Abstract
The effect of extracellular ATP on adipogenesis was investigated using the mouse 3T3-L1 cell line. Incubation of cells with ATP (1–100 μM) for 5 min induced actin filament reorganization and membrane ruffling mediated through P2Y receptors. Enhancement of preadipocyte migration into fat cell clusters is one of the essential processes of adipose tissue development in vivo and cell migration assays revealed that stimulation of P2Y receptors enhanced chemokinesis (migration) in a concentration dependent manner. In this cell line, growth arrest is required before initiation of differentiation and growth-arrested post-confluent cells can be converted into adipocytes by the presence of the adipogenic hormones dexamethasone, 3-isobutyl-1-methylxanthine and insulin. On the other hand, those hormones alone do not trigger differentiation in proliferating cells. ATP did not induce differentiation when applied alone to either proliferating or postconfluent cells. By contrast, proliferating cells (density <50%) preincubated with ATP for 5 min and subsequently given the adipogenic hormones in the continued presence of ATP, underwent adipocyte differentiation mediated through phospholipase C-coupled P2Y receptors. These adipocytes were found to show very similar characteristics, including morphology and intracellular triacylglycerol accumulation compared with adipocytes differentiated from post-confluent preadipocytes with those adipogenic hormones. When proliferating cells were preincubated with ATP before the addition of the adipogenic hormones, gene expression of aP2 (adipose protein 2) was markedly increased within 6 days, whereas without ATP pretreatment the expression level stayed very low. These results suggest that extracellular ATP renders preadipocytes responsive to adipogenic hormones during the growth phase.
Keywords: adipocyte, ATP, cell migration, differentiation, membrane ruffling, P2Y receptor
Abbreviations: aP2, adipose protein 2; DMEM, Dulbecco's modified Eagle's medium; G0/G1, G0/G1 switch gene; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; KRBH, Krebs–Ringer/bicarbonate Hepes; DIC, differential interference contrast; RT, reverse transcription
INTRODUCTION
Formation of vascularized primitive fat tissue is the initial step in adipose tissue development, followed by the migration of adipose precursor cells (preadipocytes) into fat cell clusters [1]. The body fat mass increase of adulthood is thus caused by both increasing adipocyte size (enlarging existing adipocytes) and increasing numbers (formation of new adipocytes from precursor cells), so enhancement of preadipocyte migration may be one of the factors that induces adipocyte hyperplasia. It has been reported that some cytokines, such as plasminogen activator inhibitor-1 in human preadipocytes [2] and macrophage inflammatory protein-related protein-2 in 3T3-L1 preadipocytes [3], act as chemotactic agents for preadipocytes, potentially contributing to the increased tissue mass during adipogenesis.
Differentiation of adipocytes has been investigated using preadipocyte cell lines and primary cultures of adipose-derived precursor cells. The 3T3-L1 cell line derived from mouse embryo is a well-established and commonly utilized in vitro model for adipocyte differentiation and function [4]. In this cell line, cells undergo preadipocyte-to-adipocyte conversion after progression from a rapidly dividing to a confluent and growth-arrested state in the presence of adipogenic hormones dexamethasone, 3-isobutyl-1-methylxanthine and insulin. After growth arrest at confluence, preadipocytes undergo mitotic clonal expansion followed by gene expression changes that produce the adipocyte phenotype [5]. Fully differentiated adipocytes express proteins involved in fatty acid binding, lipogenesis, lipolysis and insulin-sensitive glucose uptake, which are not expressed or only weakly expressed in undifferentiated preadipocytes [6].
ATP acts both as an intracellular energy source and as an extracellular messenger [7]. Extracellular ATP elicits diverse physiological effects by binding to the ligand-gated cation channels P2X receptors or the heterotrimeric G-protein-coupled P2Y receptors on the plasma membrane [8]. In addition to the short-term effects of extracellular nucleotides on cell functions, there is increasing evidence that such purinergic signalling can have long-term effects on cell-proliferation, -differentiation and -death [9]. Previously, it has been demonstrated that P2 receptors play a role in the migration of arterial smooth muscle cells [10,11] and microglia [12], proliferation of HaCaT keratinocytes [13] and differentiation of human epidermal keratinocytes [14] and rat skeletal muscle cells [15]. A number of studies have reported that extracellular ATP induces cellular functions in both white and brown fat cells. In white fat cells, external ATP affects glucose transport [16], lipolysis [17], insulin processing [18], glycogen synthase [19], cytosolic Ca2+ [20] and membrane capacitance [21], and it also modulates membrane trafficking [22], cytosolic Ca2+ [23–25], cell proliferation [26] and cytoskeletal organization [25] in brown adipose cells. However, the functional effect of external ATP on preadipocytes has not been investigated.
The aim of the present study was to examine the effect of extracellular ATP on adipogenesis. Our results indicate that ATP appears to increase the sensitivity of preadipocytes to adipogenic hormones even before confluence in 3T3-L1 cells.
EXPERIMENTAL
Materials
3T3-L1 cells were purchased from Dainippon Pharmaceutical Co., Ltd (Osaka, Japan). Swiss albino 3T3 fibroblasts were generously provided by Dr Hirokazu Inoue (Department of Microbiology, Shiga University of Medical Science, Japan). Foetal serum was obtained from Gibco BRL (Grand Island, NY, U.S.A.). Insulin, adenosine 5′-triphosphate (ATP, disodium salt), adenosine 5′-diphosphate (ADP, sodium salt), uridine-5′-triphosphate (UTP, sodium salt) and poly-L-lysine were from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Fraction V BSA was purchased from Intergen (Purchase, NY, U.S.A.) and Alexa Fluor® 488-phalloidin from Molecular Probes (Eugene, OR, U.S.A.). Suramin sodium salt was obtained from Wako Pure Chemicals Industries Ltd (Osaka, Japan). Y-27632, U-73122 and U-73343 were from Calbiochem-Novabiochem. Corp. (San Diego, CA, U.S.A.). Oil Red O was from Chroma (Muenster, Germany) and haematoxylin monohydrate was from Merck (Darmstadt, Germany). All other reagents were purchased from Nacalai Tesque (Kyoto, Japan).
Culture and differentiation of 3T3-L1 cells
3T3-L1 preadipocytes and Swiss albino 3T3 fibroblasts were grown in DMEM (Dulbecco's modified Eagle's medium) containing 4.5 g/l glucose, 10% (w/v) foetal bovine serum, 100 μg/ml penicillin and 0.1 mg/ml streptomycin (complete culture medium) at 37 °C in 5% CO2. The cells taken for microscopic experiments were seeded on glass coverslips pre-coated with 0.1% poly-L-lysine. Subculture of the cells (passage) was performed 2 or 3 times a week before cells reached confluence. Differentiation of 3T3-L1 cells was carried out by hormone-induced protocols [27]. Three kinds of differentiation protocols, (i) conventional protocol, (ii) ATP-stimulated protocol (addition of ATP and the hormones before confluence) and (iii) negative control protocol (addition of the hormones before confluence), were performed. Day 0 was defined as the day when 3T3-L1 preadipocytes were subcultured onto a new culture plate. The cells proliferated rapidly and mostly reached a confluent state on day 6.
In the conventional protocol, 3 day post-confluent cells (day 9) were exposed to a cocktail of adipogenic hormones containing 0.5 μM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine and 5 μg/ml insulin in the medium and cultured for 3 days (days 9–12, differentiation). The cells were then cultured for an additional 3 days in the medium containing 3 μg/ml insulin (days 12–15, maturation). Subsequently, cells were given the complete medium with exchanges 2 or 3 times a week (after day 15).
In the ATP-stimulated protocol, cells at <50% confluence (day 3) were exposed to 100 μM ATP in the culture medium for 5 min at 37 °C and subsequently we added the cocktail of adipogenic hormones to the medium in the continued presence of ATP. The cells were cultured for 3 days in this medium (days 3–6) and then incubated for an additional 3 days in the medium containing 3 μg/ml insulin (days 6–9). After day 9, cells were given the complete medium with exchanges 2 or 3 times a week.
