Abstract
CD16b is unique in that it is the only Fc receptor linked to the plasma membrane by a GPI (glycosylphosphatidylinositol) anchor. GPI-anchored proteins often preferentially localize to DRMs (detergent-resistant membranes) that are rich in sphingolipids and cholesterol and play an important role in signal transduction. Even though the responses to CD16b engagement have been intensively investigated, the importance of DRM integrity for CD16b signalling has not been characterized in human neutrophils. We provide direct evidence that CD16b constitutively partitions with both low- and high-density DRMs. Moreover, upon CD16b engagement, a significant increase in the amount of the receptor is observed in high-density DRMs. Similarly to CD16b, CD11b also resides in low- and high-density DRMs. In contrast with CD16b, the partitioning of CD11b in DRMs does not change in response to CD16b engagement. We also provide evidence for the implication of Syk in CD16b signalling and its partitioning to DRMs in resting and activated PMNs (polymorphonuclear neutrophils). Additionally, DRM-disrupting agents, such as nystatin and methyl-β-cyclodextrin, alter cellular responses to CD16b receptor ligation. Notably, a significant increase in the mobilization of intracellular Ca2+ and in tyrosine phosphorylation of intracellular substrates after CD16b engagement is observed. Altogether, the results of this study provide evidence that high-density DRMs play a role in CD16b signalling in human neutrophils.
Keywords: CD16b, detergent-resistant membranes, Fc receptor, neutrophil, signal transduction, tyrosine phosphorylation
Abbreviations: DFP, di-isopropyl fluorophosphates; DRM, detergent-resistant membrane; DRM-H, high-density DRM; DRM-L, low-density DRM; EGF, epidermal growth factor; GPI, glycosylphosphatidylinositol; HBSS, Hanks' balanced salt solution; HRP, horseradish peroxidase; mAb, monoclonal antibody; NP40, Nonidet P-40; PMN, polymorphonuclear neutrophil; RLB, modified relaxation buffer
INTRODUCTION
The PMN (polymorphonuclear neutrophil) is a phagocytic cell that plays a pivotal role in acute inflammatory responses. PMN involvement in a local inflammatory episode begins with a chemotactic response and migration to the site of injury. This is accompanied by the priming and activation of PMNs, resulting in adhesion to endothelial cells and eventual extravasation through the blood vessel wall and into the tissue. On arrival at the site of inflammation, PMNs engulf and destroy the initiating agent.
Phagocytosis at the inflammatory site involves, in part, the participation of Fc receptors [1]. Fc receptors recognize and bind the Fc region of immunoglobulins that opsonize the antigen. Two different types of Fc receptors for IgG are constitutively expressed on the surface of human PMNs, namely, FcγRIIA (CD32a) and FcγRIIIB (CD16b) [2,3]. CD16b is exclusively expressed on PMNs. A third type of Fc receptor, FcγRI (CD64), is strongly expressed following PMN activation [4].
CD16b is unique amongst Fc receptors since it is linked to the plasma membrane by a GPI (glycosylphosphatidylinositol) anchor [5]. Proteins attached to the cell surface by GPI anchors are thought to preferentially reside in dynamic assemblies of cholesterol and sphingolipids that are located in the exoplasmic leaflet of the membrane bilayer known as DRMs (detergent-resistant membranes) (reviewed in [6,7]). Evidence has been obtained indicating that, in some cases, DRMs behave like platforms within which protein complexes form and transmit signals across the plasma membrane upon receptor engagement.
Despite the lack of a physical link between DRMs and the inner leaflet of the plasma membrane, GPI proteins trigger signalling events within cells [8]. Multivalent cross-linking of CD16b, for instance, elicits downstream signals, including the mobilization of intracellular Ca2+ [9,10] and the phosphorylation of the Src kinase Hck [11], ERK (extracellular signal regulated kinase) [12,13], p38 [12,13] and the tyrosine kinase Pyk2 [13]. The effector functions observed after CD16b cross-linking include actin filament assembly [14], degranulation [15], phagocytosis of concanavalin A-opsonized erythrocytes [16], activation of respiratory burst [17,18], killing of chicken erythrocytes opsonized with anti-CD16b-Fab [4] and the recruitment of neutrophils in immune-complex-mediated inflammation [19].
A possible mechanism through which signals are transduced across the plasma membrane upon engagement of GPI-linked proteins is through lateral membrane interactions with transmembrane receptors [20]. Previous reports provide evidence for a possible role for CR3 (CD11b/CD18) as a transmembrane partner of CD16b [21]. Several studies revealed that CD11b is in physical proximity to CD16b on the plasma membrane of resting PMNs [22,23]. Upon prolonged CD11b or CD16b cross-linking, these two receptors co-cap on the surface of the cell [24]. Moreover, CD16b-mediated phagocytosis in transfected fibroblasts is dependent on the surface expression of CR3 [25]. CD16b may also associate with other transmembrane receptors for signalling across the plasma membrane, one of which may be CD32a. Several reports provide evidence for a functional relationship between CD32a and CD16b [11,17,26–28]. The co-cross-linking of CD16b and CD32a, for instance, induces a synergistic enhancement of Ca2+ transients and phagocytosis [29].
Much effort has been made to characterize the signalling pathways activated by the CD16b receptor. As yet, however, the importance of DRM integrity for CD16b signalling has been largely unexplored. Moreover, the distribution of CD16b in DRMs of PMNs has not been fully characterized. It has only been reported that superoxide generation induced by opsonized zymosan in neutrophils through the co-operation of CD16b and CR3 is resistant to methyl-β-cyclodextrin [30].
The present study was initiated to examine the distribution of the CD16b receptor, as well as its presumed co-receptor (CR3) and an associated signalling molecule, Syk, in DRMs in resting and activated PMNs. By employing a gradient that extends beyond the density range normally used to isolate DRMs, we report the partitioning of the majority of CD16b receptors with DRM-H (high-density DRMs) in human PMNs and the translocation of the receptor to these membrane-skeleton-associated signalling domains upon receptor activation. In contrast with CD16b, the distribution of CD11b and Syk in DRMs does not change upon CD16b cross-linking. The effect of cholesterol-disturbing agents on CD16b signalling was also investigated. The results of the present study provide evidence for the involvement of DRMs in CD16b signalling in human PMNs.
MATERIALS AND METHODS
Antibodies
The mAb (monoclonal antibody) used to cross-link CD16b in all our experiments was 3G8 F(ab′)2 (Ancell, catalogue number 165-520). The unconjugated AffiniPure F(ab′)2 fragment of goat anti-mouse IgG, F(ab′)2 fragment-specific (catalogue number 115-006-072) was purchased from Jackson ImmunoResearch Laboratories. The anti-CD16 mAb 7.5.4, raised against recombinant CD16b extracellular domains, was prepared as described previously [31] and was used for immunoblotting. The anti-phosphotyrosine mAb (catalogue number 05-321, clone 4G10) was purchased from UBI, the mAb anti-flotillin-1 (catalogue number 610820) from BD Biosciences and the anti-Syk mAb (MAB88906) from Chemicon International. Rabbit anti-CD11b antibodies were raised against a synthetic peptide (KRQYKDMMSEGGPPG) corresponding to amino acids 1134–1148 of the CD11b chain and purified by Protein A chromatography. The secondary antibody, HRP (horseradish peroxidase)-labelled sheep anti-mouse IgG (NXA931), was obtained from Amersham Biosciences, and the donkey anti-rabbit HRP (catalogue number 711-035-152) was from Jackson ImmunoResearch Laboratories.
Chemicals
Dextran T-500, Sephadex G-10 and Protein A–Sepharose beads were purchased from Amersham Biosciences. Ficoll-Paque was obtained from Wisent. DFP (di-isopropyl fluorophosphate) was purchased from Serva Electrophoresis and the enhanced chemiluminescence detection kit was obtained from PerkinElmer. Nystatin, methyl-β-cyclodextrin, sodium orthovanadate, Triton X-100 and OptiPrep density gradient medium were purchased from Sigma–Aldrich. Aprotinin and leupeptin were obtained from Roche Diagnostics. NP40 (Nonidet P-40) was purchased from Calbiochem. Fura-2-acetoxymethyl ester was obtained from Molecular Probes.
Isolation of PMNs
Blood was obtained from healthy adult volunteers in tubes containing heparin. After sedimentation of erythrocytes in 2% dextran, PMNs were purified by centrifugation at 350 g for 20 min on Ficoll-Paque cushions under aseptic conditions. Contaminating erythrocytes were removed by hypotonic lysis and cells were resuspended in Mg2+-free HBSS (Hanks balanced salt solution) containing 1.6 mM CaCl2.
Stimulation of cells with an anti-CD16b antibody
PMNs were incubated for 10 min at 22 °C with 1 mM DFP before an incubation with 0.5 μg/ml 3G8 F(ab′)2 mAb per 2×106 cells. Cross-linking was subsequently performed with 5.8 μg/ml F(ab′)2, goat anti-mouse F(ab′)2 at the temperatures and for the times indicated.
