Abstract
Peptidases of parasitic protozoans are emerging as novel virulence factors and therapeutic targets in parasitic infections. A trypanosome-derived aminopeptidase that exclusively hydrolysed substrates with Glp (pyroglutamic acid) in P1 was purified 9248-fold from the plasma of rats infected with Trypanosoma brucei brucei. The enzyme responsible was cloned from a T. brucei brucei genomic DNA library and identified as type I PGP (pyroglutamyl peptidase), belonging to the C15 family of cysteine peptidases. We showed that PGP is expressed in all life cycle stages of T. brucei brucei and is expressed in four other blood-stream-form African trypanosomes. Trypanosome PGP was optimally active and stable at bloodstream pH, and was insensitive to host plasma cysteine peptidase inhibitors. Native purified and recombinant hyper-expressed trypanosome PGP removed the N-terminal Glp blocking groups from TRH (thyrotrophin-releasing hormone) and GnRH (gonadotropin-releasing hormone) with a kcat/Km value of 0.5 and 0.1 s−1·μM−1 respectively. The half-life of TRH and GnRH was dramatically reduced in the plasma of trypanosome-infected rats, both in vitro and in vivo. Employing an activity-neutralizing anti-trypanosome PGP antibody, and pyroglutamyl diazomethyl ketone, a specific inhibitor of type I PGP, we demonstrated that trypanosome PGP is entirely responsible for the reduced plasma half-life of TRH, and partially responsible for the reduced plasma half-life of GnRH in a rodent model of African trypanosomiasis. The abnormal degradation of TRH and GnRH, and perhaps other neuropeptides N-terminally blocked with a pyroglutamyl moiety, by trypanosome PGP, may contribute to some of the endocrine lesions observed in African trypanosomiasis.
Keywords: African trypanosome, aminopeptidase, gonadotropin-releasing hormone (GnRH), hypothyroidism, pyroglutamyl peptidase (PGP) type I, thyrotrophin-releasing hormone (TRH)
Abbreviations: AMC, 7-amino-4-methylcoumarin (or its amidated form when conjugated); AMT, acetate-Mes-Tris; ANF, atrial natriuretic factor; DCI, 3,4-dichloroisocoumarin; DMK, diazomethyl ketone; E-64, trans-epoxysuccinyl-L-leucylamido-(4-guanidino)butane; Glp, pyroglutamic acid; Glp-AMC, L-pyroglutamyl-7-amido-4-methylcoumarin; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; MALDI–TOF, matrix-assisted laser-desorption ionization–time-of-flight; PGP, pyroglutamyl peptidase; pNA, p-nitroanilide; RT, reverse transcriptase; TCEP, tris(2-carboxyethyl)phosphine; TPP, three-phase partitioning; TRH, thyrotrophin-releasing hormone
INTRODUCTION
African trypanosomes of the genus Trypanosoma cause wide-spread disease in humans and livestock throughout sub-Saharan Africa [1]. Infection causes severe febrile illness characterized by dyspnoea, oedema, cardiomyopathy and endocrine dysfunction [2]. The pathogenic mechanisms underlying the development of these lesions have not been elucidated, but are often attributed to biologically active products released by trypanosomes into the host circulation [2].
Trypanosomes release peptidases extracellularly both in vitro [3] and in vivo [4]. It has been proposed that these peptidases are responsible for creating the abnormal degradation products of TRH (thyrotrophin-releasing hormone) and GnRH (gonadotropin-releasing hormone) that are observed in plasma from trypanosome-infected hosts [5]. Degradation of these peptides is believed to cause a reduction in their circulating levels [5,6], promoting dysregulation of the hypothalamic–pituitary–thyroid and hypothalamic–pituitary–gonadal axes, and thus contributing to the endocrine lesions observed after trypanosome lysis in the host bloodstream [7].