In the negative control protocol, the cocktail of adipogenic hormones was added to the cells at <50% confluence (day 3) and the cells were cultured for 3 days in this medium (days 3–6) and then incubated for an additional 3 days in the medium containing 3 μg/ml insulin (days 6–9). After day 9, cells were given the complete medium with exchanges 2 or 3 times a week.
Actin staining
Actin filaments were stained with Alexa Fluor® 488 labelled phalloidin. Incubation of the cells was performed in KRBH buffer (Krebs–Ringer bicarbonate/Hepes buffer) supplemented with 5.6 mM glucose and 0.05% BSA (standard buffer). KRBH buffer contained (mM): 120 NaCl, 4 KH2PO4, 2 CaCl2, 1 MgSO4, 10 NaHCO3, 30 Hepes (pH adjusted to 7.4 with NaOH). The staining for actin was performed as described previously [25]. Fluorescent signals were observed using a confocal laser scanning microscope (Bio-Rad MRC-600UV) mounted on a Nikon Diaphot inverted microscope with a Nikon Fluor ×60 oil immersion objective, 488 nm excitation filter and 510 nm emission filter.
DIC (differential interference contrast) imaging of living cells
Cells cultured on coverslips were rinsed twice with the standard buffer and transferred to the recording chamber, which was maintained at 36 °C, and the solution was exchanged by perfusion. Cells were incubated with the standard buffer in the presence or absence of test reagents for desired periods before observation. DIC images were acquired using an image-capture system consisting of an inverted microscope (Olympus IX70, Olympus Optical, Tokyo, Japan) and a charged-coupled device camera with fitting equipment (Roper Industries, Duluth, GA, U.S.A.). Image data were acquired and analysed using MetaMorph™ software (Universal Imaging Corporation, Downingtown, PA, U.S.A.).
Cell migration assay
Cell migration assays were performed using a Falcon Cell Culture Insert system with 8 μm pore size in a 6.4 mm diameter insert (Becton Dickinson Labware, Franklin Lakes, NJ, U.S.A.). Briefly, 60000 cells in the culture medium were placed into the upper well and the lower well was filled with 600 μl of medium in the presence or absence of the test reagents. After incubating the cells for 4 h at 37 °C non-migrated cells were removed by scraping and the cells which had migrated across the filter were fixed and stained with Diff-Quick (International Reagents Corp., Kobe, Japan). Stained cells were counted in five high-power objective fields. Analysis was performed on three wells for each condition and each experiment was repeated at least three times.
Triacylglycerol staining and assay
Triacylglycerol accumulated in the cytoplasmic space was stained and assayed using Oil Red O. For triacylglycerol staining, cells cultured on coverslips were fixed with 3.7% formaldehyde in PBS for 30 min at room temperature and incubated with 60% propan-2-ol for 1 min. The cells were then stained using the Oil Red O solution (final concentration, 0.2 g in 40% propan-2-ol) for 10 min, incubated with 60% propan-2-ol for 2 min and washed with distilled water. Nuclei were visualized using conventional haematoxylin staining. After washing with distilled water, each coverslip was mounted on a slide glass in a 1:1 solution of PBS/glycerol and observed using bright field microscopy.
Triacylglycerol-assay was performed as described previously [28]. Cells grown and differentiated in 24 well culture plates were rinsed three times with PBS and fixed with 500 μl of 3.7% formaldehyde in PBS for 60 min at 4 °C. After removing the formaldehyde solution, the cells in each well were stained with 120 μl of Oil Red O solution for 15 min and washed three times with 150 μl of distilled water. Dye trapped in intracellular lipid droplets was eluted with 150 μl of propan-2-ol per well and absorbance analysis was performed at 540 nm. Analysis was performed on 3 wells for each condition and each experiment was repeated at least 3 times.
RT (reverse transcription)-PCR and real-time quantitative PCR
Total RNA was extracted from 3T3-L1 cells using Sepasol-RNA I Super reagent (Nacalai tesque). Single-strand cDNA was synthesized from 2.5 μg of total RNA in 20 μl volumes using RNase H− SuperScript II reverse transcriptase (Invitrogen, U.S.A.) with 1 μg of (N)9 random primer (Takara Bio Inc., Shiga, Japan). A portion of the reverse transcription products (0.4 μl) was amplified by PCR using specific primers. Specific primers for mouse aP2 (adipose protein 2), CD36 antigen, G0/G1 (G0/G1 switch gene) and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) were synthesized according to the nucleotide sequences of mouse aP2 (GenBank® accession number, K02109) [29], CD36 (L23108) [30], G0/G1 (X95280) and GAPDH (M32599) [31] respectively: aP2 sense, 5′-CCTGGAAGACAGCTCCTCCTC-5′ (nt 10–30); aP2 antisense, 5′-ATCCAGGCCTCTTCCTTTGCTC-3′ (nt 463–441); CD36 sense, 5′-CACATTTCCTACATGCAAGTCCAG-3′ (nt 1312–1335); CD36 antisense, 5′-CCAATCCCAAGTAAGGCCATCTC-3′ (nt 1636–1614); G0/G1 sense, 5′-GCCTGACCTTCTCCAGCAAGTG-3′ (nt 57–78); G0/G1 antisense, 5′-TCATGATCTGTGTCTCCCTTCTC-3′ (nt 482–460); GAPDH sense, 5′-ACCACAGTCCATGCCATCAC-3′ (nt 566–585); GAPDH antisense, 5′-TCCACCACCCTGTTGCTGTA-3′ (nt 1017–998). The PCR reaction included 0.05 μg of template cDNA, 0.25 units of KOD dash polymerase (Toyobo Biochemicals, Osaka, Japan), 1 mM KCl, 6 mM (NH4)2SO4, 0.1% Triton X-100, 10 μg/ml BSA, 0.2 mM each of deoxynucleotide triphosphates and 4 pmol primers in 20 μl of 120 mM Tris/HCl buffer (pH 8.0). The amplification was performed with 30 cycles of denaturation at 98 °C for 10 s, annealing at 55 °C for 2 s and elongation at 74 °C for 30 s. The PCR products were analysed by electrophoresis on 2% agarose gel with ethidium bromide. DNA sequencing of the PCR products was performed by the dideoxy chain termination method using an ABI PRISM 310 Genetic Analyzer (Applied Biosystems, Foster City, CA, U.S.A.).
Real-time quantitative PCR was performed using a LightCycler™ system (Roche Diagnostics, Mannheim, Germany). Reverse transcription products (0.4 μl) were amplified by the Hot Start PCR method in 20 μl glass capillaries using 4 pmole of specific primers and a LightCycler Fast Start DNA MasterPLUS SYBR Green I (Roche Diagnostics) kit including Fast Start Taq DNA polymerase and double-stranded DNA-specific fluorescent dye SYBR Green I. Fast Start Taq DNA polymerase was activated at 95 °C for 10 min and PCR reaction was performed with 45 cycles of denaturation at 95 °C for 10 s, annealing at 55 °C for 20 s and extension at 72 °C for 18 s. Real-time PCR product accumulation was monitored using intercalation dye SYBR Green I. The standard DNA was prepared by RT-PCR: after analysing the products by electrophoresis on 2% agarose gel with ethidium bromide, the signals of interest were cut out from the agarose and PCR products were then extracted from the gel and purified by ethanol precipitation. Specificity of fluorescence-induced SYBR Green I binding was determined by melting point analysis as described by the manufacturer: denaturation at 95 °C for a nominal 0 s, annealing at 65 °C for 15 s and melting at 95 °C (slope: 0.1 °C/s) for a nominal 0 sec.
Statistics
The results are expressed as means±S.E.M. and n=number of experiments. Statistical comparisons were made using Student's t test or one-way ANOVA followed by Bonferroni's test, and differences were considered to be significant at P<0.05.