To determine the effect of nystatin on PMN signalling, PMNs at 107 cells/ml were incubated in HBSS containing 30 μg/ml nystatin per 5×106 cells for 1 h at 37 °C before CD16b receptor engagement. Incubation of PMNs with 3G8 F(ab′)2 and the cross-linking antibody were both performed at 37 °C. Since the stock solution of nystatin was prepared in DMSO, PMNs were also incubated in DMSO alone before CD16b receptor engagement as a negative control. Similar experimental conditions were used for methyl-β-cyclodextrin which was incubated for 30 min at 37 °C with PMNs (107 cells/ml) in HBSS at a final concentration of 10 mM.
Isolation of DRMs
DRMs were isolated by sucrose-gradient ultracentrifugation essentially as described by Robbins et al. [32]. Briefly, 2×107 PMNs were pelleted at 400 g for 2 min at 4 °C, resuspended and lysed in 1 ml of cold MBS buffer [25 mM MES (pH 6.5), 150 mM NaCl, 2.5 mM orthovanadate, 10 μg/ml leupeptin and 10 μg/ml aprotinin] containing 1% Triton X-100 for 30 min on ice. The PMN lysate was then adjusted to 40% sucrose by adding an equal volume of 80% sucrose in MBS buffer. An 800 μl aliquot of lysate was overlaid with a discontinuous sucrose density gradient (2.8 ml of 30% sucrose, then 0.4 ml of MBS lysis buffer) and centrifuged at 43000 rev/min for 16 h in a SW60 rotor. As described in [33], 300 μl fractions were collected from the bottom of the gradient, and proteins were precipitated. Precipitated proteins from each fraction were resuspended in 2×Laemmli's sample buffer [1× is 62.5 mM Tris/HCl (pH 6.8), 4% (w/v) SDS, 5% (v/v) 2-mercaptoethanol, 8.5% (v/v) glycerol, 2.5 mM sodium orthovanadate, 10 μg/ml leupeptin, 10 μg/ml aprotinin and 0.025% (w/v) Bromophenol Blue]. The pellet of the gradient was washed twice with cold HBSS and resuspended in 300 μl of 2×Laemmli's sample buffer. Samples were then heated for 7 min at 100 °C before SDS/PAGE analysis.
To analyse the lipid raft components in the pellet of the sucrose gradient, lysis of PMNs was performed in cold MBS lysis buffer containing 0.1 M sodium carbonate (pH 11). The PMN lysate was then passed ten times through a 22G needle to shear the DNA, before the preparation of the sucrose gradient.
For the OptiPrep gradient experiments, 2×107 PMNs were pelleted at 400 g for 2 min at 4 °C, resuspended and lysed in 500 μl of cold lysis buffer [10 mM Hepes (pH 7.4), 150 mM NaCl, 2.5 mM sodium orthovanadate, 10 μg/ml leupeptin and 10 μg/ml aprotinin] containing 1% (v/v) Triton X-100 for 30 min on ice. The PMN lysate was then adjusted to 40% (v/v) OptiPrep with a stock solution (59.4% OptiPrep and 10 mM Hepes, pH 7.4). Aliquots (700 μl) were transferred to 4 ml centrifuge tubes and overlaid with 700 μl of ice-cold solutions of 35, 30, 25, 20 and 0% OptiPrep. The gradients were centrifuged at 348000 g (80000 rpm) for 3 h at 4 °C in a TLA 100.4 rotor. A total of 13 fractions of 300 μl were collected from the top of the gradients. Proteins were chloroform/methanol precipitated as described previously [33]. Precipitated proteins from each fraction were resuspended in 2×Laemmli's sample buffer. Samples were then heated for 7 min at 100 °C before SDS/PAGE analysis.
Isolation and stimulation of plasma membrane preparations
Isolated neutrophils were resuspended at 6–8×107 cells/ml in RLB (modified relaxation buffer) and lysed by nitrogen cavitation as described previously [34]. Purified membranes were resuspended in RLB at 2–2.5×107 cell equivalents/ml and then incubated with 5 μg/ml 3G8 F(ab′)2 antibody for 5 min at 4 °C. A buffer composed of 2 mM ATP and 400 μM MnCl2 (final concentrations), was added at 22 °C 3 min before the addition of 2 mM sodium orthovanadate and 50 μg/ml goat F(ab′)2 directed against mouse F(ab′)2. The reaction mixture was then incubated for 5 min at 22 °C. Plasma membranes were then chilled on ice for 5 min and then incubated with 1% (v/v) NP40 for 20 min on ice. The solubilized membranes were then centrifuged at 100000 g for 1 h and the supernatants and pellets analysed by SDS/PAGE (10 % gels).
Immunoprecipitation
The CD16b receptor was cross-linked for 15 s at 37 °C on the surface of PMNs after an incubation with 3G8 F(ab′)2 for 1 min at 37 °C. The immunoprecipitation was performed under denaturing conditions as described previously [35]. Briefly, non-stimulated or stimulated PMNs were lysed directly in modified 2×Laemmli's sample buffer [125 mM Tris/HCl (pH 6.8) 6% (w/v) SDS, 2% (v/v) 2-mercaptoethanol, 17% (v/v) glycerol, 5 mM sodium orthovanadate, 20 μg/ml aprotinin, 20 μg/ml leupeptin and 0.05% (w/v) Bromophenol Blue] and heated for 7 min at 100 °C. Lysates were then filtered through a Sephadex G-10 column to remove the denaturing and reducing agents. The filtered lysates were adjusted to 1% (v/v) NP40, 0.005% (w/v) BSA, 2 mM sodium orthovanadate, 10 μg/ml aprotinin and 10 μg/ml leupeptin. The antibodies (1.25 μg of anti-Syk/incubation) were bound to Protein A–Sepharose by incubation in a 30% slurry of Sepharose for 1 h at 4 °C. The antibody/Sepharose mixture was washed once in washing buffer [62.5 mM Tris/HCl (pH 6.8), 1% (v/v) NP40, 1% (v/v)glycerol, 2.0 mM sodium orthovanadate, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 137 mM NaCl, and 0.001% (w/v) BSA] and added to the lysates for immunoprecipitation. Following immunoprecipitation for 3 h at 4 °C, the beads were washed three times in washing buffer and solubilized in 60 μl of sample buffer.
Immunoblotting
Samples were loaded on to SDS/7.5–20% polyacrylamide, gradient gels. Separated proteins were transferred from the gels on to Immobilon PVDF membranes as previously described [36]. The membranes were blocked with 2% (w/v) gelatin for the anti-Syk and 4G10, and 5% (w/v) dried milk powder for anti-CD11b and anti-flotillin-1, antibodies for 30 min at 37 °C. The immunoblotting procedure for the 7.5.4 mAb involved blocking in 10% (w/v) dried milk powder for 30 min at 37 °C. All the blocking agents were dissolved in TBS-Tween [25 mM Tris/HCl (pH 7.8), 190 mM NaCl and 0.15% (v/v) Tween 20]. The anti-Syk, 4G10 and anti-flotillin-1 antibodies were diluted in TBS-Tween and incubated for 1 h at 37 °C. The anti-CD11b and anti-CD16 antibodies were diluted in 1% (w/v) dried milk powder and incubated for 1 h at 37 °C. The membranes were then rinsed three times in TBS-Tween and incubated with an HRP-labelled secondary antibody for 30 min at 37 °C. After three washes in TBS-Tween, membranes were developed using the enhanced chemiluminescence detection system (Amersham Biosciences).
The antibody dilutions used were as follows, 1/500 for the antiflotillin-1 (0.25 μg/ml final concentration), anti-CD16b and anti-CD11b antibodies, the anti-Syk antibody was diluted 1/2000 (0.1 μg/ml final concentration), and the 4G10 antibody was diluted 1/4000 (0.25 μg/ml final concentration). The secondary antibodies, HRP-labelled sheep anti-mouse IgG and donkey anti-rabbit IgG, were used at a final concentration of 1/20000.
Measurement of Ca2+ mobilization
PMNs at a concentration of 107 cells/ml were incubated at 37 °C with 1 μM fura-2-acetoxymethyl ester for 30 min. Extracellular probe was removed by washing in HBSS and cells were resuspended at a concentration of 5×106 cells/ml. Cells were stimulated with 2 μg of 3G8 F(ab′)2 mAb/107 cells followed by cross-linking with 80 μg of F(ab′)2 goat anti-mouse F(ab′)2/107 cells. Fluorescence was monitored at 37 °C in a fluorescence spectrophotometer (SLM 8000C) at an excitation wavelength of 340 nm and an emission wavelength of 510 nm. The internal Ca2+ concentrations were calculated as described [37].
RESULTS
A fraction of CD16b constitutively resides in DRMs in human PMNs
CD16b is capable of transducing signals across the plasma membrane as shown by the phosphorylation of intracellular substrates [11–13] and mobilization of Ca2+ [9,10] upon its engagement. To determine whether CD16b signals through DRMs due to its GPI-anchor, we began by determining the distribution of CD16b in DRMs in resting PMNs.
