Abstract
Preliminary studies indicated that the potent insecticidal lectin, Gleheda, from the leaves of Glechoma hederacea (ground ivy) preferentially agglutinates human erythrocytes carrying the Tn (GalNAcα1-Ser/Thr) antigen. However, no details have been reported yet with respect to the fine specificity of the lectin. To corroborate the molecular basis of the insecticidal activity and physiological function of Gleheda, it is necessary to identify the recognition factors that are involved in the Gleheda–glycotope interaction. In the present study, the requirement of high-density multivalent carbohydrate structural units for Gleheda binding and a fine-affinity profile were evaluated using ELLSA (enzyme-linked lectinosorbent assay) with our extended glycan/ligand collections, a glycan array and molecular modelling. From the results, we concluded that a high-density of exposed multivalent Tn-containing glycoproteins (natural armadillo and asialo ovine salivary glycoproteins) were the most potent factors for Gleheda binding. They were, on a nanogram basis, 6.5×105, 1.5×104 and 3.1×103 times more active than univalent Gal (galactose), GalNAc (N-acetylgalactosamine) and Tn respectively. Among mono- and oligo-saccharides examined, simple clustered Tn (molecular mass <3000 Da) from ovine salivary glycoprotein was the best, being 37.5 and 1.7×103 times better than GalNAc and Gal respectively. GalNAc glycosides were significantly more active than Gal glycosides, indicating that the N-acetamido group at C-2 plays an important role in Gleheda binding. The results of glycan array support the conclusions drawn with respect to the specificity of Gleheda based on the ELLSA assays. These findings combined with the results of the molecular modelling and docking indicate the occurrence of a primary GalNAcα1-binding site in the Gleheda monomer. However, the extraordinary binding feature of Gleheda for glycoproteins demonstrates the importance of affinity enhancement by high-density multivalent glycotopes in the ligand–lectin interactions in biological processes.
Keywords: carbohydrate specificity, combining site, Gleheda, glycoprotein binding, lectin, multivalency
Abbreviations: ABA, Agaricus bisporus agglutinin; Ara, arabinose; BSM, bovine submandibular glycoprotein; ELLSA, enzyme-linked lectinosorbent assay; Fuc, fucose; Gal, galactose; GalN, galactosamine; GalNAc, N-acetylgalactosamine; Glc, glucose; GlcNAc, N-acetylglucosamine; Gleheda, Glechoma hederacea lectin; gp, glycoprotein; HSM, hamster submaxillary mucin; native ASG-Tn, native armadillo salivary gland Tn (GalNAcα1-Ser/Thr) gp; OSM, ovine submandibular gp; PSM, porcine salivary mucin; S/N, signal to noise ratio; SSL, Salvia sclarea lectin; TBS, Tris-buffered saline; VVLB4, Vicia villosa isolectin B4. The mammalian carbohydrate structural units in glycans used to define binding properties of Gleheda are: A, GalNAcα1-3Gal, human blood group A-specific disaccharide; Ah, GalNAcα1-3(L-Fucα1-2)Gal, human blood group A-specific trisaccharide containing crypto H determinant; AL, GalNAcα1-3Galβ1-4Glc; B, Galα1-3Gal, human blood group B-specific disaccharide; E, Galα1-4Gal; F, GalNAcα1-3GalNAc; H, L-Fucα1-2Gal, human blood group H-specific disaccharide; I, Galβ1-3GlcNAc, human blood group type I precursor sequence; II, Galβ1-4GlcNAc, human blood group type II precursor sequence; L, Galβ1-4Glc; P, GalNAcβ1-3Gal; S, GalNAcβ1-4Gal; T, Thomsen–Friedenreich disaccharide Galβ1-3GalNAc; Tα, Galβ1-3GalNAcα1-; Tn, GalNAcα1-Ser/Thr
INTRODUCTION
An agglutinin with a potent insecticidal activity towards the larvae of the Colorado beetle (Leptinotarsa decemlineata) was isolated from the leaves of Glechoma hederacea (ground ivy). The lectin, called Gleheda, exhibited a marked specificity towards polyagglutinable human erythrocytes carrying the Tn (GalNAcα1-Ser/Thr) antigen [1,2]. Biochemical analyses and molecular cloning revealed that Gleheda is a tetrameric protein composed of four subunits linked pairwise through an interchain disulphide bridge and is structurally and evolutionarily related to the legume lectins.
Tn is one of the terminal α-anomeric GalNAc (N-acetylgalactosamine) residues (GalNAcα1-) present in three glycotopes (the carbohydrate determinants of Tn, human blood group A and Forssman antigens). The Tn determinant, normally cryptic in mucin-type O-glycans, is one of the most specific human tumour markers [3–5]. Expressed early in transformed cells during carcinogenesis, the density of the Tn antigen is correlated directly to carcinoma aggressiveness [6]. The Forssman antigen (GalNAcα1-3GalNAcβ1-3Galα1-4Galβ1-4Glcβ1-1′ceramide) commonly occurs as a heterophile antigen and is thought to be absent from most humans. However, it has been demonstrated that the Forssman antigen is definitely present in several forms of human cancer, including gastric, colon and lung cancers [7–11]. This antigen is one of the tumour-associated glycolipid antigens with blood group A-like epitopes. Since the terminus of the antigen shares a sugar residue, GalNAcα1-, with the blood group A terminal saccharide as well as Tn antigen, the unusual enhancement of activity of the blood group A-like antigen has been associated with carcinogenesis. Consequently, an assay for this antigen in tissue sections and in circulating plasma would be of great value to detect colon cancer [12]. Several plant lectins [13–15], monoclonal antibodies [16,17], glycosyltransferases [18] and human macrophage lectins [19] have been reported to bind to the Tn antigen and are proposed as potential detection probes.
In earlier papers, studies of the carbohydrate specificity of Gleheda were limited to the inhibition of monosaccharides and a few GalNAcα1-related oligosaccharides [1,2]. Further characterization with natural multivalent glycotopes in macromolecules and mammalian disaccharide structural units may provide a better understanding of the carbohydrate recognition factors of Gleheda. Thus, in the present study, we systematically analysed the glycan affinity of Gleheda at macromolecular level by ELLSA (enzyme-linked lectinosorbent assay) and our developed inhibition assay [20,21] using our collection of glycans. In a parallel approach, the fine specificity of Gleheda was assessed using the glycan array available through the Consortium for Functional Glycomics (http://www.functionalglycomics.org/static/consortium/) and by molecular docking. From the results obtained, we conclude that: (i) a high-density of exposed Tn is the most potent recognition factor for binding; (ii) cryptoforms of Tn and human blood group precursor equivalent gps (glycoproteins) which have I/II (Galβ1-3/4GlcNAc) sequences as major glycotopes and some Tn and T (Galβ1-3GalNAc) glycotopes may also be active ligands; (iii) the configuration of Gal (galactose) is most essential and the cluster form of Gal with -NHCH3CO at C-2 (high-density Tn cluster) enhanced the reactivities by more than 6.5×105 times over univalent Gal; (iv) the combining site of Gleheda is of a cavity type with a primary GalNAcα1-binding site; (v) substitution of either an N-acetyl group or a fucosyl group for the hydroxy group at the C-2 position of the penultimate galactose reduces the lectin-binding reactivity. The results of glycan array support the conclusions drawn with respect to the specificity of Gleheda from the ELLSA assays. This distinct binding feature of Gleheda for gps establishes a valuable concept of affinity enhancement by high-density multivalencies of glycotopes that is relevant to ligand–lectin recognition in biological processes.
EXPERIMENTAL
Lectin preparation and labelling
Gleheda was purified from Glechoma hederacea leaves by affinity chromatography as described previously [1]. For biotinylation by biotinamidocaproate-N-hydroxysuccinimide ester (biotin ester; Sigma Chemical Co.), the lectin [200 μg/250 μl of PBS (0.14 M NaCl, 0.027 M KCl, 0.081 M Na2HPO4 and 0.0014 M KH2PO4, pH 7.3)] was mixed with 400 μl of the biotin ester solution (100 μg of biotin ester per 200 μg of lectin) and left for 30 min at room temperature (20 °C). The biotinylated lectin was dialysed for 2–3 h against double-distilled water and overnight against TBS (Tris-buffered saline; 0.05 M Tris/HCl and 0.15 M NaCl, pH 7.35). After dialysis, the sample volume was adjusted to 1 ml with TBS, and 20 μl of 5% sodium azide was added (yielding a 200 μg/ml solution of Gleheda in 0.1% sodium azide) [20,21].
For the glycan array screening experiments, the lectin was labelled at a concentration of 2 mg/ml with an Alexa Fluor® 488 Protein Labeling kit (Molecular Probes) according to the manufacturer's protocol. The labelled lectin was applied to a PD-10 column (Sephadex G-25, Amersham Biosciences) and separated from the free label. Protein concentration was determined by Lowry determination and Alexa Fluor® 488 labelling efficiency was determined at an excitation wavelength of 485 nm and an emission wavelength of 535 nm. The Gleheda–Alexa Fluor® 488 was applied to the Consortium streptavidin/biotin array for binding specificity determination. Gps and polysaccharides used for interaction and inhibition studies are described in the Supplementary Material at http://www.BiochemJ.org/bj/393/bj3930331add.htm.