RESULTS
Stimulation of P2Y receptors induces plasma membrane ruffling in 3T3-L1 preadipocytes
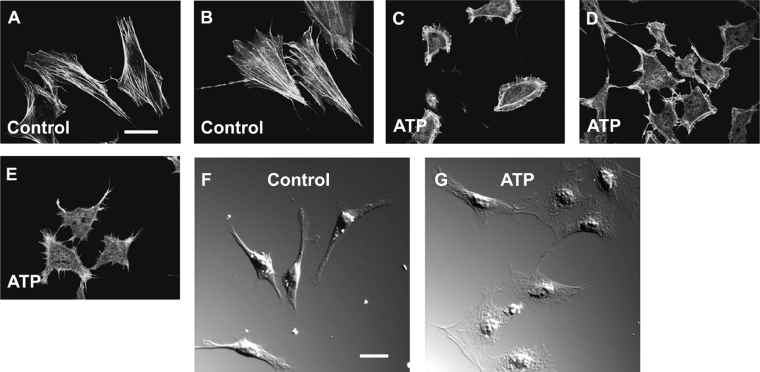
Extracellular ATP has been shown to induce peripheral accumulation of actin filaments in round-shaped brown adipocytes in rat [25]. We investigated whether extracellular ATP induces a change in the distribution of cytoskeletal actin in 3T3-L1 preadipocytes. In control cells, phalloidin staining revealed a network of actin stress fibres running through the cytoplasm constructing a cellular frame as is typically observed in fibroblasts (Figures 1A and 1B). After the cells were incubated with 10 μM ATP for 5 min at 37 °C, actin filament structures underwent a significant change in distribution, forming a peripheral meshwork of broad, sheet-like structures called lamellipodia (membrane ruffling) and surface projections of thin, needle-like structures called filopodia (Figures 1C–1E).
Figure 1. Reorganization of actin filaments by extracellular ATP in 3T3-L1 preadipocytes.
(A–E) Confocal laser scanning microscopy of 3T3-L1 preadipocytes stained with Alexa Fluor® 488 labelled phalloidin. Cells were incubated with the standard buffer for 5 min at 37 °C as controls (A and B) or with 10 μM ATP in the standard buffer (C–E) before the staining as described in the Experimental section. (F and G) DIC images of living 3T3-L1 cells. Cells were incubated with (G) or without (F) 10 μM ATP in the standard buffer for 5 min at 37 °C before observation. Results are representative of 10 experiments. Scale bar, 10 μm.
Cytoplasmic stress fibre structures typical of resting cells were no longer observed in ATP-treated cells. DIC images also showed a broad, flattened plasma membrane in most of the ATP-treated cells (Figure 1G) compared with the untreated cells (Figure 1F). Such membrane ruffling (lamellipodia) was observed after incubating cells with ATP at concentrations of 1–100 μM and with other purinergic agonists: 100 μM ADP and 100 μM UTP (results not shown).
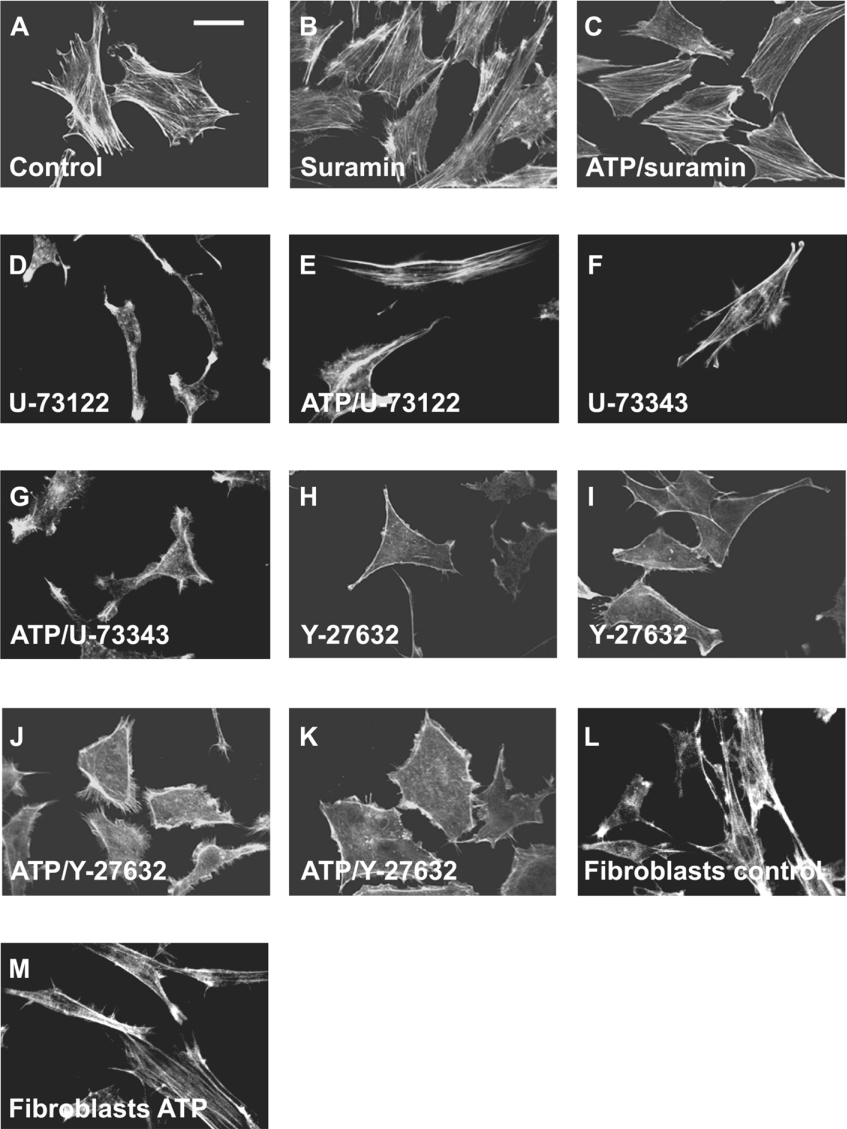
We next investigated the effect of the P2 receptor antagonist suramin [8] on ATP-induced reorganization of actin filaments. The actin filament structures in the cells incubated with 100 μM suramin for 7 min at 37 °C (Figure 2B) demonstrated staining patterns similar to those in control cells (Figure 2A), and in the presence of suramin actin fibre structures were well maintained even after incubation with ATP, without the formation of lamellipodia and filopodia (Figure 2C). This implies that extracellular ATP triggers the rearrangement of actin filaments by binding to P2 receptors in 3T3-L1 preadipocytes. Since stimulation of G-protein-coupled P2Y receptors activates phospholipase C, the effect of a widely utilized phospholipase C inhibitor U-73122 [32] was investigated. Cell incubated with 5 μM U-73122 displayed narrow shapes and less stress fibre structures (Figure 2D), implying that normal actin stress fibre structures are phospholipase C-induced. ATP did not induce marked formation of ruffling in the majority of U-73122-pretreated cells (Figure 2E). The inactive derivative U-73343 (5 μM) [33] did not appreciably affect the stress fibre structure (Figure 2F) nor ATP-induced membrane ruffling in 3T3-L1 cells (Figure 2G). These results suggest that ATP-induced reorganization of cytoskeletal actin is mediated through the activation of phospholipase C.
Figure 2. Effects of suramin, U-73122, U-73343 and Y-27632 on ATP-induced actin reorganization.