While most parasite peptidases are inactivated by inhibitors present in the host circulation [8], two trypanosome peptidases have recently emerged, oligopeptidase B [9] and prolyl oligopeptidase [10], that are insensitive to host plasma peptidase inhibitors. These peptidases therefore retain their catalytic activity in the plasma of infected hosts. Indeed, oligopeptidase B inactivates the peptide hormone ANF (atrial natriuretic factor) in the plasma of trypanosome-infected hosts [11]. However, neither TRH nor GnRH is a substrate for oligopeptidase B, suggesting that another peptidase released by trypanosomes acts on these substrates. In the present paper, we identify this peptidase as a trypanosome-derived type I PGP (pyroglutamyl peptidase).
Regulatory neuropeptides are often ‘blocked’ at their N-termini with Glp (pyroglutamic acid) to promote in vivo stability, since this blocking group resists the action of plasma aminopeptidases, and thus prolongs the plasma half-life of the peptide. We demonstrated in the present study that trypanosome PGP, which efficiently removed the N-terminal Glp from TRH and GnRH in vitro, was released into the plasma of infected rats where it retained catalytic activity, dramatically reducing the in vitro and in vivo plasma half-life of TRH and GnRH. Our results lend solid support to the proposed role for trypanosome-derived peptidases in modulating peptide hormone dynamics in the plasma of infected hosts, and have significant implications for our understanding of the endocrine lesions observed in African trypanosomiasis.
EXPERIMENTAL
Parasites
Bloodstream-form Trypanosoma brucei brucei ILTat 1.1, T. brucei rhodesiense IL3953, T. brucei gambiense IL3250, T. congolense IL3000 and T. evansi IL3298 were passaged in rats, and procyclic T. brucei brucei ILTat 1.1 (tsetse fly midgut stage) were cultured and isolated as described previously [11]. Experimental animal procedures were approved by local and national authorities.
Detection of PGP activity and immunoreactive PGP in infectious plasma
PGP activity was measured with Glp-AMC (L-pyroglutamyl-7-amido-4-methylcoumarin; Sigma) in 50 mM Tris/HCl/1 mM EDTA (pH 7.4) supplemented with the monothiol reducing agent TCEP [tris(2-carboxyethyl)phosphine, 1 mM; Fluka] at 37 °C in a Hitachi F-2000 spectrofluorimeter (λex=320 nm and λem=405 nm). Plasma was harvested from male Sprague–Dawley rats (300 g; n=3) infected with T. brucei when parasitaemia was >1×108 trypanosomes·ml−1, and processed as described previously [11,12]. Using intracellular markers (acid phosphatase and acetylesterase), we have previously shown that this procedure yields parasite- and cell-free plasma without destruction of parasites in the plasma. There is no artifactual extracellular release of intracellular trypanosome proteins during plasma processing [12]. Plasma similarly prepared from healthy rats was used as a control. Plasma PGP activity was assayed as described above. Assays were conducted after preincubation (5 min, 37 °C) with DCI (3,4-dichloroisocoumarin, 1 mM; Sigma), E-64 [trans-epoxysuccinyl-L-leucylamido-(4-guanidino)butane, 10 μM; Sigma], 1,10-phenanthroline (1 mM; Sigma) or pepstatin A (10 μM; Sigma), to identify serine, cysteine, metallo and aspartic peptidases respectively. Infectious plasma was preincubated with Glp-DMK (L-pyroglutamyl-diazomethyl ketone, synthesized as described previously [13], 100 μM), preimmune antibodies, or activity-neutralizing anti-rat PGP or anti-trypanosome PGP antibodies (100 μg·ml−1) to confirm the activity of PGP type I enzyme. For detection of immunoreactive PGP in infected plasma, whole plasma yielded poorly resolved bands on a Western blot; therefore plasma was partially fractionated by TPP (three-phase partitioning) and Cibacron Blue F3GA–Sepharose chromatography as described below, and concentrated in Centricon™ concentrators (10 kDa cut-off; Millipore), prior to electrophoresis. Fractionated plasma was resolved on a Tris/Tricine SDS/15% polyacrylamide gel, transferred on to a PVDF (Amersham Biosciences) membrane, and probed with anti-trypanosome PGP antibodies.
