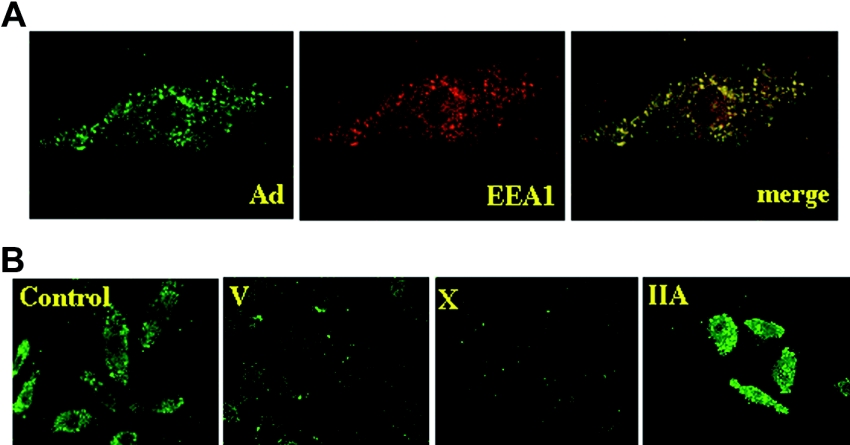
To assess whether adenovirus entry into the cells was indeed perturbed in cells expressing sPLA2-V or -X, we performed immunocytostaining of adenovirus-infected BEAS-2B cells with an antibody against adenoviral hexon protein. When control BEAS-2B cells were incubated with adenovirus, adenovirus signals exhibited a punctate pattern in the cytoplasm, which was largely colocalized with the endosomal marker EEA1 (Figure 2A). As shown in Figure 2(B), endosomal localization of adenovirus particles was markedly decreased in sPLA2-V- or -X-expressing cells compared with that in control cells, implying that adenoviral entry into cells is prevented by sPLA2-V or -X. By contrast, such a prevention of adenoviral entry was not appreciably observed in sPLA2-IIA-expressing cells (Figure 2B).
Figure 2. Immunostaining of adenoviral particles in BEAS-2B cells.
(A) Endocytosis of adenoviral particles into endosomes. BEAS-2B cells were incubated with adenovirus (MOI=20) for 6 h and were immunostained with anti-(adenoviral hexon) (left panel) and anti-EEA1 (middle panel) antibodies. A merged image is shown in the right panel. (B) Control BEAS-2B cells and cells expressing sPLA2-V, -X or -IIA were incubated with adenovirus (MOI=20) for 6 h and were immunostained with anti-adenoviral hexon antibody.
Exogenous sPLA2-V and -X prevent adenoviral infection into host cells
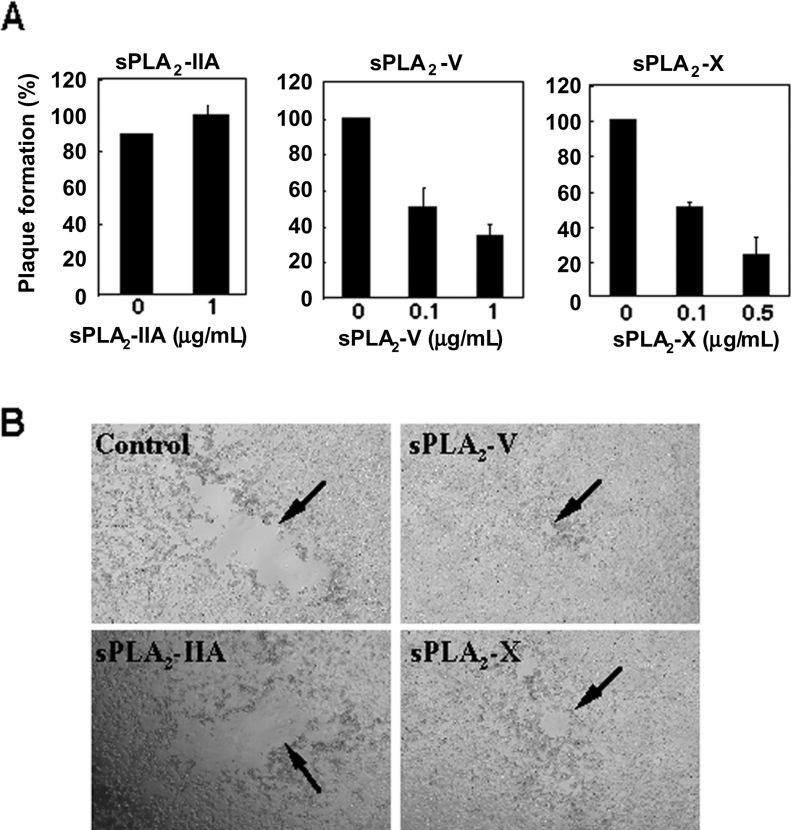
To further explore the inhibitory effect of sPLA2-V and -X on adenoviral infection, we conducted a plaque formation assay using 293A cells to determine whether or not purified recombinant sPLA2-V and -X added exogenously are capable of preventing adenoviral infection. First, in order to assess if these enzymes would inactivate adenovirus directly, the virus was preincubated with recombinant sPLA2-V or -X for various periods of time. Then the virus stock was diluted appropriately and added to 293A cells to observe the effect on adenoviral plaque formation. After this treatment (even for up to 1 week), however, we observed no appreciable inhibition of adenoviral plaque formation by these enzymes (results not shown), suggesting that adenovirus particles themselves are refractory to these enzymes. By contrast, when recombinant sPLA2-V or -X was added directly to 293A cells in the presence of adenovirus, there was an sPLA2 dose-dependent decrease in the number of adenoviral plaques formed (Figure 3A). A marked decrease in plaque number was observed using as little as 0.1 μg/ml of enzyme (Figure 3A), a concentration almost equivalent to that released from BEAS-2B or NHPF cells stably transfected with these enzymes (see Figure 1). In addition, each plaque in cells treated with recombinant sPLA2-V or -X appeared to be smaller than that observed in cells without addition of each enzyme (Figure 1B). By comparison, recombinant sPLA2-IIA failed to decrease adenoviral plaque formation in terms of number (Figure 3A) and size (Figure 3B) even at 1 μg/ml. Thus sPLA2-V and -X suppress adenoviral infection by acting on host cells rather than on adenoviral particles.