Sugars used for inhibition studies
Monosaccharides, their derivatives and oligosaccharides were purchased from or prepared by Dextra and Sigma. The Tn clusters used for the present study were mixtures of Tn-containing glycopeptides from OSM (ovine submandibular gp) in the filterable fraction (molecular mass <3000 Da) [22]. The lectinochemical assay is described in the Supplementary Material at http://www.BiochemJ.org/bj/393/bj3930331add.htm.
Glycan array screening
Labelled Gleheda was screened in binding buffer (20 mM Tris/HCl, pH 7.4, 150 mM NaCl, 2 mM CaCl2, 2 mM MgCl2, 0.05% Tween 20 and 1% BSA). The lectin was screened on the streptavidin/biotin array (EA V3) as described previously [23]. Biotinylated glycosides [24] were bound to streptavidin-coated microtitre plates in replicates of n=4. Pre-coated plates were washed three times with 100 μl of wash buffer (binding buffer without BSA) before incubation. A stock solution of Gleheda–Alexa Fluor® 488 (30 μg/ml) was added to each well and incubated at room temperature for 1 h. The plates were washed and read in 25 μl of wash buffer on a Victor-2™ 1420 Multi-label Counter (PerkinElmer Life Sciences) at an excitation wavelength of 485 nm and an emission wavelength of 535 nm.
To analyse the results on the streptavidin/biotin array, all glycans were ranked according to their S/N (signal to noise ratio) by dividing their mean relative fluorescence units by the mean background generated in control wells that lacked glycosides. This value was compared with the average S/N for all wells in the array, and the results were then ranked as high affinity (>3×mean S/N), medium affinity (>2×mean S/N) and low affinity (>mean S/N).
Molecular modelling and docking experiments
Molecular modelling of Gleheda has been performed on a Silicon Graphics O2 10000 workstation, using the programs InsightII, Homology and Discover (Accelrys) as described previously [1]. The atomic co-ordinates of VVLB4 (Vicia villosa isolectin B4) in complex with the Tn antigen (PDB accession code 1N47) [25] have been used as a template to build the three-dimensional model.
Docking of Tn and T into the binding site of Gleheda was performed with the program InsightII. The lowest apparent binding energy (Ebind expressed in kcal·mol−1) compatible with the hydrogen bonds {considering Van de Waals interactions and strong [2.5 Å<distance(D-A)<3.1 Å and 120°<angle(D-H-A)] and weak [2.5 Å<distance(D-A)<3.5 Å and 105°<angle(D-H-A)<120°] hydrogen bonds; where D is donor, A is acceptor and H is hydrogen} found in the Tn antigen–VVLB4, was calculated with the Discover3 forcefield and used to anchor the pyranose ring of the sugars into the binding site of the lectin. Cartoons were drawn with and rendered with PyMOL (DeLano Scientific; http://www.pymol.org).
RESULTS AND DISCUSSION
Gleheda–glycan interaction
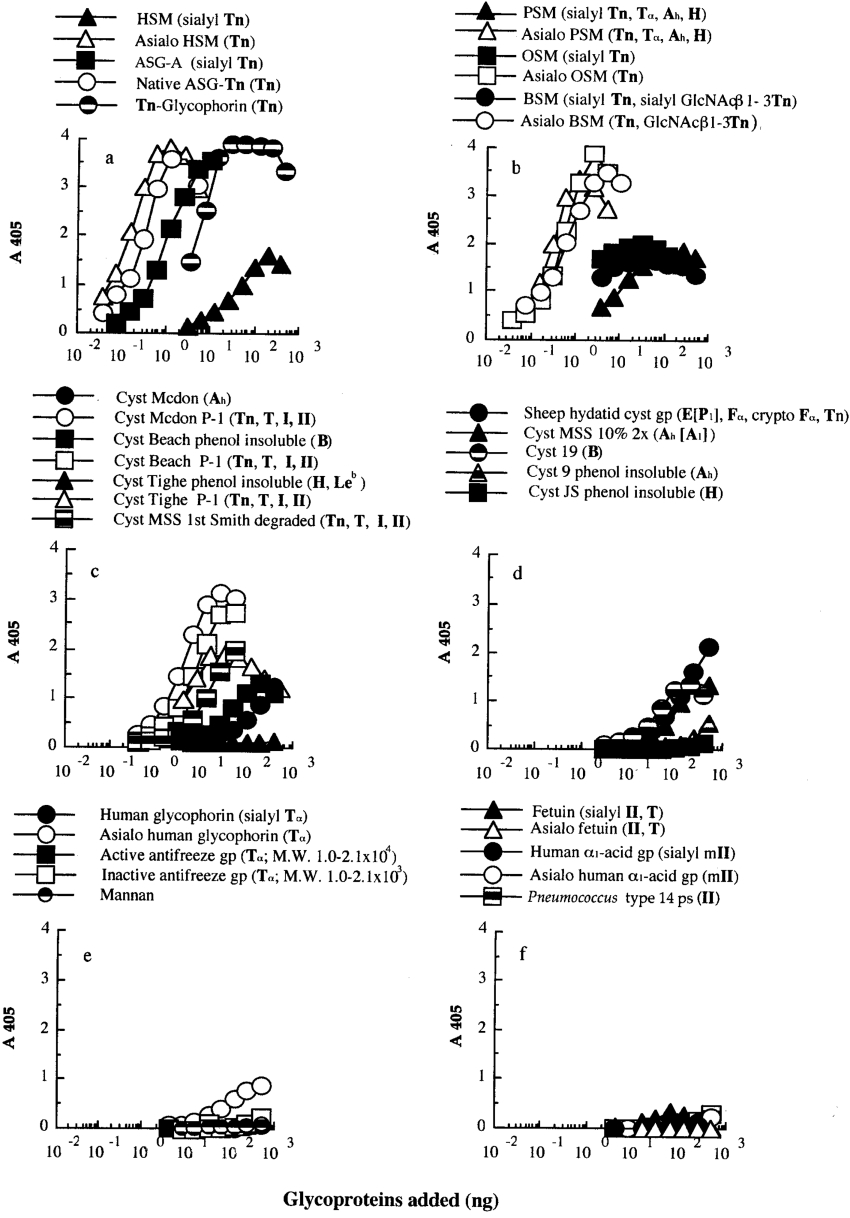
The interaction profile of Gleheda with various gps was studied by a microtitre plate ELLSA. A general overview of the results obtained with the different gps and other glycans is summarized in Table 1. Binding profiles with individual gps are illustrated in Figure 1. Among the ligands tested, Gleheda reacted most strongly with mammalian salivary mucins containing multivalent Tn-containing gps [native ASG-Tn (native armadillo salivary gland Tn gp) (Figures 1a and 2, i), asialo HSM (hamster submaxillary mucin) (Figure 1a), asialo OSM (Figure 1b), asialo PSM (porcine salivary mucin) (Figure 1b) and asialo BSM (bovine submandibular glycoprotein) (Figure 1b)]. These gps reached A405 values of >3.0 within 2 h and required less than 0.4 ng of gp for 1.5 units of absorbance. It also bound well to Tn-glycophorin prepared from human erythrocytes (Figure 1a), crypto Tn-containing gps covered by advanced glycosylation of sialic acids (e.g. ASG-A, OSM, PSM, BSM and HSM shown in Figures 1a and 1b); human blood group ABH (O) precursor equivalent gps which have I/II sequences as major glycotopes and some Tn and T glycotopes (cyst Mcdon P-1, Beach P-1, Tighe P-1 and MSS first Smith degraded gps shown in Figure 1c); and human blood group P1 active cyst gp (sheep hydatid cyst gp; Figure 1d). However, Gleheda was weakly active or inactive with ABH active gps (cyst MSS 10% 2×, cyst 9, 19, Mcdon, Beach, JS and Tighe phenol-insoluble as shown in Figures 1c and 1d), T-containing gps (native and asialo human glycophorin and active and inactive antifreeze gp shown in Figure 1e), multi-antennary II-containing N-glycans (fetuin, human α1-acid gp and their asialo products shown in Figure 1f) and mannan (Figure 1e). To prove that the differences in affinity of Gleheda for these gps are not due to discrepancies in the plate adsorption, and to corroborate the ‘cluster effect’ on binding, the binding affinity was confirmed further by each gp in solution to interfere with the Gleheda–glycan interaction.
Table 1. Binding of Gleheda to human blood group A, B, H, P1 Lea and Leb active gps, sialo and asialo gps as determined by ELLSA.
