Abstract
FLAP (5-lipoxygenase-activating protein) is a nuclear transmembrane protein involved in the biosynthesis of LTs (leukotrienes) and other 5-LO (5-lipoxygenase) products. However, little is known about its mechanism of action. In the present study, using cross-linkers, we demonstrate that FLAP is present as a monomer and a homodimer in human PMN (polymorphonuclear cells). The functional relevance of the FLAP dimer in LT biosynthesis was assessed in different experimental settings. First, the 5-LO substrate AA (arachidonic acid) concomitantly disrupted the FLAP dimer (at ≥10 μM) and inhibited LT biosynthesis. Secondly, using Sf9 cells expressing active and inactive FLAP mutants and 5-LO, we observed that the FLAP mutants capable of supporting 5-LO product biosynthesis also form the FLAP dimer, whereas inactive FLAP mutants do not. Finally, we showed that FLAP inhibitors such as MK-0591 which block LT biosynthesis in human PMN, disrupt the FLAP dimer in PMN membranes with a similar IC50. The present study demonstrates that LT biosynthesis in intact cells not only requires the presence of FLAP but its further organization into a FLAP homodimer.
Keywords: arachidonic acid (AA), 5-lipoxygenase-activating protein (FLAP), leukotriene (LT), 5-lipoxygenase (5-LO), polymorphonuclear cells (PMN), prostaglandin B2 (PGB2)
Abbreviations: AA, arachidonic acid; DFDNB, 1,5-difluoro-2,4-dinitrobenzene; FBS, foetal bovine serum; FLAP, 5-lipoxygenase-activating protein; HBSS, Hanks' balanced salt solution; 5-HETE, 5-hydroxyeicosatetraenoic acid; 5-HpETE, 5-hydroperoxyETE; LT, leukotriene; 5-LO, 5-lipoxygenase; MAPEG, membrane-associated protein in eicosanoid and glutathione metabolism; NHS-ASA, N-hydroxysuccinimidyl-4-azidosalicylic acid; NP40, Nonidet P40; PAF, platelet activating factor; PGB2, prostaglandin B2; PMN, polymorphonuclear cells; sulpho-HSAB, N-hydroxysulphosuccinimidyl-4-azidobenzoate; sulpho-SADP, N-sulphosuccinimidyl-(4-azidophenyl)-1,3′-dithiopropionate
INTRODUCTION
LTs (leukotrienes) were discovered by Samuelsson and co-workers in 1979 [1–3]. The first two steps in LT biosynthesis implicate 5-LO (5-lipoxygenase), which transforms free AA (arachidonic acid), first into 5-HpETE (5-hydroperoxyeicosatetraenoic acid) then into LTA4 [4]. These two steps are distinct and the intermediate 5-HpETE, may be enzymatically reduced to 5-HETE (5-hydroxy ETE). In intact cells, the production of LTA4 is greatly enhanced by the translocation of 5-LO to the nuclear membranes containing FLAP (5-LO-activating protein) [5–7]. Indeed, Sf9 cells expressing 5-LO produced LT only when FLAP was coexpressed [8]. The actual role of FLAP in 5-LO product biosynthesis in intact cells is not well understood. Two hypotheses either identify FLAP as a 5-LO nuclear anchor protein [6] or an AA presentation protein [9,10], both suggesting that FLAP and 5-LO are associated or lie in close proximity at the nuclear membrane level. The translocation of 5-LO to the nuclear membrane [11] and its activation [12] involves an increase in cytosolic calcium concentration and implicates the N-terminal β-barrel domain of 5-LO [13]. Other studies have also shown that phosphorylation of 5-LO may be important for translocation and activity [14–16]. Interestingly, this nuclear translocation of 5-LO was shown to be essential for LT biosynthesis in whole cells [6] but not in cell lysates [11].
Until now, no direct association between 5-LO and FLAP has been demonstrated. The only evidence of such interaction between FLAP and 5-LO and its relevance to LT biosynthesis is derived from studies using the FLAP inhibitor MK-886, which was shown to inhibit both 5-LO nuclear translocation and LT biosynthesis. Interestingly, MK-886 competes with AA for binding to FLAP, thus blocking the potential AA transfer from FLAP to 5-LO [17]. As stated previously, in Sf9 cells expressing 5-LO with or without FLAP a correlation was observed between the presence of FLAP and the formation of LTA4 [8]. This suggested that FLAP favours the formation of LT over that of 5-HETE. These and other results, illustrate that FLAP and 5-LO are at the crossroad of biosynthetic pathways which lead to the formation of very potent 5-LO-derived lipid mediators of inflammation, such as the LTs and the lipoxins [18–21].
Given the critical importance of FLAP in 5-LO product biosynthesis, experiments involving cell fractionation and cross-linkers were undertaken in an attempt to define putative molecular organization of FLAP (and other proteins) in relation to LT biosynthesis. In the present study, we provide evidence that FLAP occurs as a monomer and homodimer in human PMN (polymorphonuclear cells) and Sf9 cells expressing active FLAP mutants. We also demonstrate that FLAP inhibitors disrupt the FLAP dimer, an effect likely to be relevant to their mechanism of inhibition of LT biosynthesis. Finally, we have shown that AA, at concentrations previously shown to inhibit LT biosynthesis (≥10 μM), disrupts the dimer. These observations support the hypothesis that the organization of FLAP as a homodimer is essential for LT biosynthesis.
MATERIALS AND METHODS
Materials
Polyclonal FLAP antisera anti-H4, anti-H5, anti-H9 [22], the different baculoviruses, L-739,010, MK-0591 and MK-886 were generously given by Merck Frosst (Pointe-Claire, Canada). The BLT-1 receptor antagonist CP-105,696, the PAF (platelet activating factor) receptor antagonist BN-50,730, the FLAP inhibitors BAY-1005 and BAY-1015, and the CoA independent transacylase inhibitor SB-216,754 were obtained from Pfizer Inc. (Groton, CT, U.S.A.), Institut Henri-Beaufour (Paris), Bayer (Germany) and Smith-Kline Beecham Pharmaceuticals (King of Prussia, PA, U.S.A.) respectively. The 5-LO monoclonal antibody was from RDI (Flanders, NJ, U.S.A.). Sf9 cells were obtained from Pharmingen (Mississauga, Canada). Tumour necrosis factor α and granulocyte/macrophage colony-stimulating factor were from Peprotech (Rocky Hill, N.J., U.S.A.). LTB4, ethyl arachidonate, PGB2 (prostaglandin B2) and 19-hydroxy-PGB2 were from Cayman (Ann Arbor, MI, U.S.A.). Eicosanoic acid was from NuChek Prep Inc. (Elysian, MN, U.S.A.). Thapsigargin was from RBI (Natick, MA, U.S.A.). Cytochalasin B, AA, PAF, N-formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP), A23187, lipopolysaccharide and dexamethasone were obtained from the Sigma Chemical Co. (St. Louis, MO, U.S.A.) and sulpho-HSAB (N-hydroxysulphosuccinimidyl-4-azidobenzoate), NHS-ASA (N-hydroxysuccinimidyl-4-azidosalicylic acid), sulpho-SADP [N-sulphosuccinimidyl-(4-azidophenyl)-1,3′-dithiopropionate] and DFDNB (1,5-difluoro-2,4-dinitrobenzene) were obtained from MJS Biolynx Inc. (Brockville, Canada). TNM-FH insect medium, FBS (foetal bovine serum) Premium and gentamicin were from Wisent Inc. (St-Bruno, Canada).