Figure 3. Exogenous sPLA2-V and -X block adenoviral plaque formation in 293A cells.
(A) Effects of sPLA2s on plaque numbers. 293A cells were incubated for five days with adenovirus in the presence or absence of the indicated amounts of sPLA2s, and plaque numbers formed on 293A cell monolayers were counted. Values are expressed as percentages of the number of plaques compared with that of the control as 100% (mean±S.E.M., n=3). (B) Photographs of the plaques formed on a 293A cell monolayer after culture for five days in the presence or absence (control) of 1 μg/ml sPLA2-IIA, 1 μg/ml sPLA2-V or 0.5 μg/ml sPLA2-X.
Requirement for catalytic activity
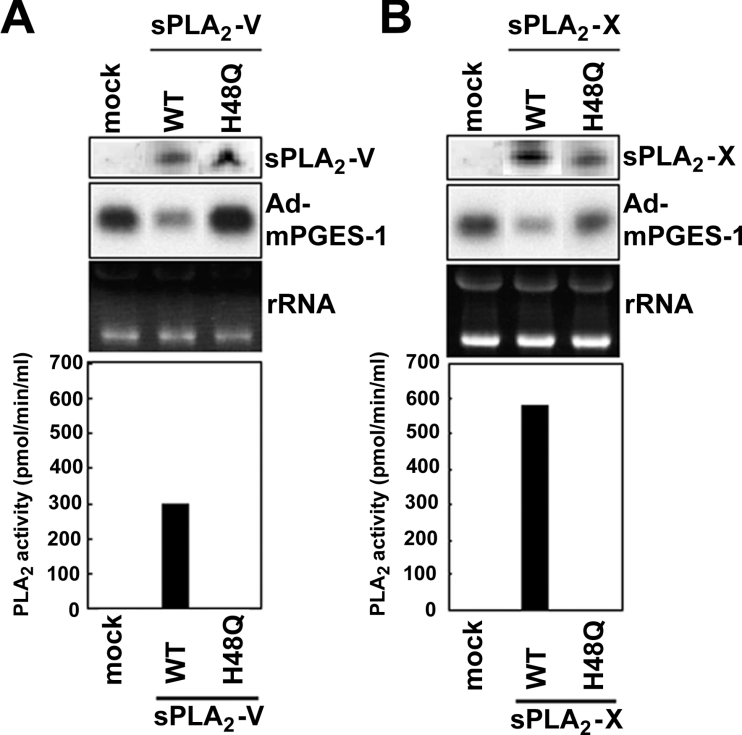
To assess whether the enzymatic activities of sPLA2-V and -X are required for their ability to block adenoviral infection, we prepared BEAS-2B transfectants stably expressing catalytically inactive sPLA2-V and -X mutants in which the catalytic centre histidine was replaced with asparagine (H48Q). We then examined the infection efficiency of Ad-mPGES-1 in these cells by monitoring mPGES-1 expression. Neither the sPLA2-V H48Q mutant (Figure 4A) or the sPLA2-X H48Q mutant (Figure 4B) decreased adenoviral expression of mPGES-1 in BEAS-2B cells under the conditions where wild-type enzymes significantly prevented it. This result implies that the catalytic activity of sPLA2-V and -X is a prerequisite for their anti-viral effect.
Figure 4. Requirement for sPLA2 catalytic activity.
Control BEAS-2B cells and those stably expressing wild-type enzymes (WT) or the catalytically inactive H48Q mutants of sPLA2-V (A) or sPLA2-X (B) were infected with Ad-mPGES-1 for 24 h. Then the cells were subjected to Northern-blotting to assess the expression of mPGES-1 and sPLA2s. Equal loading of RNA in each lane was verified by ribosomal RNA (rRNA) stained with ethidium bromide. Bottom graphs indicate PLA2 activity released into the supernatants, which confirmed that the H48Q mutants were catalytically inactive.
sPLA2-V and -X increase LPC levels in cells
It has been reported that LPC, a PLA2 reaction product of PC, disturbs virus-mediated membrane fusion and thereby viral infection [31–35]. In the light of the proposal that sPLA2-V and -X are much more potent than other sPLA2s in acting on the PC-rich external leaflet of the plasma membrane [4–6], we hypothesized that the hydrolysis of membrane PC, and thereby increased production of LPC, by sPLA2-V or -X might be functionally linked to their inhibitory effects on adenoviral infection. To corroborate this possibility, we investigated whether LPC or other lysophospholipids added exogenously could prevent adenoviral infection in BEAS-2B cells. As shown in Figure 5(A), infection of Ad-mPGES-1 in BEAS-2B cells, as revealed by mPGES-1 expression, was markedly decreased when the cells were incubated with exogenous LPC containing relatively long sn-1 fatty acyl chains (C14:0, C16:0 and C18:0, but not C12:0) (Figure 5A). By contrast, none of the other lysophospholipids (LPE, LPS and LPA with C16:0 at sn-1) (Figure 5A) or AA (Figure 5B) affected the adenoviral delivery of mPGES-1, thus revealing LPC specificity. The inhibitory effect of LPC on the adenoviral delivery of mPGES-1 was dependent on the concentration (>10 μM) of this lysophospholipid added (Figure 5C).
