Abstract
The aniline-assimilating bacterium Rhodococcus sp. AN-22 was found to constitutively synthesize CatB (cis,cis-muconate cycloisomerase) and CatC (muconolactone isomerase) in its cells growing on non-aromatic substrates, in addition to the previously reported CatA (catechol 1,2-dioxygenase). The bacterium maintained the specific activity of the three enzymes at an almost equal level during cultivation on succinate. CatB and CatC were purified to homogeneity and characterized. CatB was a monomer with a molecular mass of 44 kDa. The enzyme was activated by Mn2+, Co2+ and Mg2+. Native CatC was a homo-octamer with a molecular mass of 100 kDa. The enzyme was stable between pH 7.0 and 10.5 and was resistant to heating up to 90 °C. Genes coding for CatA, CatB and CatC were cloned and named catA, catB and catC respectively. The catABC genes were transcribed as one operon. The deduced amino acid sequences of CatA, CatB and CatC showed high identities with those from other Gram-positive micro-organisms. A regulator gene such as catR encoding a regulatory protein was not observed around the cat gene cluster of Rhodococcus sp. AN-22, but a possible relic of catR was found in the upstream region of catA. Reverse transcriptase-PCR and primer extension analyses showed that the transcriptional start site of the cat gene cluster was located 891 bp upstream of the catA initiation codon in the AN-22 strain growing on both aniline and succinate. Based on these data, we concluded that the bacterium constitutively transcribed the catABC genes and translated its mRNA into CatA, CatB and CatC.
Keywords: aniline-assimilating bacterium; cis,cis-muconate cycloisomerase; constitutive expression; muconolactone isomerase; primer extension analysis; Rhodococcus
Abbreviations: CatA, catechol 1,2-dioxygenase; CatB, cis,cis-muconate cycloisomerase; CatC, muconolactone isomerase; CatD, 3-oxoadipate enol-lactone hydrolase; CatIJ, 3-oxoadipate:succinyl-CoA transferase; CatR, regulatory protein of the catechol-degrading pathway; IPTG, isopropyl β-D-thiogalactoside; ORF, open reading frame; RT, reverse transcriptase; TCA, tricarboxylic acid
INTRODUCTION
Aniline is a major industrial raw material and an intermediate used for the production of dyes, plastics, herbicides, etc. Studies of the biodegradation of aniline as an environmental pollutant were made by Lyons et al. [1]. We have isolated many bacteria [2] from soil that grew on aniline as the sole carbon, energy and nitrogen source, and have investigated their metabolic pathways [3], enzyme and gene systems [4–7]. It was revealed that all the isolated bacteria degrade aniline via catechol, which is then metabolized by the enzymes encoded by the cat gene cluster [8].
Through these investigations, we found that the aniline-assimilating bacterium Rhodococcus sp. AN-22 constitutively synthesized CatA (catechol 1,2-dioxygenase; EC 1.13.11.1) in cells grown on a medium without aniline [9], as well as on aniline medium, although other aniline-assimilating bacteria metabolized aniline with inducible enzymes including CatA [10]. In micro-organisms, CatA is a key enzyme in the ortho-cleavage pathway of aromatic compounds. This enzyme catalyses the ring fission of catechol with the consumption of 1 mol of oxygen per mol of substrate and produces cis,cis-muconate. A variety of CatAs are distributed to a large extent in micro-organisms that metabolize aromatic compounds through the ortho-cleavage pathway. Almost all of the previously reported enzymes are inducible.
Some reports have suggested the existence of the constitutive CatAs in the 3-aminophenol-assimilating bacterium Arthrobacter sp. mA3 [11], a p-nitrophenol-assimilating phototrophic bacterium [12] and a phenol-assimilating bacterium Acinetobacter radioresistens S13 [13]. However, the level of constitutive synthesis of the CatAs was markedly low, and the constitutive enzyme production was not characterized. On the other hand, aniline-assimilating Rhodococcus sp. AN-22 produced more CatA on 11 non-aromatic substrate media, such as sugars, and amino and organic acids, than that on the aniline medium. The constitutive enzyme CatA was then purified and characterized [9].
In the present study, our objective was to examine whether or not other enzymes responsible for the catechol catabolism in the AN-22 strain are constitutive. For this purpose, we prepared the cell extracts from cells grown on succinate and aniline media, and estimated the CatB (cis,cis-muconate cycloisomerase; EC 5.5.1.1), CatC (muconolactone isomerase; EC 5.3.3.4), CatD (3-oxoadipate enol-lactone hydrolase; EC 3.1.1.24) and CatIJ (3-oxoadipate:succinyl-CoA transferase; EC 2.8.3.6). In addition, CatB and CatC were purified and characterized. Furthermore, we attempted the cloning and analysis of genes coding for the catabolic enzymes and regulating their expression, which will provide more definitive information for the constitutive synthesis of these enzymes.
The production, purification, characterization and gene analysis of catabolic enzymes for catechol in aniline-assimilating Rhodococcus sp. AN-22 are described in the present paper.
EXPERIMENTAL
Chemicals
Aniline hydrochloride, catechol and 4-methylcatechol were purchased from Wako Pure Chemical; 3-methylcatechol, 3-chlorocatechol, 4-chlorocatechol and 3-fluorocatechol were from Tokyo Kasei; DE52 (DEAE) cellulose was from Whatman Chemical Separation; DEAE-Toyopearl 650S, Phenyl-Toyopearl 650S and Toyopearl HW-55S were from Tosoh Corporation; and restriction endonucleases were from Takara Bio Incorporation.
Bacterial strains, plasmids and growth conditions
Rhodococcus sp. AN-22 was used throughout the present study as a producer of enzymes for the catechol catabolism and a source of their genes. Escherichia coli XL1-Blue (Stratagene) was used as a host for constructing a gene library. A pBluescript II KS+ (Stratagene) was used for the construction of a gene library and subcloning of DNA fragments.
Rhodococcus sp. AN-22 was cultured at 30 °C with shaking on the aniline medium as described previously [14] or on a succinate medium containing 0.9% (w/v) sodium succinate and 0.15% (w/v) (NH4)2SO4, instead of aniline. For the preparation of DNA and RNA, the aniline and succinate media supplemented with 0.6% (w/v) glycine were used. E. coli XL1-Blue was cultured at 37 °C with shaking on LB (Luria–Bertani) broth and, if necessary, supplemented with 100 μg/ml ampicillin, 12.5 μg/ml tetracycline, 1 mM IPTG (isopropyl β-D-thiogalactoside) and 0.04% (w/v) X-Gal (5-bromo-4-chloroindol-3-yl β-D-galactopyranoside). E. coli XL1-Blue bacteria carrying the plasmid pED14 [8] and pUC9A [15] were used as the producers of CatD and chlorocatechol 1,2-dioxygenase respectively.
Enzyme assays
The activities of CatA and CatB were spectrophotometrically measured following the methods of Aoki et al. [4] and Murakami et al. [16] respectively. The activity of CatC was measured essentially by the method of Ornston [17] in the presence of an excess of partially purified CatD, which was prepared from the cell extracts of recombinant E. coli XL1-Blue carrying the catD gene derived from another aniline-assimilating bacterium Frateuria sp. ANA-18 [8]. The molar absorption coefficient of 1.43×103 at 230 nm for muconolactone was used [18]. The activities of CatD and CatIJ were measured by the methods of Ornston [19] and Yeh and Ornston [20] respectively.
One unit of activity for CatB and C was defined as the amount of each enzyme that consumed 1 μmol of substrate per min. The specific activity was defined as units per mg of protein. Protein concentrations were measured by the method of Lowry et al. [21].