Isolation of human PMN
Venous blood was obtained from healthy donors and collected on heparin as anticoagulant. PMN were prepared as described previously [23] and resuspended in HBSS (Hanks' balanced salt solution) containing 10 mM Hepes without calcium. This study was approved by the Laval University Ethics Committee and blood donors have signed an Informed Consent Form.
Cell stimulation and 5-LO product analysis
PMN (5×106/ml) were preincubated in HBSS containing 1.6 mM CaCl2 for 5 min at 37 °C. FLAP inhibitors were added during the preincubation period. When AA was used, PMN suspensions were preincubated in the presence of adenosine deaminase (0.3 unit/ml) for 5 min, to prevent inhibition of LT biosynthesis by adenosine spontaneously released in PMN suspensions [24]. Cell suspensions were then incubated with the indicated concentrations of AA for 5 min at 37 °C and incubations were stopped by the addition of 0.5 ml of cold (0 °C) acetonitrile/methanol (1:1, v/v) containing internal standards (12.5 ng each of 19-hydroxy-PGB2 and PGB2) and stored overnight at −20 °C. The precipitates were removed by centrifugation (2000 g, 15 min at 4 °C) and the supernatants were analysed for 5-LO product content by HPLC as described previously [25].
Nonidet P40 (NP40) lysis and quantitation of 5-LO translocation
PMN (5×106/ml) were preincubated in HBSS containing 1,6 mM CaCl2 and 0.3 unit/ml adenosine deaminase for 5 min at 37 °C. Cell suspensions were then incubated with or without AA for 5 min and the cells were pelleted (300 g for 2 min, 4 °C). PMN were resuspended in 0.6 ml of lysis buffer [10 mM Tris/HCl (pH 7.4), 10 mM NaCl, 1 mM EDTA and 0.1% NP 40] containing an antiprotease cocktail (1 mM PMSF, 10 μg/ml leupeptin and 10 μg/ml aprotinin), vortexed for 15 s and left on ice for 5 min. The nuclei were then recovered by centrifugation (500 g for 10 min, at 4 °C), washed once with lysis buffer without NP 40, solubilized in 250 μl of Laemmli buffer and heated to 100 °C for 10 min. Quantitation of 5-LO was achieved by 9% SDS/PAGE and Western-blotting as described previously [26]. Quantification of band intensities (densitometry) was performed using a Multimage Light Cabinet (Alpha Innotech Corp., CA, U.S.A.) and the Alphamanager 2000 version 3.3i software. Briefly, the bands of interest were integrated and the background (intensity of an empty lane at the corresponding level) was subtracted. The values obtained for the controls were set to 1 or 10 (arbitrary units) depending on the type of experiments and intensities of other bands were normalized to that of controls. Ponceau Red staining was used to assess equal loading of samples.
Isolation of PMN cellular membranes
PMN suspensions (in Ca2+-free HBSS containing 10 mM Hepes) were pelleted and resuspended in sucrose buffer [10 mM Hepes (pH 7.4), 0.5 M sucrose and 1 mM EDTA] containing the antiprotease cocktail. Sonication was performed on ice using a Branson Sonifier 450 at minimum intensity (level 1) duration 20 s. Lysates were centrifuged (500 g for 5 min, 4 °C) to remove intact cells and large cell debris and the supernatants were subjected to ultracentrifugation (73000 g for 30 min, 4 °C). Pellets (mainly cellular membranes) were resuspended at 15×106 PMN equivalent in 250 μl of HBSS, 10 mM Hepes (pH 7.4) and 1.6 mM CaCl2, and used in cross-linking experiments.
Sf9 cell culture and baculovirus infection
Sf9 cell culture was performed according to the distributor's instructions. Briefly, cells were cultured in Hinks TNM-FH medium containing 10% FBS Premium and 0.1 μg/ml gentamicin. Confluent cells were split 1:3 and infected with the baculoviruses using a MOI (multiplicity of infection) of ≥3. Unless otherwise indicated (in Figure legend), in experiments where 5-LO and FLAP were co-expressed, the Sf9 cells were infected with the 5-LO baculovirus one day before the FLAP baculovirus, given the slower expression of the 5-LO. The Sf9 cells infected with the different baculoviruses were harvested simultaneously, 3–4 days after infection. Sf9 cells were washed once with HBSS without Ca2+ and resuspended at 5×106/ml in HBSS, 10 mM Hepes (pH 7.4), 1.6 mM CaCl2 and were sonicated on ice using a Branson Sonifier 450 at minimum intensity (level 1), duration 20 s. Lysates were used directly in cross-linking experiments without membrane enrichment, except for the experiments shown in Figure 1 which were performed on Sf9 cell membranes prepared as described above for PMN membranes.
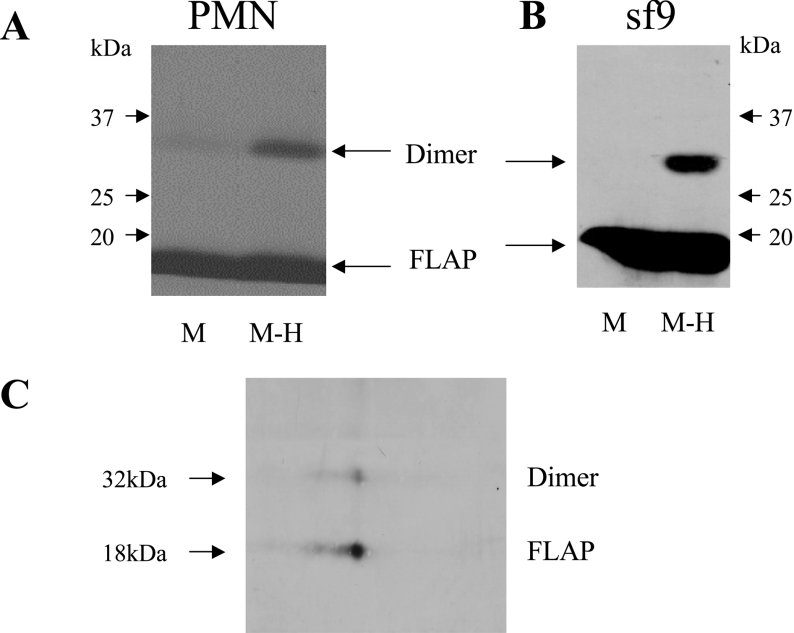
Figure 1. FLAP homodimer in PMN and Sf9 cells.
(A) Immunoblot analysis of human PMN membrane proteins with the FLAP antiserum anti-H5. Membranes from 15×106 PMN equivalent were resuspended in HBSS and treated with the cross-linker sulpho-HSAB (20 μg/ml) for 15 min. The reaction was stopped with sample buffer and proteins were analysed by SDS/PAGE using a 5–20% gradient. (B) Immunoblot analysis of Sf9 cell membrane proteins with the FLAP antiserum anti-H4. Sf9 cells were infected for 3–4 days with a baculovirus containing FLAP. Sf9 cells were sonicated in HBSS and membranes from 5×106 cell equivalents were resuspended in HBSS. Cross-linking and electrophoresis were performed as in (A). (C) Gel-strip 2D-electrophoresis of Sf9 cell membranes. The sample was prepared as in (B), treated with the cross-linker and processed as described in the Material and methods section. Proteins were separated first on a pH 3–10 gradient strip and then by 5–20% SDS/PAGE gradient. The membrane was blotted with the FLAP antiserum anti-H5. M-H, membrane treated with the cross-linker sulpho-HSAB; M, untreated membranes. Results shown are from one experiment and are representative of four different experiments.
