Abstract
A group of OPTs (oligopeptide transporters) exclusively identified in plants and fungi are proposed to transport oligopeptides and derivatives of three to six amino acids in length, but their transport mechanisms and biological functions are poorly understood. We expressed the Saccharomyces cerevisiae (yeast) OPT ScOPT1 and five Arabidopsis thaliana AtOPTs in Xenopus laevis oocytes for two-electrode voltage-clamp studies. ScOPT1 produced inward currents in response to GSH or GSSG, the phytochelatin (PC) PC2 and oligopeptides including the tetrapeptide GGFL, but not KLGL. Inward currents were dependent on the external proton and substrate concentrations, with high affinity for both. This and the inward currents evoked by substrates with net negative charges showed that ScOPT1 is a proton-coupled transporter. ScOPT1 displayed highest apparent affinity for PC2, with small differences in the maximal current among substrates. Glutathione transport by any of the tested AtOPTs, including AtOPT6, was not detected in yeast growth complementation assays. With AtOPT4, initially only small KLGL-dependent currents were recorded in batches of oocytes showing high ScOPT1 expression. AtOPT4 expression was optimized by swapping the 5′-untranslated region with that of ScOPT1. AtOPT4 displayed a higher affinity for KLGL than ScOPT1 did for any peptide. AtOPT4-mediated KLGL transport was detectable at pH 5.0, but not at pH 6.0 or 7.0. Taken together, our results demonstrate that ScOPT1 and AtOPT4 are proton-coupled OPTs with broad but distinct substrate specificities and affinities.
Keywords: Arabidopsis thaliana, oligopeptide transport, phytochelatin, Saccharomyces cerevisiae, two-electrode voltage clamping, Xenopus laevis oocyte
Abbreviations: DTNB, 5,5′-dithiobis-(2-nitrobenzoic acid); OPT, oligopeptide transporter; AtOPT, Arabidopsis thaliana OPT; ScOPT, Saccharomyces cerevisiae OPT; PC, phytochelatin; 5′-UTR, 5′-untranslated region; YNB, yeast nitrogen base
INTRODUCTION
Oligopeptide transport across the cell membrane through integral membrane proteins is a widely distributed phenomenon in both prokaryotes and eukaryotes and is mediated by three distinct families of transporters [1]. Members of the OPT (oligopeptide transporter) family mediate the transport of oligopeptides and derivatives that contain three to six amino acids [1]. OPTs were initially identified in fungi and plants [2–5], and are also found in prokaryotes, but not in animals [6]. Fungal and plant OPTs are categorized into two distinct but closely related phylogenetic clusters within the OPT family [6]. A separate cluster contains plant transporters related to maize Yellow Stripe 1 that transports iron–mugineic acid complexes [6–8]. The higher similarity between fungal and plant OPTs suggests that the function of these transporters may be similar.
Sequence analysis identified nine OPTs from Arabidopsis thaliana (AtOPTs) in the plant OPT cluster [1,5]. A T-DNA knockout allele of AtOPT3 was found to be embryo-lethal [9]. In contrast, knockout alleles of other AtOPT genes showed no phenotypes, presumably because of gene redundancy [10]. AtOPT4 rescues growth of a yeast leucine auxotroph on media containing tetra- or penta-peptides as a sole leucine source [5]. Recent studies suggested that AtOPT6 improves the uptake of glutathione in a yeast mutant deficient in glutathione uptake [11]. This finding is in contrast with previous results showing that none of the AtOPTs, including AtOPT6, expressed in yeast enhanced transport activity for radiolabelled GSH [5]. The Saccharomyces cerevisiae OPT ScOPT1 (also called ScHGT1) transports both oligopeptides [12] and GSH [4] in a pH-dependent manner.
Functional characterization of plant and fungal OPTs so far has been limited to oligopeptide-mediated growth rescue assays and radiolabelled oligopeptide uptake in yeast [3,5,11–14]. The broad substrate specificity of these transporters determined by competitor experiments does not necessarily represent actual transport of compounds since these compounds might act as blockers of OPTs. Furthermore, yeast growth assays might be misleading if the substrate is toxic or resistant to metabolic processing. The expression of heterologous transporters in Xenopus oocytes is useful to study membrane transport because it enables a direct and electrophysiological analysis with high sensitivity, and allows the testing of substrates for which radiolabelled tracers are not available. However, electroneutral transport would not be detected in oocyte two-electrode voltage-clamp experiments. The combined use of the oocyte and yeast expression systems could minimize the limitations posed by an individual system.
In the present study, we employed Xenopus laevis oocyte twoelectrode voltage clamping and growth complementation in yeast to understand the transport mechanisms of various AtOPTs. We included ScOPT1 in our analysis because it is the best-characterized OPT from any organism in terms of identified substrates. We show that ScOPT1 is a proton-coupled symporter for GSH, GSSG, the phytochelatin (PC) PC2 and tetra- and penta-peptides such as GGFL, with affinities for the different substrates ranging from 35 μM for PC2 to 211 μM for GGFL. Initially, none of the tested AtOPTs gave reproducible currents in oocytes. We focused on AtOPT4 because radiotracer uptake studies in yeast had identified KLGL as a substrate [5]. We succeeded in optimizing AtOPT4 expression by swapping the 5′-UTR (5′-untranslated region) for that of ScOPT1. With this construct, we demonstrate that AtOPT4 is a proton-coupled high-affinity transporter for oligopeptides including KLGL, a tetrapeptide that is not transported by ScOPT1, but not for GSH. In addition, none of the tested AtOPTs mediated GSH uptake in yeast. The detailed biological function of OPTs, particularly in terms of substrate specificity and kinetics, will improve our understanding of peptide-dependent functions in plants and fungi.
EXPERIMENTAL
Plasmid constructs
AtOPT1, AtOPT3, AtOPT4, AtOPT6 and AtOPT7 cDNAs from Columbia ecotype were subcloned into the yeast expression vector pDB20 [15] and the oocyte expression vector pGEM-HE [16]. Total RNA isolated from Columbia plants was used as a template for reverse transcription–PCR using random hexamer primers and Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA, U.S.A.). First-strand cDNA was amplified with Pfu DNA polymerase (Stratagene, La Jolla, CA, U.S.A.) using gene-specific primers. For AtOPT4, the following primers were used: forward, 5′-TCACACACAAACTAAACCGGAATGG-3′; and reverse, 5′-CCTTTAAGATTATTTAACCGGACAACC-3′. Amplified PCR products were subcloned into the pGEM-T Easy cloning vector (Promega, Madison, WI, U.S.A.) and sequenced to verify the absence of amplification errors.
To facilitate the subcloning of AtOPT cDNAs into both pDB20 and pGEM-HE using flanking NotI restriction sites in pGEM-T Easy, a NotI site was introduced into pGEM-HE by swapping the EcoRI–XbaI fragment with the EcoRI–XbaI multiple cloning fragment of the pCR2.1-TOPO TA cloning vector (Invitrogen). AtOPT3 and AtOPT6 cDNAs were subcloned from pGEM-T Easy into pDB20 or pGEM-HE using flanking EcoRI sites, and AtOPT1, AtOPT4 and AtOPT7 cDNAs using flanking NotI sites.
To swap 5′-UTR sequences in AtOPT4, first-strand cDNA was amplified with Pfu DNA polymerase using AtOPT4-specific primers with engineered BamHI sites at the 5′-end and appropriate 5′-UTR sequences in the forward primer (Kozak::AtOPT4 forward: 5′-GCGCGGATCCACAGCCACCATGGCCACCGCCGACGAATTC-3′; Sc5′UTR::AtOPT4 forward: 5′-GCGCGGATCCTTATATAACGTCACAGAACACATGGCCACCGCCGACGAATTC-3′). The reverse primer (5′-CCCGGATCCGAAAGCACTACCCGCATTCG-3′) was designed downstream of an endogenous BamHI site in AtOPT4. Amplified PCR products were digested with BamHI and then subcloned into the AtOPT4 construct in pGEM-HE using the BamHI sites in the pGEM-HE 5′-multiple cloning site and in AtOPT4. Sequencing confirmed the correct orientation of the 5′-end and the absence of amplification errors.
Yeast strains and growth experiments
For yeast expression, S. cerevisiae strains BY4730 (MATα leu2Δ0 met15Δ0 ura3Δ0) [17], ABC 817 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 hgt1Δ::LEU2) or ABC 822 (MATa ura3-52 leu2-Δ1 lys2-801 his3-Δ200 trp1-Δ63 ade2-101 hgt1Δ::LEU2) [4] were transformed using the lithium acetate method [18]. Transformed cells were selected by growth on 0.17% (w/v) YNB (yeast nitrogen base) plates supplemented with 2% (w/v) glucose lacking uracil. Yeast growth assays in the presence of 100 μM peptide, PC2 or GSH were performed on Difco synthetic dextrose minimal medium (BD, Franklin Lakes, NJ, U.S.A.). Ammonium sulphate was replaced with ammonium chloride for sulphur-free methionine complementation assays [13]. The peptides KLGL, KLLLG and PC2 were kindly provided by Dr Fabio Gallazzi (University of Missouri-Columbia). All other peptides were purchased from American Peptide Co. (Sunnyvale, CA, U.S.A.).