Confocal laser scanning microscopy of 3T3-L1 preadipocytes (A–K) or Swiss albino 3T3 fibroblasts (L and M) stained with Alexa Fluor® 488 labelled phalloidin. 3T3-L1 preadipocytes were incubated with the standard buffer for 30 min as controls (A), with 100 μM suramin for 7 min (B), 100 μM suramin for 2 min followed by 5 min with 10 μM ATP in the continued presence of suramin (C), 5 μM U-73122 for 30 min (D), 5 μM U-73122 for 25 min followed by 5 min with 10 μM ATP in the continued presence of U-73122 (E), 5 μM U-73343 for 30 min (F), 5 μM U-73343 for 25 min followed by 5 min with 10 μM ATP in the continued presence of U-73343 (G), 10 μM Y-27632 for 30 min (H and I) or 10 μM Y-27632 for 25 min followed by 5 min with 10 μM ATP in the continued presence of Y-27632 (J and K) before the staining procedure. Swiss albino 3T3 fibroblasts were incubated with standard buffer for 5 min at 37 °C as controls (L), or with 100 μM ATP in the standard buffer for 5 min at 37 °C (M) before the staining procedure. Results are representative of six experiments. Scale bar, 10 μm.
It seems that low-molecular-mass G-proteins (small G-proteins) of the Rho families (Rho, Rac and Cdc42) act as molecular switches to regulate a signal transduction pathway that links membrane receptors coupling with heterotrimeric G-proteins to the actin cytoskeleton [34]. We next investigated the effect of the widely utilized selective Rho kinase inhibitor, Y-27632 [35]. Phalloidin staining of cells incubated with 10 μM Y-27632 for 30 min at 37 °C only displayed the outline of the plasma membrane without showing a cytoplasmic network of stress fibre structures (Figures 2H and 2I); this implies that normal formation of actin stress-fibre structures is Rho-induced. Figures 2(J) and 2(K) demonstrate that ATP induced the assembly of peripheral lamellipodia and filopodia even in the presence of Y-27632. These results suggest that ATP-induced reorganization of cytoskeletal actin is not due to Rho/Rho kinase activity, but must result from the activation of other members of the Rho family (Rac and/or Cdc42) involving G-protein-coupled P2Y receptors.
We next investigated whether extracellular ATP also induces reorganization of actin filaments in non-adipose fibroblasts, using Swiss albino 3T3 fibroblasts as a control (Figure 2L). When the cells were incubated with 100 μM ATP for 5 min at 37 °C, the actin stress fibre structure was still visible and ATP-induced membrane ruffling was not appreciably observed in these fibroblasts (Figure 2M). This suggests that extracellular ATP-induced membrane ruffling is remarkable in the preadipocyte cell line but not obvious in non-adipose fibroblasts.
Extracellular ATP induces cell migration in 3T3-L1 preadipocytes
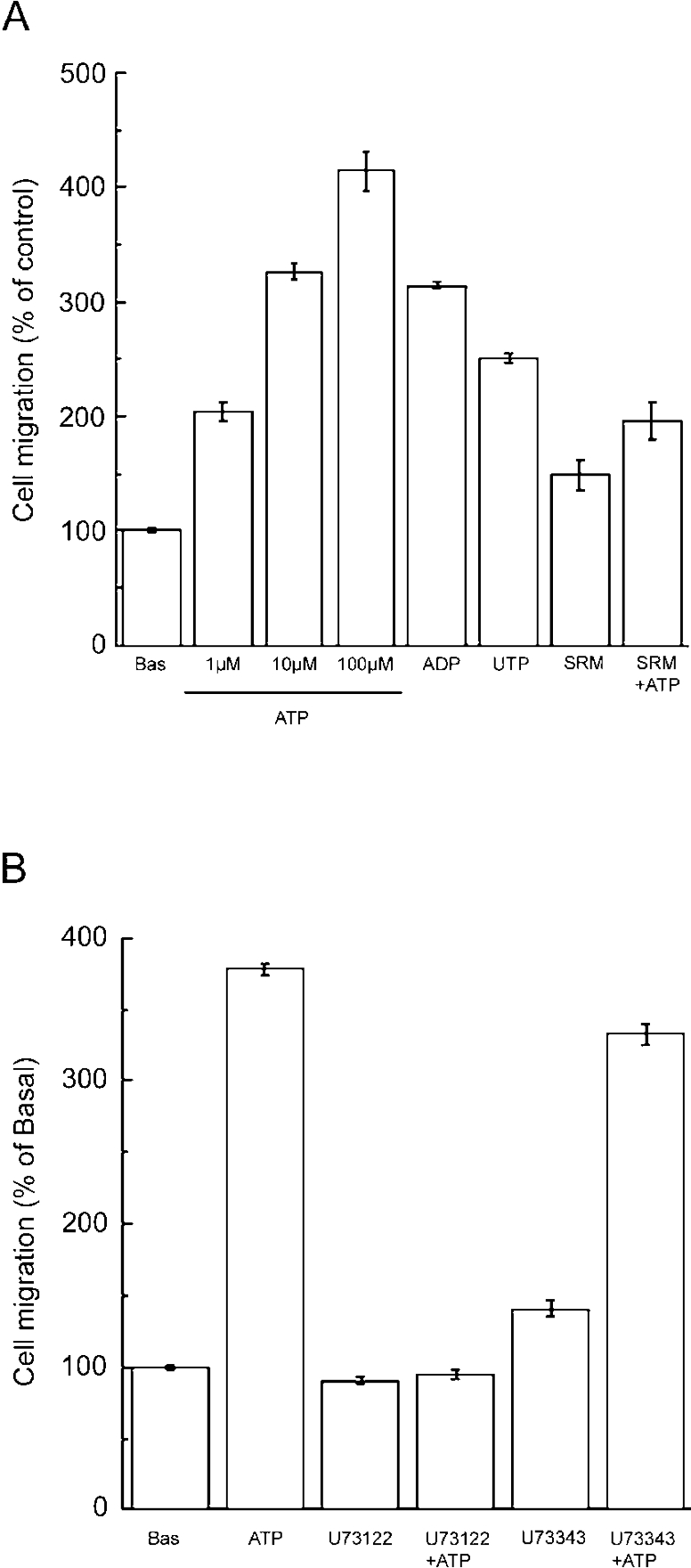
The structures of both lamellipodia and filopodia are found primarily at the plasma membrane of migrating cells [36]. To examine whether purinergic stimulation enhances the translocation of cells, a cell migration assay was performed. Basal cell-migration was defined as the number of migrated cells in the absence of nucleotide. As illustrated in Figure 3(A), extracellular ATP stimulated migration in a concentration-dependent manner, taking the basal migration rate as 100%, 1 μM, 10 μM and 100 μM ATP increased migration to 204±8.5, 326±7.2 and 414±18% compared with basal cell migration activity respectively (P<0.05).
Figure 3. Extracellular nucleotides enhance cell migration of 3T3-L1 preadipocytes.
Cell migration assay was performed by the method described in Experimental section. The number of migrated cells is indicated as a percentage of basal migration. (A) Cells were incubated in the upper well with the complete culture medium (basal; Bas), with 1–100 μM ATP, 100 μM ADP, 100 μM UTP or 100 μM suramin (SRM) for 4 h or 100 μM suramin for 30 min followed by 3.5 h with 100 μM ATP in the continued presence of suramin (SRM+ATP). (B) Cells were incubated in the upper well with the complete culture medium (basal), with 100 μM ATP, 5 μM U-73122 or 5 μM U-73343 for 4 h, or 5 μM U-73122 for 30 min followed by 3.5 h with 100 μM ATP in the continued presence of U-73122 (U-73122+ATP), or 5 μM U-73343 for 30 min followed by 3.5 h with 100 μM ATP in the continued presence of U73343 (U-73343+ATP). Each value represents the mean±S.E.M. for three separate experiments. Statistical significance was determined by one-way ANOVA followed by Bonferroni's test.
ADP (100 μM) and UTP (100 μM) also increased cell migration, to 314±3.9% and 251±3.6% of the basal level, respectively (P<0.05).
Suramin increased migration slightly, but not statistically significantly to 148±12%. Pretreatment of cells with suramin for 30 min at 37 °C significantly decreased the ATP-induced increase in cell migration to 196±16% (P<0.05, compared with ATP). These results indicate that ATP enhanced migration via P2 receptors in 3T3-L1 preadipocytes.