Cross-linking experiments
Cross-linking experiments were performed on cellular membranes obtained from 15×106 PMN or from lysates of 5×106 Sf9 cells in 250 μl and 1 ml of HBSS/Hepes buffer respectively. When used in these experiments, AA or FLAP inhibitors were added at room temperature, 5 min before treatment with the cross-linkers. Sulpho-HSAB or sulpho-SADP, two photoreactive heterobifunctional cross-linkers (solubilized in DMSO at 20 mg/ml) were added to the membrane suspensions or lysates at the final concentration of 20 μg/ml; incubations were maintained for 15 min at room temperature to allow the first step of the crosslinking process to occur. The UV dependent reaction (phenyl azide) of the cross-linking (second step) was then induced by a 10 min exposure to UV light in a Hoefer UV Crosslinker 500 at maximum intensity. DFDNB, a short (3 Å) homobifunctional cross-linker, reacting instantly (without UV) with amino groups, was specifically used in experiments aimed at detecting putative high molecular mass 5-LO- and/or the FLAP-containing complex in Sf9 cells. DFDNB was used at the final concentration of 5 μg/ml and added directly to the Sf9 cell lysates, without membrane enrichment. Sf9 cell lysates were then incubated for 15 min at room temperature to allow cross-linking to occur. After treatment with the cross-linkers, samples were solubilized by the addition of 5× Laemmli buffer (100 °C for 10 min) and stored at −20 °C (sulpho-SADP required Laemmli buffer without 2-mercaptoethanol). SDS/PAGE (10–20% gradient) and Western blot analysis was used to quantify the FLAP monomer and dimer. The immunoblot analysis of the 5-LO-containing complex and the dimer on the same membrane required the use of 5–20% gradient gels. Quantification of band intensities was performed as described above for 5-LO quantification. Ponceau Red staining was used to assess equal loading of samples.
2D electrophoresis
The Sf9 membrane preparation treated with the cross-linking reagent was desalted and concentrated using the Bligh and Dyer chloroform/methanol extraction procedure. The pellet was resuspended in 1% SDS; SDS concentration was then decreased to ≤0.25% using a 8 M urea, 2% NP 40 (w/v), 60 mM dithiothreitol, 2% Pharmalyte 3–10 and 0.002% Bromophenol Blue solution. 2D electrophoresis was performed on an Immobiline Drystrip pH 3–10, 11 cm, using an Ettan™ IPGphor™ (Amersham). Rehydration under voltage was used to load the sample (200 μl) on to the strip and isoelectric focusing was performed immediately as part of the same standard programmed protocol. The strip was then equilibrated in SDS equilibration buffer [50 mM Tris/HCl (pH 8.8), 6 M urea, 30% glycerol, 2% SDS and 0.002% Bromophenol Blue]. The second dimension was performed on a preparative 5–20% SDS/PAGE gradient.
RESULTS
Presence of FLAP homodimer in human PMN
The occurrence of FLAP multimers in human PMN was assessed using cross-linkers and Western blot analysis of FLAP in PMN membranes. Figure 1(A) shows the presence of FLAP in the membrane preparation as expected. When PMN membranes were treated with the cross-linking agent sulpho-HSAB, prior to Western blot analysis, a second band was observed at 32 kDa, compatible with the presence of a FLAP dimer. The same results were obtained with three different FLAP antisera (anti-H4, anti-H5 and anti-H9, results not shown), and the 32 kDa band was not observed using the secondary anti-(rabbit IgG) antiserum alone, confirming the presence of FLAP in the 32 kDa band. Moreover, the same results were obtained using other cross-linkers with different characteristics, NHS-ASA, sulpho-SADP and DFDNB (results not shown). FLAP immunoreactive material above the 32 kDa band was not detected in any experiments.
In order to assess the nature of the 32 kDa band as a FLAP homodimer, experiments were also carried out using Sf9 cells expressing FLAP. Whereas the native Sf9 cells did not show FLAP or the 32 kDa band (results not shown), Figure 1(B) clearly shows that the transfected Sf9 cells expressed FLAP and that treatment with the cross-linker sulpho-HSAB also resulted in a second FLAP immunoreactive band. The same samples were also used for gel-strip 2D electrophoresis (as described in the Materials and methods section) and Figure 1(C) clearly shows that FLAP and the 32 kDa band have the same apparent isoelectric point, which is strongly supportive that the latter is a FLAP homodimer.
FLAP dimer disruption by AA
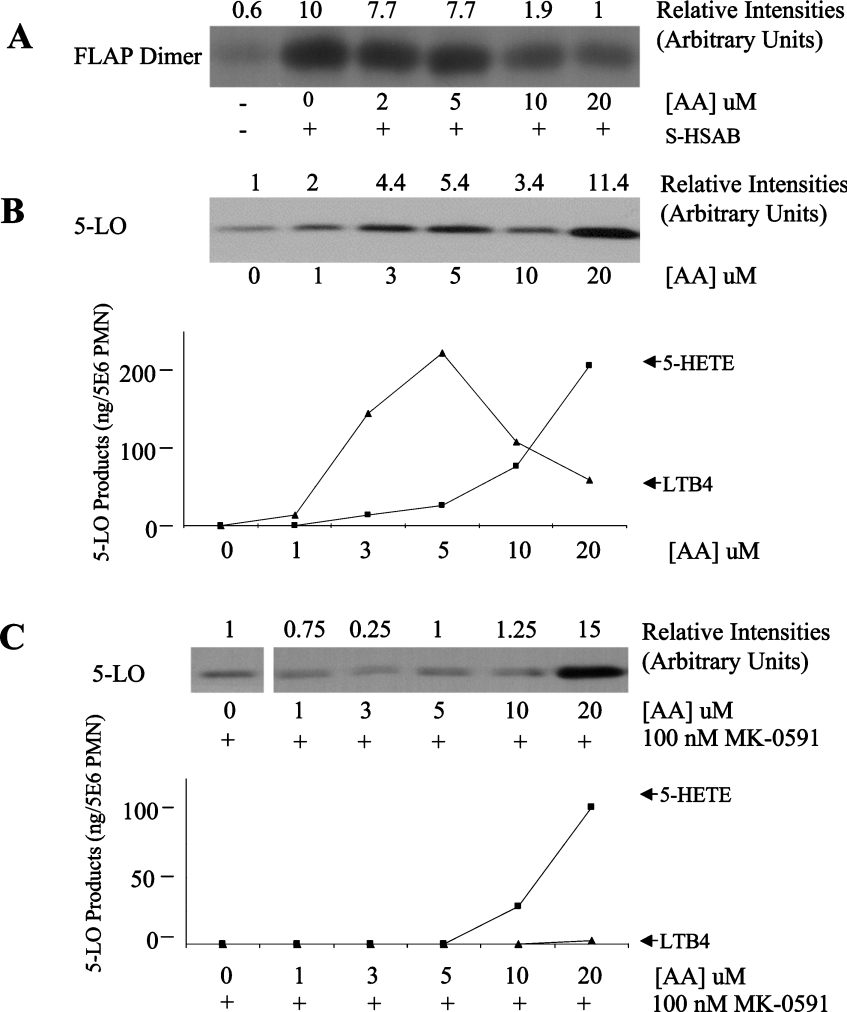
It was shown previously that FLAP binds AA. We therefore investigated the effect of AA on FLAP-dimer level in PMN membranes; these experiments were carried out on isolated PMN membranes because the various hydrosoluble cross-linkers used do not react with intracellular components efficiently in intact cells. Figure 2(A) shows that low micromolar concentrations of AA (up to 5 μM) have no effect on the FLAP-dimer content of PMN membranes. Interestingly, higher concentrations of AA (10 and 20 μM) clearly decreased the FLAP-dimer content of the membranes. Since 5-LO translocation is FLAP-dependent and critical for LT biosynthesis in human PMN, the effect of AA on these processes was also investigated in the same experimental conditions. Figure 2(B) shows that the effect of AA on 5-LO translocation is complex, with an initial stimulation at concentrations up to 5 μM, inhibition at 10 μM and a strong stimulation at 20 μM (and above). This multiphasic pattern was highly reproducible. Figure 2(B) also shows that exogenous AA, up to a concentration of 5 μM, causes an increase in LTB4, whereas the further elevation of substrate concentration results in a clear inhibition of LTB4 formation. In the same experiments, the formation of 5-HETE increased at AA concentrations 1 to 20 μM (see Discussion section). Most importantly, in these experiments the FLAP inhibitor MK0591 completely blocked 5-LO translocation and 5-LO product biosynthesis elicited at AA concentrations of 1 to 5 μM (Figure 3C), but not 5-LO translocation or 5-HETE formation induced by high AA concentration (≥20 μM), indicating that at high AA concentrations, these events are FLAP-independent. These results demonstrate that AA at concentrations of 10 μM and above causes a decrease of FLAP-dimer content, which parallels the decrease in FLAP-dependent 5-LO translocation and LTB4 biosynthesis. These experiments thus support a functional link between the FLAP dimer and LT biosynthesis in human PMN.