Purification of PGP from infectious plasma
Whole plasma (83 ml) from ten rats infected with T. brucei brucei was harvested at peak parasitaemia and diluted to 200 ml with 50 mM Tris/HCl/1 mM EDTA/1 mM TCEP/0.1% (w/v) Brij 35, pH 8 (buffer A), containing 10 μM E-64, 1 mM DCI and 50 μg·ml−1 aprotinin. TPP was performed by addition of t-butanol (86 ml) and solid (NH4)2SO4 (29 g) yielding a 30% (v/v) t-butanol, 10% (w/v) (NH4)2SO4 solution in a final volume of 286 ml. The suspension was centrifuged (10000 g, 10 min, 16 °C) to yield a 0–10% (NH4)2SO4 TPP fraction. The pellet was discarded, and a 10–30% (NH4)2SO4 TPP fraction was prepared by adding solid (NH4)2SO4 (58 g) and re-centrifuging. The 10–30% (NH4)2SO4 TPP fraction was resuspended in buffer A (100 ml) and applied to a Cibacron Blue F3GA–Sepharose column (200 mm×25 mm, 0.5 ml·min−1; Amersham Biosciences). The flow-through was applied to a HiLoad™ 26/10 Q-Sepharose column (26 mm×100 mm, 1 ml·min−1; Amersham Biosciences). Bound protein was eluted on a linear gradient of 50–500 mM NaCl in buffer A over 5 column volumes. Pooled active fractions were diluted 20-fold with buffer A containing 1 M (NH4)2SO4 and applied to a HiPrep™ 16/10 phenyl-Sepharose column (16 mm×100 mm, 1 ml·min−1; Amersham Biosciences) equilibrated in buffer A containing 1 M (NH4)2SO4. Bound protein was eluted with a linear gradient of 1–0.01 M (NH4)2SO4 in buffer A. Pooled, active fractions were diluted 30-fold with buffer A and applied to a RESOURCE™ Q-Sepharose column (6.2 mm×30 mm, 2 ml·min−1; Amersham Biosciences), and bound protein was eluted with a gradient of 0.05–1 M NaCl in buffer A over 20 column volumes. Bound active fractions were concentrated in polysulphone concentrators (10 kDa cut-off), and applied to a Sephacryl S-100 HR column (1000 mm×15 mm, 0.25 ml·min−1) in buffer A. Purified PGP was subjected to in-gel digestion with endoproteinase Lys-C, and peptides resolved by HPLC were sequenced exactly as described previously [9].
Cloning and expression of the T. brucei brucei pgp gene
Forward (5′-RCKCRTCCCNKCANTNYGGRA-3′) and reverse (5′-NCNRCCNGCRTCNYNRGARAT-3′) degenerate oligonucleotide primers were synthesized, corresponding to bp 168–188 and 418–438 respectively of the Mus musculus PGP sequence (GenBank®/EBI accession number AJ278829). A 275 bp amplicon was generated by PCR using T. brucei brucei genomic DNA as a template. This amplicon was cloned into pGEM T-Easy (Promega) to create pRM228, excised with EcoRI, and used to screen a T. brucei brucei ILTat 1.1 SalI genomic DNA library [14]. A 9 kb SalI–SalI DNA fragment contained the full T. brucei brucei PGP I (pgp) open reading frame. The T. brucei brucei pgp gene was amplified by PCR from pRM228 with PfuTurbo polymerase (Stratagene) using forward (5′-CTAGCTCGAGATGAAGCCTACAAAACCACTA-3′) and reverse (5′-CTAGCTCGAGTCATTCAACTGCTTCCATGTG-3′) primers (with internal XhoI sites in boldface). The amplicon, cloned into pGEM T-Easy to create pRM233, was subcloned into the XhoI site of pET15b (Novagen) to create pRM249. A catalytically inactive PGP variant was created by converting the active-site cysteine (Cys167) [15] into an alanine by site-directed mutagenesis using the