DRMs were isolated by sucrose gradient centrifugation after cell lysis in cold 1% (v/v) Triton X-100 as described by Barabé et al. [38]. The density range in the sucrose gradient was between 1 and 1.17 g/ml. This procedure separates the lipid-rich components from the bulk of the Triton X-100-insoluble material in cell lysates. The latter includes nuclear remnants and the cytoskeleton that pellet upon sucrose gradient ultracentrifugation. The DRM components band at a low density on a sucrose gradient, while Triton X-100 soluble proteins band at a high density. As described in other cell types [6], DRMs isolated from human PMNs could be visualized as a band in the low-density part of the sucrose gradient (results not shown).
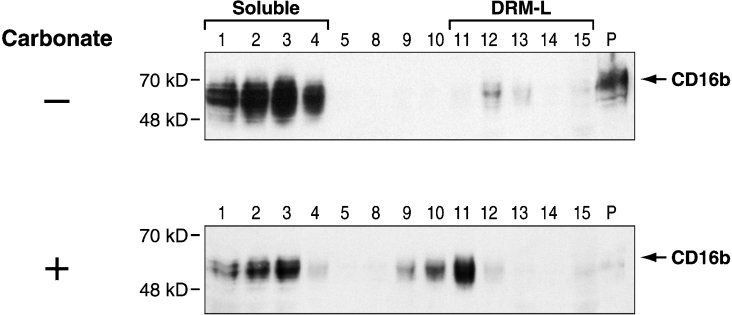
The results in Figure 1 (upper panel) show that the majority of CD16b receptors in non-stimulated PMNs reside in the soluble fractions of the gradient (fractions 1–4), a portion in the pellet (P) and a small amount in the low-density fractions of the gradient (fractions 11–15) corresponding to DRM-L (low-density DRMs). The CD16b in the pellet may localize to cytoskeletal components that are known to be insoluble in Triton X-100, to DRMs that non-specifically associate with pellet components during lysis as described by Brown and Rose [39] or to DRM-H [40].
Figure 1. Localization of CD16b to DRM-L in non-stimulated PMNs.
PMNs were lysed in cold lysis buffer containing 1% Triton X-100 in the absence (−) or presence (+) of sodium carbonate and overlaid with a discontinuous sucrose gradient (as described in the Materials and methods section). Fractions were analysed by SDS/PAGE and immunoblotted with the anti-CD16 7.5.4 mAb. Fractions 1–4 contain soluble cellular components, fractions 11–15 contain DRM-L, and the remainder of the cellular components that partition in the pellet were loaded in lane P. Molecular mass sizes are given in kDa.
To determine whether the CD16b in the pellet localizes to DRMs, cells were treated with a sodium carbonate-containing buffer that is known to release DRMs from the pellet fraction by interfering with protein–protein interactions [39]. Proteins associated with DRMs float to the top of the gradient upon sodium carbonate treatment. The results shown in Figure 1 (lower panel) show that sodium carbonate effectively releases CD16b from the pellet. The observation that the released CD16b partitions with cellular components that float in the sucrose gradient is strongly suggestive that it is associated with DRMs. Moreover, the partitioning of these DRMs in fractions of a higher density than DRM-L is indicative that they are probably DRM-H. Similarly to DRM-L, DRM-H are rich in cholesterol [40]. What distinguishes DRM-H from DRM-L is that they are enriched in membrane skeleton proteins. DRM-H are therefore a subset of signalling domains that are associated with the membrane skeleton. Owing to their density, DRM-H do not float in the standard sucrose gradient used to isolate DRM-L [41]. Instead, fractions with densities corresponding to DRM-H are recovered in the pellet of the sucrose gradient described in Figure 1. DRM-H can be more conveniently isolated using an OptiPrep, rather than a sucrose, gradient [34,40].
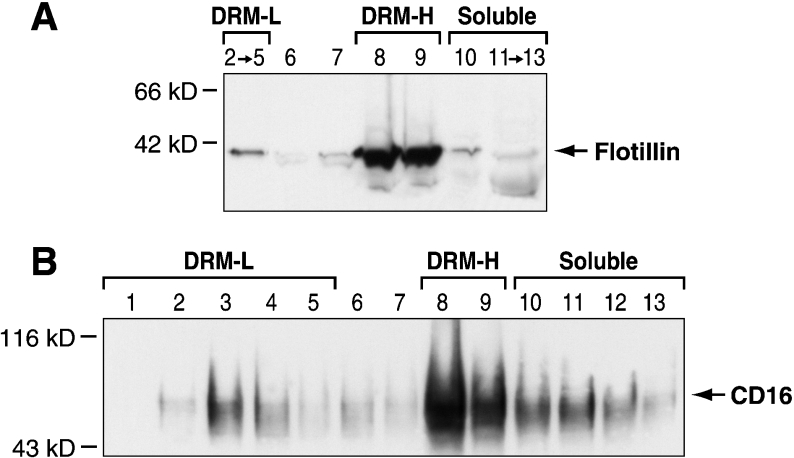
The observation that a portion of the CD16b receptors resides in DRMs of higher density than DRM-L led to the following experiments that confirm the partitioning of CD16b in DRM-H. Briefly, PMNs were lysed in Triton X-100 as described in the Materials and methods section and overlaid with an OptiPrep step gradient. To validate our OptiPrep gradient, the distribution of the lipid-raft-associated integral membrane protein flotillin-1 in the fractions collected was determined. As shown in Figure 2(A), flotillin-1 is enriched in fractions 2–5, 8 and 9. The density of these fractions, 1.08–1.15 g/ml and 1.18–1.20 g/ml respectively, indicates that DRM-L partition in fractions 2–5 and DRM-H in fractions 8 and 9. Similar densities for DRM-L and DRM-H were reported by Nebl et al. [40].
Figure 2. Localization of CD16b to DRM-L and DRM-H in non-stimulated PMNs.
PMNs were lysed in cold lysis buffer containing 1% Triton X-100 and overlaid with a discontinuous gradient of OptiPrep (as described in the Materials and methods section). Fractions were analysed by SDS/PAGE and immunoblotted with an anti-flotillin-1 antibody (A) or the anti-CD16 7.5.4 mAb (B). Fractions 2–5 contain DRM-L, fractions 8 and 9 contain DRM-H, and fractions 10–15 contain the soluble cellular components. Molecular mass sizes are given in kDa.
To determine the distribution of CD16b in DRM-L and DRM-H, fractions collected from the OptiPrep gradient were immunoblotted with the anti-CD16 mAb. The results in Figure 2(B) demonstrate that a significant amount of CD16b receptors localize to DRM-H like the DRM-H marker flotillin-1 (fractions 8 and 9). The remainder localizes to DRM-L (fractions 2–5) as well as to fractions of the gradient containing cellular components solubilized by Triton X-100 (fractions 10–13). Altogether, the above observations provide direct evidence that CD16b resides constitutively in DRM-L and DRM-H in resting human PMNs. No CD16b was found in the pellet of the OptiPrep gradient (results not shown).
CD16b translocates to DRM-H after ligation
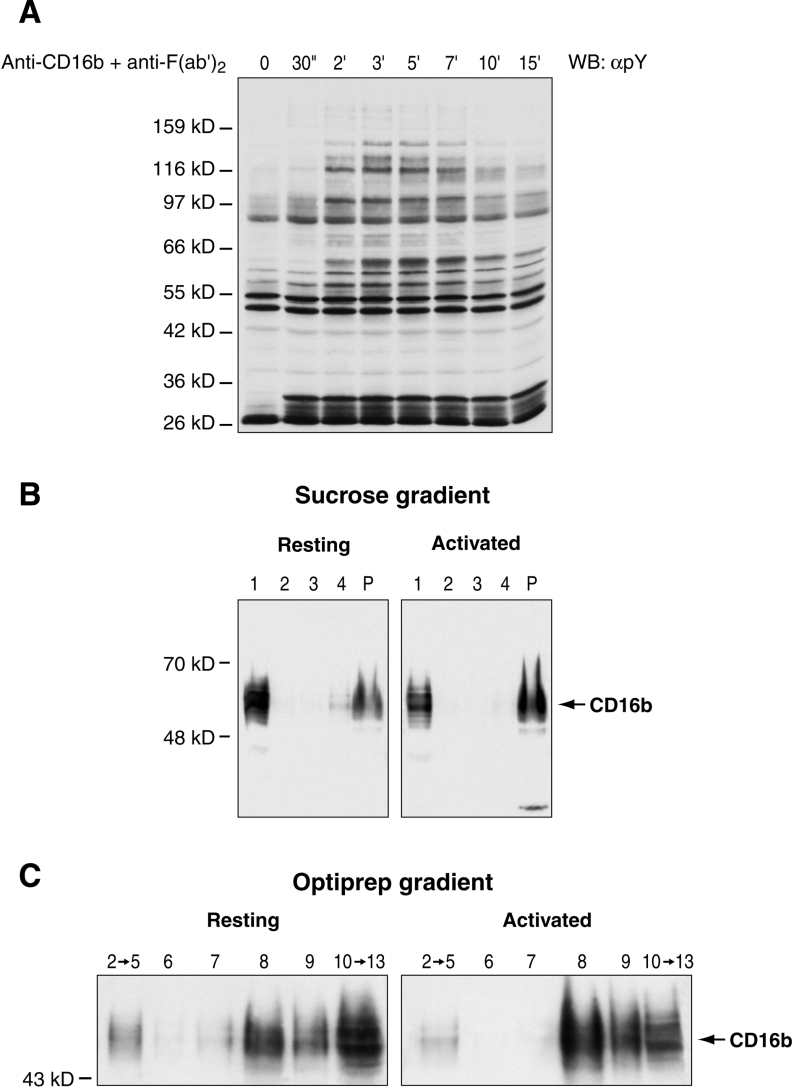
Since DRMs are thought to act as platforms for signalling upon cell activation [7], we hypothesized that the distribution of CD16b within the distinct populations of DRMs would differ after its cross-linking. As a first step to verify this hypothesis, the optimal experimental conditions for the engagement of CD16b were determined by analysing the kinetics of tyrosine phosphorylation of intracellular substrates. The results in Figure 3(A) show that the peak of tyrosine phosphorylation of intracellular substrates under our experimental conditions is reached after approx. 3 min of CD16b cross-linking. If DRMs are important for CD16b signalling, receptor translocation would occur before the maximum peak of phosphorylation. Therefore a 1 min time point was chosen to study CD16b translocation to DRMs.