Figure 2. Effects of arachidonic acid on PMN.
(A) Effect of AA on FLAP dimer level. Membranes from 15×106 PMN equivalent were pre-treated with AA for 5 min before the addition of the cross-linker sulpho-HSAB (20 μg/ml), as described in the Materials and methods section. Total proteins were analysed by SDS/PAGE (10–20% gradient) and the membrane was blotted with the FLAP antiserum anti-H5. (B) Effect of AA on 5-LO translocation and 5-LO product biosynthesis. Intact PMN (5×106) were pre-treated with adenosine deaminase (0.3 unit/ml) for 5 min at 37 °C and then stimulated with the indicated concentrations of AA for 5 min. Nuclei were obtained by 0.1% NP 40 hypotonic lysis as described in the Materials and methods section. Nuclear proteins were analysed by SDS/PAGE (5–20% gradient) and the membrane was blotted with the 5-LO antibody. For the determination of 5-LO product biosynthesis, the reactions were stopped with cold (0 °C) methanol/acetonitrile (50:50, v/v) containing internal standards. 5-LO products were measured by reverse phase HPLC. Each incubation was performed in triplicate. LTB4 represents the sum of LTB4 and its ω-oxidation products (6-trans- and 6-trans-12-epi-LTB4 are not detectable under these experimental conditions). (C) Experiments were performed as described in (B) with the exception that PMN were exposed to 100 nM MK-0591 for 5 min before stimulation with AA. Results shown are from one experiment and are representative of three different experiments.
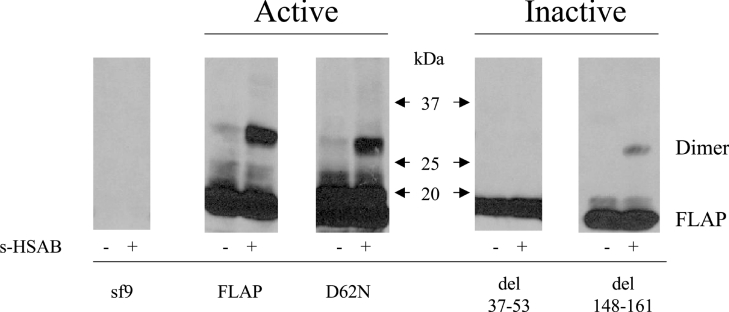
Figure 3. FLAP mutant dimers in Sf9 cells.
Sf9 cells were cultured and infected with baculoviruses. Post infection (3–4 days), Sf9 cells expressing the various FLAP mutants were sonicated and the lysates were treated with the cross-linking agent sulpho-HSAB (20 μg/ml) as described in the Materials and methods section. The reaction was stopped with the sample buffer and the proteins were analysed by SDS/PAGE using a 5–20% gradient and the FLAP antiserum anti-H5. Results shown are from one experiment and are representative of three different experiments.
Dimerization of FLAP mutants in Sf9 cells
As an alternative approach to assess the biological significance of the FLAP dimer in LT biosynthesis, we used baculovirus constructs containing either wild-type (wt) FLAP or FLAP mutants [8] in the Sf9 expression system. Figure 3 shows that in Sf9 cells FLAP forms a homodimer and that the various mutations alter this process. The FLAP mutant D62N was previously reported to support 5-LO product biosynthesis, even more efficiently than FLAP itself under given experimental conditions [8]. Interestingly, we observed that the expression of the active mutant D62N also enabled the formation of this FLAP mutant dimer. Similar analyses were performed using two other FLAP mutants previously reported to be unable to support LT biosynthesis. As seen in Figure 3, no dimer was detectable using the FLAP mutant del 37–53, in support of the concept that the FLAP dimer may be a critical structure for LT biosynthesis in intact cells. Similarly, the third mutant tested, del 148–161, also reportedly unable to support LT biosynthesis, allowed only the formation of a very small amount of the dimer.