Quantification of intracellular glutathione content in yeast cells
For determination of glutathione contents, yeast cells were grown and harvested as described in [19]. Briefly, S. cerevisiae strain BY4730 transformed with empty vector (pDB20), or with ScOPT1 or AtOPT4 in pDB20, was grown in minimal liquid Difco YNB medium (BD) lacking uracil supplemented with ammonium sulphate and 2% glucose. For GSH uptake assays, cells were inoculated at an A (absorbance) of 0.2 in YNB medium lacking methionine and uracil supplemented with ammonium chloride and 2% glucose, and containing 0 or 0.2 mM GSH. Cells were incubated for 6 h at 30 °C.
After the incubation period, cells were harvested, washed twice with water and resuspended in 0.25 ml of 5% (w/v) sulphosalicylic acid. Cells were homogenized by bead-mixing in a bead mixer (Tomy Seiko, Tokyo, Japan) at 4 °C for a total of 4 min with intermittent pulses. After centrifugation at 15000 g for 5 min, 0.2 ml of the supernatant was used for the quantification of glutathione. Total intracellular glutathione (GSH plus GSSG) contents were determined enzymatically using the DTNB [5,5′-dithiobis-(2-nitrobenzoic acid)]–GSSG reductase recycling assay [20].
Oocyte isolation and two-electrode voltage clamping
For oocyte expression, vectors were linearized with appropriate restriction enzymes, and capped cRNA was transcribed in vitro using the T7 mMessage mMachine RNA transcription kit (Ambion, Austin, TX, U.S.A.). X. laevis frogs were purchased from Xenopus Express (Plant City, FL, U.S.A.) or NASCO (Fort Atkinson, WI, U.S.A.) and kept under the control of a University of Missouri-Columbia Animal Care and Use Protocol (no. 3926). Stage V and VI oocytes were harvested and defolliculated in a Ca2+-free solution (82 mM NaCl, 20 mM MgCl2, 2 mM KCl and 5 mM Hepes, pH 7.4) containing 1 mg/ml collagenase type II A (Sigma, St. Louis, MO, U.S.A.) for 30 min at room temperature (22 °C). Oocytes were injected on the same day with 23 ng of ScOPT1 or AtOPT cRNA using a Nanoject II injector (Drummond Scientific, Broomall, PA, U.S.A.). Oocytes were incubated in ND96 Ringer solution with antibiotics [96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 5 mM Hepes, 2.5 mM sodium pyruvate, 5% (v/v) horse serum, 10 μg/ml sodium penicillin, 10 μg/ml streptomycin sulphate and 50 μg/ml gentamicin sulphate, pH 7.4] at 14 °C.
Oocytes were voltage-clamped 5 or 6 days after cRNA injection in a bath solution containing 90 mM N-methyl-D-glucamine chloride, 1 mM MgCl2, 1.8 mM CaCl2 and 5 mM Mes/Tris (pH 5.0) unless otherwise noted. Voltage clamping was controlled and currents were recorded with a TEV-200A amplifier (Dagan, Minneapolis, MN, U.S.A.) and pCLAMP 6.0 or Axotape 2.0 software (Axon Instruments, Union City, CA, U.S.A.).
RESULTS
Peptide uptake by yeast expressing OPTs
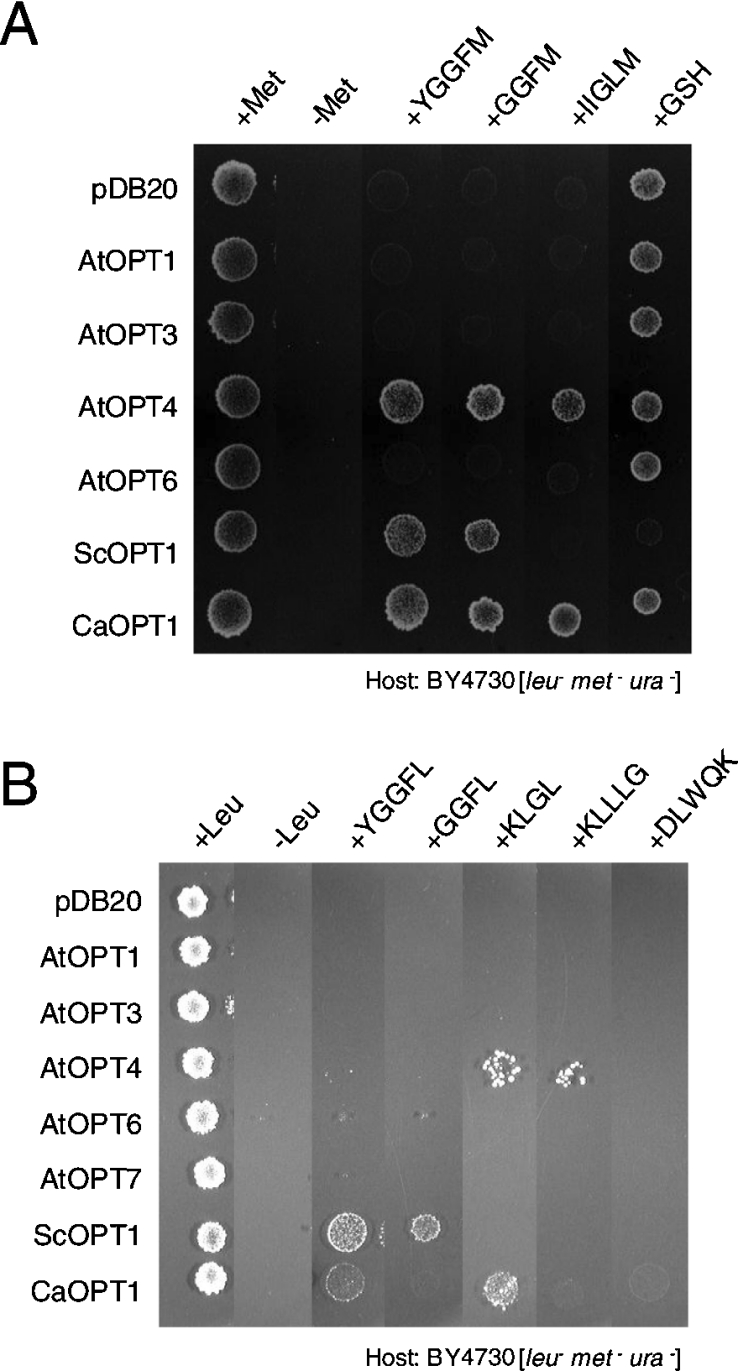
ScOPT1 is one of the few OPTs for which permeable substrates have been identified. A yeast mutant with wild-type low expression of ScOPT1 is unable to grow on a nutrient agar plate supplemented with [Leu]- or [Met]-enkephalins (YGGFL or YGGFM) as a sole source of leucine or methionine respectively. In contrast, yeast that overexpress ScOPT1 driven by the ADH promoter are able to use enkephalins as sole leucine or methionine sources [12]. In addition, GSH uptake is abolished in a ScOPT1 deletion strain [4]. To enlarge the range of possible peptide substrates, we expressed ScOPT1, the Candida albicans CaOPT1 gene and the A. thaliana OPT genes AtOPT1, AtOPT3, AtOPT4, AtOPT6 and AtOPT7 in yeast strain BY4730 [12]. We identified GGFM, YGGFM and IIGLM as new substrates for AtOPT4, and GGFM and GGFL for ScOPT1 (Figures 1A and 1B). Consistent with previous reports, uptake of KLGL was most robust with AtOPT4 among the AtOPTs (Figure 1B) [5].
Figure 1. Rescue of yeast growth with peptides and GSH as sole amino acid sources.
(A) Methionine-containing peptides and GSH were supplied at 100 μM in minimal media lacking sulphur and methionine. (B) Leucine-containing peptides were supplied at 100 μM in minimal media lacking leucine.
