Abstract
Intracellular aggregates of α-syn (α-synuclein) represent pathoanatomical hallmarks of neurodegenerative disorders (synucleinopathies). The molecular mechanisms underlying α-syn aggregation into filamentous inclusions may involve oxidation and nitration of the protein. Whereas the effects of oxidants and nitrating species on soluble α-syn have been studied in detail, the effect of these reactive species on α-syn associated with lipids is still unknown. In the present paper, we report that α-syn bound to small unilamellar liposomes composed of phosphatidylcholine/phosphatidic acid is resistant to oxidation and nitration when compared with soluble α-syn. Additionally, increasing concentrations of unsaturated fatty acids diminished the oxidation and nitration of α-syn upon exposure to fluxes of peroxynitrite (8–20 μM·min−1). To investigate the effect of oxidized lipids on α-syn, the protein was incubated with the bifunctional electrophile 4-HNE [4-hydroxy-2(E)-nonenal]. MS analysis showed the formation of three major products corresponding to the native protein and α-syn plus one or two 4-HNE molecules. Trypsin digestion of the modified protein followed by peptide ‘finger-printing’ revealed that 4-HNE modified the peptide E46GVVHGVATVAEK58. Further analysis of the peptides with liquid chromatography–tandem MS identified the modified residue as His50. The data indicate that the association of α-syn with biological membranes protects the protein from oxidation and nitration and thus diminishes the formation of protein molecules capable of forming aggregates. However, products of lipid peroxidation can also modify α-syn, generating novel protein adducts that could serve as biomarkers for documenting oxidative processes in human as well as animal and cellular models of α-syn aggregation and pathology.
Keywords: lipid–protein adduct, neurodegenerative disorder, Parkinson's disease, peroxynitrite, α-synuclein, nitration
Abbreviations: ESI–MS, electrospray ionization MS; 4-HNE, 4-hydroxy-2(E)-nonenal; LB, Lewy body; LC–MS/MS, liquid chromatography–tandem MS; PA, phosphatidic acid; PAsat, 1,2-dipalmitoyl-PA; PAuns, 1,2-dilinoleoyl-PA; PC, phosphatidylcholine; PD, Parkinson's disease; α-syn, α-synuclein
INTRODUCTION
Wild-type α-syn (α-synuclein) is a major component of Lewy bodies (LBs) in sporadic Parkinson's disease (PD). A number of neurodegenerative diseases, collectively called synucleinopathies, are also characterized by proteinaceous accumulation of α-syn [1,2]. The insoluble α-syn fibrils observed in LBs have an increased content of β-sheet typical of amyloid structures [3]. However, upon binding to acidic phospholipid vesicles, α-syn folds into an α-helical conformation [4]. The N-terminus of α-syn has a series of amphipathic α-helical domains that resemble those found in the class A2 apolipoproteins [5]. Whereas most α-syn is thought to be in its soluble form, a certain portion is associated with membranes.
Aggregated and nitrated α-syn is found in PD brain tissues and other synucleinopathies [6]. We have previously demonstrated that tyrosine residues in α-syn can be oxidized and nitrated by nitrating oxidants such as peroxynitrite and myeloperoxidase/H2O2/nitrite [7]. Nitrating agents also mediate dityrosine formation, leading to the formation and stabilization of filaments either in vitro or in vivo [8]. In particular, nitration of Tyr39 decreases the ability of α-syn to adopt an α-helical conformation thus decreasing its binding to phospholipid vesicles [9]. Despite significant progress in understanding the functional alterations induced by oxidative and nitrative modification of α-syn, the effect of α-syn oxidation and nitration when associated with phospholipid membranes has only been partially addressed [7,9].
Polyunsaturated lipid oxidation breakdown products, like hydroperoxides and aldehydes, modify free amino groups of protein to form lipid–protein adducts [10,11]. Membrane association and the presence of lysine and histidine residues in the amphipathic domains of α-syn may favour lipid–protein adduct formation. In the present paper, we show that α-syn nitration and oxidation mediated by peroxynitrite is modulated by the lipid environment, which may promote the formation of α-syn–lipid adducts as a novel additional post-translational α-syn modification.
EXPERIMENTAL
Materials
Egg PC (phosphatidylcholine), 1,2-dipalmitoyl-PA (phosphatidic acid) (PAsat) and 1,2-dilinoleoyl-PA (PAuns) were obtained from Avanti Polar Lipids (Pelham, AL, U.S.A.). All other reagents were obtained at the highest purity available from standard supply sources.
Preparation of peroxynitrite
Peroxynitrite was synthesized from sodium nitrite and H2O2 using a quenched-flow reactor as described previously [12,13]. Residual H2O2 was eliminated with MnO2 and the remaining nitrite measured was <20% of the peroxynitrite content. Peroxynitrite concentration was determined spectrophotometrically at 302 nm [molar absorption coefficient ϵ=1.67 mM−1·cm−1]. Peroxynitrite first decomposed in 100 mM sodium phosphate buffer (pH 7.4) was tested as a control.
α-Syn overexpression and purification
Human α-syn cDNAs subcloned into the bacterial expression vector pRK172 were expressed in Escherichia coli BL21 (DE3) and purification was performed as described previously [9]. Briefly, after induction with isopropyl β-D-thiogalactoside, bacterial pellets were resuspended in lysis buffer (0.75 M NaCl, 100 mM Mes and 1 mM EDTA, pH 7.0) containing protease inhibitors, heated at 100 °C for 10 min and centrifuged at 7000 g for 30 min. The supernatants were dialysed against 10 mM Tris/HCl (pH 7.5), applied to a Mono Q column and eluted with a 0–0.5 M NaCl gradient. α-Syn fractions were loaded on to a gel filtration column (Superdex 200; Amersham Biosciences) to confirm purity and achieve final buffer exchange.
Preparation of liposomes and α-syn–liposome complexes
Liposomes composed of PC/PAsat or PC/PAuns in 1:1 ratios were prepared as reported previously [4]. From lipid stock solution, chloroform was removed by evaporation with argon, and phospholipids were resuspended in 10 mM sodium phosphate buffer (pH 7.4). The lipid suspension was subjected to ten freeze–thaw cycles using liquid nitrogen and a 37 °C bath, and unilamellar vesicles were prepared with an extrusion device. In order to obtain α-syn–liposome complexes, the protein was incubated with liposomes of different composition at room temperature (23 °C) for 18 h at a 20:1 mass ratio of phospholipids/α-syn [4,14]. Complex formation was evaluated by changes in electrophoretic mobility under native conditions [12].