Effect of FLAP inhibitors on the FLAP homodimer
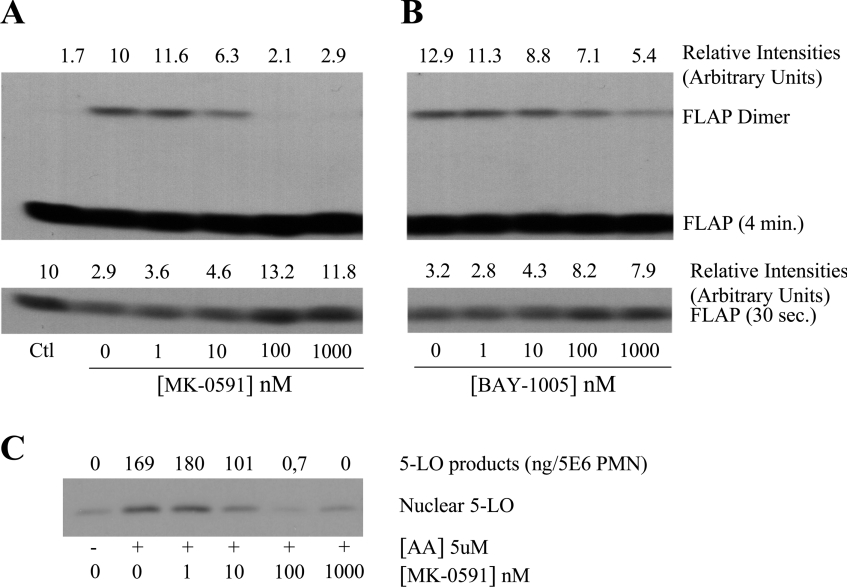
The next series of experiments was undertaken to determine if the FLAP inhibitors affect the level of FLAP homodimer in human PMN. Figures 4(A) and 4(B) show that exposure of PMN membranes to the FLAP inhibitors MK-0591 and BAY-1005 before treatment with the cross-linker results in a dose-dependent decrease in the amount of FLAP homodimer in PMN membranes. As expected, when the PMN membranes were treated first with the cross-linker and then exposed to a FLAP antagonist, the drug did not affect the level of the dimer (results not shown). The potency of the FLAP inhibitors for blocking LT biosynthesis (IC50≈10 and 150 nM for MK-0591 and BAY-1005 respectively), was similar to their potency at causing disruption of the FLAP dimer. The lower panels of Figures 4(A) and 4(B) show the levels of the FLAP (monomer) in the PMN membranes (as revealed by a shorter 30 s exposure time); these results clearly show that treatment of the PMN membranes with the cross-linker not only results in appearance of the FLAP dimer but also a fall in the FLAP level in the PMN membranes as expected. Moreover, the addition of the FLAP antagonists, which causes the disappearance of the dimer, also resulted in the complete recovery of FLAP levels. Figure 4(C) shows the effect of the FLAP inhibitor MK-0591 in human PMN activated with 5 μM AA. MK-0591 caused concentration-dependent and parallel inhibition of both 5-LO translocation to nuclear structures and 5-LO product biosynthesis, as previously reported.
Figure 4. Effects of FLAP inhibitors on FLAP dimer and 5-LO product biosynthesis in PMN.
(A) and (B) membranes from 15×106 PMN equivalent were exposed to MK-0591 or BAY-1005 for 5 min before treatment with sulpho-HSAB (20 μg/ml), as described in the Materials and methods section. The reaction was stopped with sample buffer and proteins were analysed by SDS/PAGE using a 5–20% gradient and the FLAP antiserum anti-H5. Membranes were exposed for 30 s and 4 min for quantification of FLAP and FLAP-dimer respectively. (C) Intact PMN (10×106/ml) were pretreated with various concentrations of the FLAP inhibitor MK-0591 in the presence of adenosine deaminase (0.3 unit/ml) for 10 min at 37 °C and then stimulated with 5 μM AA for 5 min. The incubations were stopped by addition of 1 vol. of cold (0 °C) HBSS and then centrifuged (1000 g for 1 min, 4 °C). The supernatants were denatured by addition of 0.5 vol. of cold (0 °C) methanol/acetonitrile (50:50, v/v) containing internal standards and 5-LO products were analysed as described in the Materials and methods section. The cell pellets were processed for nuclear 5-LO analysis as described in Figure 2(B) (legend). 5-LO products represent the sum of LTB4 and its ω-oxidation products and 5-HETE. Results shown are from one experiment and are representative of three different experiments.
Formation of a 5-LO-containing complex in the Sf9 cell expression system
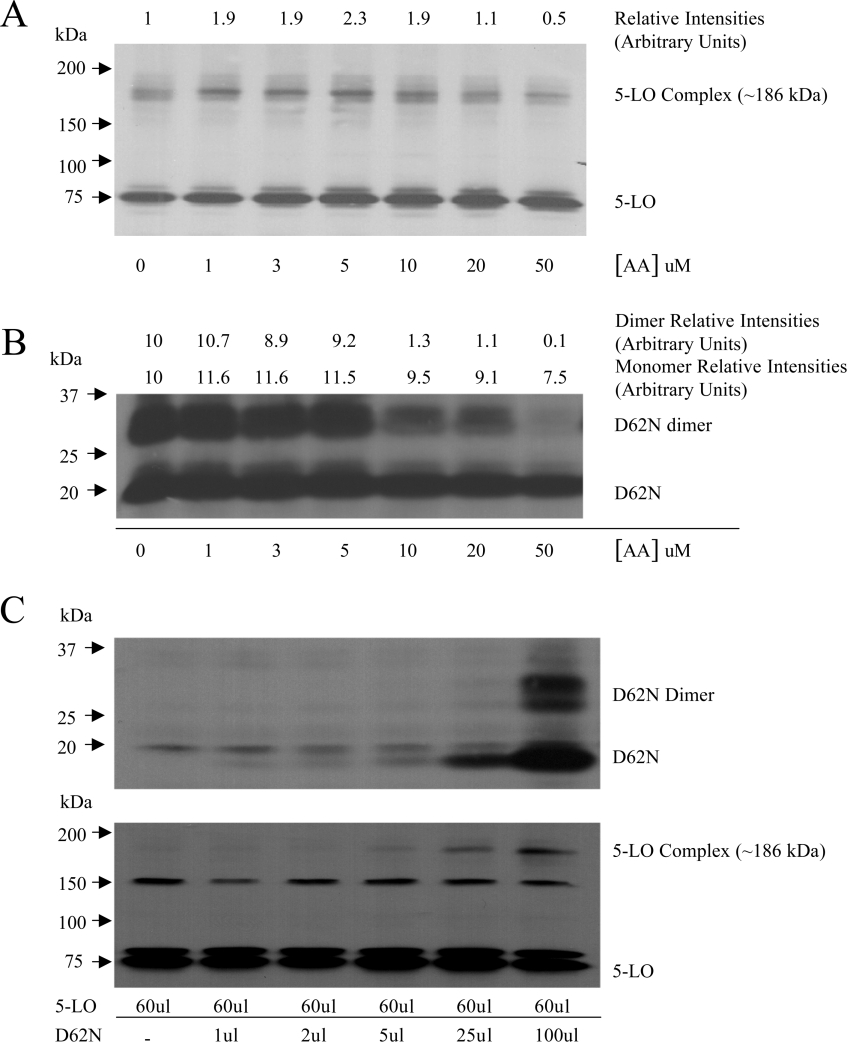
The pivotal role of FLAP in LT biosynthesis has led to the suggestion that FLAP and 5-LO may form a molecular complex. This was investigated using Sf9 cells co-expressing FLAP and 5-LO and the cross-linker DFDNB (spacer arm 3.0 Å). As shown in Figure 5(A), Western blot analysis of 5-LO in transfected Sf9 cell lysates, revealed the presence (in addition to 5-LO itself) of a band at approx. 186 kDa. Western blot analysis of FLAP using three different FLAP antisera raised against different parts of the molecule did not allow the demonstration of FLAP immunoreactivity of this 186 kDa band. Figure 5(A) also shows that detection of the 5-LO-containing complex is strongly enhanced by addition of AA, up to 5 μM, which is then decreased at 10 μM and above. Interestingly, as shown in Figure 5(B), the formation of the FLAP dimer was unaffected by AA at concentrations up to 5 μM, but was also strongly inhibited at 10 μM and above. To further investigate the putative role of FLAP in the formation of the 5-LO-containing complex, we produced Sf9 cells expressing 5-LO protein and increasing amounts of FLAP D62N mutant. Figure 5(C) shows Western blot analysis of 5-LO and FLAP in Sf9 cells infected with the same amount of 5-LO baculovirus and increasing amounts of FLAP D62N mutant baculovirus. As expected, the cells expressed similar amounts of 5-LO and the level of D62N increased with additive amounts of FLAP D62N mutant baculovirus. As shown in Figure 5(C), the 5-LO-containing complex was undetectable in absence of D62N and progressively increased in amount in Sf9 cells expressing D62N and the D62N dimer. Except for the 186 kDa 5-LO-containing complex, which was undetectable in Sf9 cells not treated with the cross-linker, immunoblot analysis (with the 5-LO antibody) of lysates treated or without the cross-linker were identical (results not shown). The two bands at approx. 80 and 150 kDa (Figure 5C, lower panel) are Sf9-cell proteins (detected in non-transfected Sf9 cells); their detection with the 5-LO antibody was highly variable (e.g bands were not observed in the experiment shown in Figure 5A) and independent of treatment with a cross-linker. Finally, it should be noted that with the exception of the two unspecific bands at approx. 80 and 150 kDa that were occasionally detected (unrelated to 5-LO expression in the Sf9 cells), no 5-LO immunoreactive material could be detected between 75 kDa (5-LO) and 186 kDa (5-LO-containing complex) in our experiments using Sf9 cells expressing both 5-LO and FLAP (Figure 5A). These results indicate that the presence of FLAP is required for the formation of the 5-LO-containing complex. These results do not, however, conclusively demonstrate that FLAP is present in the complex, nor do they exclude the possibility that the 5-LO-containing complex might contain other components.