Figure 5. LPC blocks adenoviral infection into BEAS-2B cells.
(A) and (B) BEAS-2B cells were incubated with Ad-mPGES-1 at MOI=10 (A) and at MOI=2 or 10 (B) in the presence or absence (control) of various lysophospholipids or AA (each at 50 μM) for 24 h. In the case of LPC, molecular species having C12:0, C14:0, C16:0 and C18:0 at sn-1 were used. (C) The cells were incubated with Ad-mPGES-1 at MOI=10 in the presence or absence of the indicated concentrations of LPC with C18:0 at sn-1. The cells were then harvested and subjected to Northern-blotting with probes against mPGES-1 and G3PDH.
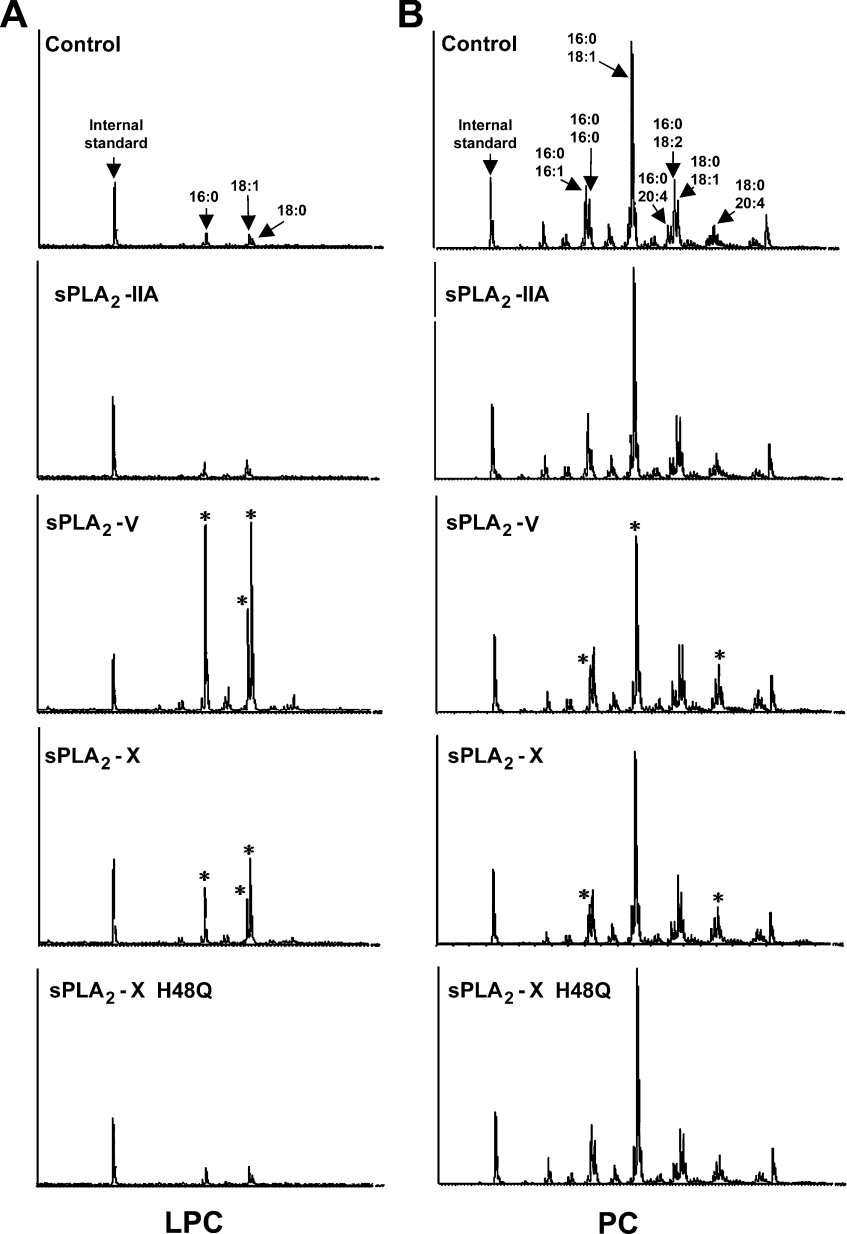
We then investigated whether sPLA2-V and -X are indeed capable of increasing levels of endogenous LPC in cells. ESI/MS analysis of phospholipids extracted from BEAS-2B cells, with or without sPLA2 transfection, revealed that there were marked increases in the amount of endogenous LPC molecular species with C16:0, C18:0 and C18:1 in sPLA2-V-expressing cells (19-, 39- and 10-fold increases respectively, as evaluated by peak areas) and in sPLA2-X-expressing cells (4-, 12- and 3-fold increases respectively) compared with those in control cells (Figure 6A). Concomitantly, there were approx. 32% and 24% decreases in the amount of PC species with C16:0–C16:1 and C16:0–C18:1 respectively, in sPLA2-V-expressing cells and an approx. 40% decrease in the amount of PC containing C16:0–C16:1 in sPLA2-X-expressing cells as compared with those in control cells (Figure 6B). Thus the increased amount of LPC containing C16:0 in sPLA2-V- or -X-expressing cells may be derived from these decreased PC species with C16:0 at sn-1. Irrespective of marked increases in the amount of LPC with C18:0 and C18:1, however, no appreciable changes in the number of PC species with C18:0 or C18:1 at sn-1 were found in sPLA2-V- or -X-expressing cells. Although the reason for this discrepancy is unknown, it may reflect accelerated membrane remodelling (hydrolysis by sPLA2s followed by rapid reacylation) of PC species with C18:0 or C18:1 at sn-1 during culture. Supporting this idea, PC containing C18:0–C20:4 was significantly increased in sPLA2-V- and -X-expressing cells (2- and 1.6-fold increases respectively) compared with that in control cells (Figure 6B). No alterations in LPC (Figure 6A) or PC (Figure 6B) species were observed in cells transfected with sPLA2-IIA or sPLA2-X H48Q, which failed to suppress adenoviral infection (see above), compared with those in control cells.