Biotinylated Gleheda (10 ng) was added to various gp concentrations ranging from 0.01 ng to 1 μg/ml.
| Panel in Figure 1 | Gp (lectin determinants*; blood group specificity) | Mass for 1.5 units of A405 (ng) | Maximum A405 reading† | Binding intensity† |
|---|---|---|---|---|
| Exposed multivalent Tn gps | ||||
| a | Asialo HSM (Tn) | 0.07 | 3.8 | +++++ |
| a | Native ASG-Tn (Tn) | 0.2 | 3.6 | +++++ |
| b | Asialo PSM (Tn, Tα, Ah, H) | 0.2 | 3.3 | +++++ |
| b | Asialo OSM (Tn) | 0.3 | 3.8 | +++++ |
| b | Asialo BSM (Tn, GlcNAcβ1-3Tn) | 0.4 | 3.4 | +++++ |
| a | Tn-Glycophorin (Tn) | 3.6 | 3.9 | +++++ |
| Cryptic multivalent Tn gps | ||||
| a | ASG-A (sialyl Tn) | 0.6 | 3.5 | +++++ |
| b | OSM (sialyl Tn) | 2.0 | 2.0 | ++++ |
| b | BSM (sialyl Tn, sialyl GlcNAcβ1-3Tn) | 10.0 | 1.8 | +++ |
| b | PSM (sialyl Tn, Tα, Ah, H) | 20.0 | 1.8 | +++ |
| a | HSM (sialyl Tn, Tn) | 130.0 | 1.6 | +++ |
| Blood group precursor equivalent gps (mixtures of exposed Tn, T, I, II) | ||||
| c | Cyst Mcdon P-1 | 2.5 | 3.1 | +++++ |
| c | Cyst Beach P-1 | 5.8 | 2.7 | +++++ |
| c | Cyst Tighe P-1 | 6.0 | 1.9 | +++ |
| c | Cyst MSS first Smith degraded | 22.0 | 2.0 | ++++ |
| Blood group ABH-active gps | ||||
| d | Sheep hydatid cyst gp (E[P1], Fα, crypto Fα and Tn) | 180.0 | 2.1 | ++++ |
| d | Cyst MSS 10% 2× (Ah [A1]) | − | 1.4 | ++ |
| d | Cyst 19 (B) | − | 1.3 | ++ |
| c | Cyst Mcdon (Ah) | − | 1.2 | ++ |
| c | Cyst Beach phenol-insoluble (B) | − | 1.2 | ++ |
| d | Cyst 9 phenol-insoluble (Ah) | − | 0.5 | + |
| d | Cyst JS phenol-insoluble (H) | − | 0.1 | − |
| c | Cyst Tighe phenol-insoluble (H, Leb) | − | 0.0 | − |
| T, crypto T, multi-antennary II-containing gps and polysaccharide | ||||
| e | Human glycophorin (sialyl Tα) | − | 0.1 | − |
| e | Asialo human glycophorin (Tα) | − | 0.9 | + |
| e | Inactive antifreeze gp [Tα; molecular mass (2.6–3.8)×103] | − | 0.2 | ± |
| e | Active antifreeze gp [Tα; molecular mass (1.0–2.1)×104] | − | 0.2 | ± |
| f | Pneumococcus type 14 polysaccharide (II) | − | 0.2 | ± |
| f | Human α1-acid gp (sialyl mII) | − | 0.1 | − |
| f | Asialo human α1-acid gp (mII) | − | 0.2 | ± |
| f | Fetuin (sialyl II, T) | − | 0.3 | ± |
| f | Asialo fetuin (II, T) | − | 0.0 | − |
| e | Mannan | − | 0.1 | − |
* The symbol in parentheses indicates the human blood group activity and/or lectin determinants [43]. m, multivalent.
† Results were interpreted according to the measured A405 after 2 h of incubation as follows: +++++, A405≥2.5; ++++, 2.5>A405≥2.0; +++, 2.0>A405≥1.5; ++, 1.5>A405≥1.0; +, 1.0>A405≥0.5; ±, 0.5>A405≥0.2 and −, A405<0.2.
Figure 1. Binding of Gleheda to microtitre plates coated with serially diluted human blood group A, B, H, Lea and Leb active gps, sialo- and asialo-gps.
The amount of lectin used was 10 ng/well. The total volume of the assay was 50 μl. A405 was recorded after 2 h of incubation.
Figure 2. Carbohydrate structural units in salivary gps.
(i) Native ASG-Tn and asialo HSM [45,46]. (ii) Asialo OSM (over 75% Tn) [47].
Inhibition of the Gleheda–glycan interaction by various multivalent glycotopes (gps)
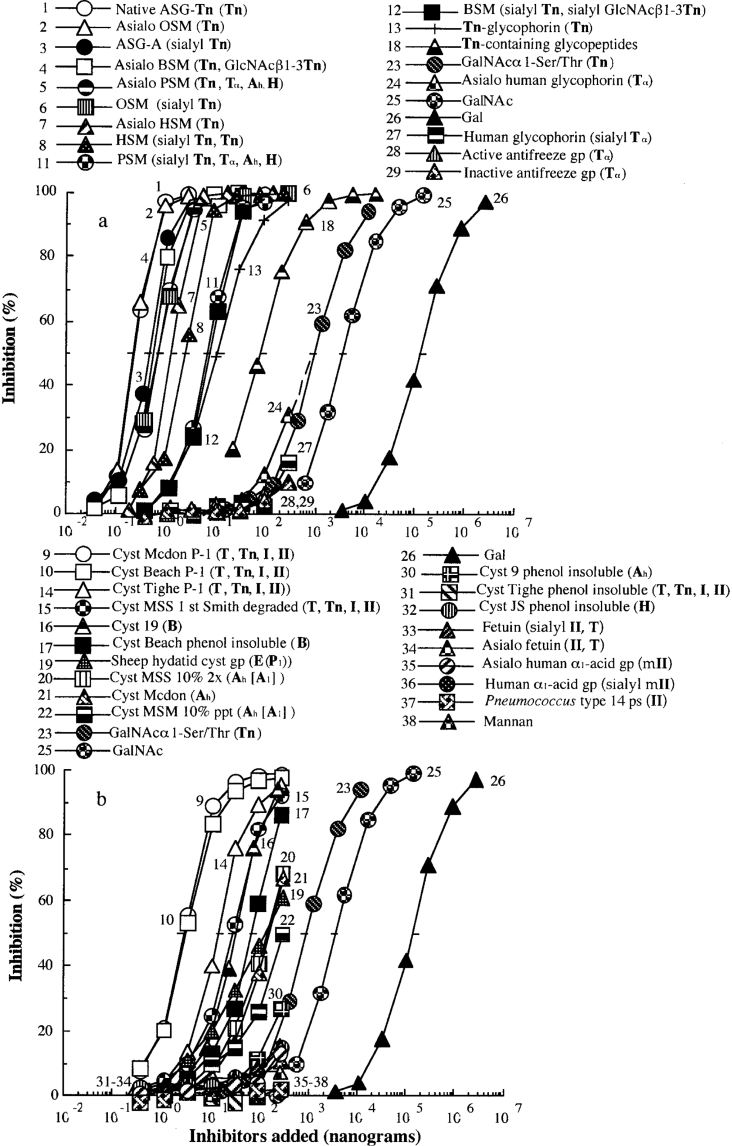
The ability of various gps to inhibit the binding of Gleheda to a Tn-containing gp (asialo PSM), expressed as the concentration (in ng/well) required for 50% inhibition, was analysed by ELLSA with the representative panel of glycans listed in Table 2. A summary of the results is given in Table 2, whereas details of the inhibition by individual gps are shown in Figure 3. Among the glycans tested for inhibition of interaction, seven mammalian salivary Tn-containing gps (native ASG-Tn, ASG-A, OSM and asialo OSM, asialo BSM, asialo PSM and asialo HSM) were the most potent inhibitors, requiring less than 1.2 ng to inhibit 50% of the interaction. They were 6.5×105, 1.5×104 and 3.1×103 times more active than Gal, GalNAc and GalNAcα1-Ser/Thr respectively (curves 1–7 compared with curves 26, 25 and 23 in Figure 3a). The Gleheda–glycan interaction was also inhibited by most of the other high-density multivalent Tn, with their cryptoforms masked by sialic acids, I/II-containing gps and blood group determinants. The active ligands include cryptic Tn glycans (curves 8, 11 and 12 in Figure 3a), Tn-glycophorin (curve 13 in Figure 3a), I/II cluster-containing gps (human blood group precursors in curves 9, 10, 14 and 15) and blood group substances (curves 16, 17 and 19–22 in Figure 3b). Tn-containing glycopeptide fraction (molecular mass <3000 Da) from ovine salivary gp was approx. 8 and 37 times more active than Tn monomer and GalNAc (Figure 3 and Table 2; curve 18 compared with curves 23 and 25), and was 410 times less active than the corresponding high-density multivalent Tn glycans (curve 1 compared with 18 in Figure 3). These consistent inhibition results confirmed the results of the direct binding studies. To confirm further that these bindings are epitope-specific, specific glycotope units and monosaccharide derivatives were used to inhibit the interaction of Gleheda with gps.
Table 2. Amount of different gps giving 50% inhibition of binding of Gleheda (5 ng/50 μl) to asialo PSM (2 ng/50 μl).