Figure 5. 5-LO-containing complex in Sf9 cells and effects of arachidonic acid.
(A) and (B) Sf9 cells were infected with 5-LO baculoviruses one day before infection with the FLAP mutant D62N baculoviruses to insure adequate expression of the 5-LO, as described in the Materials and methods section. After infection (3 days) with the FLAP mutant baculoviruses, cells were exposed to various concentrations of AA for 5 min, sonicated and the lysates were treated directly with the cross-linker DFDNB (5 μg/ml) for 15 min (membrane enrichment procedures strongly decreased the level of 5-LO complex). The reaction was stopped with sample buffer and the proteins were analysed by SDS/PAGE using a 5–20% gradient. (A) Shows immunoblot analysis using the 5-LO antibody. (B) Shows immunoblot analysis using the FLAP antiserum anti-H5. (C) Sf9 cells were infected with the baculovirus containing 5-LO, or with the baculovirus containing 5-LO, and increasing amounts of the baculovirus containing the FLAP mutant D62N. The presence of D62N, D62N-dimer, 5-LO and 5-LO-containing complex in the infected Sf9 cells was analysed as described above (A) and (B). The amount of baculoviruses used for Sf9 infection is expressed as μl of baculovirus suspension added to the Sf9 cells. The bands at approx. 80 and 150 kDa are Sf9 cell proteins (see Results section) and their detection in immunoblot analysis using the 5-LO antibody was highly variable (e.g. bands not seen in 5A). Results shown are from one experiment and are representative of three different experiments.
DISCUSSION
Several studies have demonstrated a role for FLAP in 5-LO product biosynthesis [6–9]. The results of the present study are fully compatible with an extension of this finding that would thus implicate the FLAP dimer rather than the monomer. Indeed, we demonstrate in the present study that FLAP is present in PMN membranes as monomers and homodimers, and the functional importance of the dimer (as opposed to the monomer) in LT biosynthesis was demonstrated using three distinct approaches. Since AA was previously reported to bind to FLAP [17] and to inhibit LT biosynthesis (at concentrations ≥10 μM) [24], we investigated the impact of the fatty acid on the level of the dimer. Our results show that exogenous AA at concentrations up to 5 μM did not enhance the levels of FLAP dimer but that high AA concentrations (≥10 μM) decrease FLAP dimer levels both in PMN and Sf9 cells. This supports the possibility that AA, when present in excessive amounts (≥10 μM), could disrupt or destabilize the FLAP dimer, an effect which correlates with the suppression of 5-LO product biosynthesis by the fatty acid. Accordingly, eicosanoic acid (C20:0) and the ethyl ester derivative of AA, which were shown unable to bind to FLAP [27], did not affect the level of the FLAP dimer or inhibit 5-LO product biosynthesis at concentrations up to 30 μM (results not shown). Thus these results provide the first evidence for the relevance of the FLAP dimer as an important element in the 5-LO pathway for the formation of LT and 5-HETE.
In these experiments, it was intriguing that at high AA concentrations (10–20 μM), 5-HETE formation and 5-LO translocation progressively increased, whereas FLAP dimer levels and LTB4 formation were inhibited. A logical explanation for these contrasting findings could be that at the high AA concentrations tested, the formation of 5-HETE and massive 5-LO translocation are largely FLAP-independent. This was confirmed by using the FLAP antagonist MK-0591, which at high AA concentrations, completely inhibited LTB4 biosynthesis but only partially inhibited 5-HETE formation. In agreement with this, 5-LO translocation observed at 20 μM AA was not inhibited by MK-0591 and thus also appeared to be FLAP-independent of 5-LO binding to nuclear structures. It must be pointed out, however, that the biological significance of the biosynthesis of 5-HETE under such conditions of high substrate concentration (10–20 μM) is unclear.
Further assessment of the functional significance of the FLAP dimer was achieved using FLAP mutants. We investigated one active (D62N) and two inactive (del 37–53 and del 148–161) FLAP mutants. The FLAP homodimer was observed in Sf9 cells expressing FLAP and the active FLAP mutant D62N, but not in FLAP mutant del 37–53 and to a much lesser extent in cells expressing the inactive FLAP mutant del 148–161. The results obtained using the inactive mutants del 37–53 and del 148–161 suggest that the deleted amino acids are important for FLAP dimer formation, which in turn appears critical to LT biosynthesis. Interestingly, our studies using mutant del 37–53, which does not contain the FERV sequence (amino acids 50–53), a conserved sequence of FLAP among various species and among several human MAPEG (membrane-associated protein in eicosanoid and glutathione metabolism) proteins, support the putative importance of this sequence in FLAP function [28].
Our experiments using the FLAP inhibitors MK-0591 and BAY-1005 clearly showed the ability of these compounds to disrupt the FLAP homodimer in human PMN membranes. MK-886 and BAY-1015, two other FLAP inhibitors, also caused FLAP dimer disruption (results not shown). Several other compounds tested in parallel, such as CP-105,696 (a BLT-1 antagonist), BN-50,730 (a PAF receptor antagonist), L-739,010 (a 5-LO inhibitor) and SB-216,754 (an inhibitor of CoA independent transacylase) had no effect on the level of FLAP homodimer in PMN membranes (results not shown). These results demonstrate that disruption of the dimer is specific to FLAP inhibitors and could be a feature of their mechanism of action, and further support the possibility that the FLAP dimer is essential to LT biosynthesis in intact cells. Our experiments using MK-0591 have also shown that the drug inhibits, at similar concentration–response curves, dimer formation, 5-LO translocation and 5-LO product biosynthesis, thus raising the intriguing possibility that the putative interaction of 5-LO with FLAP may instead implicate the FLAP dimer, and that the inhibitory effect of FLAP inhibitors on 5-LO translocation may be a direct consequence of the homodimer disruption. Finally, this observation is interesting in view of the recent report that BAY-1005 was able to block proteinuria in diabetic rats, an effect shown to be unrelated to its inhibitory effects on LT biosynthesis [29]. Our findings raise the possibility that the inhibitory effect of BAY-1005 on this novel function of FLAP might involve dimer disruption.