Figure 3. Distribution of CD16b receptors in DRM-L and DRM-H in activated PMNs.
(A) The kinetics of tyrosine phosphorylation after CD16b engagement were determined by incubating PMNs for 15 min at 4 °C with the 3G8 F(ab′)2 mAb followed by cross-linking with an anti-F(ab′)2 antibody for various periods of time at 22 °C. Samples were analysed by SDS/PAGE and immunoblotted with the 4G10 anti-phosphotyrosine mAb (αpY). (B) PMNs were activated for 1 min at 22 °C, as described in (A), lysed and overlaid with a sucrose gradient. (C) PMNs were incubated for 1 min at 37 °C with 3G8 F(ab′)2, cross-linked for 1 min at 37 °C, lysed in cold lysis buffer and overlaid with a discontinuous OptiPrep gradient. Fractions of non-activated (Resting) and activated (Activated) PMN were pooled (as described in the Results section), analysed by SDS/PAGE and immunoblotted with the anti-CD16 7.5.4 mAb. Molecular mass sizes are given in kDa. WB, Western blotting.
The distribution of CD16b in DRM-L and DRM-H upon CD16b cross-linking was examined using both the sucrose and OptiPrep gradients. To simplify the experimental procedure, fractions were pooled. For the sucrose gradient, fractions 1–5 (lane 1), 6–9 (lane 2), 10 and 11 (lane 3), and 12 –15 (lane 4) were pooled. For the OptiPrep gradient, fractions 2–5 and 10–13 were pooled. The results in Figure 3 show that, upon CD16b cross-linking, there is a significant increase in CD16b in the pellet of the sucrose gradient (Figure 3B) and DRM-H (fractions 8 and 9) in the OptiPrep gradient (Figure 3C). A slight decrease in CD16b in DRM-L and the soluble fractions in both gradients was also observed. The above observations reveal that DRM-H are enriched in CD16b upon receptor engagement, suggestive of a role of this subset of DRMs in CD16b signalling.
To determine whether the increase in CD16b in DRM-H occurs at the plasma membrane or on intracellular membranes, CD16b cross-linking was performed on isolated PMN plasma membrane preparations. The results in Figure 4 provide direct evidence that a translocation of CD16b to a detergent-insoluble fraction can be observed upon its cross-linking on plasma membranes isolated from PMNs. Reblots of the same samples with an anti-CD32a antibody revealed that the cross-linking of CD16b is not associated with an equivalent insolubilization of CD32a (results not shown).
Figure 4. Distribution of CD16b at the plasma membrane of resting PMNs upon receptor engagement.
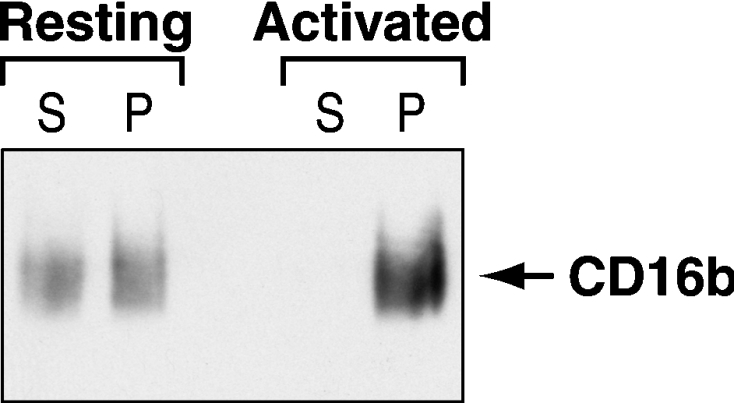
Plasma membranes were isolated from PMNs and incubated without (Resting) or with (Activated) the 3G8 F(ab′)2 mAb and goat F(ab′2) anti-mouse F(ab′)2 as described in the Materials and methods section. Following the solubilization of the plasma membranes in 1% NP40 and centrifugation at 100000 g, the pellet (P) and supernatant (S) were analysed by SDS/PAGE and immunoblotted with the anti-CD16 7.5.4 mAb.
Partitioning of CD11b to DRMs in human PMNs
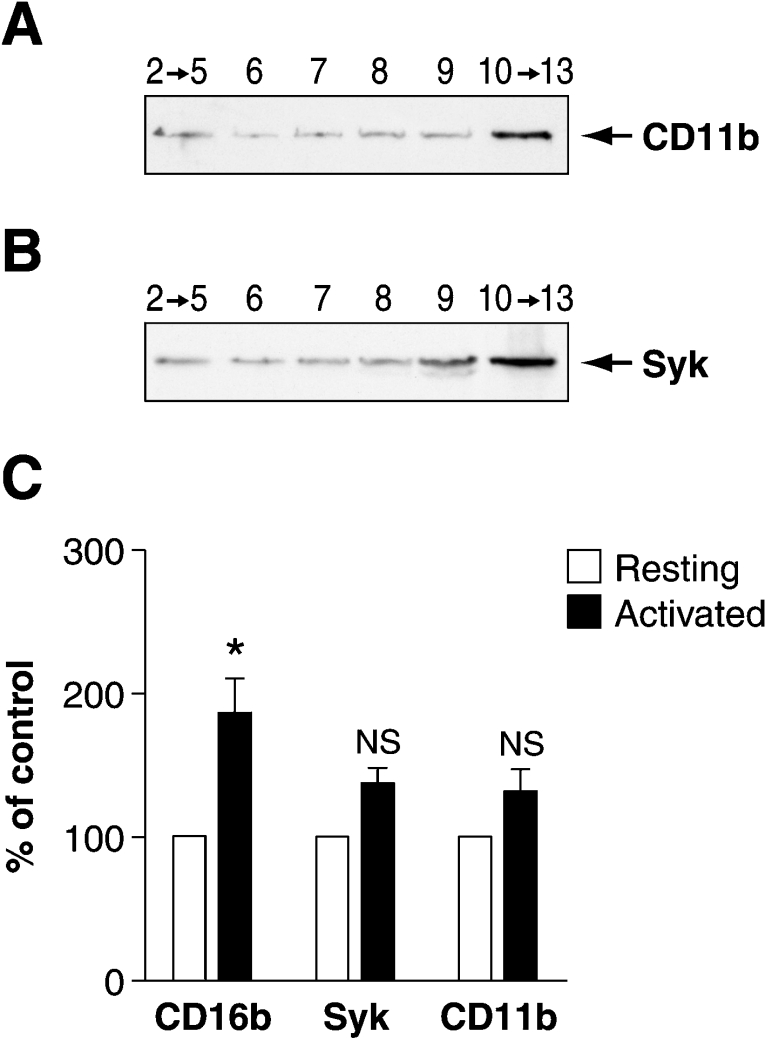
Since CD11b has been hypothesized to be one of the transmembrane transducers of CD16b [21], we sought to determine whether it also resides in DRMs in resting PMNs. For these experiments, the OptiPrep gradient was used since DRM-L and DRM-H are easily distinguishable with this experimental approach. Immunoblotting of the pooled fractions, collected from an OptiPrep gradient of resting PMNs, with an anti-CD11b antibody revealed that the majority of CD11b resides in the soluble fractions (10–13) of the gradient (Figure 5A). A small quantity of CD11b, however, is detectable in DRM-L and DRM-H. Upon engagement of CD16b, no significant increase in CD11b is observed in either DRM fraction (Figure 5C).
Figure 5. Distribution of CD11b and Syk in DRMs in resting and activated human PMNs.
DRMs were isolated using an OptiPrep gradient from resting PMNs as described in Figure 2. Fractions were pooled (as described in the Results section) and analysed by SDS/PAGE and immunoblotted with the anti-CD11b (A) or anti-Syk (B) antibody. (C) The distribution of CD16b, Syk and CD11b in DRM-H was determined in non-activated (Resting) and activated (Activated) PMNs as described in Figure 3(C). The open bars represent DRM-H isolated from resting PMNs and the closed bars show DRM-H from activated PMNs. This Figure represents the results of six independent experiments. The statistical analysis was performed using Student's paired t test (P=0.041). NS, statistically non-significant; *, statistically significant.