Interestingly, overexpression of ScOPT1 in BY4730 led to GSH hypersensitivity (Figure 1A) and to excess accumulation of glutathione in yeast cells (Table 1). This conclusion is supported by previous results showing that excess uptake of GSH by ScOPT1 causes growth inhibition in yeast, which is thought to be the result of toxic effects of an altered cellular redox state [11,21]. None of the other OPT genes, including AtOPT6, affected yeast growth on GSH (Figure 1A), and expression of AtOPT4 did not lead to glutathione accumulation in yeast cells (Table 1). Similar yeast growth results were obtained with the strain ABC 822 (results not shown), which lacks ScOPT1 but is capable of methionine synthesis and was reported to show complementation of GSH uptake by AtOPT6 [11]. However, consistent with this previous report, we observed complementation of GSH uptake by expression of ScOPT1, but not any of the other OPT genes, in the yeast strain ABC 817 (results not shown), which is defective in both GSH transport and methionine synthesis [4,11].
Table 1. Glutathione content of yeast cells expressing ScOPT1 and AtOPT4.
For GSH uptake assays, yeast strain BY4730 transformed with empty vector plasmid pDB20, or with ScOPT1 or AtOPT4 in pDB20, was left untreated (−GSH) or treated with 0.2 mM GSH (+GSH) for 6 h. Total intracellular glutathione (GSH+GSSG) content was determined enzymatically using DTNB as described in the Experimental section. Values represent the means±S.D. for three replicate samples.
| Total glutathione content (nmol/107 cells) | ||
|---|---|---|
| Plasmid | −GSH | +GSH |
| pDB20 | 0.3±0.0 | 2.1±0.1 |
| ScOPT1 | 0.3±0.1 | 36.4±5.3 |
| AtOPT4 | 0.3±0.1 | 2.9±1.0 |
PC is transported by ScOPT1 in yeast
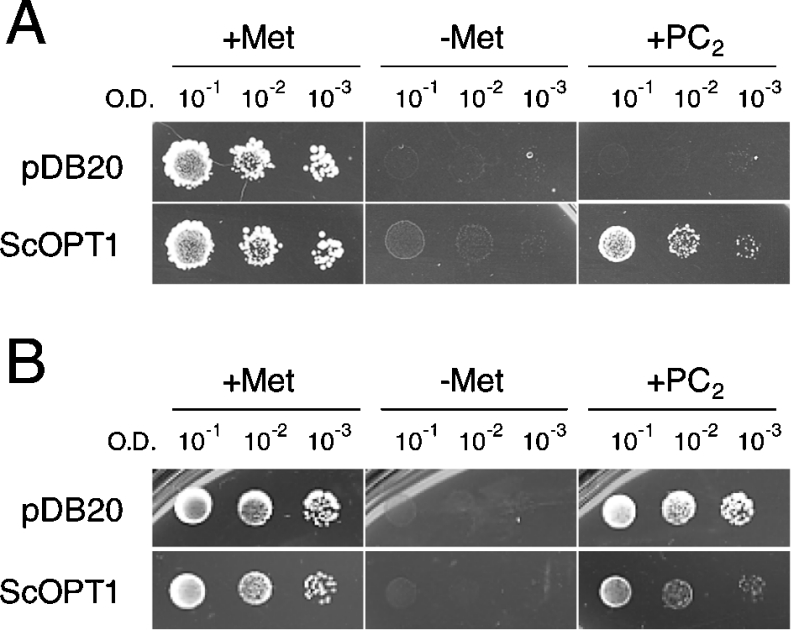
PCs are peptide polymers with the general structure (γ-Glu-Cys)n-Gly, for which GSH serves as a precursor [22–24]. Because of the structural relatedness between GSH and PCs, we tested whether ScOPT1 and AtOPTs are permeable to PCs. Growth of the yeast strain ABC 817 was evaluated on plates supplemented with PC2 [(γ-Glu-Cys)2-Gly], as a sole sulphur source. Addition of 100 μM PC2 in sulphur-free medium improved the growth of ABC 817 carrying ScOPT1 under control of the constitutive ADH promoter (Figure 2A). In contrast, ABC 817 carrying the empty vector failed to grow (Figure 2A), as did ABC 817 cells expressing AtOPT1, AtOPT3, AtOPT4, AtOPT6 or AtOPT7 (results not shown). However, constitutive expression of ScOPT1 driven by the ADH promoter repressed growth in the presence of PC2 (Figure 2B) in the yeast strain BY4730, in which the endogenous ScOPT1 gene is not disrupted [12]. This suggests that, similar to GSH, excess uptake of PC2 by ScOPT1 leads to hypersensitivity. Expression of the AtOPT genes in the BY4730 strain did not lead to PC2 hypersensitivity (results not shown).
Figure 2. PC2 is a substrate for ScOPT1 in yeast.
Yeast cells transformed with empty vector plasmid pDB20 (upper rows) or ScOPT1 in pDB20 (lower rows) were grown on sulphur-free minimal media without (left and middle) or supplemented with 100 μM PC2 (right). A600 (O.D.) of the yeast suspension is given as a measure of the number of cells plated. (A) Growth rescue by PC2 in the yeast strain ABC 817 (met15 opt1). (B) Growth suppression by PC2 in the yeast strain BY4730 (met15 OPT1).
ScOPT1-mediated transport is electrogenic
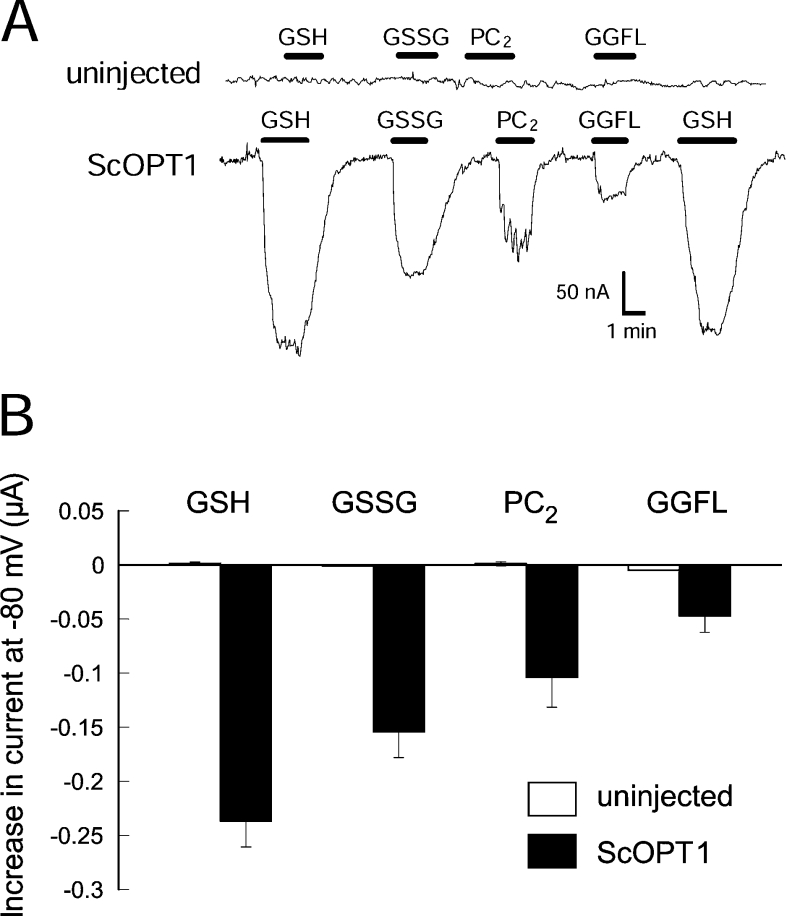
To understand how ScOPT1 transports different substrates, we tested the transport properties of ScOPT1 for GSH, GSSG, PC2 and the tetrapeptide GGFL in Xenopus oocytes with two-electrode voltage clamping. Oocytes injected with ScOPT1 cRNA exhibited an inward current in the presence of GSH in the bath solution at pH 5.0 (Figure 3A). Similarly, the addition of GGFL, GSSG or PC2 in the bath solution induced inward currents, while uninjected oocytes showed no significant current changes in response to these compounds (Figure 3A). Figure 3(B) shows a summary for each substrate from three oocytes from the same batch (oocytes from separate batches showed the same proportional behaviour, but had different absolute values). Therefore ScOPT1-mediated transport of multiple substrates is electrogenic. Since the net charge of several of these substrates is negative, we concluded that ScOPT1 functions as a co-transporter, with transport coupled with a cation.
Figure 3. Electrogenic transport by ScOPT1 expressed in oocytes.
(A) Currents elicited by various substrates applied to uninjected oocytes (top), and oocytes expressing ScOPT1. Presence of the indicated substrate is indicated by a black bar. The membrane potential was held at −80 mV. Substrate concentrations were 500 μM for GGFL, GSSG and PC2 and 100 μM for GSH at pH 5.0. (B) Average currents elicited by the indicated substrates at a membrane potential of −80 mV. Results shown are the means±S.E.M. for three oocytes.