Production and estimation of CatA, CatB, CatC, CatD and CatIJ
Rhodococcus sp. AN-22 was cultured with shaking on 67 ml of aniline or succinate medium in a 500-ml flask. At appropriate intervals, the turbidity of the culture was monitored at 660 nm. Cells were harvested by centrifugation at 8000 g for 10 min at 4 °C and washed twice with 0.8% (w/v) NaCl. The washed cells were suspended in buffer A (20 mM Tris/HCl, pH 8.0) and disrupted with a Kubota 201M ultrasonic oscillator (Kubota Shoji, Tokyo, Japan) at 180 W for 5 min. After centrifugation at 20000 g for 20 min at 4 °C (the conditions used for all centrifugation steps, unless otherwise specified), the supernatant was used as the cell extracts. Streptomycin sulphate (20%, w/v) was added to the cell extracts with stirring to a final concentration of 1% (w/v). The solution was stirred for 30 min and centrifuged. The supernatant was recovered and used for the enzyme assays of CatA, CatB, CatC, CatD and CatIJ.
Purification of CatB
Cells used for the enzyme purification were cultured on the succinate medium. All operations for the purification were carried out at 0–4 °C.
Preparation of cell extracts
The obtained frozen cells (30 g, wet weight) were thawed and suspended in 300 ml of buffer A. The cells suspended in 30-ml batches were disrupted with the ultrasonic oscillator as mentioned above. After removing the intact cells and cell debris by centrifugation, the supernatant, designated as the cell extracts, was collected (fraction 1; 310 ml).
Streptomycin sulphate treatment
Streptomycin sulphate (20%, w/v) was added to fraction 1 with stirring to a final concentration of 1% (w/v). The solution was then stirred for 30 min and centrifuged. The supernatant (320 ml) was recovered (fraction 2), and the precipitate was discarded.
(NH4)2SO4 fractionation
Fraction 2 was brought to 30% saturation with (NH4)2SO4. The mixture was stirred for 30 min and centrifuged, the supernatant was collected, and the precipitate was discarded. (NH4)2SO4 was added to the supernatant to 60% saturation. After stirring for 30 min, the precipitate was collected by centrifugation and dissolved in buffer A. The solution was dialysed against buffer A with two changes of buffer. The final volume of the dialysed solution (fraction 3) was 30 ml.
Chromatography on DE52 cellulose
Fraction 3 was applied to a column (2 cm×22 cm) of DE52 cellulose equilibrated with buffer A. Proteins were eluted with a linear gradient (0–0.4 M) of NaCl in 1.4 litre of buffer A; the protein concentration and enzyme activity of the fractions were then assayed. Fractions with a high specific activity were pooled to yield fraction 4 (210 ml).
Chromatography on DEAE-Toyopearl 650S
Fraction 4 was dialysed against buffer A, and the dialysed solution was applied to a column (2 cm×12 cm) of DEAE-Toyopearl 650S equilibrated with buffer A. Proteins were eluted with a linear gradient (0–0.35 M) of NaCl in 800 ml of buffer A, and the protein concentration and enzyme activity of the fractions were assayed. Fractions with a high specific activity were pooled to yield fraction 5 (40 ml).
Chromatography on Toyopearl HW-55S
Fraction 5 was concentrated to 0.5 ml with a collodion bag (Sartorius). The concentrated sample was placed on top of a column (3 cm×60 cm) of Toyopearl HW-55S equilibrated with buffer A containing 0.2 M NaCl. Proteins were eluted with buffer A containing 0.2 M NaCl. After estimating the protein concentration and enzyme activity, fractions with a high specific activity were pooled to yield fraction 6 (8 ml).
Chromatography on phenyl-Toyopearl 650S
To fraction 6, (NH4)2SO4 was added to make a 30% (w/w) solution. The enzyme solution was applied to a column (1.7 cm×12 cm) of Phenyl-Toyopearl 650S equilibrated with buffer A containing 30% (w/w) (NH4)2SO4. Proteins were eluted with a linear gradient (30–0%, w/w) of (NH4)2SO4 in 400 ml of buffer A, and the protein concentration and enzyme activity of the fractions were assayed. The purity of the enzyme in each fraction was determined by PAGE. Fractions showing a single protein band on the gel were pooled (fraction 14 ml).
Purification of CatC
Frozen cells (9.6 g, wet weight) were thawed and suspended in 96 ml of buffer A. The preparations of the cell extracts (fraction 1, 100 ml), streptomycin sulphate treatment (fraction 2, 103 ml), (NH4)2SO4 fractionation (fraction 3, 30 ml), chromatographies on DE52 cellulose (fraction 4, 18 ml), DEAE-Toyopearl 650S (fraction 5, 20 ml) and Phenyl-Toyopearl 650S (fraction 6, 3 ml) were carried out essentially by the same methods as used for the purification of CatB.
Determination of molecular masses
The molecular masses of the native enzymes were measured by gel filtration, and those of the enzyme subunits were measured by SDS/PAGE [22]. Size markers used for gel filtration were those in the calibration proteins gel chromatography kit from Boehringer Mannheim. The electrophoresis calibration kit LMW (Pharmacia) and the LMW calibration kit for SDS/electrophoresis (Amersham Biosciences) were used as size markers for SDS/PAGE.
Determination of N-terminal and inner amino acid sequences
Purified CatB and CatC were electroblotted using the method of Matsudaira [23], and then the N-terminal amino acid sequences were determined by automated Edman degradation with a Shimadzu PPSQ-10 protein sequencer. The inner amino acid sequences of CatB were also determined with the sequencer after fractionation of CNBr-digested peptides on an SDS/polyacrylamide gel.
Substrate specificity
Methyl, chloro and fluoro derivatives of cis,cis-muconate were enzymatically synthesized with chlorocatechol 1,2-dioxygenase from Ralstonia eutropha NH9 as described previously [24]. The following molar absorption coefficients were used: 17100 M−1·cm−1 for 2-methyl-cis,cis-muconate, 12400 M−1·cm−1 for 3-methyl-cis,cis-muconate, 18000 M−1·cm−1 for 2-chloro-cis,cis-muconate, 13900 M−1·cm−1 for 3-chloro-cis,cis-muconate and 14900 M−1·cm−1 for 2-fluoro-cis,cis-muconate [25]. Km and Vmax values of the purified enzyme were calculated by non-linear regression with the Enzfitter program (Biosoft).
Gene manipulation and construction of gene library
Standard methods were used for the plasmid DNA purifications, restriction enzyme digestions and E. coli transformations [26]. The total DNA of Rhodococcus sp. AN-22 was prepared according to the protocol described by DiLella and Woo [27]. The purified DNA (11 μg) was partially digested with Sau3AI, and the DNA fragments were fractionated by ultracentrifugation at 40000 rev./min for 15 h at 20 °C using an MLA130 rotor (Beckman Coulter) through sucrose gradients. A gene library was constructed by ligating 5–10-kb fragments to a pBluescript II KS− vector digested with BamHI. E. coli XL1-Blue was transformed with the ligated plasmids. Subcloning experiments were performed using conventional techniques [26].
PCR
On the basis of N-terminal and inner amino acid sequences of CatB, the two degenerate primers catB-N (5′-ACIACIATHATHGAYGT-3′) and catB-I (5′-CCCCAIGCRTCRTGCAT-3′) were synthesized. The cycling of primers was performed at 95 °C (1 min), 43 °C (1 min) and 72 °C (1 min) for 30 cycles and additionally at 72 °C (2 min) for one cycle to complete the amplifying reaction.