The inhibitory activity was estimated from the inhibition curve in Figure 3 and is expressed as the amount of inhibitor giving 50% inhibition. Total volume was 50 μl. Relative potency=quantity of Gal (curve 26) required for 50% inhibition is taken as 1.0/quantity of sample required for 50% inhibition.
| Curve or identification number | Panel in Figure 3 | Gp/glycan | Quantity giving 50% inhibition (ng) | Relative potency |
|---|---|---|---|---|
| 1 | a | Native ASG-Tn (Tn) | 0.2 | 6.5×105 |
| 2 | a | Asialo OSM (Tn) | 0.2 | 6.5×105 |
| 3 | a | ASG-A (sialyl Tn) | 0.4 | 3.3×105 |
| 4 | a | Asialo BSM (Tn) | 0.5 | 2.6×105 |
| 5 | a | Asialo PSM (Tn, Tα, Ah, H) | 0.7 | 1.8×105 |
| 6 | a | OSM (sialyl Tn) | 0.7 | 1.8×105 |
| 7 | a | Asialo HSM (Tn) | 1.2 | 1.1×105 |
| 8 | a | HSM (sialyl Tn, Tn) | 2.4 | 5.4×104 |
| 9 | b | Cyst Mcdon P-1 (I, II, T, Tn) | 3.1 | 4.1×104 |
| 10 | b | Cyst Beach P-1 (I, II, T, Tn) | 3.1 | 4.1×104 |
| 11 | a | PSM (sialyl Tn, Tα, Ah, H) | 7.1 | 1.8×104 |
| 12 | a | BSM (sialyl Tn, sialyl GlcNAcβ1→3Tn) | 8.0 | 1.6×104 |
| 13 | a | Tn-glycophorin (Tn) | 9.9 | 1.3×104 |
| 14 | b | Cyst Tighe P-1 (I/II) | 14.0 | 9.3×103 |
| 15 | b | Cyst MSS first Smith degraded (I, II, T, Tn) | 28.0 | 4.6×103 |
| 16 | b | Cyst 19 (B) | 34.0 | 3.8×103 |
| 17 | b | Cyst Beach phenol insoluble (B) | 70.0 | 1.8×103 |
| 18 | a | Tn-containing glycopeptides | 81.2 | 1.6×103 |
| 19 | b | Sheep hydatid cyst gp (E[P1]) | 120.0 | 1.1×103 |
| 20 | b | Cyst MSS 10% 2× (Ah [A1]) | 125.0 | 1.0×103 |
| 21 | b | Cyst Mcdon (Ah) | 130.0 | 1.0×103 |
| 22 | b | Cyst MSM 10% ppt (Ah [A2]) | 277.0 | 4.7×102 |
| 23 | a, b | GalNAcα1-Ser/Thr (Tn) | 616.0 | 2.1×102 |
| 24 | a | Asialo human glycophorin (Tα) | 800.0* | 1.6×102 |
| 25 | a, b | GalNAc | 3.0×103 | 43.3 |
| 26 | a, b | Gal | 1.3×105 | 1.0 |
| 27 | a | Human glycophorin (sialyl Tα) | >2.8×102 (5.6%)† | − |
| 28 | a | Active antifreeze gp [Tα; molecular mass (1.0–2.1)×104] | >2.8×102 (10.3%) | − |
| 29 | a | Inactive antifreeze gp [Tα; molecular mass (2.6–3.8)×103] | >2.8×102 (10.0%) | − |
| 30 | b | Cyst 9 phenol-insoluble (Ah) | >2.8×102 (26.9%) | − |
| 31 | b | Cyst Tighe phenol-insoluble (H, Leb) | >2.8×102 (2.2%) | − |
| 32 | b | Cyst JS phenol-insoluble (H) | >2.8×102 (14.9%) | − |
| 33 | b | Fetuin (sialyl II, T) | >2.8×102 (14.9%) | − |
| 34 | b | Asialo fetuin (II, T) | >2.8×102 (6.9%) | − |
| 35 | b | Asialo human α1-acid gp (II) | >2.8×102 (12.8%) | − |
| 36 | b | Human α1-acid gp (sialyl II) | >2.8×102 (0.3%) | − |
| 37 | b | Pneumococcus type 14 polysaccharide (II) | >2.8×102 (1.5%) | − |
| 38 | b | Mannan | >2.8×102 (10.8%) | − |
* Extrapolation value.
† The inhibitory potency of inactive gps are expressed as the maximum amount of gps tested that yield inhibition (in parentheses) below 50%.
Figure 3. Inhibition of Gleheda binding to asialo PSM coated on ELLSA plates by various gps.
The quantity of gp in the coating solution was 2 ng/well. The quantity of lectin used for inhibition assay was 10 ng/well. The final Gleheda content was 5 ng/well. Total volume was 50 μl. A405 was recorded after 2 h of incubation. The amount (ng) of gp required to induce 50% inhibition was determined.
Inhibition of the Gleheda–glycan interaction by mono- and oligo-saccharides
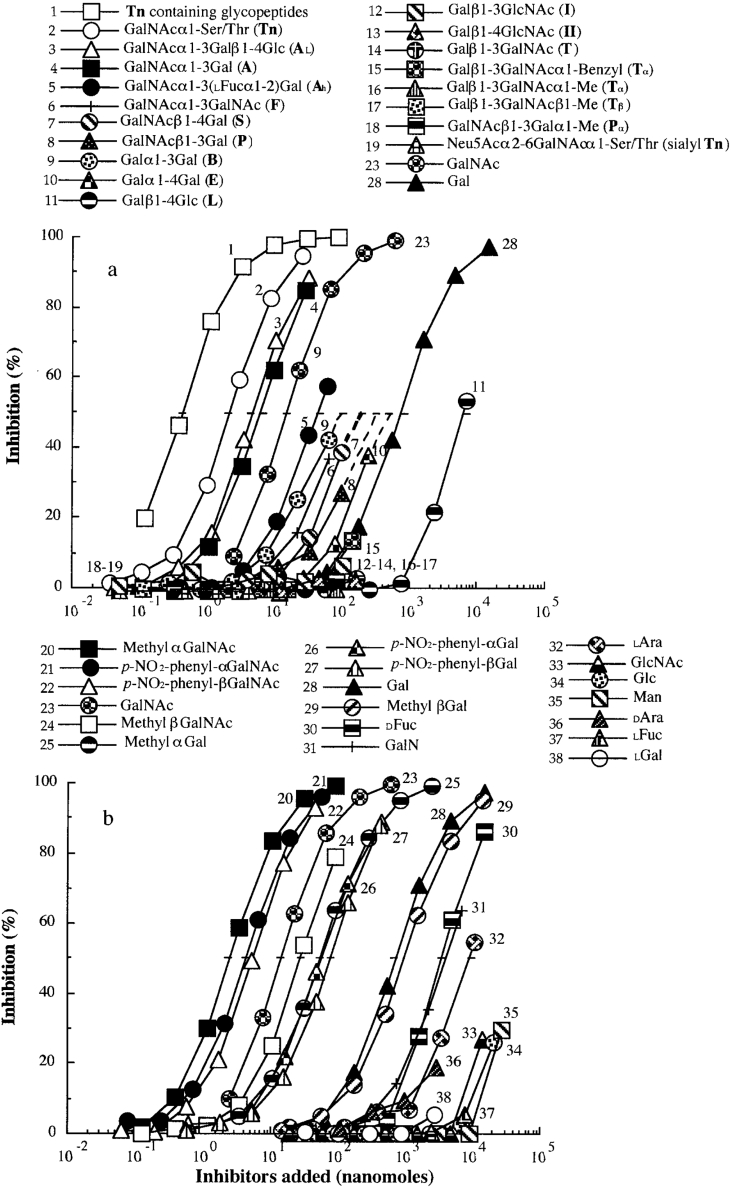
The ability of various sugar ligands to inhibit the binding of Gleheda to a Tn-containing gp (asialo PSM) was determined by ELLSA. Amounts (in nmol) required for 50% inhibition of the binding of Gleheda to asialo PSM are listed in Table 3. Details of the inhibition by individual mono- and oligo-saccharides are presented in Figure 4. Among the monosaccharides and oligosaccharides studied, the Tn-containing glycopeptide fraction (molecular mass <3000 Da) from OSM gp was the best inhibitor being 5, 37.5 and 1.7×103 times more active than Tn, GalNAc and Gal respectively (curve 1 compared with curves 2, 23 and 28 in Figure 4). This fraction was approx. 12.5–1250 times more active than other GalNAc/Gal α/β-related ligands: AL (GalNAcα1-3Galβ1-4Glc), A (GalNAcα1-3Gal), Ah [GalNAcα1-3(L-Fucα1-2)Gal, where Fuc is fucose], F (GalNAcα1-3GalNAc), S (GalNAcβ1-4Gal), P (GalNAcβ1-3Gal), B (Galα1-3Gal), E (Galα1-4Gal) (curves 3–10 in Figure 4), while I (Galβ1-3GlcNAc), II (Galβ1-4GlcNAc), T (Galβ1-3GalNAc), Tα (Galβ1-3GalNAcα1-benzyl), Tα (Galβ1-3GalNAcα1-methyl), Tβ (Galβ1-3GalNAcβ1-methyl), Pα (GalNAcβ1-3Galα1-methyl) (curves 12–18 in Figure 4) were poor inhibitors. The blood type A trisaccharide [GalNAcα1-3(L-Fucα1-2)Gal, curve 5] and Forssman disaccharide (GalNAcα1-3GalNAc, F, curve 6) were 6.7 and 25 times less potent respectively than the A disaccharide containing non-reducing terminal GalNAcα1-3Gal residues (curve 4). These results demonstrate that the free hydroxy group at C-2 of the penultimate galactose group is important for lectin binding and that replacement of this C-2 hydroxy group with an acetamido group as in the Forssman disaccharide or with an L-fucosyl group as in the A-trisaccharide reduces lectin binding. Similar results were reported for another Gal/GalNAc-specific lectin from Dutch Iris (Iris hollandica) bulbs [26].