Biochemical analysis of α-syn modifications
α-Syn–liposome complexes in 100 mM phosphate buffer (pH 7.2) and 100 μM diethylenetriaminepenta-acetic acid at 25 °C were exposed to low controlled fluxes of peroxynitrite (8–20 μM·min−1) for 1 h as described previously [12,13]. Nitration and oxidation of α-syn were evaluated by SDS/PAGE and Western blotting with polyclonal anti-nitrotyrosine [6] and monoclonal anti-α-syn antibodies [7]. Lipid–protein adduct formation was determined by fluorescence spectroscopy as described previously [12]. Briefly, lipid/protein mixtures before and after peroxynitrite addition were solubilized with 1% deoxycholate and the emission spectra were obtained with an AMINCO-Bowman Y-3057 spectrofluorimeter using an excitation lambda of 365 nm [12]. Finally, we studied the possible formation of lipid–protein adducts using 4-HNE [4-hydroxy-2(E)-nonenal] as a lipid oxidation product that can modify protein residues. We incubated α-syn (75 μM) with 4-HNE at a 20:1 molar ratio of 4-HNE/α-syn at 37 °C for 2 h. Excess 4-HNE was removed with a YM-5000 filter (Millipore) followed by two washes with 10% (v/v) ethanol and one wash with either 20% (v/v) acetonitrile and 0.1% formic acid for ESI–MS (electrospray ionization MS) or 100 mM NH4HCO3 (pH 8.5) for tryptic digestion.
Identification of amino acid residues modified by 4-HNE in α-syn
To investigate the site(s) of modification in α-syn by 4-HNE, the native and modified proteins were resuspended in 50 μl of 100 mM NH4HCO3 buffer (pH 8.5) and incubated overnight at 37 °C with sequence-grade trypsin or V8E (Promega, Madison, WI, U.S.A.) in a 1:50 (w/w) ratio of protease/protein. LC–MS/MS (liquid chromatography–tandem MS) analyses were performed on an LCQ classic ion trap mass spectrometer (ThermoFinnigan, San Jose, CA, U.S.A.). LC separation was performed using a BDS Hypersil C-18 column (100 mm×1.0 mm internal diameter; 120 Å, 5 μm; 1 Å=10−10 m) with a flow rate of 50 μl·min−1 using a Waters Alliance 2690 HPLC system equipped with an autosampler, vacuum degasser and column heater (Waters, Milford, MA, U.S.A.). For this purpose, we used 0.1% formic acid in water (solvent A) and 0.1% formic acid in acetonitrile (solvent B). A linear gradient was employed as follows: 0 min, 1% B; 90 min, 91% B; 92 min, 100% B; 94 min, 1% B; and 120 min, 1% B. ESI–MS was conducted using a needle voltage of 4.5 kV. Nitrogen was used as the sheath (60 p.s.i.; 1 p.s.i.=6.9 kPa) and auxiliary (5 units) gas with the heated capillary at 180 °C. Collision-induced dissociation experiments employed helium with a collision energy at 35% (1 V).
RESULTS
Peroxynitrite-mediated α-syn aggregation and nitration are modulated by phospholipid membranes
Previous work demonstrated that α-syn binds to unilamellar liposomes of at least 30% acidic phospholipids [4,14]. To obtain α-syn–liposome complexes, we incubated the protein with PC and PC/PA liposomes and then analysed complex formation by native electrophoresis (Figure 1). α-Syn binds to liposomes that present acidic phospholipids (lane 3) as observed by an increase in electrophoretic mobility compared with control (lane 1). In accordance with that reported in the literature [4,14], α-syn could not bind to PC liposomes (lane 2). Similar results were obtained when complex formation was analysed with gel filtration chromatography (results not shown).
Figure 1. α-Syn interacts with acidic phospholipids.
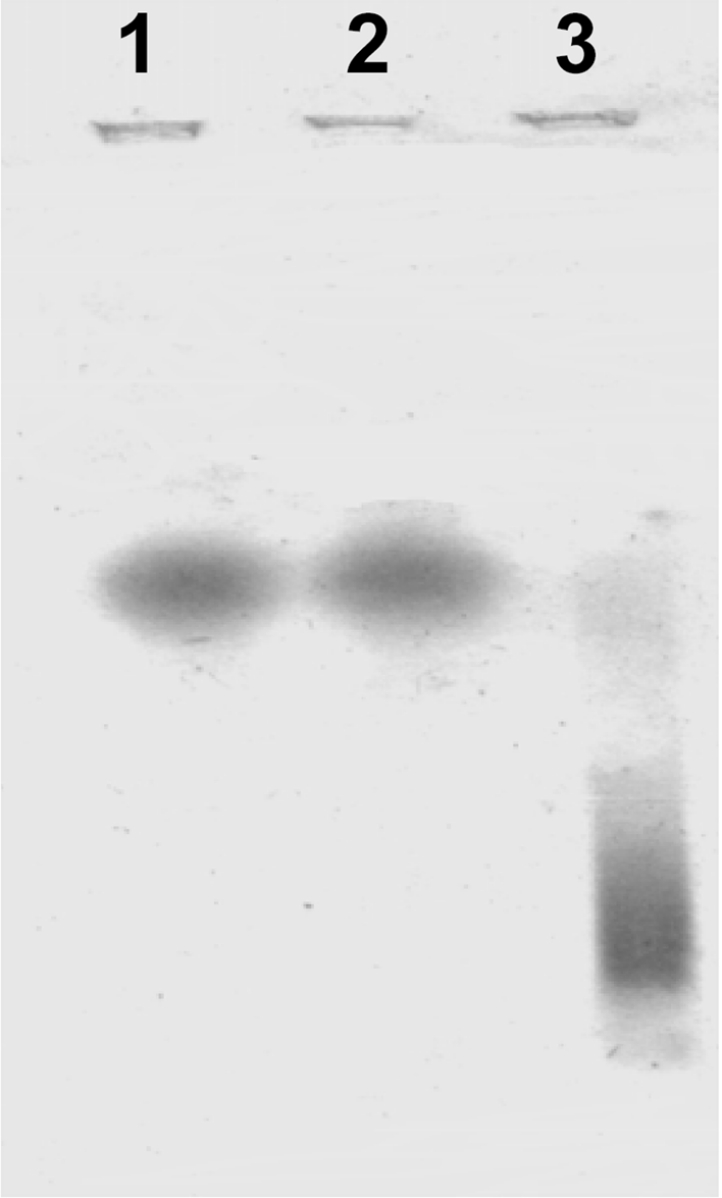
Unilamellar liposomes of PC or PC/PA were prepared as explained in the Experimental section. In order to obtain α-syn–liposome complexes, the protein (0.2 mg·ml−1) was incubated with phospholipid membranes (2 mg·ml−1) for 18 h at room temperature and then analysed for complex formation by native electrophoresis in agarose with Coomassie Blue staining. Lane 1, native α-syn; lane 2, α-syn–PC liposomes; lane 3, α-syn–PC/PA liposomes.