Figure 6. ESI-MS analyses of LPC and PC species.
Lipids extracted from control and sPLA2-expressing BEAS-2B cells (100 μg protein equivalents) were subjected to ESI-MS to assess the composition of LPC (A) and PC (B) species. LPC with C12:0 at sn-1 and PC with C14:0–C14:1 were added to samples as internal standards before lipid extraction. Asterisks indicate molecular species for which amounts were markedly changed in comparison with those in control cells. A representative result of two reproducible experiments is shown.
Expression of sPLA2-V is induced by virus-related stimuli
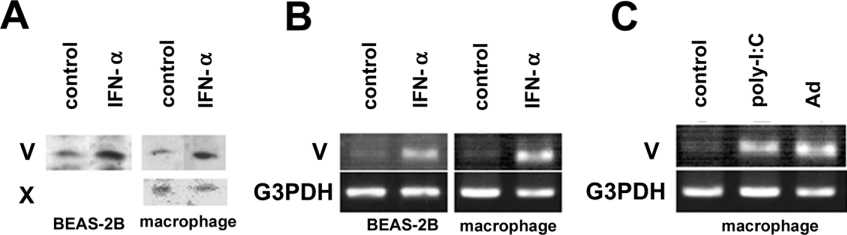
We next examined whether the expression levels of endogenous sPLA2-V and -X are altered in cells treated with certain stimuli related to viral infection. Treatment of BEAS-2B cells and mouse peritoneal macrophages with IFN-α, an anti-viral cytokine [36], resulted in a significant increase in the expression of sPLA2-V at both protein (Figure 7A) and mRNA (Figure 7B) levels, as assessed by immunoblotting and RT-PCR respectively. Increased expression of sPLA2-V was also observed in macrophages after stimulation with poly(I:C), a synthetic ligand for a Toll-like receptor (TLR3) that binds to double-stranded viral nucleic acid and activates the type I IFN response [37], and even after infection with adenovirus (Figure 7C). In contrast with sPLA2-V, sPLA2-X was expressed in macrophages at an almost constant level following treatment with IFN-α (Figure 7A), poly-I:C or adenovirus (results not shown). Expression of sPLA2-X protein was very faint and hard to detect in BEAS-2B cells under all conditions tested (results not shown) even though mRNA was weakly and constantly detectable as assessed by high sensitivity RT-PCR [23].
Figure 7. Effects of virus-related stimuli on the expression of endogenous sPLA2-V and -X.
(A and B) Human BEAS-2B cells and mouse peritoneal macrophages were incubated with or without 0.2 μg/ml IFN-α for 24 h. Then the cells were subjected to immunoblotting with anti-sPLA2-V and anti-X antibodies (A) or to RT-PCR for sPLA2-V and G3PDH (B). (C) Macrophages were incubated with or without adenovirus (MOI=20) or poly-I:C (100 μg/ml) for 24 h and then subjected to RT-PCR for sPLA2-V or G3PDH. Representative results of three independent experiments are shown.
sPLA2-V and -X mRNAs are expressed in human respiratory tract
In situ hybridization of the bronchial epithelium from human lungs affected by pneumonia with sPLA2-specific cRNA antisense, but not sense, probes revealed the existence of sPLA2-V and -X mRNAs in epithelial cells (see Supplementary Figure 1S at http://www.BiochemJ.org/bj/393/bj3930097add.htm). sPLA2-IID mRNA was also detected, whereas signals for sPLA2-IIA and -IIF mRNAs were very weak in epithelium (see Supplementary Figure 1S). These results are in agreement with our recent immunohistochemical study in which immunoreactive sPLA2-V, -X and -IID proteins were detected in the epithelium of human respiratory tract [23].
DISCUSSION
The role of sPLA2s (sPLA2-IIA in particular) in host defence against bacterial infection has been well documented by recent studies [11–14]. By contrast, the contribution of sPLA2s to innate immunity against viral infection is poorly understood. In the present study, we have provided evidence that sPLA2-V and -X, two sPLA2s that possess potent ability among sPLA2s to hydrolyse mammalian cell membranes [4–6], prevent host cells from being infected with adenoviruses, which often causes infectious respiratory diseases. Unlike the anti-bacterial function of sPLA2s, which depends on their hydrolytic action on bacterial membranes [11–14], the anti-viral action of sPLA2-V and -X depends on their ability to act on host cell membranes. Thus these two sPLA2s hydrolyse phospholipids in the plasma membrane, eventually leading to a decrease in adenoviral entry into cells. Since sPLA2-V and -X are expressed in the human respiratory tract at both protein [23] and mRNA (Supplementary Figure 1) levels, it can be speculated that these two sPLA2s may function as part of the anti-viral arsenal to protect the respiratory (and possibly other) organs against adenoviral invasion in particular pathological circumstances.