As illustrated in Figure 3(B), incubating cells with U-73122 alone slightly decreased migration (90.4±2.9% compared with basal activity) and preincubation of cells with U-73122 completely prevented ATP-induced enhancement of migration (94.2±3.2%). On the contrary, the inactive derivative U-73343 slightly increased the cell migration activity to 141±5.7% and did not statistically significantly interfere with ATP-enhanced migration (333±7.1%). These results indicate that extracellular ATP stimulates migration in 3T3-L1 cells primarily by binding to G-protein-coupled P2Y receptors. In this system, the concentration gradient of the nucleotide between upper and lower wells was not clear or was not maintained throughout the incubation period. The nucleotide-enhanced cell migration was observed when the stimuli were added in either the lower, or the upper, or both wells (results not shown). These results imply that the extracellular nucleotides induce chemokinesis in 3T3-L1 preadipocytes.
Extracellular ATP stimulates hormone-induced adipocyte differentiation in 3T3-L1 cells
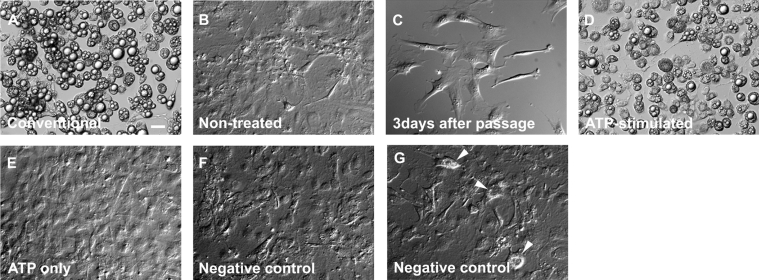
In a conventional differentiation protocol, 3 days post-confluent cells (i.e. at day 9) were given the cocktail of adipogenic hormones as described in the Experimental section. Figure 4(A) shows a DIC image of those cells at day 18 differentiated using the conventional protocol and showing marked accumulation of lipid droplets in their intracellular space. Cells cultured in the complete medium without adipogenic hormones for the same period as in Figure 4(A) did not undergo differentiation (Figure 4B), indicating that adipocyte differentiation requires the activation of the signalling pathway by these hormones.
Figure 4. Effect of extracellular ATP on the differentiation of 3T3-L1 adipocytes.
DIC images of living cells. Differentiation protocols are described in the Experimental section. (A) Day 18, cells were treated with conventional protocol. (B) Day 18, cells were cultured for the same period as in (A) without adding adipogenic hormones and insulin. Cells in (A) and (B) were subcultured on the same day. (C) Day 3, 3 days after passage. (D) Day 18, cells were treated with the ATP-stimulated protocol. (E) Day 18, cells were solely exposed to 100 μM ATP on day 3 without adding adipogenic hormones and were then treated with insulin on day 6. (F and G) Day 18, cells were treated with the negative control protocol. Arrowheads in (G) indicate the cells containing lipid droplets. Cells in (C) to (G) were subcultured on the same day. Results are representative of six experiments. Scale bar, 20 μm.
To investigate the effect of extracellular ATP on adipocyte differentiation, we firstly tested whether ATP itself causes the differentiation. However, similar growth-arrested 3 days post-confluent cells (day 9) did not differentiate when exposed only to 100 μM ATP (results not shown), suggesting that purinergic stimulation does not directly activate the transcription processes for conversion.
In addition application of 100 μM ATP to 3 days post-confluent cells (day 9), before adding the adipogenic hormones, did not result in any additional effects on differentiation (results not shown). These results indicate that purinergic stimulation does not affect growth-arrested cells proceeding to differentiation steps.
We next investigated whether extracellular ATP can cause adipocyte differentiation when applied to cells in the proliferating state. Figure 4(C) shows some cells just before application of ATP (on day 3). In the ATP-stimulated protocol, cells (day 3, <50% density) were incubated with 100 μM ATP for 5 min and then given the cocktail of adipogenic hormones in the continued presence of ATP. These cells continued in culture for 3 days, and subsequently were incubated with insulin for a further 3 days before the maturation period in which well differentiated fat cells acquired lipids. Cells from day 18 obtained by this ATP-stimulated protocol appeared as round shapes with lipid droplets accumulated in their cytoplasmic space (Figure 4D), similar to the view observed in the cells differentiated by the conventional protocol (Figure 4A). Cells treated with ATP on day 3 without adding the adipogenic hormones, and then treated with insulin on day 6, proliferated to reach a confluent state just as the control cells did, but did not show any sign of differentiation during that time (Figure 4E). When the adipogenic hormone ‘cocktail’ alone was added to the cells at low cell-density (day 3, <50%) and then insulin was added on day 6 (negative control protocol), these cells did not differentiate to adipocytes (Figure 4F), although some experiments showed that a small number of the cells contained small lipid droplets (Figure 4G, arrowheads). These results indicate that extracellular ATP did not initiate differentiation, but rendered cells sensitive to adipogenic hormones even before reaching confluence in 3T3-L1 preadipocytes.
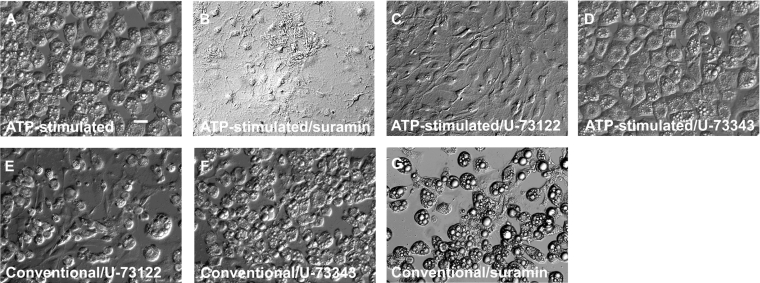
Next we determined whether the stimulatory effect of ATP on hormone-induced adipocyte differentiation is mediated through P2 receptors. Figure 5(A) shows fat cells (day 15) differentiated by an ATP-stimulated protocol. ATP (100 μM) did not stimulate hormone-induced adipocyte differentiation in the presence of 100 μM suramin (Figure 5B), indicating that ATP affects hormone-induced differentiation by binding to P2 receptors in 3T3-L1 cells. We then tested whether blocking of P2 receptors has any effects on the conventional differentiation protocol. As indicated in Figure 5(G), preincubation of post-confluent, growth-arrested cells (day 9) with suramin in the absence of extracellular ATP did not affect the differentiation procedure, which suggests that P2 receptors do not play a role in cells in which the differentiation cascade is already in progress.
Figure 5. Effects of phospholipase C inhibitors on ATP-stimulated hormone-induced adipocyte differentiation.
DIC images of living cells on day 15. (A) Cells were treated with the ATP-stimulated protocol. (B) Cells preincubated with 100 μM suramin for 2 min before ATP-stimulated protocol. (C) Cells preincubated with 5 μM U-73122 for 30 min before the ATP-stimulated protocol. (D) Cells preincubated with 5 μM U-73343 for 30 min before the ATP-stimulated protocol. (E) Post-confluent cells were exposed to 5 μM U-73122 for 30 min before the conventional protocol. (F) Post-confluent cells were exposed to 5 μM U-73343 for 30 min before the conventional protocol. (G) Post-confluent cells were exposed to 100 μM suramin for 30 min before the conventional protocol. Results are representative of six experiments. Scale bar, 20 μm.
We next tested the effect of phospholipase C inhibitor U-73122 on ATP-induced differentiation. Preincubation of cells with U-73122 for 30 min completely blocked the stimulatory effect of ATP on hormone-induced differentiation (Figure 5C), whereas an inactive derivative of U-73343 did not interfere with ATP-stimulated hormone-induced adipocyte differentiation (Figure 5D).