FLAP is a member of the MAPEG family which includes several proteins such as LTC4 synthase, the microsomal glutathione transferase-1 and the microsomal PGE synthase-1. Interestingly, all these proteins have been shown to form homodimers or homotrimers [30–32], which in some cases were shown to be the active forms of these enzymes. Recently, it was also demonstrated in a rat basophilic cell line (RBL-2H3), that FLAP was able to form multimers by itself or with the LTC4 synthase, suggesting a potential role for these molecular complexes in LT biosynthesis [33]. In the present study, in both human PMN and FLAP-transfected Sf9 cells, we did not observe immunoreactive material corresponding to a FLAP trimer. Among all known MAPEG proteins, the LTC4 synthase has the highest structural similarity with FLAP; interestingly, by analogy with our observations indicating a functional role for FLAP dimer in LT biosynthesis, the catalytically active LTC4 synthase has been shown to be a homodimer [30].
In the course of the present studies, experiments using the cross-linker DFDNB led to the detection of a 186 kDa complex reactive to 5-LO antibody, but not detectable using FLAP antisera, in Sf9 cells expressing 5-LO and FLAP. However, the 186 kDa complex was absent in Sf9 cells expressing 5-LO only, suggesting that FLAP may be a constituent of the 186 kDa 5-LO-containing complex or is essential to its formation. The observation that the 5-LO-containing complex was detectable only when FLAP was co-expressed with 5-LO in Sf9 cells, indicates a putative involvement of the 5-LO-containing complex in LT biosynthesis in intact cells. Interestingly, the apparent molecular mass of the complex (approx. 186 kDa) is compatible with structures containing two 5-LO and two FLAP, or one 5-LO and six FLAP. However, this latter arrangement would have been very likely to show a strong reactivity to FLAP antisera and a ladder pattern of FLAP on SDS/PAGE analysis, which were not seen. Conversely, in support of a tetrameric structure, it is distinctly possible that two large 5-LO molecules may produce a steric hindrance preventing the interaction of antibodies with FLAP. It is also interesting that the absence of an intermediate structure between 75 and 186 kDa, may indicate that the 5-LO monomer does not form a stable complex with the FLAP monomer or dimer. Our observation, that low concentrations of AA (1–5 μM) strongly enhanced the formation of the 5-LO-containing complex in Sf9 cells, suggests that AA promotes the formation or increases the stability of the 5-LO-containing complex. It is tempting to speculate that this effect of AA (1–5 μM) on 186 kDa-complex formation may be related to an effect of the fatty acid on 5-LO translocation (as shown in Figure 2B), the presence of 5-LO at the nuclear membrane allowing the association of 5-LO with membrane components and the formation of the 5-LO-containing complex. These results do not exclude the possibility of the presence of another component in the 5-LO-containing complex and conclusive characterization of the 5-LO-containing complex will await further structural analysis.
These results further our understanding of 5-LO product biosynthesis in human PMN. The results also provide a mechanism for the inhibition of LT biosynthesis by high concentrations of AA and FLAP inhibitors, involving disruption of the FLAP homodimer. This latter observation may be of importance for the further development of 5-LO inhibitors. Finally, our demonstration of a 5-LO-containing complex, suggests a further level of molecular organization in the 5-LO pathway involving 5-LO and other proteins, possibly FLAP.
Acknowledgments
We thank the Canadian Institutes for Health Research for financial support.
References
- 1.Murphy R. C., Hammarstrom S., Samuelsson B. Leukotriene C: a slow-reacting substance from murine mastocytoma cells. Proc. Natl. Acad. Sci. U.S.A. 1979;76:4275–4279. [PubMed] [Google Scholar]
- 2.Borgeat P., Samuelsson B. Metabolism of arachidonic acid in polymorphonuclear leukocytes. Structural analysis of novel hydroxylated compounds. J. Biol. Chem. 1979;254:7865–7869. [PubMed] [Google Scholar]
- 3.Borgeat P., Samuelsson B. Arachidonic acid metabolism in polymorphonuclear leukocytes: unstable intermediate in formation of dihydroxy acids. Proc. Natl. Acad. Sci. U.S.A. 1979;76:3213–3217. doi: 10.1073/pnas.76.7.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rouzer C. A., Matsumoto T., Samuelsson B. Single protein from human leukocytes possesses 5-lipoxygenase and leukotriene A4 synthase activities. Proc. Natl. Acad. Sci. U.S.A. 1986;83:857–861. doi: 10.1073/pnas.83.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woods J. W., Evans J. F., Ethier D., Scott S., Vickers P. J., Hearn L., Heibein J. A., Charleson S., Singer I. I. 5-lipoxygenase and 5-lipoxygenase-activating protein are localized in the nuclear envelope of activated human leukocytes. J. Exp. Med. 1993;178:1935–1946. doi: 10.1084/jem.178.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon R. A., Diehl R. E., Opas E., Rands E., Vickers P. J., Evans J. F., Gillard J. W., Miller D. K. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature (London) 1990;343:282–284. doi: 10.1038/343282a0. [DOI] [PubMed] [Google Scholar]
- 7.Rouzer C. A., Ford-Hutchinson A. W., Morton H. E., Gillard J. W. MK886, a potent and specific leukotriene biosynthesis inhibitor blocks and reverses the membrane association of 5-lipoxygenase in ionophore-challenged leukocytes. J. Biol. Chem. 1990;265:1436–1442. [PubMed] [Google Scholar]
- 8.Mancini J. A., Waterman H., Riendeau D. Cellular oxygenation of 12-hydroxyeicosatetraenoic acid and 15-hydroxyeicosatetraenoic acid by 5-lipoxygenase is stimulated by 5-lipoxygenase-activating protein. J. Biol. Chem. 1998;273:32842–32847. doi: 10.1074/jbc.273.49.32842. [DOI] [PubMed] [Google Scholar]
- 9.Miller D. K., Gillard J. W., Vickers P. J., Sadowski S., Leveille C., Mancini J. A., Charleson P., Dixon R. A., Ford-Hutchinson A. W., Fortin R. Identification and isolation of a membrane protein necessary for leukotriene production. Nature (London) 1990;343:278–281. doi: 10.1038/343278a0. [DOI] [PubMed] [Google Scholar]
- 10.Abramovitz M., Wong E., Cox M. E., Richardson C. D., Li C., Vickers P. J. 5-lipoxygenase-activating protein stimulates the utilization of arachidonic acid by 5-lipoxygenase. Eur. J. Biochem. 1993;215:105–111. doi: 10.1111/j.1432-1033.1993.tb18012.x. [DOI] [PubMed] [Google Scholar]