Several lines of evidence support the idea that sPLA2-V and -X inhibit adenoviral infection by hydrolysing the plasma membrane of host cells. Among sPLA2s tested so far, this anti-viral effect was observed only with sPLA2-V and -X (Figure 1), the two most potent plasma membrane-acting sPLA2s [4–6]. Immunofluorescence microscopy revealed that these sPLA2s prevent adenoviral entry into the endosomes of host cells (Figure 2). By comparison, parvovirus-associated sPLA2 (as opposed to host sPLA2-V and -X), which is essential for effective infection of the virus acts on a later step; transfer of the viral genome from the endosomes into the nucleus [15,16]. Although cPLA2α released more AA and PGE2 than did sPLA2-V and -X, it was unable to suppress adenovirus-mediated gene delivery (Figure 1), indicating that no arachidonate metabolite participates in the anti-viral effect of sPLA2-V and -X. Furthermore, considering that cPLA2α-mediated AA release occurs in the perinuclear region [38,39], it is unlikely that hydrolysis of the perinuclear membrane would affect viral entry.
More direct evidence for the anti-viral effect of sPLA2-V and -X was obtained from experiments using pure recombinant enzymes in the plaque-forming assay (Figure 3). Adenovirus amplification occurring in 293A cells was markedly suppressed by adding exogenous sPLA2-V and -X to the culture, whereas preincubation of adenovirus with these recombinant enzymes did not decrease the capacity of the virus to infect cells. As little as 100 ng/ml recombinant sPLA2-V and -X was able to decrease adenoviral plaque formation by ≥50%. In the case of sPLA2-V, this concentration is almost comparable with that reported to be sufficient for this enzyme to exert its bactericidal activity [12,14,40]. By contrast, exogenous sPLA2-IIA failed to suppress plaque formation even at 1 μg/ml, probably because it hardly acts on the plasma membrane of host cells [5]. Although the in vivo concentrations of sPLA2-V or -X under physiological and pathological conditions are unclear, it is known that the levels of sPLA2-IIA in severely inflamed sites often reach the order of μg/ml [41]. This fact, together with the intense immunoreactivity of sPLA2-V and -X in the respiratory epithelium of human lungs affected by pneumonia [23], suggests that local concentrations reached in the respiratory tract could be high enough to elicit their anti-viral effect, a possibility that should be confirmed by future studies.
Mutagenesis studies have revealed that the ability of sPLA2-V and -X to suppress adenoviral infection depends on their catalytic activity (Figure 4). This is in contrast with the suppressive action of bee venom sPLA2 on HIV entry into host cells; this function depends on the binding of the enzyme to cells, and a peptide of 15 amino acids that corresponds to a surface-exposed loop of the enzyme displays anti-HIV activity through interacting with the HIV coreceptor CXCR4 [17,18]. Nonetheless, the requirement of catalytic activity for the anti-viral effect of sPLA2-V and -X was further supported by the findings that LPC, a PLA2-hydrolytic product of PC that is richly present in the outer leaflet of the plasma membrane, suppressed adenoviral infection (Figure 5A), and that substantial amounts of LPC species were indeed produced in cells transfected with these enzymes (Figure 5B). To our knowledge, this is the first quantitative demonstration by ESI/MS that sPLA2-V and -X produce LPC from the cellular membrane. Since LPC binds to serum albumin, serum in medium already contains substantial amounts of albumin-bound LPC [42,43], and LPC undergoes further metabolizing such as reacylation, it was difficult to precisely evaluate the actual working concentration of LPC and the amounts of LPC produced by these sPLA2s in cells. We speculate that the local spatiotemporal concentration of LPC produced by these sPLA2s in the plasma membrane may be sufficient to facilitate their suppressive effect on adenovirus entry.
LPC has been shown to inhibit membrane fusion elicited by a variety of viruses, such as influenza, simian immunodeficiency, Sendai and rabies [31–35]. This ‘inverted-cone’ shape lysophospholipid, when present in the outer leaflet of the plasma membrane, prevents the formation of stalks, a common step in viral entry into host cells [34,35]. Alternatively, LPC could interact directly with viral fusion proteins [31–33]. For instance, inhibition of influenza virus-induced membrane fusion by LPC is increased with increasing acyl chain length and is caused by the binding of LPC to fusion peptides [32]. It is thus speculated that LPC-mediated inhibition of adenoviral infection might involve similar machinery, although the precise mechanisms for LPC action need further investigation. As another possibility, LPC, by acting as a ligand for putative LPC receptor(s) [42], might generate particular signals that lead to prevention of adenovirus internalization.
In the context of this study, increased expression of endogenous sPLA2-V in response to stimuli related to viral infection (Figure 7) may represent a host response directed towards anti-viral defence. On the contrary, the expression of sPLA2-X was relatively constant after viral-related stimuli, in agreement with previous observations that the expression of this enzyme is poorly affected by proinflammatory stimuli [22,23,44]. Conceivably, sPLA2-X may be regulated by cleavage of the N-terminal pro-peptide by certain proteases that are induced or activated during pathological processes, a possibility that is now under investigation. If the anti-viral effect of these two sPLA2s is mainly if not solely mediated by the production of LPC, which suppresses infection by various viruses [31–35], the innate immune action of these sPLA2s may be directed not only at adenovirus but also at other infectious viruses. In support of this, we have recently shown that the expression and localization of sPLA2-V in the liver of patients with viral hepatitis are closely correlated with those of hepatitis C antigen [45]. Thus although it has been proposed that the control of particular sPLA2s may have advantages over the inhibition of selective lipid mediator pathways and of other biological events in the treatment of pathological states, one should consider that pharmacological inhibition of sPLA2s would hamper their anti-microbial roles, possibly causing disadvantageous side-effects.