QuikChange™ system (Stratagene) with forward (5′-GGGCGATATTACGCCAACTATGCACTG-3′) and reverse (5′-CAGTGCATAGTTGGCGTAATATGCCCC-3′) primers (the mutated codon is underlined, base-pair changes are in boldface) and pRM249 as a template, creating pRM253. Plasmids were transformed into Escherichia coli BL21(λDE3), and N-terminal polyhistidine-tagged fusion proteins were expressed as described previously [16]. Polyhistidine tags were removed using a thrombin cleavage capture kit (Novagen) as per the manufacturer's instructions. Stage-specific expression of the pgp gene was investigated by RT (reverse transcriptase)–PCR using RNA from bloodstream-form T. brucei brucei trypomastigote and cultured promastigote [16], and forward (5′-ATGAAGCCTACAAAACCACTA-3′) and reverse (5′-TCATTCAACTGCTTCCATGTG-3′) primers spanning the full T. brucei brucei pgp open reading frame. RNA equivalence was determined by amplifying a 1500 bp fragment from the T. brucei brucei opdB gene with forward (5′-ATGCAAACTGAACGTGGTCCA-3′) and reverse (5′-ATACGGCAGGAACCGTGAGTT-3′) primers corresponding to base-pairs 1–21 and 1480–1500 respectively of the T. brucei brucei opdB sequence [9].
Enzymatic analysis of T. brucei brucei PGP
The pH activity and stability optima for native PGP purified from infectious plasma were determined by preincubating PGP (10 nM, active concentration by mass, 37 °C, 5 min) in constant-ionic-strength AMT (acetate-Mes-Tris) buffer (50 mM acetic acid/50 mM Mes/100 mM Tris/HCl, pH 4–10.5, with 1 mM TCEP), prior to the addition of Glp-AMC substrate, as described previously [11]. Substrate specificity of native and recombinant PGP was explored using a series of pNA (p-nitroanilide)-derived and AMC-derived substrates. Substrate specificity was determined by preincubation of PGP (2–10 nM, active concentration by mass, 37 °C, 5 min) in 50 mM Tris/HCl/1 mM TCEP (pH 7.4) prior to the addition of substrate. The Km, Vmax and kcat values were determined as described previously [16]. Active enzyme concentration was calculated assuming 100% activity. The inhibition constant, Ki, for reversible inhibitors and the second-order inhibition rate constant, kass, were calculated as described previously [9], but using the buffer system described above. Inclusion of PGP by α2-macroglobulin was investigated by gel filtration as described in [8]. Hydrolysis of the neuropeptides TRH and GnRH was monitored by HPLC on a μBondapack C18 column (Waters) essentially as described in [17], except that samples were acidified by the addition of acetic acid to 10% (v/v) prior to application to the column, and were eluted with a linear gradient of 5–65% (v/v) acetonitrile containing 0.15% (v/v) trifluoroacetic acid over 32 min (0.6 ml·min−1). The GnRH degradation products were identified by MALDI–TOF (matrix-assisted laser-desorption ionization–time-of-flight) MS on a Voyager-DE™ STR (Applied Biosystems) using α-cyano-4-hydroxycinnamate as matrix, as described previously [11]. The TRH MALDI–TOF spectrum was superimposed upon that of the matrix background; therefore TRH degradation products were identified by the inclusion of standards [TRH, Glp-His-Pro-OH, cyclo(His-Pro) and Glp; Sigma] in the HPLC run.