- 11.Rouzer C. A., Samuelsson B. Reversible, calcium-dependent membrane association of human leukocyte 5-lipoxygenase. Proc. Natl. Acad. Sci. U.S.A. 1987;84:7393–7397. doi: 10.1073/pnas.84.21.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammarberg T., Provost P., Persson B., Radmark O. The n-terminal domain of 5-lipoxygenase binds calcium and mediates calcium stimulation of enzyme activity. J. Biol. Chem. 2000;275:38787–38793. doi: 10.1074/jbc.M006136200. [DOI] [PubMed] [Google Scholar]
- 13.Chen X. S., Funk C. D. The N-terminal ‘β-barrel’ domain of 5-lipoxygenase is essential for nuclear membrane translocation. J. Biol. Chem. 2001;276:811–818. doi: 10.1074/jbc.M008203200. [DOI] [PubMed] [Google Scholar]
- 14.Lepley R. A., Muskardin D. T., Fitzpatrick F. A. Tyrosine kinase activity modulates catalysis and translocation of cellular 5-lipoxygenase. J. Biol. Chem. 1996;271:6179–6184. doi: 10.1074/jbc.271.11.6179. [DOI] [PubMed] [Google Scholar]
- 15.Werz O., Klemm J., Samuelsson B., Radmark O. 5-lipoxygenase is phosphorylated by p38 kinase-dependent MAPKAP kinases. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5261–5266. doi: 10.1073/pnas.050588997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Werz O., Burkert E., Fischer L., Szellas D., Dishart D., Samuelsson B., Radmark O., Steinhilber D. Extracellular signal-regulated kinases phosphorylate 5-lipoxygenase and stimulate 5-lipoxygenase product formation in leukocytes. FASEB J. 2002;16:1441–1443. doi: 10.1096/fj.01-0909fje. [DOI] [PubMed] [Google Scholar]
- 17.Mancini J. A., Abramovitz M., Cox M. E., Wong E., Charleson S., Perrier H., Wang Z., Prasit P., Vickers P. J. 5-lipoxygenase-activating protein is an arachidonate binding protein. FEBS Lett. 1993;318:277–281. doi: 10.1016/0014-5793(93)80528-3. [DOI] [PubMed] [Google Scholar]
- 18.Aliberti J., Hieny S., Reis e Sousa C., Serhan C. N., Sher A. Lipoxin-mediated inhibition of IL-12 production by DCs: a mechanism for regulation of microbial immunity. Nat. Immunol. 2002;3:76–82. doi: 10.1038/ni745. [DOI] [PubMed] [Google Scholar]
- 19.Levy B. D., Clish C. B., Schmidt B., Gronert K., Serhan C. N. Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 20.Levy B. D., De S. G., Devchand P. R., Kim E., Ackerman K., Schmidt B. A., Szczeklik W., Drazen J. M., Serhan C. N. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4) Nat. Med. 2002;8:1018–1023. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- 21.Serhan C. N., Takano T., Chiang N., Gronert K., Clish C. B. Formation of endogenous “antiinflammatory” lipid mediators by transcellular biosynthesis. Lipoxins and aspirin-triggered lipoxins inhibit neutrophil recruitment and vascular permeability. Am. J. Respir. Crit. Care Med. 2000;161:S95–S101. doi: 10.1164/ajrccm.161.supplement_1.ltta-19. [DOI] [PubMed] [Google Scholar]
- 22.Vickers P. J., Adam M., Charleson S., Coppolino M. G., Evans J. F., Mancini J. A. Identification of amino acid residues of 5-lipoxygenase-activating protein essential for the binding of leukotriene biosynthesis inhibitors. Mol. Pharmacol. 1992;42:94–102. [PubMed] [Google Scholar]
- 23.McDonald P. P., McColl S. R., Naccache P. H., Borgeat P. Studies on the activation of human neutrophil 5-lipoxygenase induced by natural agonists and Ca2+ ionophore A23187. Biochem. J. 1991;280:379–385. doi: 10.1042/bj2800379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surette M. E., Krump E., Picard S., Borgeat P. Activation of leukotriene synthesis in human neutrophils by exogenous arachidonic acid: inhibition by adenosine A2a receptor agonists and crucial role of autocrine activation by leukotriene B4. Mol. Pharmacol. 1999;56:1055–1062. doi: 10.1124/mol.56.5.1055. [DOI] [PubMed] [Google Scholar]
- 25.Borgeat P., Picard S., Vallerand P., Bourgoin S., Odeimat A., Sirois P., Poubelle P. E. Automated on-line extraction and profiling of lipoxygenase products of arachidonic acid by high-performance liquid chromatography. Methods Enzymol. 1990;187:98–116. doi: 10.1016/0076-6879(90)87014-t. [DOI] [PubMed] [Google Scholar]
- 26.Pouliot M., McDonald P. P., Krump E., Mancini J. A., McColl S. R., Weech P. K., Borgeat P. Colocalization of cytosolic phospholipase A2, 5-lipoxygenase, and 5-lipoxygenase-activating protein at the nuclear membrane of A23187-stimulated human neutrophils. Eur. J. Biochem. 1996;238:250–258. doi: 10.1111/j.1432-1033.1996.0250q.x. [DOI] [PubMed] [Google Scholar]
- 27.Charleson S., Evans J. F., Leger S., Perrier H., Prasit P., Wang Z., Vickers P. J. Structural requirements for the binding of fatty acids to 5-lipoxygenase-activating protein. Eur. J. Pharmacol. 1994;267:275–280. doi: 10.1016/0922-4106(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 28.Jakobsson P. J., Morgenstern R., Mancini J., Ford-Hutchinson A., Persson B. Common structural features of MAPEG – a widespread superfamily of membrane associated proteins with highly divergent functions in eicosanoid and glutathione metabolism. Protein Sci. 1999;8:689–692. doi: 10.1110/ps.8.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valdivielso J. M., Montero A., Badr K. F., Munger K. A. Inhibition of 5-lipoxygenase activating protein decreases proteinuria in diabetic rats. J. Nephrol. 2003;16:85–94. [PubMed] [Google Scholar]
- 30.Nicholson D. W., Ali A., Vaillancourt J. P., Calaycay J. R., Mumford R. A., Zamboni R. J., Ford-Hutchinson A. W. Purification to homogeneity and the N-terminal sequence of human leukotriene C4 synthase: a homodimeric glutathione S-transferase composed of 18-kDa subunits. Proc. Natl. Acad. Sci. U.S.A. 1993;90:2015–2019. doi: 10.1073/pnas.90.5.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt-Krey I., Mitsuoka K., Hirai T., Murata K., Cheng Y., Fujiyoshi Y., Morgenstern R., Hebert H. The three-dimensional map of microsomal glutathione transferase 1 at 6 Å resolution. EMBO J. 2000;19:6311–6316. doi: 10.1093/emboj/19.23.6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thoren S., Weinander R., Saha S., Jegerschold C., Pettersson P. L., Samuelsson B., Hebert H., Hamberg M., Morgenstern R., Jakobsson P. J. Human microsomal prostaglandin E synthase-1: purification, functional characterization, and projection structure determination. J. Biol. Chem. 2003;278:22199–22209. doi: 10.1074/jbc.M303227200. [DOI] [PubMed] [Google Scholar]
- 33.Mandal A. K., Skoch J., Bacskai B. J., Hyman B. T., Christmas P., Miller D., Yamin T. T., Xu S., Wisniewski D., Evans J. F., Soberman R. J. The membrane organization of leukotriene synthesis. Proc. Natl. Acad. Sci. U.S.A. 2004;101:6587–6592. doi: 10.1073/pnas.0308523101. [DOI] [PMC free article] [PubMed] [Google Scholar]