Syk is tyrosine-phosphorylated upon CD16b cross-linking and resides in DRMs
During the course of the present study, we also investigated the possibility that Syk is involved in CD16b signalling. Syk is known to participate in CD32a signalling upon CD32a cross-linking [42] or heterotypic cross-linking of CD32a and CD16b [43]. CD16b, however, can transmit signals independently of CD32a [9]. To determine whether Syk is tyrosine-phosphorylated upon CD16b cross-linking, the tyrosine-phosphorylation status of Syk was determined in PMNs before and after stimulation with the anti-CD16b antibody. As shown in Figure 6 (upper panel), Syk phosphorylation could be detected after CD16b receptor engagement. The mechanism(s) through which Syk is tyrosine-phosphorylated in response to the engagement of CD16b, a GPI-anchored protein, remains to be established. It is highly likely that Syk is phosphorylated by a transmembrane receptor that interacts with CD16b.
Figure 6. Tyrosine-phosphorylation status of Syk upon CD16b cross-linking.
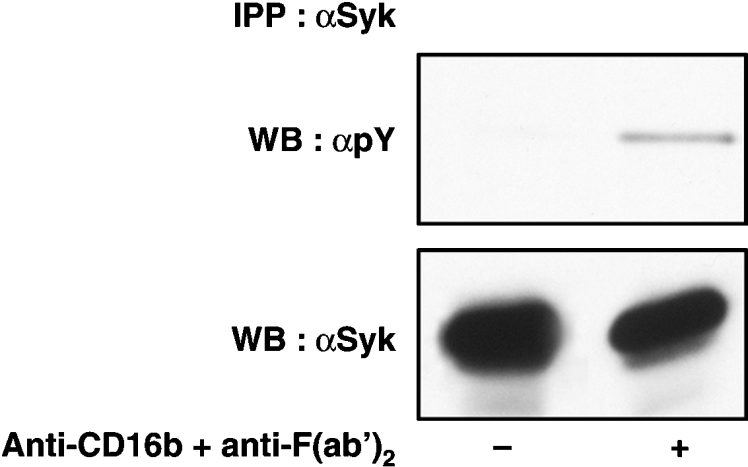
Resting PMNs (−) or PMNs activated with the 3G8 F(ab′)2 mAb (+) were lysed and immunoprecipitated with an anti-Syk antibody under denaturing conditions (as described in the Materials and methods section). Immunoprecipitated proteins were analysed by SDS/PAGE and immunoblotted with the anti-phosphotyrosine 4G10 (αpY) or anti-Syk (αSyk) antibodies. IPP, immunoprecipitation; WB, Western blot.
Analysis of our OptiPrep gradient fractions with an anti-Syk mAb revealed that Syk indeed resides in DRM-L and DRM-H (Figure 5B) and that its distribution in DRMs remains the same upon CD16b receptor engagement (Figure 5C).
Cholesterol-sequestering and -depleting agents enhance CD16b signalling
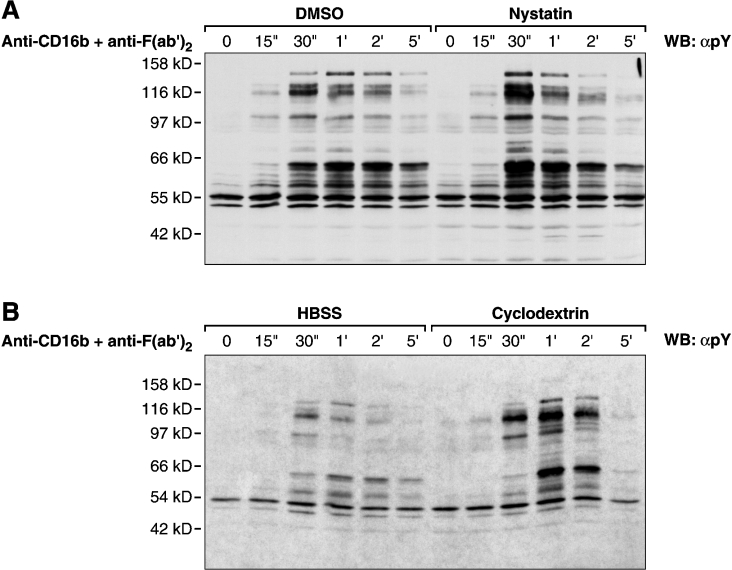
The disruption of the integrity of DRMs with nystatin and methyl-β-cyclodextrin is an approach that is often used to investigate the role of these membrane domains in receptor signalling. We therefore next determined the effect of nystatin and methyl-β-cyclodextrin on CD16b signalling. Nystatin is a cholesterol-sequestering agent, and methyl-β-cyclodextrin is a cholesterol-depleting agent [6]. The results in Figure 7(A) show that PMNs, treated with nystatin before CD16b engagement, exhibit a significant increase in the tyrosine-phosphorylation of the intracellular pool of proteins shortly after CD16b engagement. Similar observations were made when methyl-β-cyclodextrin was used (Figure 7B).
Figure 7. Effects of nystatin and β-cyclodextrin on the tyrosine phosphorylation of intracellular proteins after CD16b engagement.
(A) PMNs pre-incubated in HBSS containing 0.1% (v/v) DMSO or 30 μg/ml nystatin for 1 h at 37 °C were stimulated for various periods of time by cross-linking CD16b (1 min at 37 °C, as described in the Materials and methods section). The cross-linking reaction was stopped by lysing whole-cells directly in boiling 2×Laemmli's sample buffer. Samples were analysed by SDS/PAGE and immunoblotted with an anti-phosphotyrosine 4G10 mAb (αpY). (B) Similar experimental conditions as in (A) were used, with the exception that PMNs were incubated with 10 mM β-cyclodextrin at 37 °C for 30 min before CD16b cross-linking. Molecular mass sizes are given in kDa. WB, Western blot.
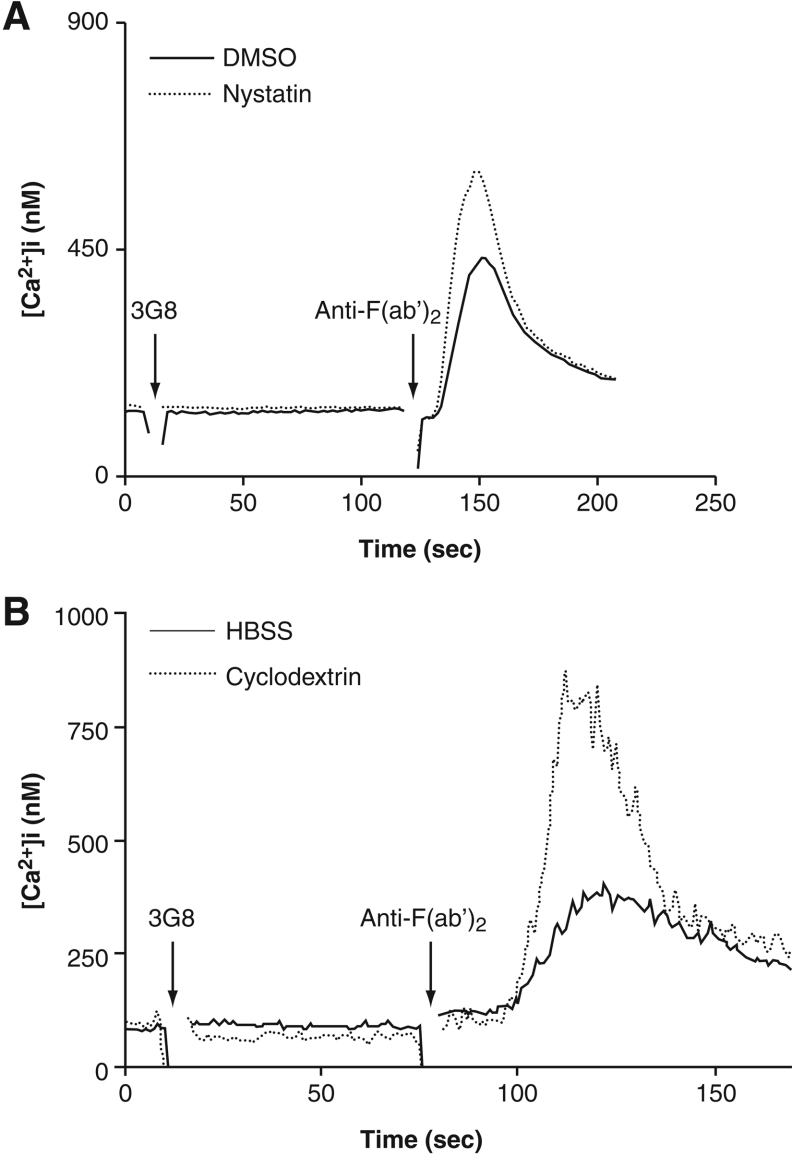
Since CD16b cross-linking is known to mobilize Ca2+ [10], we next investigated the effect of nystatin on the Ca2+ response. In accordance with the effect of nystatin on the phosphorylation of intracellular substrates, the results in Figure 8(A) show that cross-linking of CD16b rapidly increased the concentration of intracellular free Ca2+ and that this response was also enhanced in the presence of nystatin. A similar increase in cytoplasmic-free Ca2+ was observed in PMNs treated with methyl-β-cyclodextrin (Figure 8B).