Preparation of [α-32P]dCTP-labelled probe and colony hybridization
Amplified DNA fragments were ligated to a pGEM-T vector (Promega) and completely sequenced to ensure that the ligated fragments constituted a portion of the catB gene. After sequencing, the recombinant plasmid constituting the catB gene was digested with EcoRI. The inserted DNA was purified and labelled with a random primer DNA labelling kit version 2 (Takara) and [α-32P]dCTP (ICN). Recombinant plasmids of transformants from a gene library of Rhodococcus sp. AN-22 were fixed on a Hybond-N+ membrane (Amersham Biosciences) according to the manufacturer's instructions. Colony hybridization was performed under standard conditions [26].
DNA sequencing
A FlexiPrep kit (Amersham Biosciences) was used for preparing the double-stranded DNA for sequencing. Sequencing reactions were performed using a Thermo Sequenase primer cycle sequencing kit with 7-deaza dGTP (Amersham Biosciences). Reaction mixtures were run on a Shimadzu DSQ-2000L sequencer.
Preparation of total RNA, RT (reverse transcriptase)-PCR and primer extension analysis
Total RNA was prepared from cells of Rhodococcus sp. AN-22 grown on the aniline and succinate media using an RNeasy mini kit (Qiagen). On the basis of the nucleotide sequences of the catABC genes, the two primers OF (5′-CACCTGATGGTGAAGGCTCC-3′) and OR (5′-GCGCTGCAGATCCTGACTGT-3′) were synthesized (Figure 1). cDNA was synthesized with a RevertAid M-MuLV reverse transcriptase (MBI Fermentas) using the OR primer and total RNA of Rhodococcus sp. AN-22. The cycling of the primers was performed at 94 °C (1 min), 58 °C (1 min) and 72 °C (1 min) for 30 cycles and additionally at 72 °C (2 min) for one cycle to complete the amplifying reaction. For the primer extension analysis, the FITC-labelled primer PE (5′-CCCGAATGGGTCGGGATGTG-3′) was designed. Reverse transcriptional reactions were performed as mentioned above. After incubation for 1 h, the synthesized cDNA was precipitated with ethanol and prepared for electrophoresis and sequencing, according to the method reported previously [28].
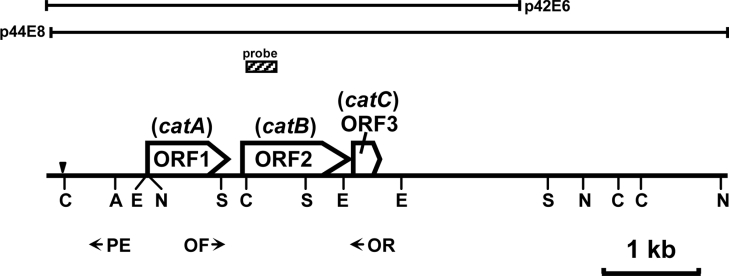
Figure 1. Location of cloned DNA fragments and cat genes.
Open arrows indicate ORFs and the directions of their transcripts. The probe synthesized by PCR using catB-N and catB-I primers was used for colony hybridization. The primers used for RT-PCR and primer extension analyses are indicated by horizontal arrows. The downward solid triangle indicates a transcriptional start site of cat gene cluster. A, ApaI; C, ClaI; E, EcoNI; N, NcoI; S, SacI.
RESULTS
Constitutive synthesis of CatB and CatC
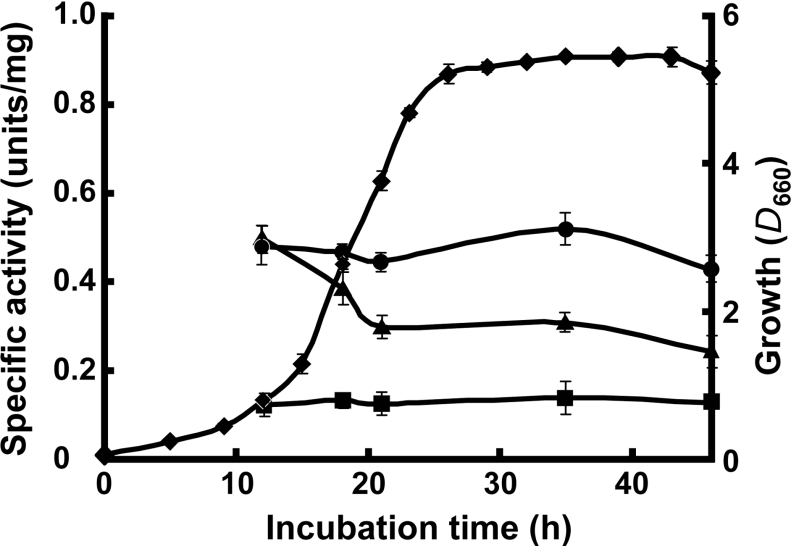
Table 1 shows that Rhodococcus sp. AN-22 synthesized CatB and CatC in its cells grown on all the non-aromatic substrates examined, as seen in the case of CatA [9]. The cells grown on D-mannose, L-malate and L-leucine produced higher specific activities of CatB and CatC than those grown on aniline. When the bacterium was incubated with acetate, which is a metabolic member of the glyoxylate cycle, the two enzyme activities markedly decreased, although satisfactory cell growth was observed. The strain maintained the specific activities of CatA, CatB and CatC at an almost equal level during cultivation on the succinate medium (Figure 2).
Table 1. Constitutive synthesis of CatB and CatC.
All values are indicated as means±S.D. for five separate experiments.
| Substrate | D660 | CatB activity (units/mg) | CatC activity (units/mg) |
|---|---|---|---|
| Aniline | 1.2 | 0.052±0.010 | 0.76±0.36 |
| D-Mannose | 1.4 | 0.087±0.015 | 0.96±0.05 |
| D-Glucose | 1.9 | 0.080±0.005 | 0.67±0.04 |
| Sucrose | 1.5 | 0.019±0.003 | 0.47±0.01 |
| L-Malate | 2.0 | 0.20±0.01 | 0.82±0.03 |
| Succinate | 1.9 | 0.12±0.02 | 0.91±0.07 |
| Acetate | 2.2 | 0.016±0.002 | 0.22±0.01 |
| L-Isoleucine | 1.2 | 0.051±0.007 | 0.77±0.05 |
| L-Leucine | 1.5 | 0.10±0.01 | 1.1±0.1 |
Figure 2. Production of CatA, CatB and CatC from Rhodococcus sp. AN-22 on succinate medium.
The bacterium was incubated at 30 °C with shaking. At appropriate intervals, cell growth (◆), CatA (▲), CatB (■) and CatC (●) were measured. Results are indicated as means±S.D. for three determinations.
On the other hand, the specific activities of CatD and CatIJ in cells grown on succinate were less than 1/8 and 1/25 respectively than those in cells obtained from the aniline medium, indicating that the two enzymes were synthesized essentially in the presence of aniline or its metabolites.