Online Data
Acknowledgments
We would like to thank Dr G. Lambeau (CNRS-UPR 411, Sophia Antipolis, France) and Dr M. H. Gelb (University of Washington, Seattle, WA, U.S.A.) for providing sPLA2 cDNAs, proteins and antibodies, Dr K. Komiyama (Nihon University, Tokyo, Japan) for technical help during in situ hybridization studies, and Dr Y. Takanezawa, Dr J. Aoki and Dr H. Arai (University of Tokyo, Tokyo, Japan) for technical assistance and helpful discussions about ESI/MS. This work was supported by a grant-in aid for scientific research from the Ministry of Education, Science, Culture, Sports and Technology of Japan.
References
- 1.Murakami M., Kudo I. Diversity and regulatory functions of mammalian secretory phospholipase A2s. Adv. Immunol. 2001;77:163–194. doi: 10.1016/s0065-2776(01)77017-4. [DOI] [PubMed] [Google Scholar]
- 2.Kudo I., Murakami M. Phospholipase A2 enzymes. Prostaglandins and Other Lipid Mediat. 2002;68–69:3–58. doi: 10.1016/s0090-6980(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 3.Murakami M., Kambe T., Shimbara S., Kudo I. Functional coupling between various phospholipase A2s and cyclooxygenases in immediate and delayed prostanoid biosynthetic pathways. J. Biol. Chem. 1999;274:3103–3115. doi: 10.1074/jbc.274.5.3103. [DOI] [PubMed] [Google Scholar]
- 4.Murakami M., Koduri R. S., Enomoto A., Shimbara S., Seki M., Yoshihara K., Singer A., Valentin E., Ghomashchi F., Lambeau G., et al. Distinct arachidonate-releasing functions of mammalian secreted phospholipase A2s in human embryonic kidney 293 and rat mastocytoma RBL-2H3 cells through heparan sulfate shuttling and external plasma membrane mechanisms. J. Biol. Chem. 2001;276:10083–10096. doi: 10.1074/jbc.M007877200. [DOI] [PubMed] [Google Scholar]
- 5.Bezzine S., Koduri R. S., Valentin E., Murakami M., Kudo I., Ghomashchi F., Sadilek M., Lambeau G., Gelb M. H. Exogenously added human group X secreted phospholipase A2 but not the group IB, IIA, and V enzymes efficiently release arachidonic acid from adherent mammalian cells. J. Biol. Chem. 2000;275:3179–3191. doi: 10.1074/jbc.275.5.3179. [DOI] [PubMed] [Google Scholar]
- 6.Murakami M., Kambe T., Shimbara S., Higashino K., Hanasaki K., Arita H., Horiguchi M., Arita M., Arai H., Inoue K., Kudo I. Different functional aspects of the group II subfamily (types IIA and V) and type X secretory phospholipase A2s in regulating arachidonic acid release and prostaglandin generation: implication of cyclooxygenase-2 induction and phospholipid scramblase-mediated cellular membrane perturbation. J. Biol. Chem. 1999;274:31435–31444. doi: 10.1074/jbc.274.44.31435. [DOI] [PubMed] [Google Scholar]
- 7.Murakami M., Kambe T., Shimbara S., Yamamoto S., Kuwata H., Kudo I. Functional association of type IIA secretory phospholipase A2 with the glycosylphosphatidylinositol-anchored heparan sulfate proteoglycan in the cyclooxygenase-2-mediated delayed prostanoid biosynthetic pathway. J. Biol. Chem. 1999;274:29927–29936. doi: 10.1074/jbc.274.42.29927. [DOI] [PubMed] [Google Scholar]
- 8.Kim Y. J., Kim K. P., Rhee H. J., Das S., Rafter J. D., Oh Y. S., Cho W. Internalized group V secretory phospholipase A2 acts on the perinuclear membranes. J. Biol. Chem. 2002;277:9358–9365. doi: 10.1074/jbc.M110987200. [DOI] [PubMed] [Google Scholar]
- 9.Mounier C. M., Ghomashchi F., Lindsay M. R., James S., Singer A. G., Parton R. G., Gelb M. H. Arachidonic acid release from mammalian cells transfected with human groups IIA and X secreted phospholipase A2 occurs predominantly during the secretory process and with the involvement of cytosolic phospholipase A2α. J. Biol. Chem. 2004;279:25024–25038. doi: 10.1074/jbc.M313019200. [DOI] [PubMed] [Google Scholar]
- 10.Lambeau G., Lazdunski M. Receptors for a growing family of secreted phospholipases A2. Trends Pharmacol. Sci. 1999;20:162–170. doi: 10.1016/s0165-6147(99)01300-0. [DOI] [PubMed] [Google Scholar]
- 11.Weinrauch Y., Elsbach P., Madsen L. M., Foreman A., Weiss J. The potent anti-Staphylococcus aureus activity of a sterile rabbit inflammatory fluid is due to a 14-kD phospholipase A2. J. Clin. Invest. 1996;97:250–257. doi: 10.1172/JCI118399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koduri R. S., Gronroos J. O., Laine V. J., Le Calvez C., Lambeau G., Nevalainen T. J., Gelb M. H. Bactericidal properties of human and murine groups I, II, V, X, and XII secreted phospholipases A2. J. Biol. Chem. 2002;277:5849–5857. doi: 10.1074/jbc.M109699200. [DOI] [PubMed] [Google Scholar]