Kinetic properties of ScOPT1
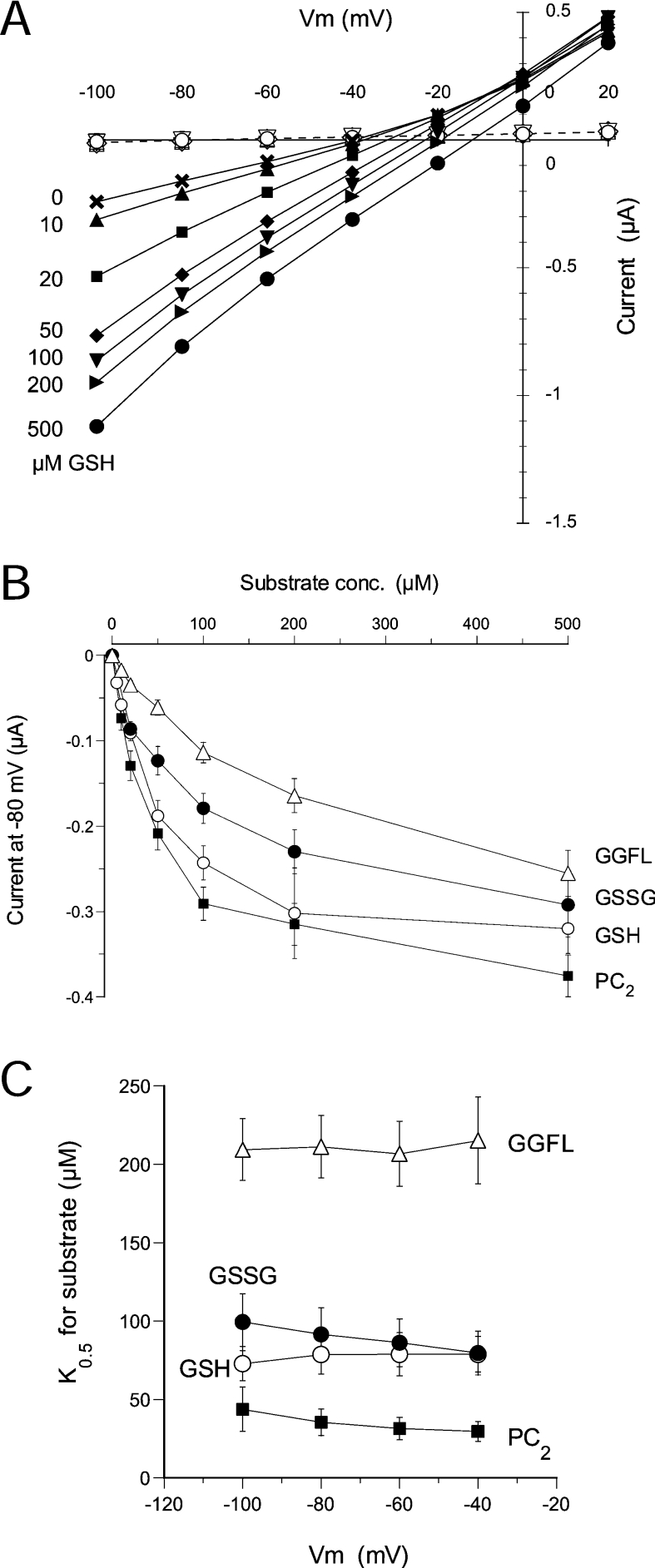
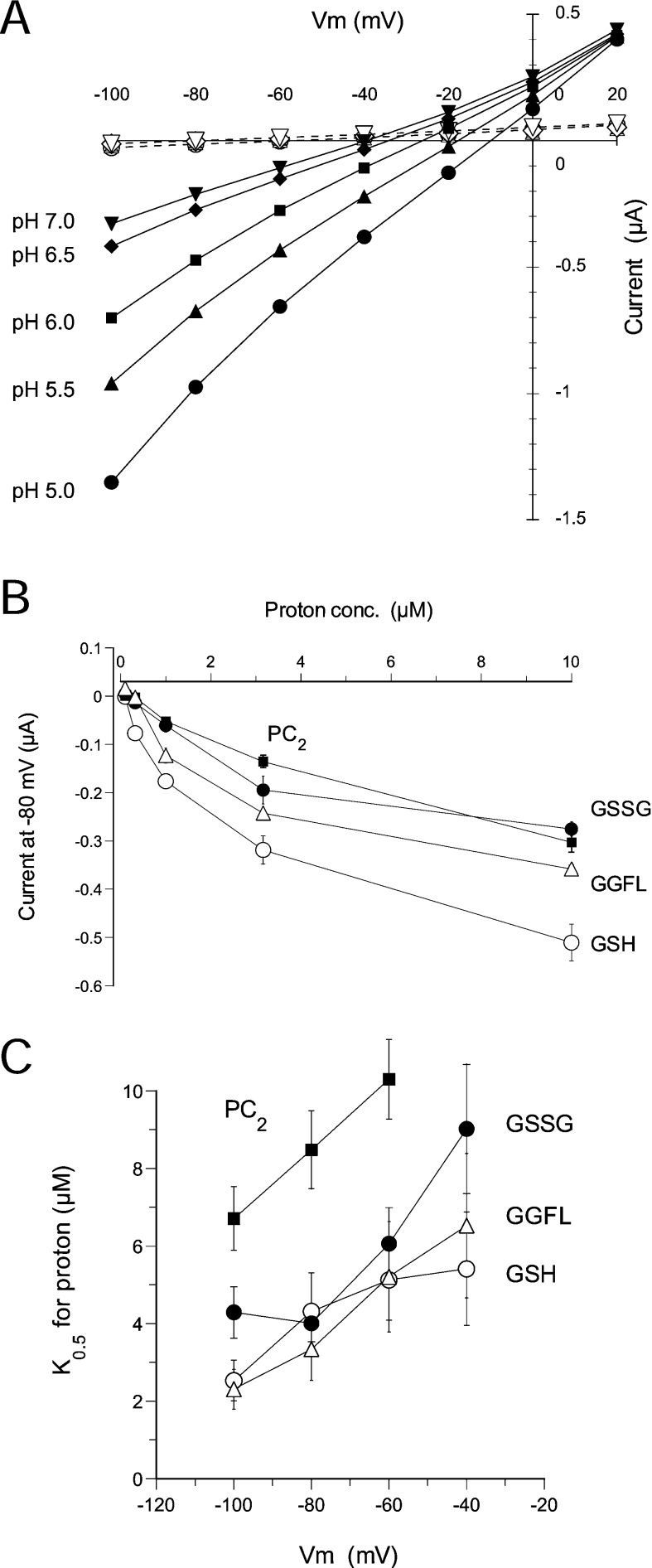
Next we obtained the kinetic properties of ScOPT1 for the four tested substrates. Oocytes expressing ScOPT1 were exposed to each substrate at different concentrations ranging from 0 to 500 μM at pH 5.0, and steady-state currents were measured at membrane voltages in the range −100 to +20 mV with 20 mV increments. Figure 4(A) shows results with GSH. Currents induced by GSH and other substrates were largely linear in the relationship to membrane voltage, indicating that ScOPT1 is not strongly regulated by voltage. In addition, increasing substrate concentrations shifted the reversal potential to more positive values (Figure 4A and results not shown), indicating that these substrates are indeed transported by ScOPT1. ScOPT1-mediated currents evoked by all four substrates were dependent on the substrate concentration and saturable (Figure 4B), with maximal currents (Imax) ranging from 0.24 μA for GGFL to 0.42 μA for GSH (Table 2). The substrate concentration at which current was half-maximal (K0.5) also was similar for these substrates, with highest apparent affinity for PC2 at 35 μM and lowest for GGFL at 211 μM (Table 2). The K0.5 values were not highly voltage-dependent (Figure 4C).
Figure 4. Kinetics of ScOPT1 transport of GSH, GSSG, GGFL and PC2.
(A) Representative current–voltage relationships of ScOPT1 for GSH at indicated concentrations and a constant pH of 5.0. For comparison, currents in uninjected oocytes are shown in the presence of 0–500 μM GSH indicated by the corresponding open symbols. Oocytes were clamped at a membrane potential of −40 mV and pulsed for 120 ms to −100 to +20 mV in +20 mV increments. (B) Concentration dependence of substrate-evoked currents for GSH, GSSG, GGFL and PC2. Steady-state currents at −80 mV were obtained from ScOPT1-expressing oocytes at pH 5.0. (C) Voltage dependence of apparent affinities for GSH, GSSG, GGFL and PC2. In (B, C), results shown are the means±S.E.M. for at least six oocytes.
Table 2. Apparent affinities and maximal currents of ScOPT1 for oligopeptide and oligopeptide-like substrates, and for H+.