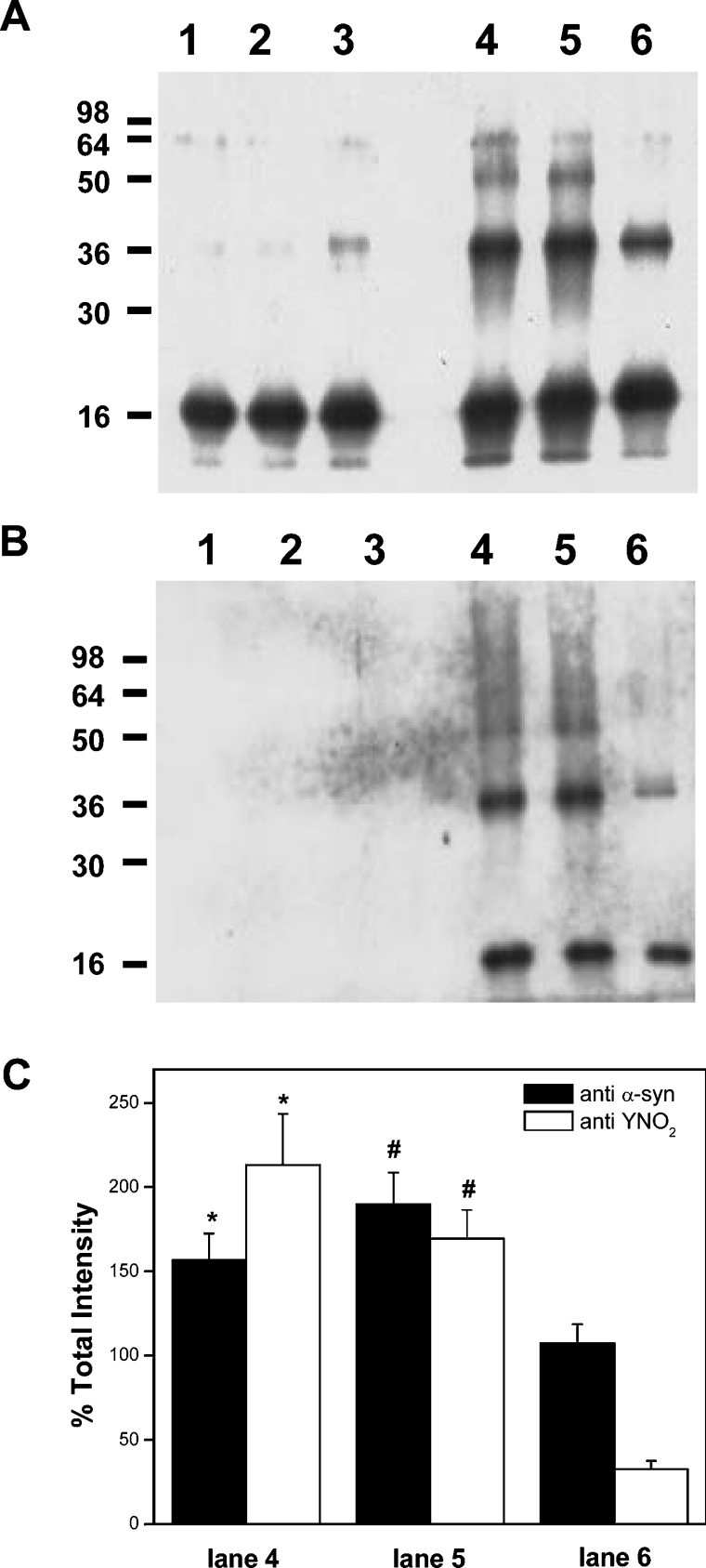
We first analysed α-syn oxidation and nitration by different fluxes of peroxynitrite. Peroxynitrite was added for 1 h at a constant rate to either native protein or to α-syn–liposome solution, and then formation of heat and SDS stable oligomers (Figure 2A) and nitration (Figure 2B) were evaluated by Western blotting. The soluble form of the protein and the α-syn/PC liposome mixture showed similar levels of oligomers after treatment with peroxynitrite (Figure 2A, lanes 4 and 5 respectively). Diminished formation of stable oligomers was observed when peroxynitrite was applied to α-syn–PC/PA liposome complexes (Figure 2A, lane 6). However, a small amount of aggregation was evident when α-syn–PC/PA liposome complexes were incubated in the absence of peroxynitrite (Figure 2A, lane 3). These results are in accordance with previous reports that show aggregation of α-syn after overnight incubations with acidic phospholipids [15]. α-Syn nitration was also decreased in the presence of α-syn–PC/PA liposome complexes (Figure 2B). Oligomer formation and nitration occurred at comparable levels in controls and PC liposome complexes, but nitration decreased proportionally more than oligomerization in α-syn–PC/PA liposome complexes (Figure 2C).
Figure 2. α-Syn binding to PC/PA liposomes modulates protein aggregation and nitration.
α-Syn–liposome complexes were incubated at 25 °C in phosphate buffer and oxidized with peroxynitrite fluxes (20 μM·min−1) for 1 h; samples were taken and analysed for protein oligomer formation (A) and tyrosine nitration (B) by Western blotting using monoclonal anti-α-syn and polyclonal anti-nitrotyrosine antibodies respectively. Lane 1, native α-syn; lane 2, α-syn–PC liposomes; lane 3, α-syn–PC/PA liposomes; lane 4, α-syn, peroxynitrite; lane 5, α-syn–PC liposomes, peroxynitrite; lane 6, α-syn–PC/PA liposomes, peroxynitrite. (C) Densitometry of bands was performed for lanes 4–6, 100% being the darkest, and total intensities were plotted. *P<0.05 and #P<0.05 compared with the condition with α-syn–PA liposome complex.