Purification and characterization of CatB
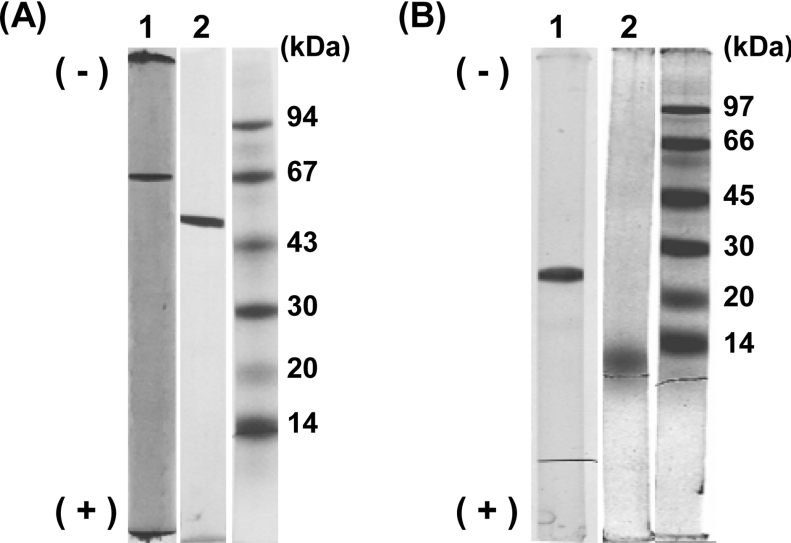
Table 2 is a summary of a typical enzyme purification for CatB from cells of Rhodococcus sp. AN-22 grown on succinate. The specific activity of the final preparation of CatB was 52 units/mg, with an overall recovery of 18%. The final enzyme preparation had a 370-fold increase in the specific activity and showed a single protein band on both native and denaturing polyacrylamide gels (Figure 3A).
Table 2. Purification of CatB from succinate-grown cells of Rhodococcus sp. AN-22.
| Fraction | Total activity (units) | Total protein (mg) | Specific activity (units/mg) | Recovery (%) |
|---|---|---|---|---|
| Cell extract | 240 | 1800 | 0.14 | 100 |
| Streptomycin sulphate | 200 | 2600 | 0.078 | 82 |
| Ammonium sulphate | 220 | 470 | 0.48 | 92 |
| DE52 | 140 | 51 | 2.7 | 57 |
| DEAE-Toyopearl 650S | 140 | 6.4 | 22 | 58 |
| HW-55S | 80 | 1.6 | 50 | 33 |
| Phenyl-Toyopearl 650S | 42 | 0.81 | 52 | 18 |
Figure 3. Native PAGE (lanes 1) and SDS/PAGE (lanes 2) of CatB (A) and CatC (B) from Rhodococcus sp. AN-22.
Lanes 1, native PAGE. The purified enzymes (5 μg each) were run on 7.5% (w/v) gels of pH 8.9 at 2 mA/tube for 2.5 h in a running buffer (pH 8.3) of Tris/glycine. Lanes 2, SDS/PAGE. The purified enzymes (4 μg each) denatured with SDS were run on 7.5% (w/v) gels containing 0.1% (w/v) SDS at 6 mA/tube for 3 h (A) or 2 h (B) in a running buffer (pH 7.2) of 0.1% (w/v) SDS/0.1 M sodium phosphate. Molecular-mass size markers used for SDS/PAGE were α-lactalbumin (14 kDa), trypsin inhibitor (20 kDa), carbonic anhydrase (30 kDa), ovalbumin (43 and 45 kDa), BSA (67 and 66 kDa) and phosphorylase b (94 and 97 kDa). The gels used for the native PAGE and SDS/PAGE were stained with 0.25% (w/v) Coomassie Brilliant Blue R-250 in a solvent of ethanol/ethanoic (acetic) acid/water (9:2:9, v/v). The molecular masses of three markers were different in (A) and (B), because they were purchased from Pharmacia and Amersham Biosciences respectively. See the text for details.
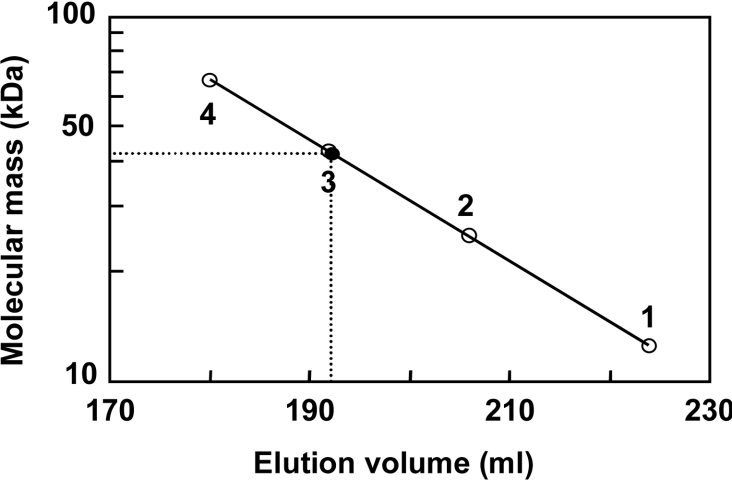
The apparent molecular mass of CatB was determined to be 43 kDa by gel filtration (Figure 4) and 44 kDa by SDS/PAGE (Figure 3A). These findings indicate that the enzyme is a monomer. The purified enzyme showed a maximal activity at pH 8.0. It was stable between pH 6.0 and 9.0. The enzyme maintained 90% activity up to 55 °C after 10 min of incubation at pH 8.0, and the enzyme activity was lost at 60 °C.
Figure 4. Determination of molecular mass of CatB by gel filtration.
The enzyme (24 units) and markers (2 mg of each) were placed on the top of a column (3.0 cm×60 cm) of Toyopearl HW-55S, and then eluted with buffer A containing 0.2 M NaCl. 1, Cytochrome c (12.5 kDa); 2, chymotrypsinogen A (25 kDa); 3, ovalbumin (45 kDa); 4, BSA (68 kDa); ●, CatB.
Table 3 shows that the activity of CatB for cis,cis-muconate increased in the presence of Mn2+, Co2+, Mg2+ and Ni2+. Of the metals tested, Co2+ produced the highest CatB activity.
Table 3. Effects of bivalent metal ions on the activity of CatB.
The enzyme reaction was performed by the standard assay methods in the presence of various bivalent ions instead of manganese ion.
| Metal ion | Relative activity (%) |
|---|---|
| None | 43 |
| Mn2+ | 100 |
| Co2+ | 170 |
| Mg2+ | 75 |
| Ba2+ | 40 |
| Ca2+ | 34 |
| Ni2+ | 55 |
| Zn2+ | 30 |
The effects of metal salts and thiol and chelating agents on the activity of CatB were tested (Table 4). HgCl2, CuSO4 and iodoacetic acid inhibited the enzyme. Other thiol and chelating agents tested did not evidently affect the activity.
Table 4. Effects of various compounds on the activity of CatB.
The enzyme (0.7 units) was incubated with various compounds in 1.0 ml of buffer A at 24 °C for 10 min. An aliquot (0.1 ml) of the incubation mixture was subjected to enzyme assay using cis,cis-muconate as a substrate. PCMB, p-chloromercuribenzoic acid.
| Compound | Concentration (mM) | Remaining activity (%) |
|---|---|---|
| None | – | 100 |
| HgCl2 | 1.0 | 20 |
| CuSO4·5H2O | 1.0 | 65 |
| Iodoacetic acid | 0.2 | 58 |
| PCMB | 0.1 | 92 |
| α,α′-Dipyridyl | 1.0 | 93 |
| N-Ethylmaleimide | 1.0 | 90 |
| Tiron | 1.0 | 100 |
| EDTA | 1.0 | 101 |
| o-Penanthroline | 0.05 | 101 |
| NaN3 | 1.0 | 96 |
The CatB of Rhodococcus sp. AN-22 showed substantial activities for the cyclization of 2-methyl-, 3-methyl- and 2-chloro-cis,cis-muconate, in addition to the original substrate cis,cis-muconate (Table 5). The turnover number (kcat) of CatB for cis,cis-muconate was higher than those for methyl and 2-chloro-derivatives, while the affinity for cis,cis-muconate was lower than the others on the basis of Km values.
Table 5. Substrate specificity of CatB.