To investigate whether phospholipase C inhibitor affects the conventional differentiation protocol when added to confluent cells, post-confluent growth-arrested cells (day 9) were exposed to these inhibitors for 30 min before the addition of the adipogenic hormones. As shown in Figure 5(E), cells pretreated with U-73122 were converted to fat cells by the ATP-stimulated protocol, but the number of living cells was slightly decreased. U-73343 did not show any inhibitory effects on adipocyte differentiation (Figure 5F).
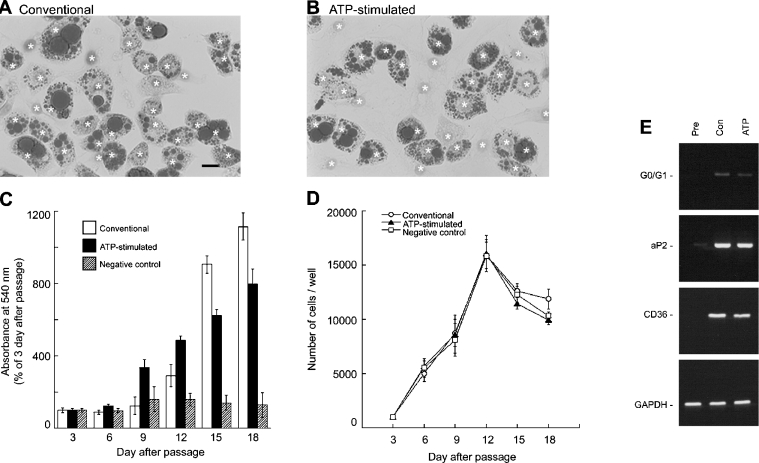
We next investigated the characteristics of the fat cells differentiated by an ATP-stimulated protocol, compared with those differentiated by the conventional protocol. Oil Red O-staining demonstrated that fat cells differentiated by either method contain numerous triacylglycerol droplets in their intracellular space (Figures 6A and 6B). Quantification of intracellular triacylglycerol was performed by Oil Red O-assay (Figure 6C). Cells (1000) were subcultured in 24 well plates on day 0, and Oil Red O-assay was performed every 3 days until day 18. In the conventional protocol, the amount of cell triacylglycerol was significantly increased after insulin-treatment (day 9); the amounts on days 12, 15 and 18 were 292±61%, 906±47% and 1114±75% respectively compared with day 3 (P<0.05). Also in the ATP-stimulated protocol, cell triacylglycerol was increased significantly after insulin-treatment (day 6); the amounts on days 9, 12, 15 and 18 were 337±42%, 488±21%, 623±35% and 795±86% respectively, compared with day 3 (P<0.05). Triacylglycerol amounts did not statistically significantly increase in the cells treated with the negative control protocol. Figure 6(D) indicates that preadipocytes of all groups progressed from rapidly dividing to a confluent and contact-inhibited state, and several rounds of cell division occurred before initiating the adipocyte conversion, which is termed clonal expansion [5]. These results indicate that extracellular ATP does not interfere with the proliferation or clonal expansion of 3T3-L1 cells.
Figure 6. Characterization of adipocytes differentiated by ATP-stimulated hormone-induced protocol.
(A and B) Triacylglycerol staining of 3T3-L1 adipocytes by Oil Red O. Day 15 for fat cells converted by the conventional (A) or ATP-stimulated (B) protocol. *, haematoxylin-positive nucleus. Scale bar, 20 μm. (C and D) Approx. 1000 cells were subcultured in 24 well plates on day 0 to determine triacylglycerol content by Oil Red O assay (C) and to count the number of cells (D). Triacylglycerol-assay was performed as described in the Experimental section and absorbance measured at 540 nm on day 3 was used as a control value. Open columns and open circles indicate the cells treated with the conventional differentiation protocol. Closed columns and closed triangles indicate those treated with the ATP-stimulated protocol. The hatched columns and open squares indicate the cells treated with the negative control protocol. For counting cell number, cells were suspended in PBS after trypsinization and observed with a haemocytometer. Each value represents the mean±S.E.M. for six separate experiments. Statistical significance compared with day 3 was determined by Student's t-test. (E) RT-PCR analysis of the expression of P2Y receptor subtypes genes in 3T3-L1 cells. Pre, preadipocytes; Con, adipocytes differentiated by the conventional protocol; ATP, adipocytes converted by the ATP-stimulated protocol. mRNAs encoding G0/G1, aP2, and CD36 were detected by RT-PCR analysis using GAPDH as a control. The fragments of amplified DNA were found to be identical to the sequences reported previously.
We next investigated the expression of the adipocyte marker protein genes G0/G1, aP2 and CD36 in 3T3-L1 adipocytes, by RT-PCR analysis using GAPDH as a control (Figure 6E). Total RNA was extracted from preadipocytes (<70% density) and adipocytes converted by the conventional or ATP-stimulated protocols. In preadipocytes, no expression of the G0/G1 and CD36 genes was detected. During amplification of the aP2 gene in preadipocytes, the signal was found to be very weak. On the other hand, in adipocytes differentiated by either method, amplification of G0/G1, aP2, and CD36 and GAPDH genes resulted in single bands around the predicted sizes of 425, 453, 324 and 451 bp respectively. These results indicate that 3T3-L1 adipocytes differentiated by the ATP-stimulated protocol express the general marker protein genes for mature fat cells, in patterns similar to that observed in fat cells differentiated by the conventional method.
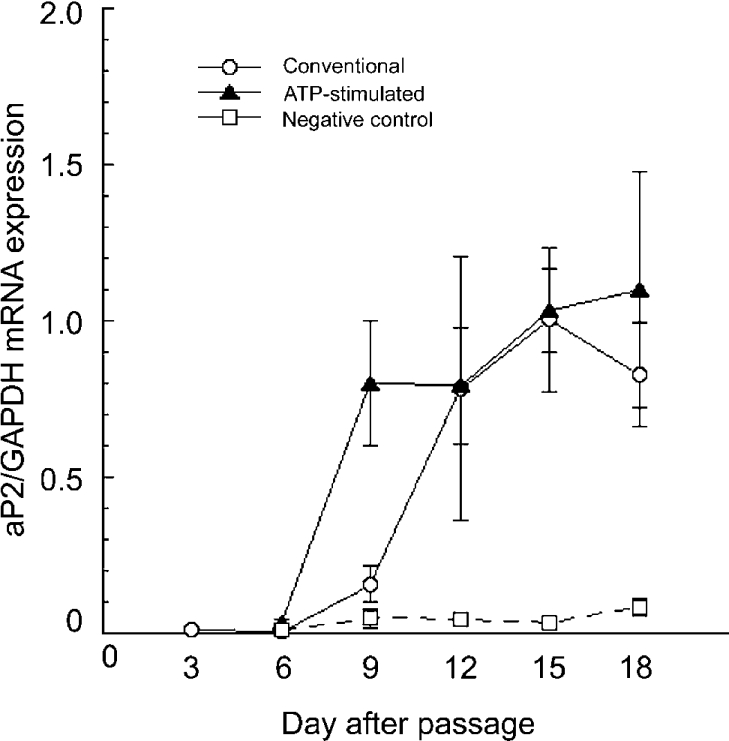
To determine whether extracellular ATP facilitates the expression of marker protein genes in differentiating cells, we further investigated the real-time quantitative PCR analysis of aP2 gene expression, using GAPDH as a control. As indicated in Figure 7, the mRNA expression of aP2 was very low until 3 days after passage (day 3, 0.009±0.004). In the conventional protocol, post-confluent cells before the addition of the adipogenic hormones (day 9) showed a small but not statistically significant increase in aP2 expression (0.158±0.056). After addition of the adipogenic hormones (day 3), mRNA expression of aP2 was markedly increased (day 12, 0.782±0.422) and the high expression levels continued until day 18 (day 15, 1.004±0.229; day 18, 0.826±0.166). On the contrary in the ATP-stimulated protocol, a significant increase in aP2 expression was observed 3 days after addition of ATP and adipogenic hormones (day 9, 0.801±0.202) and it remained high until day 18 (1.035±0.132). In the negative control protocol, aP2 expression was not appreciably increased in cells even after treatment with the adipogenic hormones on day 3 (day 9, 0.048±0.029; day 18, 0.083±0.029). These results are consistent with the time-dependent triacylglycerol accumulation demonstrated in Figure 6(C), which suggests that extracellular ATP rendered the cells sensitive to the adipogenic hormones in the early proliferating stage, stimulating adipocyte differentiation.