K0.5 and Imax values were determined by linearizing substrate concentration/current curves obtained at −80 mV. Values for substrate were obtained at pH 5.0 and those for H+ at 500 μM substrate. Values represent the means±S.E.M. for at least five oocytes.
| Substrate | H+ | |||
|---|---|---|---|---|
| Substrate | K0.5 (μM) | Imax (μA) | K0.5 (μM) | Imax (μA) |
| GSH | 76±13 | 0.42±0.05 | 4.4±1.0 | 0.86±0.10 |
| GGFL | 211±40 | 0.24±0.02 | 3.3±0.8 | 0.49±0.05 |
| GSSG | 92±17 | 0.34±0.04 | 4.0±0.5 | 0.41±0.03 |
| PC2 | 35±9 | 0.36±0.04 | 8.5±1.0 | 0.55±0.07 |
Primary energization of the plasma membrane in both fungi and plants is achieved through the action of a plasma membrane proton pump, which generates both an electrical and a proton gradient across the membrane (negative and high pH within the cell) [25]. Consequently, the vast majority of co-transporters in fungi and plants are proton-coupled. To examine the pH dependence of ScOPT1 currents, steady-state currents were measured in the presence of 500 μM GSH (Figure 5A), GSSG, GGFL or PC2 at pH 5.0, 5.5, 6.0, 6.5 and 7.0. Similar to an increase in substrate, ScOPT1 currents increased with increasing proton concentrations, and reversal potentials were shifted to more positive potentials (Figure 5A and results not shown). Increases in current were largely saturable (Figure 5B). However, this was not as marked as with increasing substrate concentrations (see Figure 4B), especially for PC2. Unlike for substrate, the apparent K0.5 for protons markedly depended on membrane voltage in the presence of all four substrates (Figure 5C), as expected for the binding of a positively charged co-substrate to a transporter. Taken together, these results showed that ScOPT1 is a proton-coupled transporter with broad substrate specificity.
Figure 5. Kinetics of ScOPT1 H+ transport.
(A) Representative current–voltage relationships of ScOPT1 at the indicated pH and a constant GSH concentration of 500 μM. For comparison, currents in uninjected oocytes are shown at the same pH values indicated by the corresponding open symbols in the presence of 500 μM GSH. Oocytes were clamped at a membrane potential of −40 mV and pulsed for 120 ms to −100 to +20 mV in +20 mV increments. (B) Concentration dependence of substrate-evoked currents for H+. Steady-state currents at −80 mV were obtained from ScOPT1-expressing oocytes at 500 μM GSH. (C) Voltage dependence of apparent affinities for H+. In (B, C), results shown are the means±S.E.M. for at least five oocytes.
ScOPT1-mediated proton leak currents
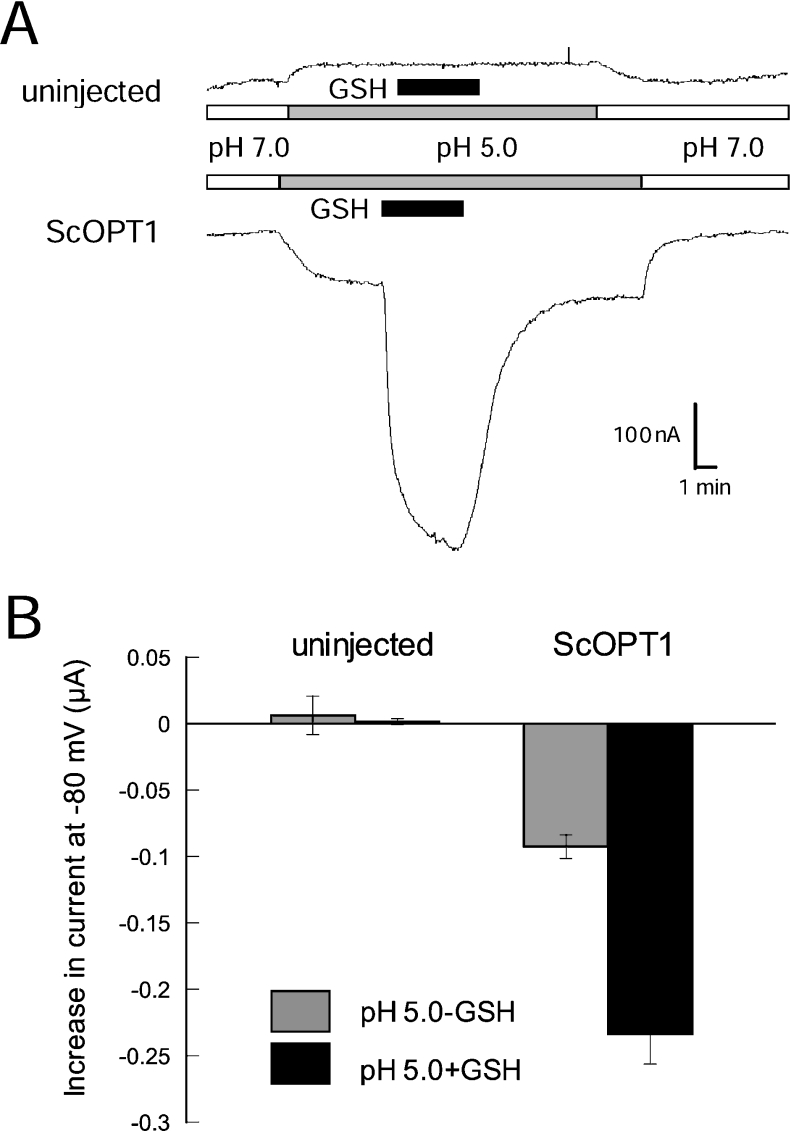
The less pronounced current saturation in response to external pH may be caused by pH-dependent changes in the abundance of a particular ionic form of the transported substrate. In addition, low external pH has been shown to induce inward currents in the absence of co-substrate with several proton-coupled transporters [26–29]. Similar to these transporters, we observed proton leak currents in oocytes expressing ScOPT1 (Figures 6A and 6B). Proton-induced currents were absent from uninjected oocytes (Figures 6A and 6B). We concluded that ScOPT1 shows proton leak currents and therefore loose coupling of substrate with proton transport.
Figure 6. ScOPT1-mediated H+ leak currents in the absence of substrate.
(A) Current responses in uninjected (top) and ScOPT1-expressing oocytes to acidification of the bath medium from pH 7.0 (white bar) to pH 5.0 (grey bar). The presence of 100 μM GSH is indicated by black bars. (B) Average current increases in uninjected and ScOPT1-expressing oocytes in response to acidification (difference between absolute current levels at pH 7.0 and 5.0; grey bars) and to addition of 100 μM GSH at pH 5.0 (difference between absolute current levels at pH 5.0 without GSH and pH 5.0 with GSH; black bars) at −80 mV. Results shown are the means±S.E.M. for at least three oocytes for the uninjected controls and eight oocytes for ScOPT1.