Km and Vmax are indicated as means±S.D. for five determinations. kcat values were calculated on the basis of the molecular mass of 44 kDa.
| Substrate | Km (μM) | Vmax (units·mg−1) | kcat (min−1) | kcat/Km (min−1·μM−1) |
|---|---|---|---|---|
| cis,cis-Muconate | 660±70 | 390±30 | 17000±1000 | 26 |
| 2-Methyl-cis,cis-muconate | 380±70 | 8.5±1.4 | 330±60 | 1.1 |
| 3-Methyl-cis,cis-muconate | 310±20 | 24±2 | 1100±100 | 3.1 |
| 2-Chloro-cis,cis-muconate | 300±60 | 6.0±2.5 | 260±110 | 1.4 |
| 3-Chloro-cis,cis-muconate | (<0.01)* | |||
| 2-Fluoro-cis,cis-muconate | (<0.01)* |
* Detection limit for specific activity under the experimental conditions.
Purification and characterization of CatC
Table 6 shows a summary of a typical enzyme purification for CatC from succinate-grown cells. The specific activity of the final preparation was 860 units/mg, with an overall recovery of 2.7%. The final enzyme preparation had a 930-fold increase in the specific activity and showed a single protein band on both native and denaturing polyacrylamide gels (Figure 3B).
Table 6. Purification of CatC from succinate-grown cells of Rhodococcus sp. AN-22.
| Fraction | Total activity (units) | Total protein (mg) | Specific activity (units/mg) | Recovery (%) |
|---|---|---|---|---|
| Cell extract | 440 | 480 | 0.92 | 100 |
| Streptomycin sulphate | 500 | 480 | 1.0 | 110 |
| Ammonium sulphate | 320 | 71 | 4.5 | 73 |
| DE52 | 100 | 7.2 | 14 | 23 |
| DEAE-Toyopearl 650S | 75 | 1.8 | 42 | 17 |
| Phenyl-Toyopearl 650S | 12 | 0.014 | 860 | 2.7 |
The apparent molecular masses of CatC were 100 kDa by gel filtration (results not shown) and 12 kDa by SDS/PAGE (Figure 3B). These findings indicate that the enzyme is a homo-octamer.
The optimal activity of the purified CatC was not determined, because the reaction mixture for the enzyme assay contained another enzyme that affected the optimal pH for CatC. It was stable between pH 7.0 and 10.5. The enzyme maintained more than 80% activity up to 90 °C after 10 min of incubation at pH 8.0. In addition, it maintained 64% activity at 95 °C. The kinetic parameters of CatC for muconolactone were as follows: Km, 640±70 μM; Vmax, 330±20 units·mg−1; and kcat, 4000±300 min−1.
Cloning and sequence analysis of cat genes from Rhodococcus sp. AN-22
The N-terminal amino acid sequences of purified CatB and CatC were SDPDLKIASVTTTIIDVPLIRPHKFAT and ALFHVRMDVD respectively. The inner amino acid sequences of a certain peptide of CatB were HDAWGALGVPVAXLLGGA. On the basis of these amino acid sequences, catB-N and catB-I primers were synthesized. When the catB-N and catB-I primers and total DNA purified from Rhodococcus sp. AN-22 as a template were incubated, a 320-bp DNA fragment (Figure 1; probe) was amplified. The sequencing of both strands of the amplified fragment showed that this fragment encoded a portion of CatB. The sequenced fragment was labelled with [α-32P]dCTP. The labelled fragment was used as a probe for colony hybridization. Among the 4600 transformants of a gene library from the AN-22 strain, two transformants harbouring plasmids p42E6 and p44E8 were positive (Figure 1). The DNA fragments of the two plasmids were subcloned and sequenced.
The DNA fragments inserted into p42E6 and p44E8 covered 7187 bp. Three complete ORFs (open reading frames) (ORF1–ORF3) were found in the determined nucleotide sequences. ORF1 (positions 1067–1915) consisted of 849 bp encoding 283 amino acid residues. The deduced amino acid sequences were identical with the inner amino acid sequences of the purified CatA from Rhodococcus sp. AN-22 [9]. The molecular mass of a polypeptide encoded by ORF1 was calculated to be 31 kDa, which corresponded to the value of 30 kDa estimated by SDS/PAGE. The deduced amino acid sequences of ORF1 showed high identities with those of CatAs from Rhodococcus rhodochrous NCIMB 13259 (86%, GenBank® accession number AF043741) [29], Rhodococcus erythropolis AN-13 (74%, GenBank® accession number D83237) [6] and Rhodococcus opacus 1CP (69%, GenBank® accession number X99622) [30]. These results show that ORF1 was a gene encoding the CatA from Rhodococcus sp. AN-22; we named the gene catA.
The deduced amino acid sequences of ORF2 (positions 2068–3192) were identical with the N-terminal and inner amino acid sequences of CatB. The deduced molecular mass of an ORF2 product was 40 kDa, which was almost the same as that of the purified CatB. The deduced amino acid sequences of ORF2 showed high identities with those of the CatBs from R. erythropolis AN-13 (81%, GenBank® accession number D83237) [6] and R. opacus 1CP (76%, GenBank® accession number X99622) [30], but low identities with those of the CatBs from Gram-negative bacteria; ORF2 was named catB.
ORF3 (positions 3233–3514) consisted of 282 bp. The deduced N-terminal amino acid sequences corresponded to those of CatC. The deduced molecular mass of an ORF3 product was 12 kDa, which corresponded to the subunit value (12 kDa) of CatC. The deduced amino acid sequences of ORF3 showed high identities with those of CatCs from R. opacus 1CP (76%, GenBank® accession number X99622) [30], from Streptomyces setonii ATCC 39116 (68%, GenBank® accession number AF277051) [31] and CatC1 from Frateuria sp. ANA-18 (62%, GenBank® accession number AB009343) [8]. These data showed that ORF3 encoded CatC; ORF3 was named catC.
In the region upstream of catA of Rhodococcus sp. AN-22, a possible relic of catR (gene encoding a regulatory protein of the catechol-degrading pathway) was found as shown in Supplementary Figure S1 (http://www.BiochemJ.org/bj/393/bj3930219add.htm). The reduced amino acid sequences of the relic of catR had high identity with those of catR from R. opacus 1CP [32] and R. erythropolis AN-13 [6]. The relic of catR neighboured a homologous sequence of IS204 (positions 800–1), an insertion sequence from Nocardia asteroides YP21 [33]. The IS204-like sequence of the AN-22 strain contained a predicted inverted repeat, but not ORFs encoding significant proteins such as transposase.
In the 3672-bp region downstream of catC, no ORFs encoding significant proteins were found.