Table 3. Amount of various saccharides giving 50% inhibition of binding of Gleheda (5 ng/50 μl) to asialo PSM (2 ng/50 μl).
The inhibitory activity was estimated from the inhibition curve in Figure 4 and is expressed as the amount of inhibitor giving 50% inhibition. Total volume was 50 μl. Relative potency=quantity of Gal (curve 28) required for 50% inhibition is taken as 1.0/quantity of sample required for 50% inhibition.
| Curve number | Panel in Figure 4 | Saccharide | Quantity giving 50% inhibition (nmol) | Relative potency |
|---|---|---|---|---|
| Tn, simple Tn cluster and GalNAc-containing saccharide | ||||
| 1 | a | Tn-containing glycopeptides (molecular mass <3.0×103) | 0.4 | 1750.0 |
| 2 | a | GalNAcα1-Ser/Thr (Tn) | 2.0 | 350.0 |
| 3 | a | GalNAcα1-3Galβ1-4Glc (A tri- or active L, AL) | 5.0 | 140.0 |
| 4 | a | GalNAcα1-3Gal (A) | 6.0 | 116.7 |
| 5 | a | GalNAcα1-3(L-Fucα1-2)Gal (Ah) | 40.0 | 17.5 |
| 6 | a | GalNAcα1-3GalNAc (F) | 150.0* | 4.7 |
| 7 | a | GalNAcβ1-4Gal (S) | 150.0* | 4.7 |
| 8 | a | GalNAcβ1-3Gal (P) | 220.0* | 3.2 |
| Other mammalian carbohydrate structural units | ||||
| 9 | a | Galα1-3Gal (B) | 100.0* | 7.0 |
| 10 | a | Galα1-4Gal (E) | 500.0* | 1.4 |
| 11 | a | Galβ1-4Glc (L) | 6000.0 | 0.1 |
| 12 | a | Galβ1-3GlcNAc (I) | >290.0 (6.5%)† | − |
| 13 | a | Galβ1-4GlcNAc (II) | >435.0 (3.0%) | − |
| 14 | a | Galβ1-3GalNAc (T) | >173.3 (3.3%) | − |
| 15 | a | Galβ1-3GalNAcα1-benzyl (Tα1-Benzyl) | >140.8 (14.0%) | − |
| 16 | a | Galβ1-3GalNAcα1-Me (Tα1-Methyl) | >83.3 (0.0%) | − |
| 17 | a | Galβ1-3GalNAcβ1-Me (Tβ1-Methyl) | >83.3 (0.0%) | − |
| 18 | a | GalNAcβ1-3Galα1-Me (Pα1-Methyl) | >83.3 (3.2%) | − |
| 19 | a | Neu5Acα2-6GalNAcα1-Ser/Thr (sialyl Tn) | >36.7 (0.0%) | − |
| Monosaccharides and their derivatives | ||||
| 20 | b | Methyl αGalNAc | 2.5 | 280.0 |
| 21 | b | p-Nitrophenyl αGalNAc | 4.0 | 175.0 |
| 22 | b | p-Nitrophenyl βGalNAc | 5.0 | 140.0 |
| 23 | a, b | GalNAc | 15.0 | 46.7 |
| 24 | b | Methyl βGalNAc | 28.0 | 25.0 |
| 25 | b | Methyl αGal | 58.0 | 12.1 |
| 26 | b | p-Nitrophenyl αGal | 58.0 | 12.1 |
| 27 | b | p-Nitrophenyl βGal | 80.0 | 8.8 |
| 28 | a, b | Gal | 700.0 | 1.0 |
| 29 | b | Methyl βGal | 1000.0 | 0.7 |
| 30 | b | D-Fuc | 4000.0 | 0.2 |
| 31 | b | GalN | 4000.0 | 0.2 |
| 32 | b | L-Ara | 8100.0 | 0.1 |
| 33 | b | GlcNAc | >14350.0 (27.1%) | − |
| 34 | b | Glc | >21000.0 (25.8%) | − |
| 35 | b | Man | >27500.0 (29.8%) | − |
| 36 | b | DAra | >3095.0 (18.6%) | − |
| 37 | b | L-Fuc | >7742.0 (5.2%) | − |
| 38 | b | L-Gal | >2775.0 (5.2%) | − |
* Extrapolation value.
† The inhibitory potency of inactive saccharides are expressed as the maximum amount of sugars tested that yield inhibition (in parentheses) below 50%.
Figure 4. Inhibition of Gleheda binding to asialo PSM coated on ELLSA plates by various saccharides.
The amount of gp in the coating solution was 2 ng/well. The lectin (10 ng/well) was pre-incubated with an equal volume of serially diluted inhibitor. The final Gleheda content was 5 ng/well. Total volume was 50 μl. A405 was recorded after 2 h of incubation.
Of the monosaccharides studied, GalNAc glycosides were significantly more active than Gal glycosides (curves 20 and 24 compared with curves 25 and 29, and curves 21 and 22 compared with curves 26 and 27 in Figure 4b), indicating possible interactions of the N-acetamido group at C-2 of GalNAc in the binding site of the lectin. For the Gal/GalNAc residue, α-linked glycosides, in the form of either methyl or p-nitrophenyl derivatives, appeared to be more active than β-anomers (curve 25 compared with curve 29, curve 26 compared with curve 27, curve 20 compared with curve 24, and curve 21 compared with curve 22 in Figure 4), indicating that the α-anomer is important for binding. Furthermore, p-nitrophenyl β-derivatives of Gal/GalNAc were 5.6 and 12.6 times more potent than their methyl β-analogues (curve 22 compared with curve 24, and curve 27 compared with curve 29 in Figure 4), indicating that hydrophobicity surrounding β-anomeric Gal/GalNAc is important for binding. In the present study, GalNAc was 46.7 times more active than Gal, indicating that the N-acetyl group at C-2 significantly enhances the lectin binding (curve 23 compared with curve 28 in Figure 4). D-Fuc (curve 30) showed 1/5 of Gal activity (curve 28), suggesting that the hydroxy group on C-6 participates in binding; L-Ara (arabinose) (curve 32), which has the same configuration as D-Gal, but lacks the hydroxymethyl group of C-6, was 10-fold less potent than Gal, indicating that the hydroxymethyl group of C-6 is required for binding. Furthermore, a weak inhibition of the Gleheda–asialo PSM interaction was observed with GalN (galactosamine), demonstrating that configuration of Gal at C-4 plays a role in the binding interaction [Gal versus Glc (glucose)] and that the binding activity was reduced by removal of the acetyl group from GalNAc to form GalN (Figure 4b, curve 31). GlcNAc (N-acetylglucosamine), Glc, Man (mannose), D-Ara, L-Fuc and L-Gal were tested at concentrations from 2775 to 27500 nmol, but only a weak or no inhibition of lectin binding was observed (Figure 4b, curves 33–38, and Table 3).
Verification of ELLSA results by glycan array screening
The affinities of Gleheda for glycotopes were determined further using the glycan array available through the Consortium for Functional Glycomics. Gleheda showed high affinity for glycan no. 135, also known as A disaccharide (110.03 S/N binding) and for glycan no. 8 (α-GalNAc) (101.02 S/N binding) respectively (Table 4 and Supplementary Figure 1 at http://www.BiochemJ.org/bj/393/bj3930331add.htm). Other glycans, such as glycan no. 30 (Galα1-4Galβ1-4Glcβ-), 4 (α-D-Gal-), 64 (B disaccharide), 193 (Galα1-3Galβ1-4Glcβ), 9 (β-GalNAc-), 31 (Galα1-4Galβ1-4GlcNAcβ-), 134 (Galα1-2Galβ-), 167 (Galα1-3Galβ1-4(Fucα1-3)GlcNAc β-), 138 (Galα1-3Galβ1-4GlcNAcβ-), 65 (Galβ1-2Galβ-), 22 (GalNAcβ1-4GlcNAcβ-) and 82 [GalNAcα1-3-(Fucα1-2)Galβ-] also showed high affinity, but their S/Ns were 1.5–3.5 times less than that of A disaccharide and α-GalNAc (Table 4 and Supplementary Figure 1 at http://www.BiochemJ.org/bj/393/bj3930331add.htm).
Table 4. Gleheda binding to the Consortium glycan (glycotope) array.