Unsaturated fatty acids affect peroxynitrite-mediated α-syn oxidation
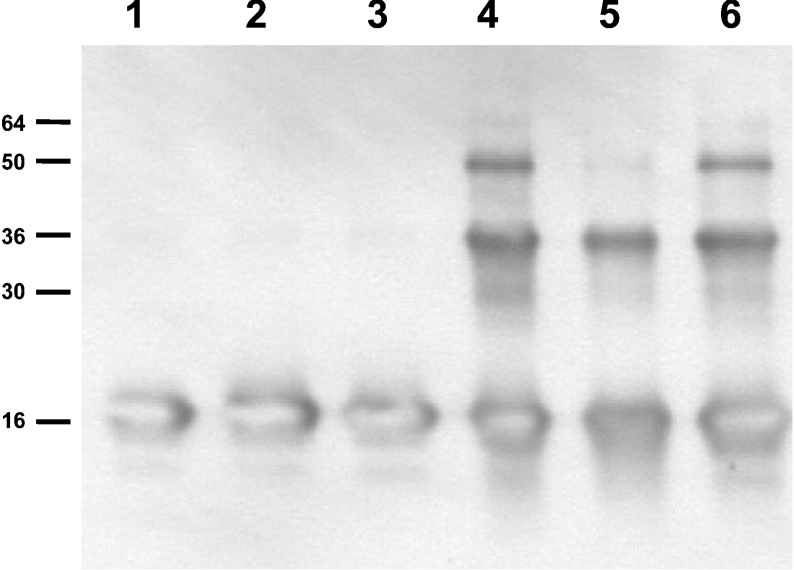
Peroxynitrite-derived radicals (•OH and •NO2) react with unsaturated fatty acids, initiating lipid oxidation reactions [16,17]. The presence of unsaturated fatty acids could either compete with α-syn for peroxynitrite or its oxidation products react with the protein, affecting α-syn aggregation and nitration. To test this hypothesis, we incubated α-syn with liposomes whose acidic phospholipids were esterified with saturated C16:0 fatty acids (PAsat) or with unsaturated C18:2 fatty acids (PAuns) and then oxidized with peroxynitrite. In the presence of saturated PA (Figure 3, lane 6), α-syn exhibited similar extents of aggregation when compared with the soluble protein (Figure 3, lane 4). In contrast, the presence of unsaturated PA diminished protein aggregation (Figure 3, lane 5) and tyrosine nitration (results not shown). Similar to the data in Figure 2(C), a 70% decline in tyrosine nitration and a 23% decline in the formation of stable oligomers were observed.
Figure 3. Unsaturated fatty acids modulate peroxynitrite-mediated α-syn aggregation.
To study the role of unsaturated fatty acids, liposomes that contained PA were prepared using PAsat or PAuns. Protein–liposome complexes were prepared as explained in the Experimental section and oxidized with peroxynitrite (8 μM·min−1) as in Figure 2; α-syn aggregation was evaluated by Western blotting. Lane 1, native α-syn; lane 2, α-syn–PC/PAuns liposomes; lane 3, α-syn–PC/PAsat liposomes; lane 4, native α-syn, peroxynitrite; lane 5, α-syn–PC/PAuns liposomes, peroxynitrite; lane 6, α-syn–PC/PAsat liposomes, peroxynitrite.
Lipid–protein adduct formation as an intermediate of α-syn aggregation
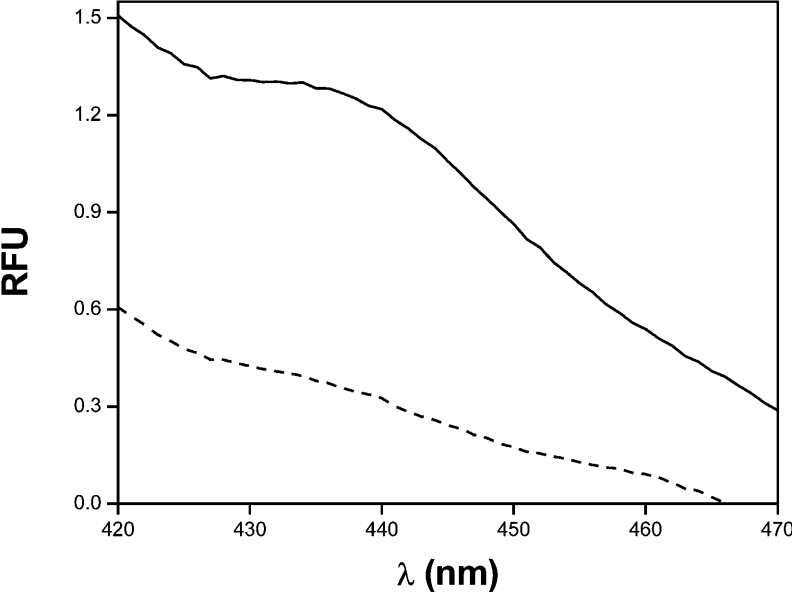
We and others [10–12] showed that lipid oxidation products (e.g. lipid hydroperoxides and aldehydes) modify proteins through lipid–protein adduct formation. These reports led us to investigate the possible formation of lipid–protein adducts. The fluorescence emission spectra of the reaction mixtures after peroxynitrite infusion were determined as described previously [12]. As shown in Figure 4, unsaturated α-syn–PC/PA liposome complexes exhibit a maximum in fluorescence near 440 nm, with an increase in fluorescence of more than 70% compared with control (Table 1). Under the same conditions, α-syn incubated with PC liposomes exhibit a lower increase in fluorescence (∼17%; Table 1). In addition, α-syn was treated with 4-HNE, a reactive product of oxidized ω-6 unsaturated fatty acids, in order to study the effect of lipid–protein adducts on protein aggregation. After 3 h incubation, an increase of soluble α-syn aggregation by 4-HNE was observed (results not shown).
Figure 4. Formation of lipid–protein adducts during peroxynitrite-mediated α-syn oxidation.
α-Syn-PC/PAuns liposomes (dashed line) were oxidized with peroxynitrite fluxes (20 μM·min−1; solid line) for 60 min. Then, deoxycholate was added and the emission spectra were determined using an excitation wavelength of 365 nm [12]. The data correspond to a representative result of three independent experiments.
Table 1. α-Syn–lipid adducts formation by peroxynitrite fluxes.