Transcriptional start site and operon structure of cat genes
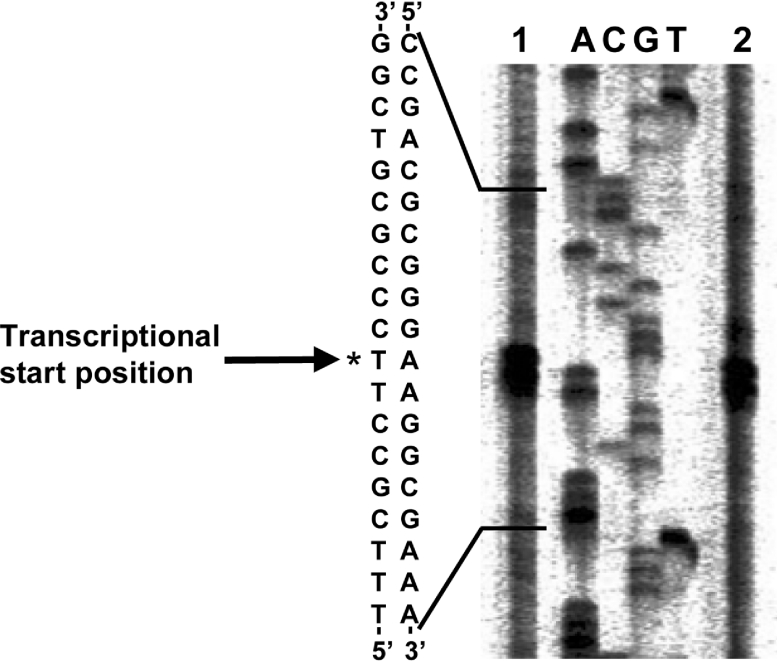
RT-PCR analyses carried out using three primer pairs on the basis of the sequences upstream of catA showed that a transcriptional start site of the cat genes existed between 531 and 159 bp upstream of a catA initiation codon (Supplementary Figure S2 at http://www.BiochemJ.org/bj/393/bj3930219add.htm). Primer extension analyses were then employed to determine the amount of single primer extension products and the exact transcriptional start site of the cat genes. Almost the same amount of each was synthesized in the presence of the PE primer and total RNA obtained from aniline- and succinate-grown cells of Rhodococcus sp. AN-22 (Figure 5). Both transcriptions started at the same position of 891 bp (thiamine residue on the coding strand) upstream of the catA initiation codon.
Figure 5. Primer extension analysis of cat gene transcripts.
Total RNA prepared from aniline- and succinate-grown cells of Rhodococcus sp. AN-22 was used for the reverse-transcription reaction with the PE primer. This primer was also used for a dideoxy sequencing reaction using plasmid p42E6 as a template. The reverse-transcription products obtained (lanes 1 and 2) corresponded in size to the thymine residue (T) marked by an asterisk (*) in the coding strand.
When RT-PCR analysis of total RNA from succinate-grown cells was carried out using a primer pair that spanned catABC, a DNA fragment was amplified, indicating that read-though transcription occurred in the region containing these three genes (Figure 6). Since no PCR product was observed in the negative-control sample without reverse transcriptase, possible DNA contamination was excluded.
Figure 6. Agarose gel electrophoresis of RT-PCR product.
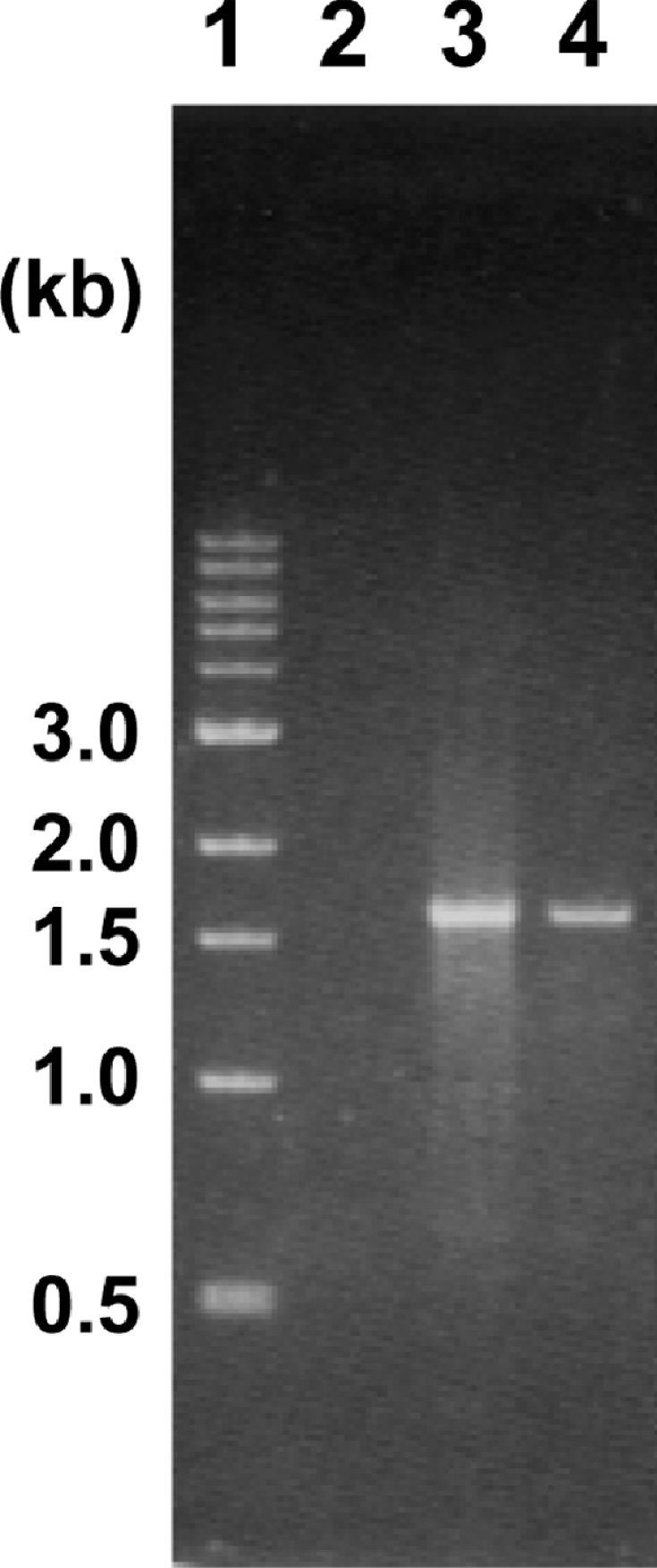
Total RNA prepared from succinate-grown cells of Rhodococcus sp. AN-22 was used for the synthesis of cDNA as a template. PCR was performed with the synthesized cDNA using OF and OR primers as amplification primers (lane 3). Control experiments were as follows: PCR was carried out using total RNA as a template without a reverse transcription step (lane 2) and using total DNA (lane 4) as a template. A 1-kb DNA ladder (New England Biolabs) was used as markers (lane 1). Sizes are given in kb.
DISCUSSION
In the present study, we found that aniline-assimilating Rhodococcus sp. AN-22 constitutively synthesized CatB and CatC in its cells, which were then purified and characterized, in addition to CatA reported previously [9]. On the other hand, CatD and CatIJ were inducibly produced. This is the first report describing the finding, purification and characterization of constitutive CatB and CatC from micro-organisms that degrade aromatic compounds. The CatB of Rhodococcus sp. AN-22 exhibited activity as a monomer, although all the inducible CatBs reported are activated by the polymerization of a subunit to a polymer with the molecular mass of 200–400 kDa. The CatB of the AN-22 strain was resistant to inhibitors such as thiol and chelating agents, compared with the enzymes inducibly produced from other micro-organisms [34]. Although the specific constant (kcat/Km) for cis,cis-muconate of the CatB from the AN-22 strain was similar to those of CatBs from Arthrobacter sp. BA-5-17 [24], Acinetobacter calcoaceticus ADP1 [35] and R. opacus 1CP [36], the kcat value of the former was higher than those from other bacterial strains. In addition, CatB showed activity in the presence of Co2+ and Mg2+, besides Mn2+. For these reasons, constitutive CatB of Rhodococcus sp. AN-22 seems to be peculiar in terms of its enzyme chemistry.
The CatC of the AN-22 strain was quite stable to heating and maintained more than 80% activity at 90 °C, although that of Pseudomonas putida rapidly lost activity at 65 °C [17]. In an SDS/polyacrylamide gel, the CatC band was extensively diffused, probably because of its small molecular mass (Figure 3B). Since the reaction mixture for the CatC assay contained CatD, CatCs from various micro-organisms including Rhodococcus sp. AN-22 have not been fully characterized [36].