Spacer definitions and entire glycan array version at http://www.functionalglycomics.org/static/consortium/. PAA, polyacrylamide conjugate; BT, biotin conjugate.
| Glycan no. | Glycan (glycotope)–spacer | Glycotope abbreviation | S/N |
|---|---|---|---|
| High affinity (S/N was greater than 3 times the average S/N for the entire array) | |||
| 135 | GalNAcα1-3Gal–Sp2 | A disaccharide | 110.03 |
| 8 | α-GalNAc–Sp2 | α-GalNAc | 101.02 |
| 30 | Galα1-4Galβ1-4Glcβ–Sp1 | Pk; (ELβ Tri-) | 75.88 |
| 4 | α-D-Gal–Sp2 | Galα1- | 75.33 |
| 64 | Galα1-3Galβ–Sp2 | B disaccharide | 71.07 |
| 193 | Galα1-3Galβ1-4Glcβ–Sp1 | Galili Tri; (BL Tri-) | 62.93 |
| 9 | β-GalNAc–Sp2 | β-GalNAc | 58.25 |
| 31 | Galα1-4Galβ1-4GlcNAcβ–Sp1 | P1; (EIIβ) | 49.07 |
| 134 | Galα1-2Galβ–Sp2 | Galα1-2Gal | 46.78 |
| 167 | Galα1-3Galβ1-4(Fucα1-3)GlcNAcβ–Sp2 | αGalLex; (BII/Lex Tetra-) | 42.14 |
| 138 | Galα1-3Galβ1-4GlcNAcβ–Sp2 | Galα1-3′LacNAc; (BIIβ Tri-) | 36.69 |
| 65 | Galβ1-2Galβ–Sp2 | Galβ1-2Gal | 35.50 |
| 22 | GalNAcβ1-4GlcNAcβ–Sp2 | Lac-di-NAc | 34.51 |
| 82 | GalNAcα1-3(Fucα1-2)Galβ–Sp7 | Atri-long; (Ah) | 31.56 |
| Medium affinity (S/N was greater than 2 times the average S/N for the entire array) | |||
| 172 | GalNAcα1-3(Fucα1-2)Galβ1-4GlcNAcβ–Sp2 | A type II; (AhII β) | 17.74 |
| 63 | 6-Su-GalNAcα–Sp2 | α-GalNAc-6-sulphate | 16.54 |
| 67 | Galβ1-4(6-O-Su)GlcNAcβ–Sp2 | 6′-O-su-LacNAc | 14.86 |
| Low affinity (S/N was greater than the average S/N for the entire array) | |||
| 112 | GalNAcβ1-4GlcNAcβ1-4Manα1-6(GalNAcβ1-4GlcNAcβ1- | Bi-LDN (remodelled from human fibrinogen) | 12.91 |
| 4Manα1-3)Manβ1-4GlcNAcβ1-4GlcNAcβ-N–Sp | |||
| 203 | GalNAcα1-3(Fucα1-2)Galβ1-4GlcNAc–SP1.BT | A-tetra type II; (AhII Tetra-) | 10.98 |
| 26 | GalNAcβ1-4(Fucα1-3)GlcNAc–Sp1 | Fucα1-3-Lac-di-NAc | 10.38 |
| 91 | α-D-Gal-PAA–Sp1 | α-D-galactose | 9.86 |
| 77 | Galα1-3(Fucα1-2)Galβ–Sp7 | Btri-long; (Bh) | 9.58 |
| 93 | α-GalNAc-PAA–Sp1 | α-GalNAc | 9.32 |
| 202 | GalNAcα1-3(Fucα1-2)Galβ1-4Glc–SP1.BT | A-tetra Lac; (AhL Tetra-) | 7.32 |
Molecular modelling and docking experiments
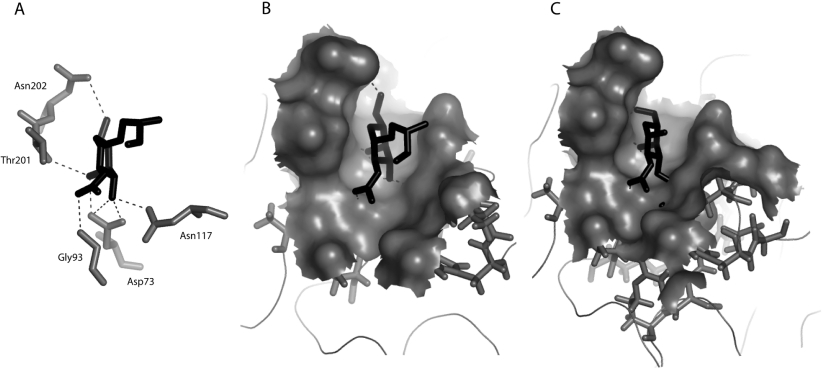
According to the modelled three-dimensional structure of Gleheda, the Tn antigen is anchored into the carbohydrate-binding site by a network of seven hydrogen bonds connecting residues Asn73, Gly93, Asp117, Thr201 and Asn202 to O3, O4, O5 and O7 of GalNAc (Figure 5A). This hydrogen-bond-mediated interaction is reinforced by a hydrophobic interaction with the imidazole ring of His115, which is located in the close vicinity of the carbohydrate-binding site and stacks against the pyranose ring of GalNAc. Importantly, no steric clash occurred upon docking of the Tn antigen into the carbohydrate-binding site (Figure 5B). In contrast, anchoring the T antigen or Tα antigen (Galβ1-3GalNAcα1-methyl) into the carbohydrate-binding site of Gleheda resulted in severe steric clashes between the GalNAc residue and Asp73, His115 and Asn117 (Figure 5C). These three separate methods (ELLSA, glycan array and molecular docking) confirmed that multivalent Tn is the most potent carbohydrate ligand for Gleheda (Tables 1–4 and Figures 1 and 3–5).
Figure 5. Docking of Tn and T antigen in the carbohydrate-binding site of Gleheda.
(A) Docking of Tn antigen in the carbohydrate-binding site. The network of hydrogen bonds (dark broken lines) connecting the sugars to residues Asp73, Gly93, Asn122, Trp117, Thr201 and Asn202 (Corey–Pauling–Koltun code) forming the monosaccharide-binding site of Gleheda. Residue His115 which stacks against the pyranose ring of the GalNAc is not shown. (B) Docking of GalNAcα1-Ser into the carbohydrate-binding cavity of Gleheda. (C) Docking of the T antigen into the carbohydrate-binding cavity of Gleheda showing the clash of the Gal residues with residues Asp73, His115 and Asn117. Both the carbohydrate-binding site and Tn or T antigen are similarly oriented in (A), (B) and (C). The Figure was drawn using PyMOL.
Effect of multivalency of glycotopes on Gleheda binding
Multivalent binding is characterized by the simultaneous interaction between multiple ligands on one entity and multiple receptors on another. Studies in other systems have demonstrated that multivalent display of carbohydrates can lead to remarkably high binding avidities [27–29]. Accumulating evidence also indicates that specificity increases when carbohydrates are presented in a multivalent form [30–35]. Hence, it is important to estimate the effect of multivalency on binding while studying the carbohydrate specificity of a lectin. In the present study, we have examined the influence of multivalency on the binding avidity of Gleheda. The results shown in Table 2 clearly demonstrate that O-linked native ASG-Tn, the carbohydrate side chains of which consist exclusively of Tn determinants, and asialo OSM which contains over 75% of Tn determinants (Figure 2) exhibit 6.5×105 and 3.1×103 times higher binding than Gal and monomeric Tn respectively (curves 1 and 2 compared with curves 26 and 23 in Figure 3). Although a mixture of Tn-containing glycopeptides was the most active ligand among various saccharides (curve 1 in Figure 4 and Table 3), it was, on a nanogram basis, only 8 times more active than monomeric Tn (curve 18 compared with 23 in Figure 3), and about 410 times less active than native ASG-Tn or asialo OSM (curves 1 and 2 in Figure 3 and Table 2). These results suggest that, when Tn is in high-density multivalent form, it is the most potent ligand and plays an essential role in binding. Based on the results of the present study, the concept of glycoside cluster effect can be classified into two groups: (i) the ‘multi-antennary or simple glycoside cluster effect’, as in galactosides with hepatic lectin [36,37] and tri-antennary II sequences as in a galectin from chicken liver (CG-16) [38] or Tn glycopeptides which is 8 times more potent than monomeric Tn (curve 18 compared with curve 23 in Figure 3 and Table 2); and (ii) the ‘High-density multivalent effect’ as in the macromolecular interaction of multivalent Tn glycotope in native ASG-Tn and asialo OSM, which generates an enhancement in affinity towards Gleheda by approx. 6.5×105 and 3.1×103 times over Gal and monomeric Tn (curve 1 compared with curves 26 and 23 in Figure 3 and Table 2). A similar phenomenon was also found in other lectins, such as ABA (Agaricus bisporus agglutinin) [33], ALA (Artocarpus lakoocha agglutinin) [34] and galectin-4 from rat gastrointestinal tract [35]. However, the multivalencies of glycotopes do not make an important contribution in all carbohydrate–protein interactions. The theory on lectin binding and the relation of the interaction of natural multivalent glycotopes has not yet been well established. For example, the potency in the interaction of Pseudomonas aeruginosa II lectin with L-Fucα1- multivalent glycans is similar or weaker than the incremental increase in carbohydrate specificity of monomers ([39], and A. M. Wu, J.-H. Liu and N. Gilboa-Garber, unpublished work). Similarly, for Anguilla anguilla agglutinin [40], although greater affinity was seen for multivalent ligands in gps than with univalent haptens, the degree of enhancement was 1.5×104 times less compared with that found in other lectins, such as ABA [33] and galectin-4 from rat gastrointestinal tract [35]. This indicates that the importance of multivalent effects on carbohydrate–protein interactions must be individually evaluated to establish rules or elaborate a theory.