α-Syn and α-syn–liposome complexes were incubated with fluxes of peroxynitrite. Samples were solubilized with 1% deoxycholate and emission spectra were recorded using an excitation wavelength of 365 nm. The data reported correspond to the means±S.D. of the increase in fluorescence at 440 nm for three independent experiments.
| Condition | Increase in fluorescence at 440 nm |
|---|---|
| α-Syn | 0.10±0.02% |
| α-Syn–PC/PC | 16.70±1.57% |
| α-Syn–PC/PA | 73.30±5.75% |
Formation of 4-HNE–α-syn adducts
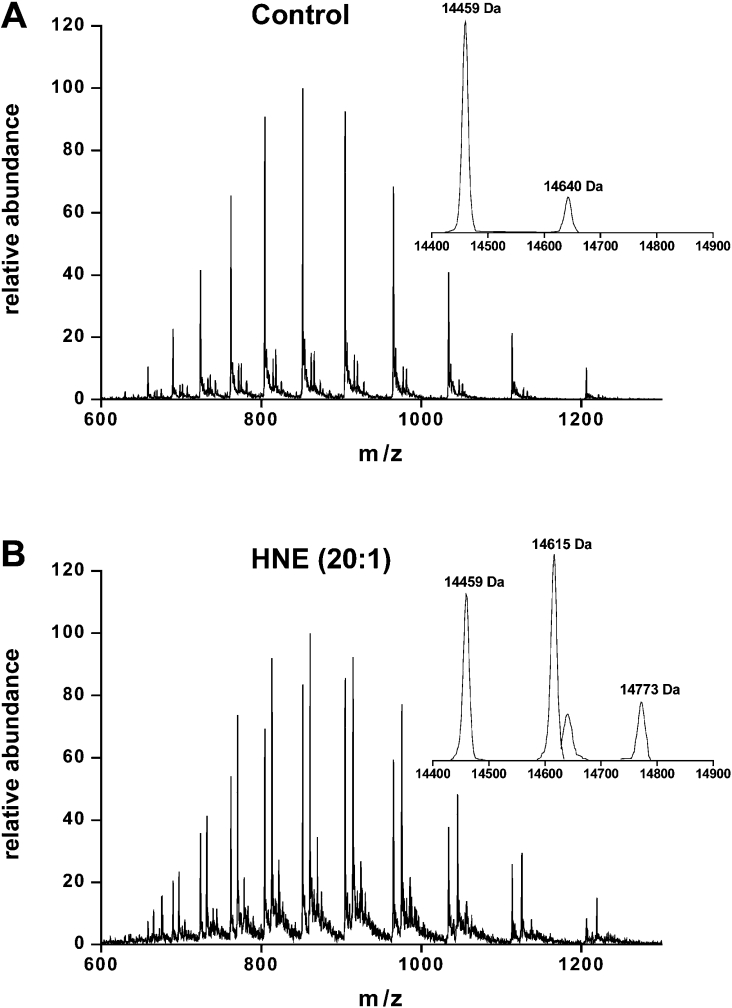
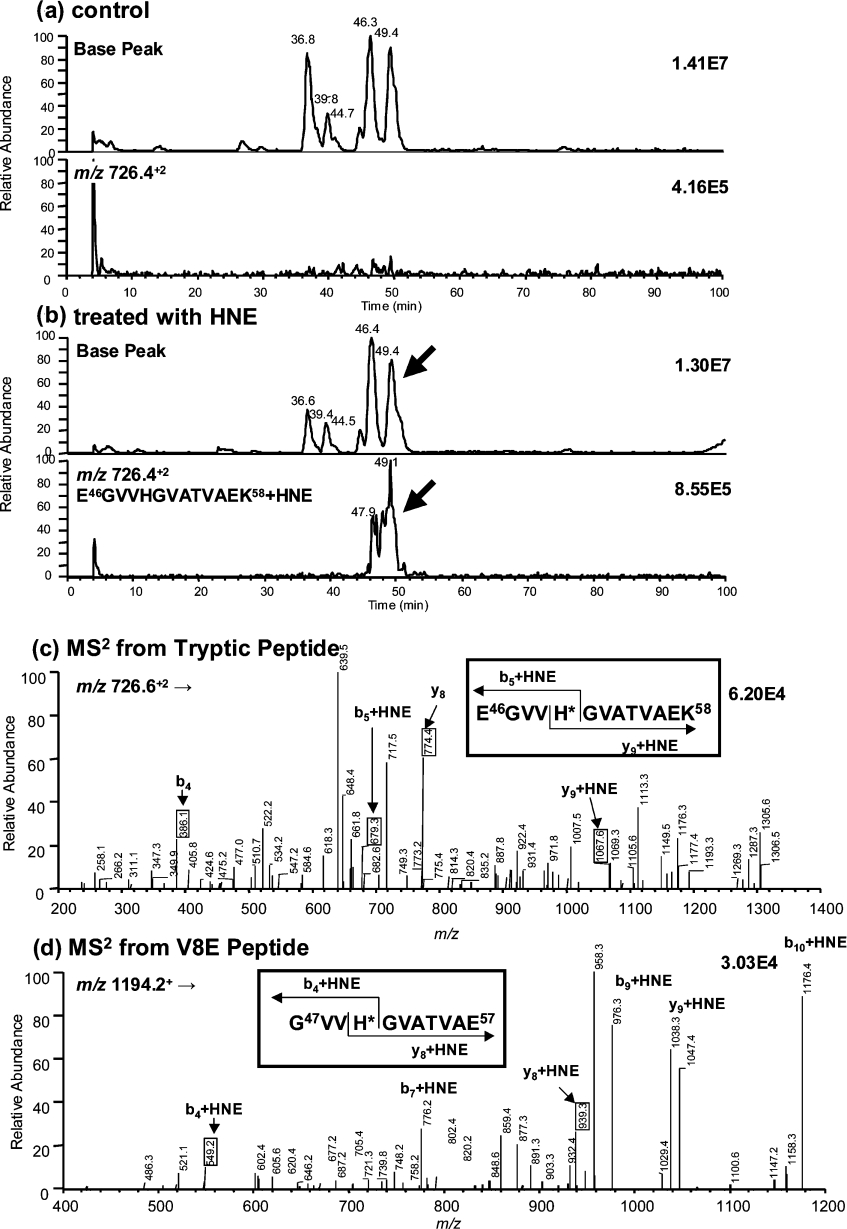
To further characterize lipid–α-syn adducts, α-syn was incubated with a 20-fold excess of 4-HNE and products were analysed by direct injection on to an ESI mass spectrometer. Deconvolution of the spectrum for the unmodified protein showed, as expected, the presence of a single protein with a 14459 Da mass (Figure 5A). Three major products of 14459, 14615 and 14773 Da were observed after the reaction of α-syn with 4-HNE (Figure 5B). The last two species corresponded to the Michael addition of one (+156 Da) and two (+314 Da) molecules of 4-HNE to α-syn respectively. To determine the sites of the 4-HNE modifications, native and 4-HNE-modified α-syn were digested by either trypsin or V8E. Mapping of the peptides generated after trypsin digestion showed the presence of a peptide peak (Figure 6b, retention time 49.1 min) in the 4-HNE-modified protein that was absent from the native protein. This peptide had an m/z ratio of 726.4, which corresponds to the doubly charged peptide of 1295.7 plus 156 Da (one 4-HNE addition). Peptide ‘fingerprinting’ indicated that the 1295.7 Da peptide matched the mass of the E46GVVHGVATVAEK58 peptide in α-syn. In order to confirm the identification of this peptide as the site of modification and to map the residue(s) that were reacted with 4-HNE, LC–MS/MS studies were performed. 4-HNE-modified α-syn was digested by either trypsin (Figure 6c) or V8E (Figure 6d) endoproteases. The de novo sequence confirmed that the E46GVVHGVATVAEK58 peptide was modified and the data unequivocally identified His50 as the 4-HNE-modified residue.