Figure 7. Effect of ATP on aP2 gene expression in adipocyte differentiation.
Quantification of the expression of the aP2 gene during the differentiation procedure was determined with real-time quantitative RT-PCR using GAPDH as a control. The day when cells were subcultured in a new plate was defined as day 0. Open circles indicate the cells treated with the conventional protocol, closed triangles indicate those treated with the ATP-stimulated protocol and open squares indicate the cells treated with the negative control protocol. Each value represents the mean±S.E.M. for three separate experiments with duplicate measurement. Statistical significance compared with day 3 was determined by Student's t-test.
DISCUSSION
In the present study, we provide evidence that extracellular ATP affects actin cytoskeletal organization and the migration of growing 3T3-L1 cells and changes the responsiveness of preadipocytes to hormonal adipogenic stimuli mediated through P2Y receptors. These results suggest that extracellular ATP renders 3T3-L1 preadipocytes sensitive to adipogenic hormones during the proliferating phase.
Reorganization of actin filaments beneath the plasma membrane is usually found at the front edge of migrating cells [34]. Migratory cells including fibroblasts, amoebae, leucocytes, and neurones can translocate and crawl across the surface of the culture plate. Migrating cells exhibit expansion of the plasma membrane caused by local actin polymerization, displaying the broad, flat, sheet-like structures of lamellipodia (membrane ruffling) and thin, cylindrical, needle-like projections of filopodia. In 3T3-L1 preadipocytes, stimulation of P2Y receptors induced the reorganization of actin filaments at the cell periphery, and cell migration (Figures 1–3).
Small G-proteins of the Rho subfamilies (Rho, Rac and Cdc42) are key regulatory molecules that link surface receptors coupling with heterotrimeric G-proteins to actin cytoskeletal organization [32]. Y-27632 is a Rho/Rho kinase inhibitor, which inhibits Rho-induced formation of stress fibres but does not prevent Rac-induced membrane ruffling [33]. Phalloidin staining of Y-27632-treated cells did not reveal the network of stress fibres running through the cytoplasm (Figures 2H and 2I), indicating that the activation of Rho kinase by growth factors in the culture medium contributes to the maintenance of actin stress fibre structures in 3T3-L1 preadipocytes. The evidence that neither ATP-induced formation of lamellipodia nor of filopodia was prevented by Y-27632 (Figures 2J and 2K) suggests that the P2Y-receptor-mediated reorganization of actin filaments is not mediated through the activation of Rho kinase but through Rac and/or Cdc42 stimulating cell migration of 3T3-L1 preadipocytes. Extracellular ATP-induced membrane ruffling and migration were sensitive to both suramin and U-73122 but not to U-73343 (Figures 2 and 3), indicating that cell migration was stimulated through the activation of G-protein-coupled P2Y receptors.
In most of the preadipocyte cell lines, as well as in primary cultured preadipocytes, initiation of adipocyte differentiation requires growth arrest induced by cell confluence, cell–cell contact, or withdrawal of serum from the medium [5]. Then the post-confluent cells are exposed to the adipogenic hormones, activating transcription factors to accomplish the differentiation process (Figures 4, 6 and 7). ATP, applied alone to the cells whether at low density or at confluence did not induce differentiation (Figure 4), suggesting that extracellular ATP is not an activator of the transcription factors governing adipocyte differentiation. ATP did not have additive effects on adipocyte differentiation when added to the post-confluent cells and suramin did not affect conventional hormone-induced differentiation (Figure 5G), indicating that P2 receptors do not play a role in the differentiation of cells which have already reached the initiation stage of the differentiation process. Real-time quantitative PCR and triacylglycerol accumulation analyses (Figures 6C and 7) also indicated that extracellular ATP advanced the onset of sensitivity to the adipogenic hormones before confluence.
Recently, there has been increasing evidence that P2X and/or P2Y receptors play a role in long-term cellular events, including proliferation and differentiation [9,13–15]. However, the effects of purinergic stimulation seem to vary between cell types. The present study demonstrates that extracellular ATP did not inhibit preadipocyte proliferation (Figures 4E and 6D), but shows there was a stimulatory effect of ATP on hormone-induced adipocyte differentiation mediated through P2Y receptors when ATP was added to the cells at low density (Figures 4–6). One possible source of complication may be differences in the function of P2 receptor subtypes in these various cells.
There are many ways by which ATP may arrive in the extracellular space from intracellular sources in vivo, including co-release with noradrenaline from sympathetic nerve terminals, secretion from endocrine cells, autocrine/paracrine release from self or neighbouring cells, release from mechanically stretched cells and leakage from damaged cells [7–9]. In microglia, it has been reported that extracellular ATP or ADP enhances chemotaxis through P2Y receptors and may be involved in cellular functions including scavenging and neuroprotective actions in pathological states [12]. Nucleotides released from mechanically stretched vascular or damaged cells have been shown to induce cell migration mediated through P2Y2 and P2Y4 receptors in arterial smooth muscle cells, which may participate in arterial wall remodelling [10,11]. These results indicate that extracellular nucleotides play important roles in pathophysiological conditions, as well as in normal physiology. It seems possible that extracellular ATP stimulates recruitment and differentiation of preadipocytes during fat cell hyperplasia in pathophysiological conditions.
Both adipocytes and myocytes are derived from common mesenchymal stem cells. Recently, Sordella et al. [37] demonstrated that activation of Rho/Rho kinase inhibits adipogenesis and that Rho GTPase acts as a key modulator of the signals which decide the cell fate of adipogenesis-myogenesis. Further experiments are needed to determine whether the activation of Rac and/or Cdc42 contributes to the P2Y signalling responsible for preadipocyte migration which may be one of the factors in the initiation of the differentiation process, and to clarify the mechanism behind the effects of ATP on adipogenesis.