Comparison with SSL (Salvia sclarea lectin)
The binding properties of Gleheda were compared with that of another Lamiaceae lectin, isolated from the seeds of Salvia sclarea (SSL) [41]. This comparison revealed the following: (i) Both Gleheda and SSL showed affinity for the α/β-anomer of Gal that was enhanced by an N-acetyl group at C-2 (GalNAcα1-) (see Supplementary Table 1 at http://www.BiochemJ.org/bj/393/bj3930331add.htm). In Gleheda, the hydrophobicity surrounding α-anomeric Gal/GalNAc is not required for binding (as in Supplementary Table 1, 2e and 2f at http://www.BiochemJ.org/bj/393/bj3930331add.htm), whereas, in SSL, the hydrophobicity of α/β-anomeric Gal (the ratio of p-nitrophenyl and methyl glycosides, as in 2f and 2h in Supplementary Table 1 at http://www.BiochemJ.org/bj/393/bj3930331add.htm) enhances the binding. (ii) The reactivity of Gleheda and SSL towards glycotopes was compared (see Supplementary Table 1 at http://www.BiochemJ.org/bj/393/bj3930331add.htm). Gleheda, together with exposed and sialylated Tn, also recognizes human blood group ABH (O) precursor equivalent gps, which have I/II sequences as major glycotopes and some T and Tn glycotopes, indicating that Gleheda can be used as a sensitive tool to detect I/II-containing O-glycans with a small amount of incomplete Tn residues [42]. In contrast, SSL has a narrow binding spectrum that is confined to exposed Tn gps, but not to their cryptoforms. (iii) The combining site of Gleheda may be a small cavity type interacting best with Tn structure, whereas that of SSL may be a shallow groove type that can accommodate from the monosaccharide of GalNAc at the terminal non-reducing end or Tn up to Forssman pentasaccharide. (iv) The affinity of Gleheda is enhanced five times by simple Tn cluster (Table 3) and 3100 times by multivalent Tn (Table 2 and Supplementary Table 1 at http://www.BiochemJ.org/bj/393/bj3930331add.htm), while SSL binding is significantly enhanced by complex multivalent Tn-containing gps, but limited by simple cluster Tn-containing glycopeptides from asialo OSM gp (molecular mass <3000 Da). The above comparison explains the evolutionary relationship of the two lectins in one family and provides a model system for comparing ligand specificity profiles.
Recognition profile of Gleheda
In conclusion, our work demonstrates that: (i) multivalent Tn-containing gps are the essential and most potent carbohydrate ligands for Gleheda, (ii) with few exceptions, Gleheda also recognizes sialylated and crypto Tn glycotopes (Tables 1 and 2), (iii) the fact that GalNAc is 46.7 times more potent than D-Gal as an inhibitor indicates that acetamido group at the C-2 position significantly enhances binding to the lectin, (iv) substitution of the hydroxy group with either an N-acetyl group or a fucosyl group at the C-2 position of the penultimate galactose reduces the lectin binding reactivity (Table 3), (v) the α-anomers of Gal and GalNAc are more active than their β-anomers, (vi) hydrophobic interactions are important for binding of the β-anomeric Gal/GalNAc (Figure 4b), (vii) the combining site of Gleheda may be a cavity type with GalNAcα1- as the major combining site, and (viii) the peculiar preference of Gleheda for clustered Tn makes it useful as a diagnostic tool for Tn tumour detection. Thus, in the present study, we identified the important recognition factors involved in Gleheda binding, with the configuration of Gal being the most essential and that the cluster form of Gal with -NHCH3CO at C-2 (high-density Tn cluster) enhances the reactivities by more than 6.5×105 times over univalent Gal. These results are important in view of our understanding of the importance of multivalency in phenomena involving the cell-surface carbohydrate ligand–lectin recognition, and enable optimizing the application of this novel structural probe in glycobiological and clinical research. Furthermore, our study supports the concept that every lectin with its unique amino acid sequence has its own binding characteristics [33–35,41,43,44].
Online Data
Acknowledgments
This work was supported by grants from the Chang-Gung Medical Research Project (CMRP no. 1028), Kwei-San, Tao-Yuan, Taiwan, and the National Science Council (NSC 92-2311-B-182-005, 92-2320-B-182-045, 92-2320-B-182-046), Taipei, Taiwan; the Research Council of Ghent University (grant no. 01109203) and the Fund for Scientific Research-Flanders (FWO grants G.0113.01 and G.0201.04). The glycan array of The Consortium for Functional Glycomics was supported by the National Institute of General Medical Sciences grant GM62116.
References
- 1.Wang W., Peumans W. J., Rougé P., Rossi C., Proost P., Chen J., Van Damme E. J. M. Leaves of the Lamiaceae species Glechoma hederacea (ground ivy) contain a lectin that is structurally and evolutionary related to the legume lectins. Plant J. 2003;33:293–304. doi: 10.1046/j.1365-313x.2003.01623.x. [DOI] [PubMed] [Google Scholar]
- 2.Wang W., Hause B., Peumans W. J., Smagghe G., Mackie A., Fraser R., Van Damme E. J. M. The Tn antigen-specific lectin from ground ivy is an insecticidal protein with an unusual physiology. Plant Physiol. 2003;132:1322–1334. doi: 10.1104/pp.103.023853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Springer G. F. T and Tn, general carcinoma autoantigens. Science. 1984;24:1189–1260. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]
- 4.Springer G. F. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J. Mol. Med. 1997;75:594–602. doi: 10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]
- 5.Itzkowitz S. H., Bloom E. J., Kokal W. A., Modin G., Hakomori S. I., Kim Y. S. Sialosyl-Tn: a novel mucin antigen associated with prognosis in colorectal cancer patients. Cancer. 1990;66:1960–1966. doi: 10.1002/1097-0142(19901101)66:9<1960::aid-cncr2820660919>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 6.Springer G. F. T and Tn pancarcinoma markers: auto-antigenic adhesion molecules in pathogenesis, pre-biopsy carcinoma detection and long-term breast-carcinoma immunotherapy. Crit. Rev. Oncogen. 1995;6:57–85. doi: 10.1615/critrevoncog.v6.i1.50. [DOI] [PubMed] [Google Scholar]
- 7.Hakomori S. Blood group glycolipid antigens and their modifications as human cancer antigens. Am. J. Clin. Pathol. 1984;82:635–648. doi: 10.1093/ajcp/82.6.635. [DOI] [PubMed] [Google Scholar]
- 8.Hakomori S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv. Cancer Res. 1989;52:257–331. doi: 10.1016/s0065-230x(08)60215-8. [DOI] [PubMed] [Google Scholar]
- 9.Hakomori S., Wang S. M., Young W. W. Isoantigenic expression of Forssman glycolipid in human gastric and colonic mucosa: its possible identity with “A-like antigen” in human cancer. Proc. Natl. Acad. Sci. U.S.A. 1977;74:3023–3027. doi: 10.1073/pnas.74.7.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taniguchi N., Yokosawa N., Narita M., Mitsuyama T., Makita A. Expression of Forssman antigen synthesis and degradation in human lung cancer. J. Natl. Cancer Inst. 1981;67:577–583. [PubMed] [Google Scholar]
- 11.Yoda Y., Ishibashi T., Makita A. Isolation, characterization, and biosynthesis of Forssman antigen in human lung and lung carcinoma. J. Biochem. 1980;88:1887–1890. [PubMed] [Google Scholar]
- 12.Ono K., Hattori H., Uemura K. I., Nakayama J., Ota H., Katsuyama T. Expression of Forssman antigen in human large intestine. J. Histochem. Cytochem. 1994;42:659–665. doi: 10.1177/42.5.7512587. [DOI] [PubMed] [Google Scholar]
- 13.Tollefsen S., Kornfeld R. Isolation and characterization of lectins from Vicia villosa: two distinct carbohydrate binding activities are present in seed extracts. J. Biol. Chem. 1983;258:5165–5171. [PubMed] [Google Scholar]
- 14.Medeiros A., Bianchi S., Calvete J. J., Balter H., Bay S., Robles A., Cantacuzéne D., Nimtz M., Alzari P. M., Osinaga E. Biochemical and functional characterization of the Tn-specific lectin from Salvia sclarea seeds. Eur. J. Biochem. 2000;267:1434–1440. doi: 10.1046/j.1432-1327.2000.01141.x. [DOI] [PubMed] [Google Scholar]
- 15.Wu A. M. Polyvalency of Tn (GalNAcα1→Ser/Thr) glycotope as a critical factor for Vicia villosa B4 and glycoprotein interactions. FEBS Lett. 2004;562:51–58. doi: 10.1016/S0014-5793(04)00180-2. [DOI] [PubMed] [Google Scholar]