Figure 5. Formation of HNE–α-syn adducts.
α-Synuclein (50 μM; A) was incubated with a 20-fold excess of 4-HNE (1 mM; B) in 100 mM potassium phosphate buffer (pH 7.4) for 2 h at 37 °C and products were measured by ESI–MS. The insets show the mass of the products obtained from deconvolution of the corresponding spectrum.
Figure 6. LC–MS and LC–MS/MS analyses of α-syn after tryptic digestion.
Control (a) and α-syn treated with 4-HNE (b). Arrows show modified peptide. In (a, b), the upper graph corresponds to the base peak chromatogram and the lower graph to the ion chromatogram for MH2+ of tryptic peptide with a modification (E46GVVHGVATVAEK58+4-HNE, m/z 726.4). MS/MS spectrum of tryptic peptide (E46GVVH*GVATVAEK58+4-HNE) from m/z 726.6 (c) and MS/MS spectrum of V8E peptide (G47VVH*GVATVAE57+4-HNE) from m/z 1194.2 (d). Asterisk indicates the amino acid modified by 4-HNE.
DISCUSSION
The present study demonstrates that acidic phospholipids modulate nitrating oxidant-mediated α-syn nitration and stable oligomer formation, particularly in the presence of high concentrations of unsaturated fatty acids. Under these experimental conditions, lipid oxidation-derived products can react with α-syn, leading to the formation of fluorescent lipid–protein adducts. Finally, MS analysis demonstrated that 4-HNE could modify α-syn at His50 within the lipid-binding domain of the protein amino acid sequence.
It is well established that α-syn forms complexes with unilamellar liposomes of at least 30% acidic phospholipids [4]. We used PC/PA liposomes of 50% acidic phospholipids and found that α-syn formed complexes with the liposomes detectable on native agarose gels (Figure 1, lanes 1 and 3). At the same time, incubations with PC liposomes were performed without observation of complex formation (Figure 1, lane 2).
α-Syn is a target for nitrating agents [1,7,18,19], and nitrated α-syn is found in LBs from PD patients [6]. Under our experimental conditions, α-syn tyrosine nitration as well as protein oligomerization was modulated by the interaction of α-syn with phospholipid vesicles (Figure 2). As can be observed in Figure 2(B), the binding of α-syn to PC/PA liposomes resulted in a decrease in tyrosine nitration mediated by peroxynitrite fluxes, compared with both the soluble protein and the α-syn–PC liposomes incubations. Similar results were obtained for the formation of α-syn stable oligomers. It is remarkable to note that in the case of α-syn–PC liposome incubations, the extent of nitration and oligomerization were similar to that of the soluble protein. This result is in accordance with Andrekopoulos et al. [20], who reported that there were no differences in nitration or aggregation of soluble α-syn in the aqueous phase of the PC liposomes when compared with water alone. However, nitration of α-syn in the hydrophobic milieu of the PC/PA liposomes decreased proportionally more than aggregation, suggesting another factor contributing to aggregation in these liposomes.
As radicals formed from the decomposition of peroxynitrite are able to induce lipid peroxidation in lipid and lipoprotein systems [12,16], the overall decrease in α-syn oxidation and nitration could result from a scavenging effect of the phospholipid fatty acids. Alternatively, it may arise from the reaction of lipid peroxidation products with α-syn. To address this issue, we first prepared lipid–protein complexes with different amounts of unsaturated lipids using acidic phospholipid vesicles esterified with saturated (PAsat) or unsaturated (PAuns) fatty acids. Data in Figure 3 show that when peroxynitrite fluxes were added to α-syn–PC/PAsat complexes, the degree of α-syn aggregation was similar to that obtained in the absence of lipids. However, a decrease in peroxynitrite-mediated aggregation when using PC/PAuns (Figure 3) was observed. These results suggest that a scavenger effect of unsaturated fatty acids, due to their reaction with peroxynitrite, diminishes the amount of oxidant reacting with the protein.