Acknowledgments
We thank Dr Hirokazu Inoue (Department of Microbiology, Shiga University of Medical Science, Japan) for generously supplying Swiss albino 3T3 fibroblasts and P. N. Vigers for critically reading the manuscript. This study was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science to M.O.-K. (16590165) and to H.M. (15590184)
References
- 1.Wasserman F. The development of adipose tissue. In: Renold A. E., Cahill G. F., editors. Handbook of Physiology. Washington, DC: America Physiological Society; 1965. pp. 87–100. [Google Scholar]
- 2.Crandall D. L., Busler D. E., McHendry-Rinde B., Groeling T. M., Kral J. G. Autocrine regulation of human preadipocyte migration by plasminogen activator inhibitor-1. J. Clin. Endocrinol. Metab. 2000;85:2609–2614. doi: 10.1210/jcem.85.7.6678. [DOI] [PubMed] [Google Scholar]
- 3.Kim C.-S., Kawada T., Yoo H., Kwon B.-S., Yu R. Macrophage inflammatory protein-related protein-2, a novel CC chemokine, can regulate preadipocyte migration and adipocyte differentiation. FEBS Lett. 2003;548:125–130. doi: 10.1016/s0014-5793(03)00728-2. [DOI] [PubMed] [Google Scholar]
- 4.Green H., Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974;3:127–133. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- 5.Gregoire F. M., Smas C. M., Sul H. S. Understanding adipocyte differentiation. Physiol. Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 6.Gerhold D. L., Liu F., Jiang G., Li Z., Xu J., Lu M., Sachs J. R., Bagchi A., Fridman A., Holder D. J., et al. Gene expression profile of adipocyte differentiation and its regulation by peroxisome proliferation-activated receptor-γ agonists. Endocrinology. 2002;143:2106–2118. doi: 10.1210/endo.143.6.8842. [DOI] [PubMed] [Google Scholar]
- 7.Burnstock G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology. 1997;36:1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- 8.Ravelic V., Burnstock G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 9.Abbrachio M. P., Burnstock G. Purinergic signalling: pathophysiological roles. Jpn. J. Pharmacol. 1998;78:113–145. doi: 10.1254/jjp.78.113. [DOI] [PubMed] [Google Scholar]
- 10.Chaulet H., Desgranges C., Renault M.-A., Dupuch F., Ezan G., Peiretti F., Loirand G., Pacaud P., Gadeau A.-P. Extracellular nucleotides induce arterial smooth muscle cell migration via osteopontin. Circ. Res. 2001;89:772–778. doi: 10.1161/hh2101.098617. [DOI] [PubMed] [Google Scholar]
- 11.Pillois X., Chaulet H., Belloç I., Dupuch F., Desgranges C., Gadeau A.-P. Nucleotide receptors involved in UTP-induced rat arterial smooth muscle cell migration. Circ. Res. 2002;90:678–681. doi: 10.1161/01.res.0000013700.98464.8e. [DOI] [PubMed] [Google Scholar]
- 12.Honda S., Sasaki Y., Ohsawa K., Imai Y., Nakamura Y., Inoue K., Kohsaka S. Extracellular ATP or ADP induces chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J. Neurosci. 2001;21:1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee W. K., Choi S. W., Lee H.-R., Lee E.-J., Lee K.-H., Kim H. O. Purinoceptor-mediated calcium mobilization and proliferation in HaCaT keratinocytes. J. Dermatol. Sci. 2001;25:97–105. doi: 10.1016/s0923-1811(00)00117-1. [DOI] [PubMed] [Google Scholar]
- 14.Greig A. V. H., Linge C., Terenghi G., McGrouther D. A., Burnstock G. Purinergic receptors are part of a functional signaling system for proliferation and differentiation of human epidermal keratinocytes. J. Invest. Dermatol. 2003;120:1007–1015. doi: 10.1046/j.1523-1747.2003.12261.x. [DOI] [PubMed] [Google Scholar]
- 15.Ryten M., Dunn P. M., Neary J. T., Burnstock G. ATP regulates the differentiation of mammalian skeletal muscle by activation of a P2X5 receptor on satellite cells. J. Cell Biol. 2002;158:345–355. doi: 10.1083/jcb.200202025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halperin M. L., Mak M. L., Taylor W. M. Control of glucose transport in adipose tissue of the rat: role of insulin, ATP and intracellular metabolites. Can. J. Biochem. 1978;56:708–712. doi: 10.1139/o78-106. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt M., Löffler G. Induction of aromatase activity in human adipose tissue stromal cells by extracellular nucleotides. Evidence for P2-purinoceptors in adipose tissue. Eur. J. Biochem. 1998;252:147–154. doi: 10.1046/j.1432-1327.1998.2520147.x. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto N., Robinson F. W., Shibata Y., Flanagan J. E., Kono T. Diversity in the effects of extracellular ATP and adenosine on the cellular processing and physiologic actions of insulin in rat adipocytes. J. Biol. Chem. 1987;262:15026–15032. [PubMed] [Google Scholar]
- 19.Tamura S., Dubler R. E., Larner J. Stimulation of maximal intracellular insulin action on glycogen synthase by preincubation of adipocytes with adenosine 5′-triphosphate. J. Biol. Chem. 1983;258:719–724. [PubMed] [Google Scholar]
- 20.Kelly K. L., Deeney J. T., Corkey B. E. Cytosolic free calcium in adipoytes. J. Biol. Chem. 1989;264:12754–12757. [PubMed] [Google Scholar]
- 21.Lee S. C., Pappone P. A. Membrane responses to extracellular ATP in rat isolated white adipocytes. Pflügers Arch. 1997;434:422–428. doi: 10.1007/s004240050416. [DOI] [PubMed] [Google Scholar]
- 22.Pappone P. A., Lee S. C. Purinergic receptor stimulation increases membrane trafficking in brown adipocytes. J. Gen. Physiol. 1996;108:393–404. doi: 10.1085/jgp.108.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Omatsu-Kanbe M., Matsuura H. Inhibition of store-operated Ca2+ entry by extracellular ATP in rat brown adipocytes. J. Physiol. 1999;521:601–615. doi: 10.1111/j.1469-7793.1999.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omatsu-Kanbe M., Isono T., Matsuura H. Multiple P2 receptors contribute to a transient increase in intracellular Ca2+ concentration in ATP-stimulated rat brown adipocytes. Exp. Physiol. 2002;87:643–652. doi: 10.1113/eph8702455. [DOI] [PubMed] [Google Scholar]
- 25.Omatsu-Kanbe M., Shibata M., Yamamoto T., Isono T., Matsuura H. Actin filaments play a permissive role in the inhibition of store-operated Ca2+ entry by extracellular ATP in rat brown adipocytes. Biochem. J. 2004;381:389–396. doi: 10.1042/BJ20040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson S. M., Barsoum M. J., Wilson B. W., Pappone P. A. Purine nucleotides modulate proliferation of brown fat preadipocytes. Cell Prolif. 1999;32:131–140. doi: 10.1046/j.1365-2184.1999.32230131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison S. A., Buxton J. M., Clancy B. M., Czech M. P. Insulin regulation of hexose transport in mouse 3T3-L1 cells expressing the human HepG2 glucose transporter. J. Biol. Chem. 1990;265:20106–20116. [PubMed] [Google Scholar]
- 28.Ramírez-Zacarías J. L., Castro-Muñozledo F., Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry. 1992;97:493–497. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- 29.Bernlohr D. A., Angus C. W., Lane M. D., Bolanowski M. A., Kelly T. J., Jr Expression of specific mRNAs during adipose differentiation: identification of an mRNA encoding a homologue of myelin P2 protein. Proc. Natl. Acd. Sci. U.S.A. 1984;81:5468–5472. doi: 10.1073/pnas.81.17.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Endemann G., Stanton L. W., Madden K. S., Bryant C. M., White R. T., Protter A. A. CD36 is a receptor for oxidized low density lipoprotein. J. Biol. Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- 31.Sabath D. E., Broome H. E., Prystowsky M. B. Glyceraldehyde-3-phosphate dehydrogenase mRNA is a major interleukin 2-induced transcript in a cloned T-helper lymphocyte. Gene. 1990;91:185–191. doi: 10.1016/0378-1119(90)90087-8. [DOI] [PubMed] [Google Scholar]
- 32.Thompson A. K., Mostafapour S. P., Denlinger L. C., Bleasdale J. E., Fisher S. K. The aminosteroid U-73122 inhibits muscarinic receptor sequestration and phosphoinositide hydrolysis in SK-N-SH neuroblastoma cells. A role for Gp in receptor compartmentation. J. Biol. Chem. 1991;266:23856–23866. [PubMed] [Google Scholar]
- 33.Smith R. J., Sam L. M., Justen J. E., Bundy G. L., Bala G. A., Bleasdale J. E. Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibition of phospholipase C-dependent processes on cell responsiveness. J. Pharmacol. Exp. Ther. 1990;253:688–697. [PubMed] [Google Scholar]
- 34.Hall A. Rho GTPases and the actin cytoskeleton. Science (Washington, D.C.) 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 35.Uehata M., Ishizaki T., Satoh H., Ono T., Kawahara T., Morishita T., Tamakawa H., Yamagami K., Inui J., Maekawa M., Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature (London) 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 36.Lauffenburger D. A., Horwitz A. F. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 37.Sordella R., Jiang W., Chen G.-C., Curto M., Settleman J. Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell. 2003;113:147–158. doi: 10.1016/s0092-8674(03)00271-x. [DOI] [PubMed] [Google Scholar]