- 16.Hirohashi S., Clausen H., Yamada T., Shimossato Y., Hakomori S. I. Blood group A cross-reacting epitope defined by monoclonal antibodies NCC-LU-35 and -81 expressed in cancer of blood group O or B individuals: its identification as Tn antigen. Proc. Natl. Acad. Sci. U.S.A. 1985;82:7039–7043. doi: 10.1073/pnas.82.20.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakada H., Numata Y., Inoue M., Tanaka N., Kitagawa H., Funakoshi I., Fukui S., Yamashina I. Elucidation of an essential structure recognized by an anti-GalNAc α-Ser(Thr) monoclonal antibody (MLS 128) J. Biol. Chem. 1991;266:12402–12405. [PubMed] [Google Scholar]
- 18.Van den Steen P., Rudd P., Dwek R., Opdenakker G. Concepts and principles of O-linked glycosylation. Crit. Rev. Biochem. Mol. Biol. 1998;33:151–208. doi: 10.1080/10409239891204198. [DOI] [PubMed] [Google Scholar]
- 19.Iida S., Yamamoto K., Irimura T. Interaction of human macrophage C-type lectin with O-linked N-acetylgalactosamine residues on mucin glycopeptides. J. Biol. Chem. 1999;274:10697–10705. doi: 10.1074/jbc.274.16.10697. [DOI] [PubMed] [Google Scholar]
- 20.Duk M., Lisowska E., Wu J. H., Wu A. M. The biotin/avidin-mediated microtiter plate lectin assay with the use of chemically modified glycoprotein ligand. Anal. Biochem. 1994;221:266–272. doi: 10.1006/abio.1994.1410. [DOI] [PubMed] [Google Scholar]
- 21.Lisowska E., Duk M., Wu A. M. Preparation of biotinylated lectins and application in microtiter plate assays and western blotting. BioMethods. 1996;7:115–129. [Google Scholar]
- 22.Wu A. M., Song S. C., Chang S. C., Wu J. H., Chang K. S. S., Kabat E. A. Further characterization of the binding properties of a GalNAc specific lectin from Codium fragile subspecies tomentosoides. Glycobiology. 1997;7:1061–1066. doi: 10.1093/glycob/7.8.1061. [DOI] [PubMed] [Google Scholar]
- 23.Bochner B. S., Alvarez R. A., Mehta P., Bovin N. V., Blixt O., Whites J. R., Schnaar R. L. Glycan array screening reveals a candidate ligand for Siglec-8. J. Biol. Chem. 2005;280:4307–4312. doi: 10.1074/jbc.M412378200. [DOI] [PubMed] [Google Scholar]
- 24.Korchagina E., Bovin N. V. Synthesis of spaced trisaccharides with blood group A and B specificity, their fragments, and structural analogs. Bioorg. Khim. 1992;18:283–298. [PubMed] [Google Scholar]
- 25.Babino A., Tello D., Rojas A., Bay S., Osinaga E., Alzari P. M. The crystal structure of a plant lectin in complex with the Tn antigen. FEBS Lett. 2003;536:106–110. doi: 10.1016/s0014-5793(03)00037-1. [DOI] [PubMed] [Google Scholar]
- 26.Mo H., Van Damme E. J. M., Peumans W. J., Goldstein I. J. Isolation and characterization of an N-acetyl-D-galactosamine-binding lectin from Dutch Iris bulbs which recognizes the blood group A disaccharide (GalNAc α1–3Gal) J. Biol. Chem. 1994;269:7666–7673. [PubMed] [Google Scholar]
- 27.Kiessling L. L., Pohl N. L. Strength in numbers: non-natural polyvalent carbohydrate derivatives. Chem. Biol. 1996;3:71–77. doi: 10.1016/s1074-5521(96)90280-x. [DOI] [PubMed] [Google Scholar]
- 28.Welply J. K., Abbas S. Z., Scudder P., Keene J. L., Broschat K., Casnocha S., Gorka C., Steininger C., Howard S. C., Schmuke J., et al. Multivalent sialyl-Lex: potent inhibitors of E-selectin-mediated cell adhesion; reagent for staining activated endothelial cells. Glycobiology. 1994;4:259–265. doi: 10.1093/glycob/4.3.259. [DOI] [PubMed] [Google Scholar]
- 29.Sigal G. B., Mammen M., Dahmann G., Whitesides G. M. Polyacrylamides bearing pendant α-sialoside groups strongly inhibit agglutination of erythrocytes by influenza virus: the strong inhibition reflects enhanced binding through cooperative polyvalent interactions. J. Am. Chem. Soc. 1996;118:3789–3800. [Google Scholar]
- 30.Lees W. J., Spaltenstein A., Kingery-Wood J. E., Whitesides G. M. Polyacrylamides bearing pendant α-sialoside groups strongly inhibit agglutination of erythrocytes by influenza A virus: multivalency and steric stabilization of particulate biological systems. J. Med. Chem. 1994;37:3419–3433. doi: 10.1021/jm00046a027. [DOI] [PubMed] [Google Scholar]
- 31.Liang R., Loebach J., Horan N., Ge M., Thompson C., Yan L., Kahne D. Polyvalent binding to carbohydrates immobilized on an insoluble resin. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10554–10559. doi: 10.1073/pnas.94.20.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horan N., Yan L., Isobe H., Whitesides G. M., Kahne D. Nonstatistical binding of a protein to clustered carbohydrates. Biochemistry. 1999;96:11782–11786. doi: 10.1073/pnas.96.21.11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu A. M., Wu J. H., Herp A., Liu J. H. Effect of polyvalencies of glycotopes on the binding of a lectin from the edible mushroom, Agaricus bisporus. Biochem. J. 2003;371:311–320. doi: 10.1042/BJ20021361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh T., Chatterjee U., Wu J. H., Chatterjee B. P., Wu A. M. Carbohydrate recognition factors of a Tα (Galβ1→3GalNAcα1→Ser/Thr) and Tn (GalNAcα1→Ser/Thr) specific lectin isolated from the seeds of Artocarpus lakoocha. Glycobiology. 2005;15:67–78. doi: 10.1093/glycob/cwh144. [DOI] [PubMed] [Google Scholar]
- 35.Wu A. M., Wu J. H., Liu J. H., Singh T., André S., Kaltner H., Gabius H.-J. Effects of polyvalency of glycotopes and natural modifications of human blood group ABH/Lewis sugars at the Galβ1-terminated core saccharides on the binding of domain-I of recombinant tandem-repeat-type galectin-4 from rat gastrointestinal tract (G4-N) Biochimie. 2004;86:317–326. doi: 10.1016/j.biochi.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Lee Y. C. Biochemistry of carbohydrate–protein interaction. FASEB J. 1992;6:3193–3200. doi: 10.1096/fasebj.6.13.1397841. [DOI] [PubMed] [Google Scholar]
- 37.Lee R. T., Lee Y. C. Affinity enhancement by multivalent lectin–carbohydrate interaction. Glycoconj. J. 2000;17:543–551. doi: 10.1023/a:1011070425430. [DOI] [PubMed] [Google Scholar]
- 38.Wu A. M., Wu J. H., Tsai M. S., Kaltner H., Gabius H.-J. Carbohydrate specificity of a galectin from chicken liver (CG-16) Biochem. J. 2001;358:529–538. doi: 10.1042/0264-6021:3580529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell E., Houles C., Sudakevitz D., Wimmerova M., Gautier C., Perez S., Wu A. M., Gillboa-Garber N., Imberty A. Structural basis for oligosaccharide-mediated adhesion of Pseudomonas aeruginosa in the lungs of cystic fibrosis patients. Nat. Struct. Biol. 2002;9:918–921. doi: 10.1038/nsb865. [DOI] [PubMed] [Google Scholar]
- 40.Wu A. M., Wu J. H., Singh T., Liu J. H., Herp A. Lectinochemical studies on the affinity of Anguilla anguilla agglutinin for mammalian glycotopes. Life Sci. 2004;75:1085–1103. doi: 10.1016/j.lfs.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 41.Wu A. M. Lectinochemical studies on the glyco-recognition factors of a Tn (GalNAcα1→Ser/Thr) specific lectin isolated from the seeds of Salvia sclarea. J. Biomed. Sci. 2005;12:167–184. doi: 10.1007/s11373-004-8180-x. [DOI] [PubMed] [Google Scholar]
- 42.Wu A. M., Kabat E. A., Nilsson B., Zopf D. A., Gruezo F. G., Liao J. Immunochemical studies on blood groups: purification and characterization of radioactive 3H-reduced di- to hexasaccharides produced by alkaline β-elimination-borohydride 3H reduction of Smith degraded blood group A active glycoproteins. J. Biol. Chem. 1984;259:7178–7186. [PubMed] [Google Scholar]
- 43.Wu A. M. Carbohydrate structural units in glycoproteins and polysaccharides as important ligands for Gal and GalNAc reactive lectins. J. Biomed. Sci. 2003;10:676–688. doi: 10.1159/000073954. [DOI] [PubMed] [Google Scholar]
- 44.Wu A. M., Wu J. H., Singh T., Hwang P.-Y., Tsai M.-S., Herp A. Lectinochemical studies on the binding properties of a toxic lectin (ricin) isolated from the seeds of Ricinus communis. Chang Gung Med. J. 2005 in the press. [PubMed] [Google Scholar]
- 45.Wu A. M., Shen F.-S., Herp A., Wu J. H. Interaction of hamster submaxillary sialyl-Tn and Tn glycoproteins with Gal, GalNAc and GlcNAc specific lectins. Mol. Immunol. 1994;31:485–490. doi: 10.1016/0161-5890(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 46.Wu A. M., Wu J. H., Shen F. Interaction of a novel Tn (GalNAcα1→Ser/Thr) glycoprotein with Gal, GalNAc and GlcNAc specific lectins. Biochem. Biophys. Res. Commun. 1994;198:251–256. doi: 10.1006/bbrc.1994.1035. [DOI] [PubMed] [Google Scholar]
- 47.Herp A., Borelli C., Wu A. M. Biochemistry and lectin binding properties of mammalian salivary mucous glycoproteins. Adv. Exp. Med. Biol. 1988;228:395–435. doi: 10.1007/978-1-4613-1663-3_15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.