Purified α-syn in solution has a random coil conformation [21], which changes to a rich α-helical conformation in the presence of lipid membranes containing acidic phospholipids [4,14,15,22]. α-Syn has a resemblance to the A2 apolipoprotein family through the 11-residue periodicity that gives rise to amphipathic α-helices. These helices participate in a variety of lipid and protein interactions, including α-syn binding to membranes [4,14,23]. This cluster is characterized by the presence of lysine residues in the non-polar–polar interface, stabilizing the lipid–protein interaction [4,14]. The presence of both lysine and histidine residues in the lipid–protein interface led us to propose the possible formation of lipid–protein adducts in our experimental conditions. These amino acids are susceptible to modification by lipid oxidation-derived products such as hydroperoxides and aldehydes [10–12,24], which could affect nitration and aggregation of membrane-bound α-syn. In order to study the formation of these adducts, we determined the fluorescence emission spectra of α-syn and α-syn–liposome complexes before and after peroxynitrite infusion. When peroxynitrite fluxes were added to α-syn–PC/PA complexes, the fluorescence emission spectra showed a widening maximum around 440 nm, characteristic of fluorescent lipid–protein adducts (Figure 4), with an increase in fluorescence of 70% compared with the condition without peroxynitrite (Table 1). When α-syn was incubated with PC liposomes, peroxynitrite induced a smaller increase in fluorescence (Table 1). This result could be due to the incapacity of α-syn to bind PC liposomes or to the lower content of unsaturated fatty acids in PC liposomes compared with PC/PA membranes. One of the most abundant final products of lipid peroxidation is 4-HNE formed by oxidation of ω-6 unsaturated fatty acids such as linoleic and arachidonic acids [11]. When α-syn was incubated with 4-HNE, α-syn aggregation occurred (results not shown). Reaction of oxidized fatty acids with α-syn could contribute to the increase in stable oligomer/nitration ratio apparent in α-syn–PC/PA liposomes (Figure 2C). However, the overall decrease in oligomer formation in α-syn–PC/PA may be due to the initial scavenging of peroxynitrite by the unsaturated fatty acids. The histochemical evidence for the presence of other types of protein adducts, such as α-syn cross-linking with advanced glycosylation end-products in LBs [25], suggests that the presence of lipid–protein adducts could be important in vivo.
The presence of 4-HNE-modified proteins has been documented in a variety of diseases [11]. Therefore 4-HNE-induced modification of α-syn was investigated as a model for lipid–protein adduct formation (Figure 5). The deconvoluted ESI–MS spectrum of unmodified α-syn consisted of a single peak at 14459 Da, corresponding to the native protein (Figure 5A). When α-syn was incubated with 4-HNE (Figure 5B), three peaks appeared at 14459, 14615 and 14773 Da. This suggested that three modified proteins had been formed. 4-HNE has a molecular mass of 156 Da, so the last two products corresponded to α-syn with the addition of one or two 4-HNE molecules respectively. The site(s) of modification in α-syn by 4-HNE were investigated by analysing the peptides generated after trypsin and V8E digestion. The 4-HNE-modified protein contained a doubly charged peptide with an increase in mass of 156 Da compared with control (1451.8 Da versus 1295.7 Da), corresponding to the E46GVVHGVATVAEK58 peptide. This sequence contains two possible sites for adduct formation, His50 and Lys58. To distinguish the sites of adduct formation, trypsin and V8E protease fragments were analysed by LC–MS/MS. An increased mass of 156 Da was identified on His50 [Figure 6(c) and 6(d) and Scheme 1] corresponding to the Michael addition product of 4-HNE to this residue. This histidine residue is situated within the α-helix-forming lipid-binding motifs of the protein. Modification of this residue may affect the conformation of α-syn and its ability to bind lipids. It has been recently demonstrated that His50 participates as a primary binding site for Cu(II) involved in copper-mediated α-syn fibrillization [26], illustrating the important role of His50 in fibril formation. Whereas His50 may be important for metal and bifunctional lipid aldehyde-mediated aggregation, it does not appear to be a site of interaction with other known chemical modifiers of α-syn fibrillization such as dopamine or oxidized intermediates of dopamine. Recent results show that mutation of His50 does not prevent the dopamine-mediated inhibition of α-syn fibrillization [27]. Collectively, these results suggest that several amino acids in α-syn could be targeted for modification by metals, oxidants and small molecules, resulting in unique protein species that differentially modulate the aggregation properties of this protein.
Scheme 1. Michael addition of 4-HNE to His50.
In the onset and progression of many pathologies, such as cardiovascular and neurodegenerative diseases, 4-HNE is implicated as a key mediator [11]. Most of the biological consequences of 4-HNE have been ascribed to the capacity of the α,β-unsaturated aldehyde to react with the nucleophilic sites in proteins and peptides to form covalently modified molecules [11,24]. α-Syn modified by 4-HNE may be a mediator in PD and other neurodegenerative diseases, inducing filament and LB formation. Future work will focus on the presence of lipid-modified α-syn in vivo and its potential involvement in the pathogenesis of synucleinopathies.
Acknowledgments
This work was supported by Comisión Sectorial de Investigación Científica (CSIC, Uruguay) to A. T., NIH grant no. R01 CA95586 to I. A. B., NIH National Institute on Aging to H. I., NIH-Fogarty and the John Guggenheim Foundation to H. R. and Third World Academy of Science, Fondo Clemente Estable and Fundación Manuel Perez to J. M. S.
References
- 1.Duda J. E., Giasson B. I., Chen Q., Gur T. L., Hurtig H. I., Stern M. B., Gollomp S. M., Ischiropoulos H., Lee V. M., Trojanowski J. Q. Widespread nitration of pathological inclusions in neurodegenerative synucleinopathies. Am. J. Pathol. 2000;157:1439–1445. doi: 10.1016/S0002-9440(10)64781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spillantini M. G., Schmidt M. L., Lee V. M., Trojanowski J. Q., Jakes R., Goedert M. Alpha-synuclein in Lewy bodies. Nature (London) 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 3.Giasson B. I., Uryu K., Trojanowski J. Q., Lee V. M. Mutant and wild type human alpha-synucleins assemble into elongated filaments with distinct morphologies in vitro. J. Biol. Chem. 1999;274:7619–7622. doi: 10.1074/jbc.274.12.7619. [DOI] [PubMed] [Google Scholar]