Modified 5′-UTRs improve AtOPT4 expression in oocytes
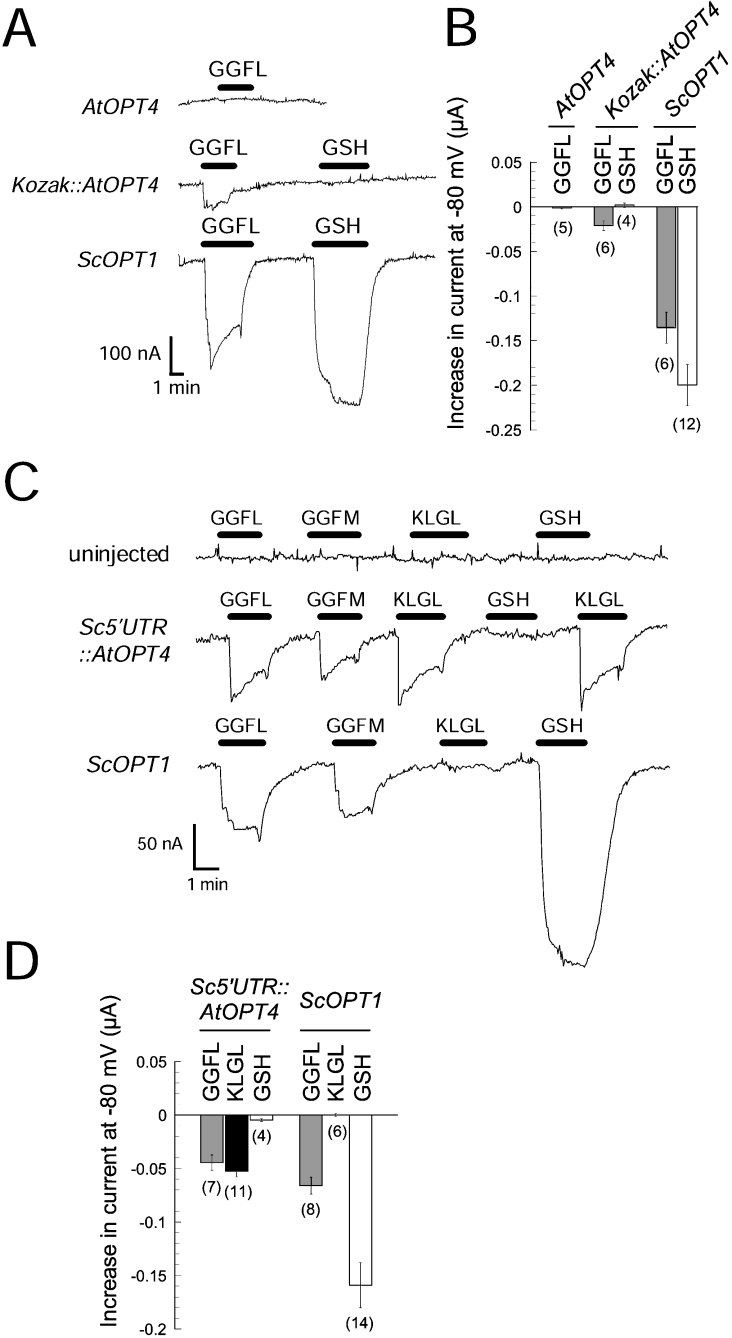
Similar to ScOPT1 (Figure 3A), we tested for activity with oocytes expressing AtOPT1, AtOPT3, AtOPT4, AtOPT6 or AtOPT7. Occasionally, we detected very small but significant peptide-induced currents, but only in AtOPT4-expressing oocytes and only in batches of oocytes that expressed ScOPT1 to unusually high levels (results not shown). Combined with yeast growth results showing KLGL as a substrate for AtOPT4, we concluded that AtOPT4 is probably a functional transporter, but that it is inefficiently expressed in oocytes. The reason could be an unfavourable AtOPT4 5′-UTR structure and low translation efficiency. To test this, the 5′-UTR just upstream of the AUG in AtOPT4 was swapped either with an optimized 6 bp Kozak sequence (Kozak::AtOPT4), which is considered to be optimal for efficient translation in mammalian systems [30], or with the 11 bp 5′-UTR upstream of the AUG in our ScOPT1 construct (Sc5′UTR::AtOPT4) (see the Experimental section). Note that these constructs do not contain alternative upstream start codons and thus do not alter the amino acid sequence of AtOPT4.
Exchange of the 5′-UTR in AtOPT4 with Kozak sequences enabled us to detect AtOPT4-mediated electrogenic GGFL transport (Figure 7A). However, the size of GGFL-evoked inward current in oocytes expressing Kozak::AtOPT4 was still significantly lower than that measured with ScOPT1 (Figures 7A and 7B). Importantly, oocytes expressing Sc5′UTR::AtOPT4 exhibited pronounced inward currents in response to GGFL, GGFM, and KLGL at pH 5.0 (Figure 7C). This result suggested that 5′-UTR sequences immediately upstream of the start codon affect the expression of OPTs in oocytes. Interestingly, although the current magnitudes evoked by GGFL or GGFM were the same for AtOPT4 and ScOPT1, KLGL-evoked currents were only observed with AtOPT4, and GSH-evoked currents only with ScOPT1 (Figure 7C). In addition, we did not observe PC2-evoked currents with AtOPT4 (results not shown). This demonstrated that ScOPT1 and AtOPT4 have overlapping but distinct substrate specificities.
Figure 7. AtOPT4-mediated currents in oocytes injected with AtOPT4 cRNA with modified 5′-UTR.
(A) Current responses in oocytes injected with unmodified AtOPT4 cRNA (top), AtOPT4 cRNA with modified Kozak sequence (Kozak::AtOPT4; middle) or ScOPT1 cRNA (bottom). Substrate (GGFL or GSH) was supplied at 500 μM for GGFL or 100 μM for GSH at times indicated by a black bar at pH 5.0. The membrane holding potential was −80 mV. (B) Average current responses in oocytes injected with cRNA used in (A) at a membrane potential of −80 mV. Results shown are the means±S.E.M., with the number of oocytes indicated in parentheses. (C) Increased expression of AtOPT4-mediated currents in an oocyte injected with AtOPT4 cRNA carrying the 5′-UTR from ScOPT1 (Sc5′UTR::AtOPT4; middle). An uninjected oocyte (top) and an oocyte expressing ScOPT1 (bottom) are shown for comparison. Substrates were applied at times indicated by black bars at a concentration of 500 μM for GGFL, GGFM and KLGL and 100 μM for GSH, at pH 5.0. (D) Average currents in oocytes injected with Sc5′UTR::AtOPT4 or ScOPT1 cRNA. Results shown are the means±S.E.M., with the number of oocytes indicated in parentheses.
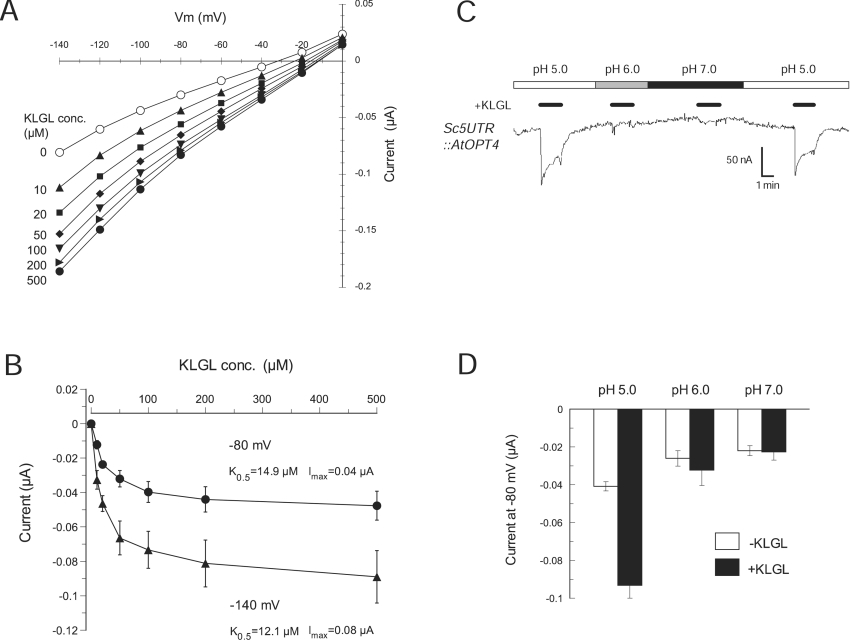
We further examined the transport properties of AtOPT4 using the Sc5′-UTR::AtOPT4 construct and KLGL as a representative oligopeptide substrate. Similar to ScOPT1, current–voltage curves for AtOPT4 were almost linear and shifted to more positive potential with increasing substrate (Figure 8A). The apparent K0.5 of AtOPT4 for KLGL was 15 μM at −80 mV (Figure 8B), which was significantly lower than the K0.5 values of ScOPT1 for GGFL or even GSH and PC2 (Table 2). These results suggested that AtOPT4 is a high-affinity OPT. To check the pH dependence of AtOPT4 currents, oocytes expressing Sc5′UTR::AtOPT4 were exposed to KLGL at pH 5.0, 6.0 and 7.0. AtOPT4-mediated KLGL transport was detectable at pH 5.0, but not at pH 6.0 or 7.0 (Figures 8C and 8D). As with ScOPT1, we observed activation of proton currents in the absence of co-substrate (Figure 8C). Together, these results showed that AtOPT4 is a proton-coupled OPT, whereas ScOPT1 may mainly function as a transporter of GSH or GSH-derived compounds [4,21].
Figure 8. Kinetics of AtOPT4-mediated oligopeptide transport in oocytes.
(A) Representative current–voltage relationships of AtOPT4 for KLGL at indicated concentrations and a constant pH of 5.0. Oocytes were clamped at a membrane potential of −40 mV and pulsed for 120 ms to −140 to 0 mV in +20 mV increments. (B) Concentration dependence of substrate-evoked currents for KLGL. Steady-state currents at −80 and −140 mV were obtained from AtOPT4-expressing oocytes at pH 5.0. (C) pH dependence of AtOPT4-mediated KLGL currents. Oocytes expressing AtOPT4 were exposed to bath media of pH 5.0 (white bar), pH 6.0 (grey bar) and pH 7.0 (black bar). KLGL (500 μM) was present at times indicated by small black bars. The membrane potential was held at −80 mV. (D) Average current values in response to pH (white bars) and KLGL (black bars) measured at −80 mV from oocytes expressing AtOPT4. In (B, D), results shown are the means±S.E.M. for three oocytes.