Figure 8. Effects of nystatin and β-cyclodextrin on the release of intracellular Ca2+ stores after CD16b engagement.
(A) The release of Ca2+ stores was determined in PMNs stimulated with 3G8 mAb F(ab′)2 after a pre-incubation of 1 h in HBSS containing 0.1% (v/v) DMSO or 30 μg/ml nystatin (as described in the Materials and methods section). (B) Similar experimental conditions as in (A) were used, with the exception that PMNs were incubated with 10 mM β-cyclodextrin at 37 °C for 30 min before CD16b cross-linking.
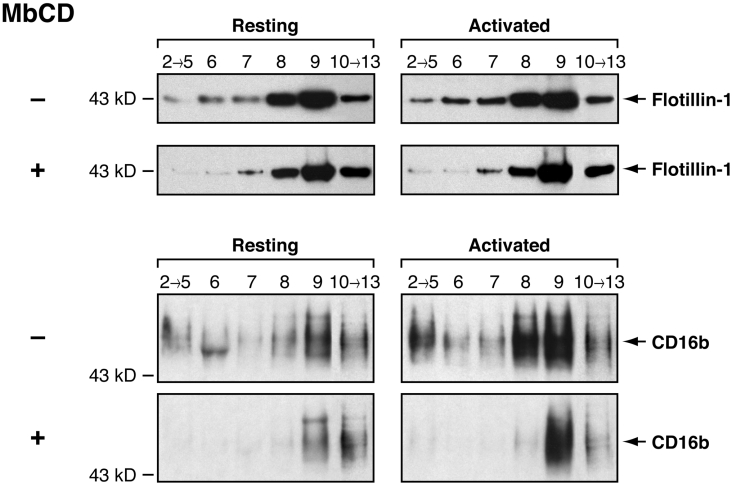
The increase in CD16b signalling in the presence of DRM-disrupting agents prompted an analysis of the effect of these drugs on DRM integrity. DRMs were isolated from resting PMNs incubated in HBSS containing methyl-β-cyclodextrin using an OptiPrep gradient. As shown in Figure 9, the amount of the DRM marker, flotillin-1, in DRM-L in PMNs incubated in the presence of methyl-β-cyclodextrin was significantly diminished in comparison with PMNs incubated in buffer alone (compare fractions 2–5 of ‘Resting’ gradients in the upper set of panels of Figure 9). Similar results were obtained for CD16b (compare fractions 2–5 of ‘Resting’ gradients in the lower set of panels of Figure 9). The decrease in the flotillin-1 and CD16b signal in DRM-L was statistically significant when the results of three independent experiments were analysed. In contrast, the loss of flotillin-1 in DRM-H is less pronounced than in DRM-L in the presence of methyl-β-cyclodextrin (compare fractions 8 and 9 of ‘Resting’ gradients in the upper set of panels of Figure 9). Statistical analysis of the results of three independent experiments revealed that the decrease in the flotillin-1 signal is not statistically significant in DRM-H in the presence of methyl-β-cyclodextrin. A similar observation was made for CD16b (compare fractions 8 and 9 of ‘Resting’ gradients in the lower set of panels of Figure 9). Although there is less CD16b signal in DRM-H in the presence of methyl-β-cyclodextrin, statistical analysis of the results of three independent experiments revealed that the decrease in signal is not statistically significant.
Figure 9. Effects of methyl-β-cyclodextrin on the distribution of flotillin-1 and CD16b receptors in DRM-L and DRM-H in resting and activated PMNs.
PMNs were incubated in HBSS (−) or in HBSS containing 10 mM methyl-β-cyclodextrin (+) at 37 °C for 30 min. CD16b was then cross-linked for 1 min at 37 °C (as described in the Materials and methods section.) with the 3G8 F(ab′)2 mAb (Activated). As a control, PMNs were also incubated in HBSS alone for 1 min at 37 °C (Resting). PMNs were then lysed in cold lysis buffer containing 1% Triton X-100 and overlaid with an OptiPrep gradient (as described in the Materials and methods section). Fractions were pooled (as described in the Results section) and analysed by SDS/PAGE and immunoblotted with an anti-flotillin-1 antibody (upper panel set) or with the anti-CD16b 7.5.4 mAb (lower panel set). MbCD, methyl-β-cyclodextrin. Molecular mass sizes are given in kDa.
The effect of methyl-β-cyclodextrin on the distribution of CD16b in DRMs after CD16b engagement was then examined. As shown in Figure 9, a decrease in CD16b signal was observed in DRM-L in PMNs after CD16b cross-linking in the presence of methyl-β-cyclodextrin (compare fractions 2–5 of ‘Activated’ gradients in the lower set of panels of Figure 9). Although a decrease in CD16b signal is observed, statistical analysis of the results of three independent experiments revealed that the decrease in CD16b signal is close to being, but is not, statistically significant. Similar results were obtained for flotillin-1 (compare fractions 2–5 of ‘Activated’ gradients in the upper set of panels of Figure 9). There was also no statistically significant difference in the CD16b signal in DRM-H in the presence of methyl-β-cyclodextrin observed in activated PMNs (compare fractions 8 and 9 of ‘Activated’ gradients in the lower set of panels of Figure 9). A similar observation was made when flotillin-1 was used (compare fractions 8 and 9 of ‘Activated’ gradients in the upper set of panels of Figure 9).
DISCUSSION
CD16b is a unique Fc receptor since it is linked to the plasma membrane by a GPI anchor and is only expressed on human neutrophils. To further our understanding of how CD16b transmits its signal from the surface to the inside of the cell, the role of DRMs in CD16b signalling was investigated.
The novelty of our observations lies in the dynamic partitioning of CD16b to DRM-H in PMNs. Owing to the higher density of DRM-H (1.16 g/ml), these domains have generally been neglected, since the density range of standard sucrose gradients is usually between 1.02 and 1.16 g/ml and DRM-H will sediment in the pellet of these preparations. Most published studies on DRMs isolated from a sucrose gradient do not report the analysis of the pellet of the gradient. DRM-H and DRM-L are distinct domains found within the PMN plasma membrane [40]. DRM-L are the DRMs that have been intensively investigated by the isolation of cellular components that float in low-density fractions in a sucrose gradient after cell lysis in cold Triton X-100. Similarly to DRM-L, DRM-H also float in a sucrose gradient but in higher-density fractions [40]. The major difference between DRM-L and DRM-H is the enrichment in cytoskeletal proteins, such as fodrin and supervillin, in the latter [40]. Flotillin-1 is also enriched in DRM-H, as shown in Figure 2(A). PMNs therefore contain distinct populations of CD16b+ DRMs of different densities that differ in protein composition. A complete characterization of these heterogeneous populations of CD16b+ DRMs in PMNs will further our understanding of the role of DRMs in CD16b signalling.
A variety of models on how lipid rafts may mediate cellular signalling have been proposed [44]. In some models, signalling occurs via receptors that constitutively reside in DRMs and in others via receptors that translocate to or out of DRMs upon cellular activation. We provide direct evidence that, first, CD16b resides in DRM-L and DRM-H in resting PMNs and, secondly, upon activation, a significant increase in CD16b receptors is observed in DRM-H. This contrasts with CD32a, a receptor that is recruited to DRM-H only after cross-linking [34]. These data strongly suggest that DRM-H are implicated in CD16b signalling. Since DRM-H are enriched in cytoskeletal proteins, it is reasonable to suggest that the association of CD16b with DRM-H may implicate these membrane domains in the polymerization of actin that is observed upon receptor activation. It has been suggested that DRM-H may be involved in the active signalling between chemotactic receptors and the actin cytoskeleton in PMNs [40].
Further evidence for the involvement of DRM-H in CD16b signalling was obtained by studying the distribution of CD11b and Syk in DRMs before and after CD16b engagement. Previous studies support the hypothesis that CD11b is a co-receptor for CD16b [22–25]. None of these studies, however, provides evidence of a link between CD16b and CD11b under conditions directly related to the initiation of signalling induced upon CD16b cross-linking. We therefore examined the distribution of CD11b in DRMs in PMNs before and shortly after the cross-linking of CD16b. Similarly to CD16b, the majority of CD11b in resting PMNs is solubilized by Triton X-100 and a portion localizes to DRM-H and DRM-L. Upon CD16b engagement, no significant increase in the quantity of CD11b in DRM-H was observed. The tyrosine kinase Syk, which is thought to be critically involved in the phagocytic process, partitions similarly to CD11b in DRM-H. Syk is constitutively present in DRM-H in resting PMNs and no increase is observed in the amount of Syk in DRM-H upon CD16b cross-linking. These observations suggest that CD16b may translocate to a subset of DRM, DRM-H, that contains the required proteins for CD16b signalling. A similar model has been proposed for the EGF (epidermal growth factor) and PDGF (platelet-derived growth factor) receptors [44].