- 4.Davidson W. S., Jonas A., Clayton D. F., George J. M. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J. Biol. Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 5.Jo E., McLaurin J., Yip C. M., St George-Hyslop P., Fraser P. E. Alpha-synuclein membrane interactions and lipid specificity. J. Biol. Chem. 2000;275:34328–34334. doi: 10.1074/jbc.M004345200. [DOI] [PubMed] [Google Scholar]
- 6.Giasson B. I., Duda J. E., Murray I. V., Chen Q., Souza J. M., Hurtig H. I., Ischiropoulos H., Trojanowski J. Q., Lee V. M. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 7.Souza J. M., Giasson B. I., Chen Q., Lee V. M., Ischiropoulos H. Dityrosine cross-linking promotes formation of stable alpha-synuclein polymers. Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J. Biol. Chem. 2000;275:18344–18349. doi: 10.1074/jbc.M000206200. [DOI] [PubMed] [Google Scholar]
- 8.Norris E. H., Giasson B. I., Ischiropoulos H., Lee V. M. Effects of oxidative and nitrative challenges on alpha-synuclein fibrillogenesis involve distinct mechanisms of protein modifications. J. Biol. Chem. 2003;278:27230–27240. doi: 10.1074/jbc.M212436200. [DOI] [PubMed] [Google Scholar]
- 9.Hodara R., Norris E. H., Giasson B. I., Mishizen-Eberz A. J., Lynch D. R., Lee V. M., Ischiropoulos H. Functional consequences of alpha-synuclein tyrosine nitration: diminished binding to lipid vesicles and increased fibril formation. J. Biol. Chem. 2004;279:47746–47753. doi: 10.1074/jbc.M408906200. [DOI] [PubMed] [Google Scholar]
- 10.Uchida K., Sakai K., Itakura K., Osawa T., Toyokuni S. Protein modification by lipid peroxidation products: formation of malondialdehyde-derived N(epsilon)-(2-propenol)lysine in proteins. Arch. Biochem. Biophys. 1997;346:45–52. doi: 10.1006/abbi.1997.0266. [DOI] [PubMed] [Google Scholar]
- 11.Uchida K. Role of reactive aldehyde in cardiovascular diseases. Free Radical Biol. Med. 2000;28:1685–1696. doi: 10.1016/s0891-5849(00)00226-4. [DOI] [PubMed] [Google Scholar]
- 12.Trostchansky A., Batthyany C., Botti H., Radi R., Denicola A., Rubbo H. Formation of lipid-protein adducts in low-density lipoprotein by fluxes of peroxynitrite and its inhibition by nitric oxide. Arch. Biochem. Biophys. 2001;395:225–232. doi: 10.1006/abbi.2001.2583. [DOI] [PubMed] [Google Scholar]
- 13.Trostchansky A., Ferrer-Sueta G., Batthyany C., Botti H., Batinic-Haberle I., Radi R., Rubbo H. Peroxynitrite-mediated LDL oxidation is inhibited by manganese porphyris in the presence of uric acid. Free Radical Biol. Med. 2003;35:1293–1300. doi: 10.1016/j.freeradbiomed.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Perrin R. J., Woods W. S., Clayton D. F., George J. M. Interaction of human alpha-synuclein and Parkinson's disease variants with phospholipids. Structural analysis using site-directed mutagenesis. J. Biol. Chem. 2000;275:34393–34398. doi: 10.1074/jbc.M004851200. [DOI] [PubMed] [Google Scholar]
- 15.Perrin R. J., Woods W. S., Clayton D. F., George J. M. Exposure to long-chain polyunsaturated fatty acids triggers rapid multimerization of synucleins. J. Biol. Chem. 2001;276:41958–41962. doi: 10.1074/jbc.M105022200. [DOI] [PubMed] [Google Scholar]
- 16.Radi R., Beckman J. S., Bush K. M., Freeman B. A. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 17.Radi R., Denicola A., Alvarez B., Ferrer G., Rubbo H. The biological chemistry of peroxynitrite. In: Ignarro L. J., editor. Nitric Oxide Biology and Pathobiology. San Diego, CA: Academic Press; 2000. pp. 57–82. [Google Scholar]
- 18.Takahashi T., Yamashita H., Nakamura T., Nagano Y., Nakamura S. Tyrosine 125 of alpha-synuclein plays a critical role for dimerization following nitrative stress. Brain Res. 2002;938:73–80. doi: 10.1016/s0006-8993(02)02498-8. [DOI] [PubMed] [Google Scholar]
- 19.Paxinou E., Chen Q., Weisse M., Giasson B. I., Norris E. H., Rueter S. M., Trojanowski J. Q., Lee V. M., Ischiropoulos H. Induction of alpha-synuclein aggregation by intracellular nitrative insult. J. Neurosci. 2001;21:8053–8061. doi: 10.1523/JNEUROSCI.21-20-08053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrekopoulos C., Zhang H., Joseph J., Kalivendi S., Kalyanaraman B. Bicarbonate enhances alpha-synuclein oligomerization and nitration: intermediacy of carbonate radical anion and nitrogen dioxide radical. Biochem. J. 2004;378:435–447. doi: 10.1042/BJ20031466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinreb P. H., Zhen W., Poon A. W., Conway K. A., Lansbury P. T., Jr NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 22.Cole N. B., Murphy D. D., Grider T., Rueter S., Brasaemle D., Nussbaum R. L. Lipid droplet binding and oligomerization properties of the Parkinson's disease protein alpha-synuclein. J. Biol. Chem. 2002;277:6344–6352. doi: 10.1074/jbc.M108414200. [DOI] [PubMed] [Google Scholar]
- 23.Segrest J. P., Jones M. K., De Loof H., Brouillette C. G., Venkatachalapathi Y. V., Anantharamaiah G. M. The amphipathic helix in the exchangeable apolipoproteins: a review of secondary structure and function. J. Lipid Res. 1992;33:141–166. [PubMed] [Google Scholar]
- 24.Uchida K., Toyokuni S., Nishikawa K., Kawakishi S., Oda H., Hiai H., Stadtman E. R. Michael addition-type 4-hydroxy-2-nonenal adducts in modified low-density lipoproteins: markers for atherosclerosis. Biochemistry. 1994;33:12487–12494. doi: 10.1021/bi00207a016. [DOI] [PubMed] [Google Scholar]
- 25.Münch G., Lüth H. J., Wong A., Arendt T., Hirsch E., Ravid R., Riederer P. Crosslinking of alpha-synuclein by advanced glycation endproducts – an early pathophysiological step in Lewy body formation? J. Chem. Neuroanat. 2000;20:253–257. doi: 10.1016/s0891-0618(00)00096-x. [DOI] [PubMed] [Google Scholar]
- 26.Rasia R. M., Bertoncini C. W., Marsh D., Hoyer W., Cherny D., Zweckstetter M., Griesinger C., Jovin T. M., Fernandez C. O. Structural characterization of copper(II) binding to alpha-synuclein: insights into the bioinorganic chemistry of Parkinson's disease. Proc. Natl. Acad. Sci. U.S.A. 2005;102:4294–4299. doi: 10.1073/pnas.0407881102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norris E. H., Giasson B. I., Hodara R., Xu S., Trojanowski J. Q., Ischiropoulos H., Lee V. M. Reversible inhibition of alpha-synuclein fibrillization by dopaminochrome-mediated conformational alterations. J. Biol. Chem. 2005;280:21212–21219. doi: 10.1074/jbc.M412621200. [DOI] [PubMed] [Google Scholar]