DISCUSSION
To gain a better understanding of OPT functions, we examined the transport properties and substrate specificities of yeast and Arabidopsis OPTs by functional expression and two-electrode voltage clamping in Xenopus oocytes. Heterologous expression in oocytes demonstrated that ScOPT1 electrogenically transports various oligopeptides and their derivatives composed of three (GSH) to six (GSSG) amino acids. Likewise, AtOPT4-mediated transport of the tetrapeptides GGFM, GGFL and KLGL was electrogenic, but we found no evidence for GSH uptake by AtOPT4 in yeast or oocytes. This indicates that AtOPT4 has a characteristic substrate specificity for oligopeptides that only partially overlaps with that of ScOPT1. The nine AtOPTs are expected to have distinct but overlapping functions, since each of these genes is expressed preferentially in vascular tissues but differentially in various organs at different developmental stages [10]. This and our results suggest that AtOPTs mainly function in peptide signalling or nitrogen mobilization during various developmental stages in different tissues.
AtOPT4 displays a transport mechanism and kinetics that are similar to those of ScOPT1 in terms of proton coupling and dependence on substrate concentration and voltage. The present study is the first demonstration that transport of oligopeptide-containing substrates by transporters belonging to clusters 4 and 5 within the larger OPT family [6] is electrogenic and proton-coupled. Recent electrophysiological studies showed that Zea mays YS1 (ZmYS1), which belongs to the more distantly related cluster 2 of the OPT family, functions as an electrogenic proton-coupled iron-phytosiderophore transporter [8]. Together, these results suggest that perhaps all members of the OPT family are proton-coupled transporters of peptides and peptide-like substrates, but that the exact substrate spectrum is difficult to predict. It is a common experience that some transporters are not functionally expressed in Xenopus oocytes, and the reasons are often unknown. We achieved expression of AtOPT4 by modifying the 5′-UTR, either by modifying the Kozak sequence or swapping the native 5′-UTR with that of ScOPT1. Neither the native nor the altered 5′-UTR sequences contained alternative upstream start codons. Thus altering the 5′-UTR did not change the amino acid sequence of AtOPT4.
Although radiolabelled substrates such as GSH [4] or YGGFL [12] were known to be transported via ScOPT1 in yeast, the precise transport kinetics and substrate specificity of ScOPT1 were less clear. In the present study, we show that ScOPT1 transports several substrates electrogenically irrespective of the net charge of the substrate. The pH dependence of currents and positive shifts of reversal potentials with higher extracellular proton concentrations show that protons are co-transported with substrates in stoichiometries that lead to a net positive charge per transport cycle irrespective of the negative charges of the substrates. The kinetics of electrogenic transport of GSH, GGFL, GSSG and PC2 by ScOPT1 in oocytes imply that ScOPT1 functions as a proton-coupled transporter with varying coupling ratios of proton to substrate, depending on the net charge of the substrate. This is consistent with findings from other proton-coupled transporters from animals and plants [26,28,31,32]. Interestingly, results with these transporters suggest that, in the case of anionic dipeptide substrates, the zwitterionic form may be transported. If also true for ScOPT1, this would explain our finding that ScOPT1 currents do not saturate completely with increasing proton concentrations. Increasing proton concentrations would concomitantly increase the concentration of zwitterionic substrates. Determination of a coupling ratio is also complicated by the presence of proton leak currents, suggesting transport slippage. This has also been found with other proton-coupled transporters from animals and plants [26–29].
Despite having a very broad substrate specificity, ScOPT1 did not transport the tetrapeptide KLGL, showing that ScOPT1 retains a level of substrate specificity among oligopeptides. Given that the net charge of KLGL is +1 at pH 5.0, it is very unlikely that we did not detect KLGL transport because transport is electroneutral. In addition, ScOPT1 did not rescue yeast growth of a leucine auxotroph on KLGL. The kinetics of ScOPT1 transport of different substrates in the present study revealed that the apparent affinity and maximal current of ScOPT1 are significantly higher for GSH than for the tetrapeptide GGFL, supporting the hypothesis that the primary function of ScOPT1 is high-affinity GSH transport [4].
Surprisingly, we found that ScOPT1 mediates the uptake of PC2 in yeast and in oocytes, with highest apparent affinity for PC2 among the four substrates tested here. PCs, a major class of phytosiderophores, form complexes with toxic metals such as Cd2+ and detoxify them [33,34]. Although PC synthase genes have been found in the fission yeast Schizosaccharomyces pombe and in nematodes, S. cerevisiae is an organism that lacks a PC synthase gene [22–24]. This novel identification of PC2 as a substrate for ScOPT1 could be useful for identifying PC transporters in plants. The transport of PCs by OPTs may reflect the relatedness of OPT cluster 4 to the more distantly related YS cluster 2 that contains Zea mays YS1 (ZmYS1) [7] and Arabidopsis YSL2 [35]. These proteins function as transporters of the peptide-like phytosiderophores mugineic acid and/or nicotianamine complexed with metals [8]. Interestingly, ZmYS1 did not transport the phytosiderophore 2′-deoxymugineic acid alone, whereas ScOPT1-mediated PC2 currents were observed in the absence of heavy metals.
Our screening of oligopeptides for growth improvement of auxotrophic yeast strains has identified the peptides GGFM and GGFL as new substrates for ScOPT1, and GGFM, YGGFM and IIGLM for AtOPT4. However, transport activity deduced from yeast growth with oligopeptides and their derivatives as amino acid sources needs to be interpreted with caution. The lack of GGFL-mediated growth enhancement of yeast expressing AtOPT4 does not correspond to our finding in oocytes that AtOPT4 mediates GGFL transport comparable with that of KLGL. Rescue of yeast growth on KLGL was partial, and GGFL contains half the amount of leucine per substrate molecule. Therefore poor expression of AtOPT4 in yeast and perhaps a more stringent requirement for leucine in leucine auxotrophs than for methionine in methionine auxotrophs may prevent complementation in yeast in some cases.
On the other hand, overexpression of ScOPT1 inhibits yeast growth on plates containing GSH, GSSG and PC2. Previous studies suggest that expression of ScOPT1 may be up-regulated at the post-translational level by sulphur-limited conditions irrespective of the promoter used for constitutive expression [4]. In the present study, however, growth reduction of yeast induced by overexpressed ScOPT1 was detected even in the presence of ammonium sulphate in minimal media (results not shown), consistent with a more recent report [21]. Our findings suggest that yeast growth assays can be misleading when substrate uptake is insufficient, or high and toxic. Recent studies using heterologous expression of plant OPTs in a yeast ScOPT1 deletion mutant identified several possible GSH transporters, including Brassica juncea BjGT1 [14], O. sativa OsGT1 [13] and AtOPT6 [11]. However, in the present study, we were unable to obtain any supportive evidence that AtOPT6 transports GSH in yeast even under sulphur-limited conditions, consistent with a lack of radiolabelled GSH uptake in yeast expressing AtOPTs [5]. This indicates that AtOPT6-mediated GSH transport is not readily reproducible and/or detectable in heterologous expression systems, possibly because of differences in expression vectors or environmental conditions.
In conclusion, our kinetic analyses of OPTs using heterologous expression systems demonstrate that ScOPT1 and AtOPT4 are proton-coupled OPTs with different substrate specificities and affinities. Defining the structural basis for AtOPT function by generating chimaeric OPTs with altered substrate specificities could provide further information on the domains that determine the function of this poorly understood group of transporters.
Acknowledgments
We thank Dr George Kracke (University of Missouri, Columbia) for supplying Xenopus oocytes, Dr Fabio Gallazzi for the synthesis of KLGL, KLLLG and PC2, Dr A. Bourbouloux (University of Poitiers, Poitiers, France) and Dr S. Delrot (University of Poitiers) for yeast strains ABC817 and ABC822 and Sharon Pike (University of Missouri, Columbia) for a critical reading of this paper. This research was supported in part by a University of Missouri-Columbia Life Sciences Postdoctoral Fellowship to H. O. and by National Science Foundation grant MCB-0235286 to G. S. and W. G.