The role of DRMs in CD16b signalling was studied further by disturbing the integrity of DRMs with nystatin or methyl-β-cyclodextrin. Neither nystatin nor methyl-β-cyclodextrin, at the concentrations and incubation time utilized, affect PMN viability, as determined by Trypan Blue exclusion as well as the ability of the cells to maintain their cytoplasmic levels of free Ca2+ within the physiological range. Exposure of PMNs to either nystatin or methyl-β-cyclodextrin before CD16b engagement enhanced CD16b signalling (tyrosine phosphorylation) as well as the mobilization of Ca2+ at an early time point after cross-linking. Conventionally, DRM-disrupting agents have principally been used to disrupt the integrity of DRM-L to examine the role of DRM-L in a biological phenomenon. Since a portion of CD16b resides in DRM-H, the effect of methyl-β-cyclodextrin on the distribution of protein markers of DRM-H was examined. As predicted, in the presence of methyl-β-cyclodextrin, there is a significant decrease in the quantity of marker protein, flotillin-1, in DRM-L in resting PMNs. In contrast, a significant amount of marker protein remained in the DRM-H fractions in the presence of methyl-β-cyclodextrin. Similar observations were made for CD16b. The persistence of CD16b in the DRM-H fraction after methyl-β-cyclodextrin treatment may, in part, explain why an increase in CD16b signalling is observed after treatment with methyl-β-cyclodextrin.
Our observations are in accordance with those of Katsumata et al. [30], who reported that methyl-β-cyclodextrin does not inhibit the CR3/CD16b-dependent production of superoxide in PMNs activated with opsonized zymosan. DRM disrupting reagents do not therefore inhibit CD16b signalling or certain effector functions, but do enhance the former upon CD16b cross-linking with antibodies.
These observations contrast with those of the majority of receptors whose signalling has been investigated in DRM-L. In the presence of DRM-disrupting agents, the function of these receptors is impaired [6]. The behaviour of CD16b, however, is not unique, since several receptors that signal through DRMs exhibit a similar increase in signalling after DRM disruption. Similarly to CD16b, in the presence of cholesterol-sequestration reagents, the release of Ca2+ from intracellular stores observed upon the engagement of the B-cell receptor is strongly enhanced [45]. Cholesterol depletion also increases the signalling response upon stimulation of CD32a [46] and of the EGF receptor [47]. Further biochemical analysis will reveal the underlying mechanisms involved in the augmentation of CD16b signalling upon cholesterol depletion in PMNs.
Our observations that DRMs are involved in CD16b signalling complement those of Green et al. [48] who demonstrated that the GPI anchor of CD16b plays a role in signalling through this receptor in fibroblasts. A synergistic rise in intracellular Ca2+ upon co-cross-linking of CD16b and CD32a is abolished when the GPI anchor of CD16b is replaced with a transmembrane domain [48]. Moreover, Seveau et al. [49] observed, by microscopy, the presence of CD16b in detergent-resistant parts of the plasma membrane in PMNs stimulated with fMLP (N-formylmethionyl-leucylphenylalanine). Overall, these observations favour the hypothesis that DRMs play a role in CD16b signalling and function.
In summary, this is the first report of the preferential partitioning of CD16b to DRM-H. Moreover, molecules involved in CD16b signalling, such as CD11b and Syk, also partition to DRMs in PMNs. Our observations lead to novel questions about the composition of DRM-H in human PMNs as well as the role of the distinct populations of CD16b+ DRMs in PMN effector functions.
Acknowledgments
We thank Miss Judith Bellemare for excellent technical assistance and Dr Beth Luna for insightful scientific discussions. This work was supported, in part, by grants from the Canadian Institutes for Health Research, the Arthritis Society of Canada, and The Canadian Arthritis Network. P. H. N. is the recipient of the Canada Research Chair in the Molecular Physiopathology of the Neutrophil.
References
- 1.Gessner J. E., Heiken H., Tamm A., Schmidt R. E. The IgG Fc receptor family. Ann. Hematol. 1998;76:231–248. doi: 10.1007/s002770050396. [DOI] [PubMed] [Google Scholar]
- 2.Hulett M. D., Hogarth P. M. Molecular basis of Fc receptor function. Adv. Immunol. 1994;57:1–127. doi: 10.1016/s0065-2776(08)60671-9. [DOI] [PubMed] [Google Scholar]
- 3.Kimberly R. P., Salmon J. E., Edberg J. C. Receptors for immunoglobulin G. Molecular diversity and implications for disease. Arthritis Rheum. 1995;38:306–314. doi: 10.1002/art.1780380303. [DOI] [PubMed] [Google Scholar]
- 4.Shen L., Guyre P. M., Fanger M. W. Polymorphonuclear leukocyte function triggered through the high affinity Fc receptor for monomeric IgG. J. Immunol. 1987;139:534–538. [PubMed] [Google Scholar]
- 5.Unkeless J. C., Shen Z., Lin C. W., DeBeus E. Function of human Fc γ RIIA and Fc γ RIIIB. Semin. Immunol. 1995;7:37–44. doi: 10.1016/1044-5323(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 6.Simons K., Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 7.Brown D. A., London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 8.Horejsi V., Drbal K., Cebecauer M., Cerny J., Brdicka T., Angelisova P., Stockinger H. GPI-microdomains: a role in signalling via immunoreceptors. Immunol. Today. 1999;20:356–361. doi: 10.1016/s0167-5699(99)01489-9. [DOI] [PubMed] [Google Scholar]
- 9.Kimberly R. P., Ahlstrom J. W., Click M. E., Edberg J. C. The glycosyl phosphatidylinositol-linked Fc γ RIIIPMN mediates transmembrane signalling events distinct from Fc γ RII. J. Exp. Med. 1990;171:1239–1255. doi: 10.1084/jem.171.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edberg J. C., Salmon J. E., Kimberly R. P. Functional capacity of Fc γ receptor III (CD16) on human neutrophils. Immunol. Res. 1992;11:239–251. doi: 10.1007/BF02919130. [DOI] [PubMed] [Google Scholar]
- 11.Zhou M. J., Lublin D. M., Link D. C., Brown E. J. Distinct tyrosine kinase activation and Triton X-100 insolubility upon Fc γ RII or Fc γ RIIIB ligation in human polymorphonuclear leukocytes. Implications for immune complex activation of the respiratory burst. J. Biol. Chem. 1995;270:13553–13560. doi: 10.1074/jbc.270.22.13553. [DOI] [PubMed] [Google Scholar]
- 12.Coxon P. Y., Rane M. J., Powell D. W., Klein J. B., McLeish K. R. Differential mitogen-activated protein kinase stimulation by Fc γ receptor IIa and Fc γ receptor IIIb determines the activation phenotype of human neutrophils. J. Immunol. 2000;164:6530–6537. doi: 10.4049/jimmunol.164.12.6530. [DOI] [PubMed] [Google Scholar]
- 13.Hazan-Halevy I., Seger R., Levy R. The requirement of both extracellular regulated kinase and p38 mitogen-activated protein kinase for stimulation of cytosolic phospholipase A2 activity by either FcγRIIA or FcγRIIIB in human neutrophils. A possible role for Pyk2 but not for the Grb2-Sos-Shc complex. J. Biol. Chem. 2000;275:12416–12423. doi: 10.1074/jbc.275.17.12416. [DOI] [PubMed] [Google Scholar]
- 14.Salmon J. E., Brogle N. L., Edberg J. C., Kimberly R. P. Fcγ receptor III induces actin polymerization in human neutrophils and primes phagocytosis mediated by Fcγ receptor II. J. Immunol. 1991;146:997–1004. [PubMed] [Google Scholar]
- 15.Huizinga T. W., Dolman K. M., van der Linden N. J., Kleijer M., Nuijens J. H., von dem Borne A. E., Roos D. Phosphatidylinositol-linked FcRIII mediates exocytosis of neutrophil granule proteins, but does not mediate initiation of the respiratory burst. J. Immunol. 1990;144:1432–1437. [PubMed] [Google Scholar]
- 16.Salmon J. E., Kapur S., Kimberly R. P. Opsonin-independent ligation of Fcγ receptors: the 3G8-bearing receptors on neutrophils mediate the phagocytosis of concanavalin A-treated erythrocytes and non-opsonized E. coli. J. Exp. Med. 1987;166:1798–1813. doi: 10.1084/jem.166.6.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hundt M., Schmidt R. E. The glycosylphosphatidylinositol-linked Fc γ receptor III represents the dominant receptor structure for immune complex activation of neutrophils. Eur. J. Immunol. 1992;22:811–816. doi: 10.1002/eji.1830220327. [DOI] [PubMed] [Google Scholar]