- 13.Laine V. J., Grass D. S., Nevalainen T. J. Resistance of transgenic mice expressing human group II phospholipase A2 to Escherichia coli infection. Infect. Immun. 2000;68:87–92. doi: 10.1128/iai.68.1.87-92.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gronroos J. O., Laine V. J., Janssen M. J., Egmond M. R., Nevalainen T. J. Bactericidal properties of group IIA and group V phospholipases A2. J. Immunol. 2001;166:4029–4034. doi: 10.4049/jimmunol.166.6.4029. [DOI] [PubMed] [Google Scholar]
- 15.Zadori Z., Szelei J., Lacoste M. C., Li Y., Gariepy S., Raymond P., Allaire M., Nabi I. R., Tijssen P. A viral phospholipase A2 is required for parvovirus infectivity. Dev. Cell. 2001;1:291–302. doi: 10.1016/s1534-5807(01)00031-4. [DOI] [PubMed] [Google Scholar]
- 16.Canaan S., Zadori Z., Ghomashchi F., Bollinger J., Sadilek M., Moreau M. E., Tijssen P., Gelb M. H. Interfacial enzymology of parvovirus phospholipases A2. J. Biol. Chem. 2004;279:14502–14508. doi: 10.1074/jbc.M312630200. [DOI] [PubMed] [Google Scholar]
- 17.Fenard D., Lambeau G., Valentin E., Lefebvre J. C., Lazdunski M., Doglio A. Secreted phospholipases A2, a new class of HIV inhibitors that block virus entry into host cells. J. Clin. Invest. 1999;104:611–618. doi: 10.1172/JCI6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenard D., Lambeau G., Maurin T., Lefebvre J. C., Doglio A. A peptide derived from bee venom-secreted phospholipase A2 inhibits replication of T-cell tropic HIV-1 strains via interaction with the CXCR4 chemokine receptor. Mol. Pharmacol. 2001;60:341–347. doi: 10.1124/mol.60.2.341. [DOI] [PubMed] [Google Scholar]
- 19.Medina-Kauwe L. K. Endocytosis of adenovirus and adenovirus capsid proteins. Adv. Drug Delivery Rev. 2003;55:1485–1496. doi: 10.1016/j.addr.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Meier O., Greber U. F. Adenovirus endocytosis. J. Gene Med. 2004;6:S152–S163. doi: 10.1002/jgm.553. [DOI] [PubMed] [Google Scholar]
- 21.Masuda S., Murakami M., Matsumoto S., Eguchi N., Urade Y., Lambeau G., Gelb M. H., Ishikawa Y., Ishii T., Kudo I. Localization of various secretory phospholipase A2 enzymes in male reproductive organs. Biochim. Biophys. Acta. 2004;1686:61–76. doi: 10.1016/j.bbalip.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Masuda S., Murakami M., Komiyama K., Ishihara M., Ishikawa Y., Ishii T., Kudo I. Various secretory phospholipase A2 enzymes are expressed in rheumatoid arthritis and augment prostaglandin production in cultured synovial cells. FEBS J. 2005;272:655–672. doi: 10.1111/j.1742-4658.2004.04489.x. [DOI] [PubMed] [Google Scholar]
- 23.Masuda S., Murakami M., Mitsuishi M., Komiyama K., Ishikawa Y., Ishii T., Kudo I. Expression of secretory phospholipase A2 enzymes in lungs of humans with pneumonia and their potential prostaglandin-synthetic function in human lung-derived cells. Biochem. J. 2005;387:27–38. doi: 10.1042/BJ20041307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masuda S., Murakami M., Takanezawa Y., Aoki J., Arai H., Ishikawa Y., Ishii T., Arioka M., Kudo I. Neuronal expression and neuritogenic action of group X secreted phospholipase A2. J. Biol. Chem. 2005;280:23203–23214. doi: 10.1074/jbc.M500985200. [DOI] [PubMed] [Google Scholar]
- 25.Degousee N., Ghomashchi F., Stefanski E., Singer A., Smart B. P., Borregaard N., Reithmeier R., Lindsay T. F., Lichtenberger C., Reinisch W., et al. Groups IV, V, and X phospholipases A2s in human neutrophils: role in eicosanoid production and gram-negative bacterial phospholipid hydrolysis. J. Biol. Chem. 2002;277:5061–5073. doi: 10.1074/jbc.M109083200. [DOI] [PubMed] [Google Scholar]
- 26.Murakami M., Masuda S., Kudo I. Arachidonate release and prostaglandin production by group IVC phospholipase A2 (cPLA2γ) Biochem. J. 2003;372:695–702. doi: 10.1042/BJ20030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murakami M., Masuda S., Ueda-Semmyo K., Yoda E., Kuwata H., Takanezawa Y., Aoki J., Arai H., Sumimoto H., Ishikawa Y., et al. Group VIB Ca2+-independent phospholipase A2 (iPLA2γ) promotes cellular membrane hydrolysis and prostaglandin production in a manner distinct from other intracellular phospholipase A2s. J. Biol. Chem. 2005;280:14028–14041. doi: 10.1074/jbc.M413766200. [DOI] [PubMed] [Google Scholar]
- 28.Murakami M., Nakashima K., Kamei K., Masuda S., Ishikawa Y., Ishii T., Ohmiya Y., Watanabe K., Kudo I. Cellular prostaglandin E2 production by membrane-bound prostaglandin E synthase-2 via both cyclooxygenases-1 and -2. J. Biol. Chem. 2003;278:37937–37947. doi: 10.1074/jbc.M305108200. [DOI] [PubMed] [Google Scholar]