References
- 1.Stacey G., Koh S., Granger C., Becker J. M. Peptide transport in plants. Trends Plant Sci. 2002;7:257–263. doi: 10.1016/s1360-1385(02)02249-5. [DOI] [PubMed] [Google Scholar]
- 2.Lubkowitz M. A., Hauser L., Breslav M., Naider F., Becker J. M. An oligopeptide transport gene from Candida albicans. Microbiology. 1997;143:387–396. doi: 10.1099/00221287-143-2-387. [DOI] [PubMed] [Google Scholar]
- 3.Lubkowitz M. A., Barnes D., Breslav M., Burchfield A., Naider F., Becker J. M. Schizosaccharomyces pombe isp4 encodes a transporter representing a novel family of oligopeptide transporters. Mol. Microbiol. 1998;28:729–741. doi: 10.1046/j.1365-2958.1998.00827.x. [DOI] [PubMed] [Google Scholar]
- 4.Bourbouloux A., Shahi P., Chakladar A., Delrot S., Bachhawat A. K. Hgt1p, a high affinity glutathione transporter from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 2000;275:13259–13265. doi: 10.1074/jbc.275.18.13259. [DOI] [PubMed] [Google Scholar]
- 5.Koh S., Wiles A. M., Sharp J. S., Naider F. R., Becker J. M., Stacey G. An oligopeptide transporter gene family in Arabidopsis. Plant Physiol. 2002;128:21–29. [PMC free article] [PubMed] [Google Scholar]
- 6.Yen M. R., Tseng Y. H., Saier M. H., Jr Maize Yellow Stripe1, an iron-phytosiderophore uptake transporter, is a member of the oligopeptide transporter (OPT) family. Microbiology. 2001;147:2881–2883. doi: 10.1099/00221287-147-11-2881. [DOI] [PubMed] [Google Scholar]
- 7.Curie C., Panaviene Z., Loulergue C., Dellaporta S. L., Briat J. F., Walker E. L. Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature (London) 2001;409:346–349. doi: 10.1038/35053080. [DOI] [PubMed] [Google Scholar]
- 8.Schaaf G., Ludewig U., Erenoglu B. E., Mori S., Kitahara T., von Wiren N. ZmYS1 functions as a proton-coupled symporter for phytosiderophore- and nicotianamine-chelated metals. J. Biol. Chem. 2004;279:9091–9096. doi: 10.1074/jbc.M311799200. [DOI] [PubMed] [Google Scholar]
- 9.Stacey M. G., Koh S., Becker J., Stacey G. AtOPT3, a member of the oligopeptide transporter family, is essential for embryo development in Arabidopsis. Plant Cell. 2002;14:2799–2811. doi: 10.1105/tpc.005629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stacey M. G., Osawa H., Gassmann W., Stacey G. Expression analyses of Arabidopsis oligopeptide transporters during seed germination, vegetative growth and reproduction. Planta. 2005 doi: 10.1007/s00425-005-0087-x. 10.1007/s00425-005-0087-x. [DOI] [PubMed]
- 11.Cagnac O., Bourbouloux A., Chakrabarty D., Zhang M. Y., Delrot S. AtOPT6 transports glutathione derivatives and is induced by primisulfuron. Plant Physiol. 2004;135:1378–1387. doi: 10.1104/pp.104.039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauser M., Donhardt A. M., Barnes D., Naider F., Becker J. M. Enkephalins are transported by a novel eukaryotic peptide uptake system. J. Biol. Chem. 2000;275:3037–3041. doi: 10.1074/jbc.275.5.3037. [DOI] [PubMed] [Google Scholar]
- 13.Zhang M. Y., Bourbouloux A., Cagnac O., Srikanth C. V., Rentsch D., Bachhawat A. K., Delrot S. A novel family of transporters mediating the transport of glutathione derivatives in plants. Plant Physiol. 2004;134:482–491. doi: 10.1104/pp.103.030940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogs J., Bourbouloux A., Cagnac O., Wachter A., Rausch T., Delrot S. Functional characterization and expression analysis of a glutathione transporter, BjGT1, from Brassica juncea: evidence for regulation by heavy metal exposure. Plant Cell Environ. 2003;26:1703–1711. [Google Scholar]
- 15.Becker D. M., Fikes J. D., Guarente L. A cDNA encoding a human CCAAT-binding protein cloned by functional complementation in yeast. Proc. Natl. Acad. Sci. U.S.A. 1991;88:1968–1972. doi: 10.1073/pnas.88.5.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liman E., Tytgat J., Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992;9:861–878. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- 17.Brachmann C. B., Davies A., Cost G. J., Caputo E., Li J. C., Hieter P., Boeke J. D. Designer deletion strains derived from Saccharomyces cerevisiae S288c: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14:115–132. doi: 10.1002/(SICI)1097-0061(19980130)14:2<115::AID-YEA204>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 18.Kaiser C., Michaelis S., Mitchell A. Plainview: Cold Spring Harbor Press; 1994. Methods in Yeast Genetics: a Laboratory Manual. [Google Scholar]
- 19.Chaudhuri B., Ingavale S., Bachhawat A. K. apd1+, a gene required for red pigment formation in ade6 mutants of Schizosaccharomyces pombe, encodes an enzyme required for glutathione biosynthesis: a role for glutathione and a glutathione-conjugate pump. Genetics. 1997;145:75–83. doi: 10.1093/genetics/145.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson M. E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- 21.Srikanth C. V., Vats P., Bourbouloux A., Delrot S., Bachhawat A. K. Multiple cis-regulatory elements and the yeast sulphur regulatory network are required for the regulation of the yeast glutathione transporter, Hgt1p. Curr. Genet. 2005;47:345–358. doi: 10.1007/s00294-005-0571-7. [DOI] [PubMed] [Google Scholar]
- 22.Clemens S., Kim E. J., Neumann D., Schroeder J. I. Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. EMBO J. 1999;18:3325–3333. doi: 10.1093/emboj/18.12.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ha S. B., Smith A. P., Howden R., Dietrich W. M., Bugg S., O'Connell M. J., Goldsbrough P. B., Cobbett C. S. Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell. 1999;11:1153–1164. doi: 10.1105/tpc.11.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vatamaniuk O. K., Mari S., Lu Y. P., Rea P. A. AtPCS1, a phytochelatin synthase from Arabidopsis: isolation and in vitro reconstitution. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7110–7115. doi: 10.1073/pnas.96.12.7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sze H., Li X., Palmgren M. G. Energization of plant cell membranes by H+-pumping ATPases: regulation and biosynthesis. Plant Cell. 1999;11:677–690. doi: 10.1105/tpc.11.4.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boorer K. J., Frommer W. B., Bush D. R., Kreman M., Loo D. D., Wright E. M. Kinetics and specificity of a H+/amino acid transporter from Arabidopsis thaliana. J. Biol. Chem. 1996;271:2213–2220. doi: 10.1074/jbc.271.4.2213. [DOI] [PubMed] [Google Scholar]
- 27.Chen X. Z., Zhu T., Smith D. E., Hediger M. A. Stoichiometry and kinetics of the high-affinity H+-coupled peptide transporter PepT2. J. Biol. Chem. 1999;274:2773–2779. doi: 10.1074/jbc.274.5.2773. [DOI] [PubMed] [Google Scholar]
- 28.Fei Y. J., Romero M. F., Krause M., Liu J. C., Huang W., Ganapathy V., Leibach F. H. A novel H+-coupled oligopeptide transporter (OPT3) from Caenorhabditis elegans with a predominant function as a H+ channel and an exclusive expression in neurons. J. Biol. Chem. 2000;275:9563–9571. doi: 10.1074/jbc.275.13.9563. [DOI] [PubMed] [Google Scholar]
- 29.Gunshin H., Mackenzie B., Berger U. V., Gunshin Y., Romero M. F., Boron W. F., Nussberger S., Gollan J. L., Hediger M. A. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature (London) 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 30.Kozak M. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J. Mol. Biol. 1987;196:947–950. doi: 10.1016/0022-2836(87)90418-9. [DOI] [PubMed] [Google Scholar]
- 31.Kottra G., Stamfort A., Daniel H. PEPT1 as a paradigm for membrane carriers that mediate electrogenic bidirectional transport of anionic, cationic, and neutral substrates. J. Biol. Chem. 2002;277:32683–32691. doi: 10.1074/jbc.M204192200. [DOI] [PubMed] [Google Scholar]
- 32.Boorer K. J., Fischer W. N. Specificity and stoichiometry of the Arabidopsis H+/amino acid transporter AAP5. J. Biol. Chem. 1997;272:13040–13046. doi: 10.1074/jbc.272.20.13040. [DOI] [PubMed] [Google Scholar]
- 33.Zenk M. H. Heavy metal detoxification in higher plants – a review. Gene. 1996;179:21–30. doi: 10.1016/s0378-1119(96)00422-2. [DOI] [PubMed] [Google Scholar]
- 34.Cobbett C. S. Phytochelatin biosynthesis and function in heavy-metal detoxification. Curr. Opin. Plant Biol. 2000;3:211–216. [PubMed] [Google Scholar]
- 35.DiDonato R. J., Jr, Roberts L. A., Sanderson T., Eisley R. B., Walker E. L. Arabidopsis Yellow Stripe-Like2 (YSL2): a metal-regulated gene encoding a plasma membrane transporter of nicotianamine-metal complexes. Plant J. 2004;39:403–414. doi: 10.1111/j.1365-313X.2004.02128.x. [DOI] [PubMed] [Google Scholar]