We cloned catA, catB and catC genes encoding the constitutive CatA, CatB and CatC respectively from Rhodococcus sp. AN-22. The three genes formed an operon and showed high identity with other corresponding genes from Gram-positive micro-organisms (see the Results section), although the CatB of the AN-22 strain had several characteristics mentioned above. The location of the catA, catB and catC genes of Rhodococcus sp. AN-22 was identical with those of two other Rhodococcus strains, which are inducibly expressed [6,18], and apparently different from those of a Streptomyces strain and Gram-negative strains (Supplementary Figure S3 at http://www.BiochemJ.org/bj/393/bj3930219add.htm). The catDIJ genes of Rhodococcus sp. AN-22 were not observed in the cloned DNA fragments in the present study, as these genes were not found in the catABC locus in most bacteria reported [8,30,31,37]. In addition, CatD and CatIJ were synthesized inducibly in Rhodococcus sp. AN-22. These facts suggest that the catDIJ genes of the AN-22 strain were distant from catABC and were expressed inducibly by another regulatory system. Since CatA, CatB and CatC are synthesized constitutively and CatD and CatIJ inducibly in the cells, 3-oxoadipate enol-lactone converted from catechol by CatA, CatB and CatC could be accumulated readily at the early phase of catechol metabolism. This intermediate metabolite is valuable as a substrate used for the assay of CatD.
Although the regulatory gene catR, which is commonly contained in the gene cluster for catechol catabolism [10,32], was absent around the catABC genes of Rhodococcus sp. AN-22, a possible relic of catR was adjacent to an IS204-like element in the region upstream of catA. These findings indicate that the catR encoding a regulator of catABC was disrupted by the transposition of IS204-like element into Rhodococcus sp. AN-22. It was reported that some sequences of the IS-like element are present in the region upstream of a constitutive 2,3-dihydroxybiphenyl 1,2-dioxygenase gene from dibenzofuran-degrading Rhodococcus sp. YK2 [38].
When Rhodococcus sp. AN-22 grew on aniline and succinate, almost equal amounts of two single primer extension products were synthesized (Figure 5). The transcription started at the same site in both cases, and putative σ70-type −10 (TTCCCC) and −35 (TCCCAT) promoter sequences were identified 8 bp upstream of the transcriptional start site. Since this site exists in the IS-like element, the promoter is not regulated by the original CatR. It seems likely that the promoter found in the element acted for the constitutive expression of catABC genes.
These data led us to the assumption that the catABC genes of this bacterium are always expressed with any growth substrate or its metabolite. The assumption was then supported by the data in the present and previous reports [9] that the CatA, CatB and CatC are synthesized on all the growth substrates examined. However, the cells cultured with acetate expressed much lower activities of CatA, CatB and CatC than the cells cultured with organic acids belonging to the TCA (tricarboxylic acid) cycle. These results suggest that possible regulation to repress the expression of catABC genes may function in the glyoxylate cycle, in competition with the TCA cycle.
The actinomycete genus Rhodococcus is of interest for the metabolic abilities of substituted hydrocarbons and other chemicals, and the solvent tolerance, because of the aliphatic chains of mycolic acid that are present in their cell walls [39,40]. For these reasons, a Rhodococcus-based host–vector system will be useful for many applications to bioremediation, biodegradation and bioconversion. Since Rhodococcus sp. AN-22 has the constitutive expression system, the constructed host–vector based on this system will be able to synthesize a target protein steadily without an inducer, such as IPTG.
Online Data
Acknowledgments
We thank Dr Ogawa, National Institute of Agro-Environmental Sciences, for providing the plasmid pUC9A with a cbnA.
References
- 1.Lyons C. D., Katz S. E., Bartha R. Mechanisms and pathways of aniline elimination from aquatic environments. Appl. Environ. Microbiol. 1984;48:491–496. doi: 10.1128/aem.48.3.491-496.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoki K., Shinke R., Nishira H. Identification of aniline-assimilating bacteria. Agric. Biol. Chem. 1982;46:2563–2570. [Google Scholar]
- 3.Aoki K., Shinke R., Nishira H. Metabolism of aniline by Rhodococcus erythropolis AN-13. Agric. Biol. Chem. 1983;48:1611–1616. [Google Scholar]
- 4.Aoki K., Konohana T., Shinke R., Nishira H. Purification and characterization of catechol 1,2-dioxygenase from aniline-assimilating Rhodococcus erythropolis AN-13. Agric. Biol. Chem. 1984;48:2087–2095. [Google Scholar]
- 5.Aoki K., Konohana T., Shinke R., Nishira H. Two catechol 1,2-dioxygenases from an aniline-assimilating bacterium, Frateuria sp. ANA-18. Agric. Biol. Chem. 1984;48:2097–2104. [Google Scholar]
- 6.Murakami S., Kodama N., Shinke R., Aoki K. Classification of catechol 1,2-dioxygenase family: analysis of a gene for the catechol 1,2-dioxygenase showing high specificity for methylcatechols from Gram+ aniline-assimilating Rhodococcus erythropolis AN-13. Gene. 1997;185:49–54. doi: 10.1016/s0378-1119(96)00629-4. [DOI] [PubMed] [Google Scholar]
- 7.Murakami S., Hayashi T., Maeda T., Takenaka S., Aoki K. Cloning and functional analysis of aniline dioxygenase gene cluster, from Frateuria species ANA-18, that metabolizes aniline via an ortho-cleavage pathway of catechol. Biosci. Biotechnol. Biochem. 2003;67:2351–2358. doi: 10.1271/bbb.67.2351. [DOI] [PubMed] [Google Scholar]
- 8.Murakami S., Takashima A., Takemoto J., Takenaka S., Shinke R., Aoki K. Cloning and sequence analysis of two catechol-degrading gene clusters from the aniline-assimilating bacterium Frateuria species ANA-18. Gene. 1999;226:189–198. doi: 10.1016/s0378-1119(98)00560-5. [DOI] [PubMed] [Google Scholar]
- 9.Matsumura E., Ooi S., Murakami S., Takenaka S., Aoki K. Constitutive synthesis, purification, and characterization of catechol 1,2-dioxygenase from the aniline-assimilating bacterium Rhodococcus sp. AN-22. J. Biosci. Bioeng. 2004;98:71–76. doi: 10.1016/S1389-1723(04)70245-5. [DOI] [PubMed] [Google Scholar]
- 10.Takashima A., Murakami S., Takenaka S., Aoki K. Regulation by two CatR proteins that differ in binding affinity to catB promoters expressing two cat gene clusters. Biosci. Biotechnol. Biochem. 2001;65:2146–2153. doi: 10.1271/bbb.65.2146. [DOI] [PubMed] [Google Scholar]
- 11.Lechner U., Straube G. Degradation of 3-aminophenol by Arthrobacter spec. mA3. J. Basic Microbiol. 1988;28:629–637. doi: 10.1002/jobm.3620280918. [DOI] [PubMed] [Google Scholar]
- 12.Roldan M. D., Blasco R., Caballero F. J., Castillo F. Degradation of p-nitrophenol by the phototrophic bacterium Rhodobacter capsulatus. Arch. Microbiol. 1998;169:36–42. doi: 10.1007/s002030050538. [DOI] [PubMed] [Google Scholar]