- 18.Walker B. A., Hagenlocker B. E., Stubbs E. B., Jr, Sandborg R. R., Agranoff B. W., Ward P. A. Signal transduction events and Fc γ R engagement in human neutrophils stimulated with immune complexes. J. Immunol. 1991;146:735–741. [PubMed] [Google Scholar]
- 19.Coxon A., Cullere X., Knight S., Sethi S., Wakelin M. W., Stavrakis G., Luscinskas F. W., Mayadas T. N. Fc γ RIII mediates neutrophil recruitment to immune complexes: a mechanism for neutrophil accumulation in immune-mediated inflammation. Immunity. 2001;14:693–704. doi: 10.1016/s1074-7613(01)00150-9. [DOI] [PubMed] [Google Scholar]
- 20.Petty H. R., Todd R. F., III Integrins as promiscuous signal transduction devices. Immunol. Today. 1996;17:209–212. doi: 10.1016/0167-5699(96)30013-3. [DOI] [PubMed] [Google Scholar]
- 21.Stockinger H. Interaction of GPI-anchored cell surface proteins and complement receptor type 3. Exp. Clin. Immunogenet. 1997;14:5–10. [PubMed] [Google Scholar]
- 22.Poo H., Krauss J. C., Mayo-Bond L., Todd R. F., III, Petty H. R. Interaction of Fc γ receptor type IIIB with complement receptor type 3 in fibroblast transfectants: evidence from lateral diffusion and resonance energy transfer studies. J. Mol. Biol. 1995;247:597–603. doi: 10.1006/jmbi.1995.0166. [DOI] [PubMed] [Google Scholar]
- 23.Kindzelskii A. L., Yang Z., Nabel G. J., Todd R. F., III, Petty H. R. Ebola virus secretory glycoprotein (sGP) diminishes Fc γ RIIIB-to-CR3 proximity on neutrophils. J. Immunol. 2000;164:953–958. doi: 10.4049/jimmunol.164.2.953. [DOI] [PubMed] [Google Scholar]
- 24.Zhou M., Todd R. F., III, van de Winkel J. G., Petty H. R. Cocapping of the leukoadhesin molecules complement receptor type 3 and lymphocyte function-associated antigen-1 with Fc γ receptor III on human neutrophils. Possible role of lectin-like interactions. J. Immunol. 1993;150:3030–3041. [PubMed] [Google Scholar]
- 25.Krauss J. C., PooH Xue W., Mayo-Bond L., Todd R. F., III, Petty H. R. Reconstitution of antibody-dependent phagocytosis in fibroblasts expressing Fc γ receptor IIIB and the complement receptor type 3. J. Immunol. 1994;153:1769–1777. [PubMed] [Google Scholar]
- 26.Boros P., Odin J. A., Muryoi T., Masur S. K., Bona C., Unkeless J. C. IgM anti-Fc γ R autoantibodies trigger neutrophil degranulation. J. Exp. Med. 1991;173:1473–1482. doi: 10.1084/jem.173.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naziruddin B., Duffy B. F., Tucker J., Mohanakumar T. Evidence for cross-regulation of Fc γ RIIIB (CD16) receptor-mediated signalling by Fc γ RII (CD32) expressed on polymorphonuclear neutrophils. J. Immunol. 1992;149:3702–3709. [PubMed] [Google Scholar]
- 28.Brunkhorst B. A., Strohmeier G., Lazzari K., Weil G., Melnick D., Fleit H. B., Simons E. R. Differential roles of Fc γ RII and Fc γ RIII in immune complex stimulation of human neutrophils. J. Biol. Chem. 1992;267:20659–20666. [PubMed] [Google Scholar]
- 29.Edberg J. C., Kimberly R. P. Modulation of Fc γ and complement receptor function by the glycosyl-phosphatidylinositol-anchored form of Fc γ RIII. J. Immunol. 1994;152:5826–5835. [PubMed] [Google Scholar]
- 30.Katsumata O., Hara-Yokoyama M., Sautes-Fridman C., Nagatsuka Y., Katada T., Hirabayashi Y., Shimizu K., Fujita-Yoshigaki J., Sugiya H., Furuyama S. Association of FcγRII with low-density detergent-resistant membranes is important for cross-linking-dependent initiation of the tyrosine phosphorylation pathway and superoxide generation. J. Immunol. 2001;167:5814–5823. doi: 10.4049/jimmunol.167.10.5814. [DOI] [PubMed] [Google Scholar]
- 31.Vely F., Gruel N., Moncuit J., Cochet O., Rouard H., Dare S., Galon J., Sautes C., Fridman W. H., Teillaud J. L. A new set of monoclonal antibodies against human Fc γ RII (CD32) and Fc γ RIII (CD16): characterization and use in various assays. Hybridoma. 1997;16:519–528. doi: 10.1089/hyb.1997.16.519. [DOI] [PubMed] [Google Scholar]
- 32.Robbins S. M., Quintrell N. A., Bishop J. M. Myristoylation and differential palmitoylation of the HCK protein-tyrosine kinases govern their attachment to membranes and association with caveolae. Mol. Cell. Biol. 1995;15:3507–3515. doi: 10.1128/mcb.15.7.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wessel D., Flugge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- 34.Rollet-Labelle E., Marois S., Barbeau K., Malawista S. E., Naccache P. H. Recruitment of the cross-linked opsonic receptor CD32A (FcγRIIA) to high-density detergent-resistant membrane domains in human neutrophils. Biochem. J. 2004;381:919–928. doi: 10.1042/BJ20031808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Shami A., Gilbert C., Barabe F., Gaudry M., Naccache P. H. Preservation of the pattern of tyrosine phosphorylation in human neutrophil lysates. J. Immunol. Methods. 1997;202:183–191. doi: 10.1016/s0022-1759(97)00004-5. [DOI] [PubMed] [Google Scholar]
- 36.Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U.S.A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 38.Barabé F., Rollet-Labelle E., Gilbert C., Fernandes M. J., Naccache S. N., Naccache P. H. Early events in the activation of Fc γ RIIA in human neutrophils: stimulated insolubilization, translocation to detergent-resistant domains, and degradation of Fc γ RIIA. J. Immunol. 2002;168:4042–4049. doi: 10.4049/jimmunol.168.8.4042. [DOI] [PubMed] [Google Scholar]
- 39.Brown D. A., Rose J. K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell (Cambridge, Mass.) 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 40.Nebl T., Pestonjamasp K. N., Leszyk J. D., Crowley J. L., Oh S. W., Luna E. J. Proteomic analysis of a detergent-resistant membrane skeleton from neutrophil plasma membranes. J. Biol. Chem. 2002;277:43399–43409. doi: 10.1074/jbc.M205386200. [DOI] [PubMed] [Google Scholar]
- 41.Brault A. B. Isolation and use of rafts. In: Coligan J. E., Kruisbeek A., Margulies D., Shevach E. M., Strober W., editors. Current Protocols in Immunology, vol. 2. New York: John Wiley and Sons; 2000. pp. 11.10.1–11.10.19.. [Google Scholar]
- 42.Desaulniers P., Fernandes M., Gilbert C., Bourgoin S. G., Naccache P. H. Crystal-induced neutrophil activation. VII. Involvement of Syk in the responses to monosodium urate crystals. J. Leukocyte Biol. 2001;70:659–668. [PubMed] [Google Scholar]
- 43.Suzuki T., Kono H., Hirose N., Okada M., Yamamoto T., Yamamoto K., Honda Z. Differential involvement of Src family kinases in Fc γ receptor-mediated phagocytosis. J. Immunol. 2000;165:473–482. doi: 10.4049/jimmunol.165.1.473. [DOI] [PubMed] [Google Scholar]
- 44.Zajchowski L. D., Robbins S. M. Lipid rafts and little caves. Compartmentalized signalling in membrane microdomains. Eur. J. Biochem. 2002;269:737–752. doi: 10.1046/j.0014-2956.2001.02715.x. [DOI] [PubMed] [Google Scholar]
- 45.Petrie R. J., Schnetkamp P. P., Patel K. D., Awasthi-Kalia M., Deans J. P. Transient translocation of the B cell receptor and Src homology 2 domain-containing inositol phosphatase to lipid rafts: evidence toward a role in calcium regulation. J. Immunol. 2000;165:1220–1227. doi: 10.4049/jimmunol.165.3.1220. [DOI] [PubMed] [Google Scholar]
- 46.Barabé F., Pare G., Fernandes M. J., Bourgoin S. G., Naccache P. H. Cholesterol-modulating agents selectively inhibit calcium influx induced by chemoattractants in human neutrophils. J. Biol. Chem. 2002;277:13473–13478. doi: 10.1074/jbc.M112149200. [DOI] [PubMed] [Google Scholar]
- 47.Roepstorff K., Thomsen P., Sandvig K., van Deurs B. Sequestration of epidermal growth factor receptors in non-caveolar lipid rafts inhibits ligand binding. J. Biol. Chem. 2002;277:18954–18960. doi: 10.1074/jbc.M201422200. [DOI] [PubMed] [Google Scholar]
- 48.Green J. M., Schreiber A. D., Brown E. J. Role for a glycan phosphoinositol anchor in Fc γ receptor synergy. J. Cell. Biol. 1997;139:1209–1217. doi: 10.1083/jcb.139.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seveau S., Eddy R. J., Maxfield F. R., Pierini L. M. Cytoskeleton-dependent membrane domain segregation during neutrophil polarization. Mol. Biol. Cell. 2001;12:3550–3562. doi: 10.1091/mbc.12.11.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]