- 29.Kamei D., Yamakawa K., Takegoshi Y., Mikami-Nakanishi M., Nakatani Y., Oh-Ishi S., Yasui H., Azuma Y., Hirasawa N., Ohuchi K., et al. Reduced pain hypersensitivity and inflammation in mice lacking microsomal prostaglandin E synthase-1. J. Biol. Chem. 2004;279:33684–33695. doi: 10.1074/jbc.M400199200. [DOI] [PubMed] [Google Scholar]
- 30.Nitta H., Kishimoto J., Grogan T. M. Application of automated mRNA in situ hybridization for formalin-fixed, paraffin-embedded mouse skin sections: effects of heat and enzyme pretreatment on mRNA signal detection. Appl. Immunohistochem. Mol. Morphol. 2003;11:183–187. doi: 10.1097/00129039-200306000-00017. [DOI] [PubMed] [Google Scholar]
- 31.Gunther-Ausborn S., Praetor A., Stegmann T. Inhibition of influenza-induced membrane fusion by lysophosphatidylcholine. J. Biol. Chem. 1995;270:29279–29285. doi: 10.1074/jbc.270.49.29279. [DOI] [PubMed] [Google Scholar]
- 32.Gunther-Ausborn S., Stegmann T. How lysophosphatidylcholine inhibits cell-cell fusion mediated by the envelope glycoprotein of human immunodeficiency virus. Virology. 1997;235:201–208. doi: 10.1006/viro.1997.8699. [DOI] [PubMed] [Google Scholar]
- 33.Martin I., Dubois M. C., Saermark T., Epand R. M., Ruysschaert J. M. Lysophosphatidylcholine mediates the mode of insertion of the NH2-terminal SIV fusion peptide into the lipid bilayer. FEBS Lett. 1993;333:325–330. doi: 10.1016/0014-5793(93)80680-s. [DOI] [PubMed] [Google Scholar]
- 34.Vogel S. S., Leikina E. A., Chernomordik L. V. Lysophosphatidylcholine reversibly arrests exocytosis and viral fusion at a stage between triggering and membrane merger. J. Biol. Chem. 1993;268:25764–25768. [PubMed] [Google Scholar]
- 35.Yeagle P. L., Smith F. T., Young J. E., Flanagan T. D. Inhibition of membrane fusion by lysophosphatidylcholine. Biochemistry. 1994;33:1820–1827. doi: 10.1021/bi00173a027. [DOI] [PubMed] [Google Scholar]
- 36.Taya Y., Devos R., Tavernier J., Cheroutre H., Engler G., Fiers W. Cloning and structure of the human immune interferon-gamma chromosomal gene. EMBO J. 1982;1:953–958. doi: 10.1002/j.1460-2075.1982.tb01277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doyle S., Vaidya S., O'Connell R., Dadgostar H., Dempsey P., Wu T., Rao G., Sun R., Haberland M., Modlin R., Cheng G. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity. 2002;17:251–263. doi: 10.1016/s1074-7613(02)00390-4. [DOI] [PubMed] [Google Scholar]
- 38.Schievella A. R., Regier M. K., Smith W. L., Lin L. L. Calcium-mediated translocation of cytosolic phospholipase A2 to the nuclear envelope and endoplasmic reticulum. J. Biol. Chem. 1995;270:30749–30754. doi: 10.1074/jbc.270.51.30749. [DOI] [PubMed] [Google Scholar]
- 39.Evans J. H., Spencer D. M., Zweifach A., Leslie C. C. Intracellular calcium signals regulating cytosolic phospholipase A2 translocation to internal membranes. J. Biol. Chem. 2001;276:30150–30160. doi: 10.1074/jbc.M100943200. [DOI] [PubMed] [Google Scholar]
- 40.Rubin B. B., Downey G. P., Koh A., Degousee N., Ghomashchi F., Nallan L., Stefanski E., Harkin D. W., Sun C., Smart B. P., et al. Cytosolic phospholipase A2-α is necessary for platelet-activating factor biosynthesis, efficient neutrophil-mediated bacterial killing, and the innate immune response to pulmonary infection: cPLA2-α does not regulate neutrophil NADPH oxidase activity. J. Biol. Chem. 2005;280:7519–7529. doi: 10.1074/jbc.M407438200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pruzanski W., Vadas P. Phospholipase A2: a mediator between proximal and distal effectors of inflammation. Immunol. Today. 1991;12:143–146. doi: 10.1016/S0167-5699(05)80042-8. [DOI] [PubMed] [Google Scholar]
- 42.Xu Y. Sphingosylphosphorylcholine and lysophosphatidylcholine: G protein-coupled receptors and receptor-mediated signal transduction. Biochim. Biophys. Acta. 2002;1582:81–88. doi: 10.1016/s1388-1981(02)00140-3. [DOI] [PubMed] [Google Scholar]
- 43.Kishimoto T., Soda Y., Matsuyama Y., Mizuno K. An enzymatic assay for lysophosphatidylcholine concentration in human serum and plasma. Clin. Biochem. 2002;35:411–416. doi: 10.1016/s0009-9120(02)00327-2. [DOI] [PubMed] [Google Scholar]
- 44.Hamaguchi K., Kuwata H., Yoshihara K., Masuda S., Shimbara S., Oh-ishi S., Murakami M., Kudo I. Induction of distinct sets of secretory phospholipase A2 in rodents during inflammation. Biochim. Biophys. Acta. 2003;1635:37–47. doi: 10.1016/j.bbalip.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Ito M., Ishikawa Y., Kiguchi H., Komiyama K., Murakami M., Kudo I., Akasaka Y., Ishii T. Distribution of type V secretory phospholipase A2 expression in human hepatocytes damaged by liver disease. J. Gastroenterol. Hepatol. 2004;19:1140–1149. doi: 10.1111/j.1440-1746.2004.03435.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.