- 13.Caposio P., Pessione E., Giuffrida G., Conti A., Landolfo S., Giunta C., Gribaudo G. Cloning and characterization of two catechol 1,2-dioxygenase genes from Acinetobacter radioresistens S13. Res. Microbiol. 2002;153:69–74. doi: 10.1016/s0923-2508(01)01290-6. [DOI] [PubMed] [Google Scholar]
- 14.Aoki K., Ohtsuka K., Shinke R., Nishira H. Isolation of aniline-assimilating bacteria and physiological characterization of aniline biodegradation in Rhodococcus erythropolis AN-13. Agric. Biol. Chem. 1983;47:2569–2575. [Google Scholar]
- 15.Liu S., Ogawa N., Miyashita K. The chlorocatechol degradative genes, tfdT-CDEF, of Burkholderia sp. strain NK8 are involved in chlorobenzoate degradation and induced by chlorobenzoates and chlorocatechols. Gene. 2001;268:207–214. doi: 10.1016/s0378-1119(01)00435-8. [DOI] [PubMed] [Google Scholar]
- 16.Murakami S., Takemoto J., Takashima A., Shinke R., Aoki K. Purification and characterization of two muconate cycloisomerase isozymes from aniline-assimilating Frateuria species ANA-18. Biosci. Biotechnol. Biochem. 1998;62:1129–1133. doi: 10.1271/bbb.62.1129. [DOI] [PubMed] [Google Scholar]
- 17.Ornston L. N. The conversion of catechol and protocatechuate to β-ketoadipate by Pseudomonas putida. III. Enzymes of the catechol pathway. J. Biol. Chem. 1966;241:3795–3799. [PubMed] [Google Scholar]
- 18.Moiseeva O. V., Solyanikova I. P., Kaschabek S. R., Groning J., Thiel M., Golovleva L. A., Schlömann M. A new modified ortho cleavage pathway of 3-chlorocatechol degradation by Rhodococcus opacus 1CP: genetic and biochemical evidence. J. Bacteriol. 2002;184:5282–5292. doi: 10.1128/JB.184.19.5282-5292.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ornston L. N. The conversion of catechol and protocatechuate to β-ketoadipate by Pseudomonas putida. II. Enzymes of the protocatechuate pathway. J. Biol. Chem. 1966;241:3787–3794. [PubMed] [Google Scholar]
- 20.Yeh W.-K., Ornston L. N. Evolutionarily homologous α2β2 oligomeric structures in β-ketoadipate succinyl-CoA transferases from Acinetobacter calcoaceticus and Pseudomonas putida. J. Biol. Chem. 1981;256:1565–1569. [PubMed] [Google Scholar]
- 21.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. Protein measurement with the Folin phenol regent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 22.Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J. Biol. Chem. 1969;244:4406–4412. [PubMed] [Google Scholar]
- 23.Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J. Biol. Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 24.Murakami S., Kohsaka C., Okuno T., Takenaka S., Aoki K. Purification, characterization, and gene cloning of cis,cis-muconate cycloisomerase from benzamide-assimilating Arthrobacter sp. BA-5-17. FEMS Microbiol. Lett. 2004;231:119–124. doi: 10.1016/S0378-1097(03)00933-9. [DOI] [PubMed] [Google Scholar]
- 25.Dorn E., Knackmuss H.-J. Chemical structure and biodegradability of halogenated aromatic compounds: substituent effects on 1,2-dioxygenation of catechol. Biochem. J. 1978;174:85–94. doi: 10.1042/bj1740085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook J., Frisch E. F., Maniatis T. 2nd edn. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1989. Molecular Cloning: a Laboratory Manual. [Google Scholar]
- 27.DiLella A. G., Woo S. L. C. Cloning large segments of genomic DNA using cosmid vectors. Methods Enzymol. 1987;152:199–212. doi: 10.1016/0076-6879(87)52021-3. [DOI] [PubMed] [Google Scholar]
- 28.Murakami S., Sawami Y., Takenaka S., Aoki K. Cloning of a gene encoding 4-amino-3-hydroxybenzoate 2,3-dioxygenase from Bordetella sp. 10d. Biochem. Biophys. Res. Commun. 2004;314:489–494. doi: 10.1016/j.bbrc.2003.12.111. [DOI] [PubMed] [Google Scholar]
- 29.Strachan P. D., Freer A. A., Fewson C. A. Purification and characterization of catechol 1,2-dioxygenase from Rhodococcus rhodochrous NCIMB 13259 and cloning and sequencing of its catA gene. Biochem. J. 1998;333:741–747. doi: 10.1042/bj3330741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eulberg D., Golovleva L. A., Schlömann M. Characterization of catechol catabolic genes from Rhodococcus erythropolis 1CP. J. Bacteriol. 1997;179:370–381. doi: 10.1128/jb.179.2.370-381.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park H.-J., Kim E.-S. An inducible Streptomyces gene cluster involved in aromatic compound metabolism. FEMS Microbiol. Lett. 2003;226:151–157. doi: 10.1016/S0378-1097(03)00585-8. [DOI] [PubMed] [Google Scholar]
- 32.Eulberg D., Schlömann M. The putative regulator of catechol catabolism in Rhodococcus opacus 1CP: an IclR-type, not a LysR-type transcriptional regulator. Antonie Van Leeuwenhoek. 1998;74:71–82. doi: 10.1023/a:1001755928637. [DOI] [PubMed] [Google Scholar]
- 33.Yao W., Yang Y., Chiao J. IS204: an insertion sequence from Nocardia asteroides (mexicana) YP21. Plasmid. 1994;32:262–269. doi: 10.1006/plas.1994.1065. [DOI] [PubMed] [Google Scholar]
- 34.Murakami S., Takemoto J., Takenaka S., Shinke R., Aoki K. Purification and characterization of muconate cycloisomerase from aniline-assimilating Rhodococcus erythropolis AN-13. J. Ferment. Bioeng. 1998;85:521–524. [Google Scholar]
- 35.Vollmer M. D., Hoier H., Hecht H. J., Schell U., Gröning J., Goldman A., Schlömann M. Substrate specificity of and product formation by muconate cycloisomerases: an analysis of wild-type enzymes and engineered variants. Appl. Environ. Microbiol. 1998;64:3290–3299. doi: 10.1128/aem.64.9.3290-3299.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solyanikova I. P., Maltseva O. V., Vollmer M. D., Golovleva L. A., Schlömann M. Characterization of muconate and chloromuconate cycloisomerase from Rhodococcus erythropolis 1CP: indications for functionally convergent evolution among bacterial cycloisomerases. J. Bacteriol. 1995;177:2821–2826. doi: 10.1128/jb.177.10.2821-2826.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Houghton J. E., Brown T. M., Appel A. J., Hughes E. J., Ornston L. N. Discontinuities in the evolution of Pseudomonas putida cat genes. J. Bacteriol. 1995;177:401–412. doi: 10.1128/jb.177.2.401-412.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iida T., Mukouzaka Y., Nakamura K., Yamaguchi I., Kudo T. Isolation and characterization of dibenzofuran-degrading actinomycetes: analysis of multiple extradiol dioxygenase genes in dibenzofuran-degrading Rhodococcus species. Biosci. Biotechnol. Biochem. 2002;66:1462–1472. doi: 10.1271/bbb.66.1462. [DOI] [PubMed] [Google Scholar]
- 39.Bell K. S., Philp J. C., Aw D. W. J., Christofi N. The genus Rhodococcus. J. Appl. Microbiol. 1998;85:195–210. doi: 10.1046/j.1365-2672.1998.00525.x. [DOI] [PubMed] [Google Scholar]
- 40.O'Brien X. M., Parker J. A., Lessard P. A., Sinskey A. J. Engineering an indene bioconversion process for the production of cis-aminoindanol: a model system for the production of chiral synthons. Appl. Microbiol. Biotechnol. 2002;59:389–399. doi: 10.1007/s00253-002-1052-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.