Preparation of trypanosome extracts, culture supernatants and subcellular fractionation
Parasite lysates were prepared as described previously [16]. Liberation of PGP into culture media was determined by incubation of T. brucei brucei (1×108 cells; 2 h, 37 °C) in Eagle's minimum essential medium (Sigma) with 0.3 g·l−1 L-glutamine/0.25 mM L-cysteine/0.01 mM bathocuproinedisulphonic acid/15% (v/v) fetal bovine serum. Aliquots were centrifuged (1500 g, 2 min, 4 °C). Trypanosome-free supernatants were assayed for activity against α-mannosidase, alanine aminotransferase [11] and PGP. Trypanosome cell bodies were fractionated into ‘nuclear’, ‘large granule’, ‘small granule’, ‘crude microsomal’ and ‘soluble supernatant’ fractions [11]. Fractions were screened for enrichment of α-mannosidase (a dense granule marker), and alanine aminotransferase (a cytosol marker) [11] and for PGP activity.
Antibodies
Anti-rat PGP antibodies were generated in rabbits as described previously [18]. Anti-T. brucei brucei PGP antibodies were also raised in rats to facilitate passive immunization of trypanosome-infected rats. Ten Sprague–Dawley rats each received two intradermal injections of recombinant PGP (50 μg per injection site, in Freund's Complete adjuvant; Sigma). Each rat received a booster immunization of 50 μg, 3 and 7 weeks post-immunization, in Freund's Incomplete adjuvant (Sigma). At week 9, rats were exsanguinated, and an antibody fraction was isolated from pooled plasma by poly(ethylene glycol) precipitation [19]. Antibody fractions were evaluated for PGP activity-neutralizing antibodies by including preimmune rat antibodies or rat anti-T. brucei brucei PGP antibodies (1–1000 μg·ml−1) in the PGP activity assay.
In vitro and in vivo degradation of TRH and GnRH in rat plasma
Either [L-His-4-3H(n),L-Pro-3,4-3H(n)]TRH (82 Ci·mmol−1; PerkinElmer) or 125I-Tyr5-GnRH (2000 Ci·mmol−1; GE Healthcare) was incubated in trypanosome- and cell-free plasma from healthy or infected rats for 5 min. At 30 s intervals, aliquots were removed, deproteinated with 2 vol. of 2% (w/v) ZnSO4 in 50% (v/v) methanol/10% (v/v) acetic acid, and resolved by HPLC as described above. Radioactivity associated with the intact peptide peak was quantified either by liquid-scintillation counting for [L-His-4-3H(n),L-Pro-3,4-3H(n)]TRH, or in a γ-counter for 125I-Tyr5-GnRH. A ratio was determined for the fraction of radiation recovered at time t (Ct) representing intact peptide versus that initially applied (Ctot). Linear regression of a semi-logarithmic plot of ln(Ct/Ctot) versus time yielded a graph with a slope=k, where k is the rate of disappearance of intact ANF. The half-life for the disappearance of the intact peptide, t1/2, is given by t1/2=(ln 2)/k. For estimation of t1/2 in infected hosts, rats were selected at midlevel parasitaemia (1×107 trypanosomes·ml−1). Rats with higher parasitaemia did not tolerate surgery and catheter placement. Rats were anaesthetized with sodium pentobarbital (50 mg·kg−1; intraperitoneal), and the left carotid artery and the right jugular vein were catheterized with PE-50 tubing. A bolus of [L-His-4-3H(n),L-Pro-3,4-3H(n)]TRH (2 μCi) or 125I-Tyr5-GnRH (1 μCi) was administered via the jugular catheter through which continuous infusion of Ringer's solution (50 μl·min−1; 160 mM NaCl, 4.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2 and 10 mM Hepes, pH 7.4) was maintained. At timed intervals (every 30 s for 5 min), arterial blood samples (0.5 ml) were extracted and processed for HPLC and radionuclide counting as described above. In some cases, either preimmune rat antibodies or rat anti-PGP antibodies were infused via the jugular catheter at a rate of 25 μg·min−1 for 2 h prior to application of the labelled peptide bolus.
Statistical treatment of data
Intergroup differences were estimated by statistical analysis of variance. Fisher's protected least-significant-difference test was used to compare individual groups. Differences within a group were evaluated with Student's t test.
RESULTS
A type I PGP is present and active in infected rat plasma
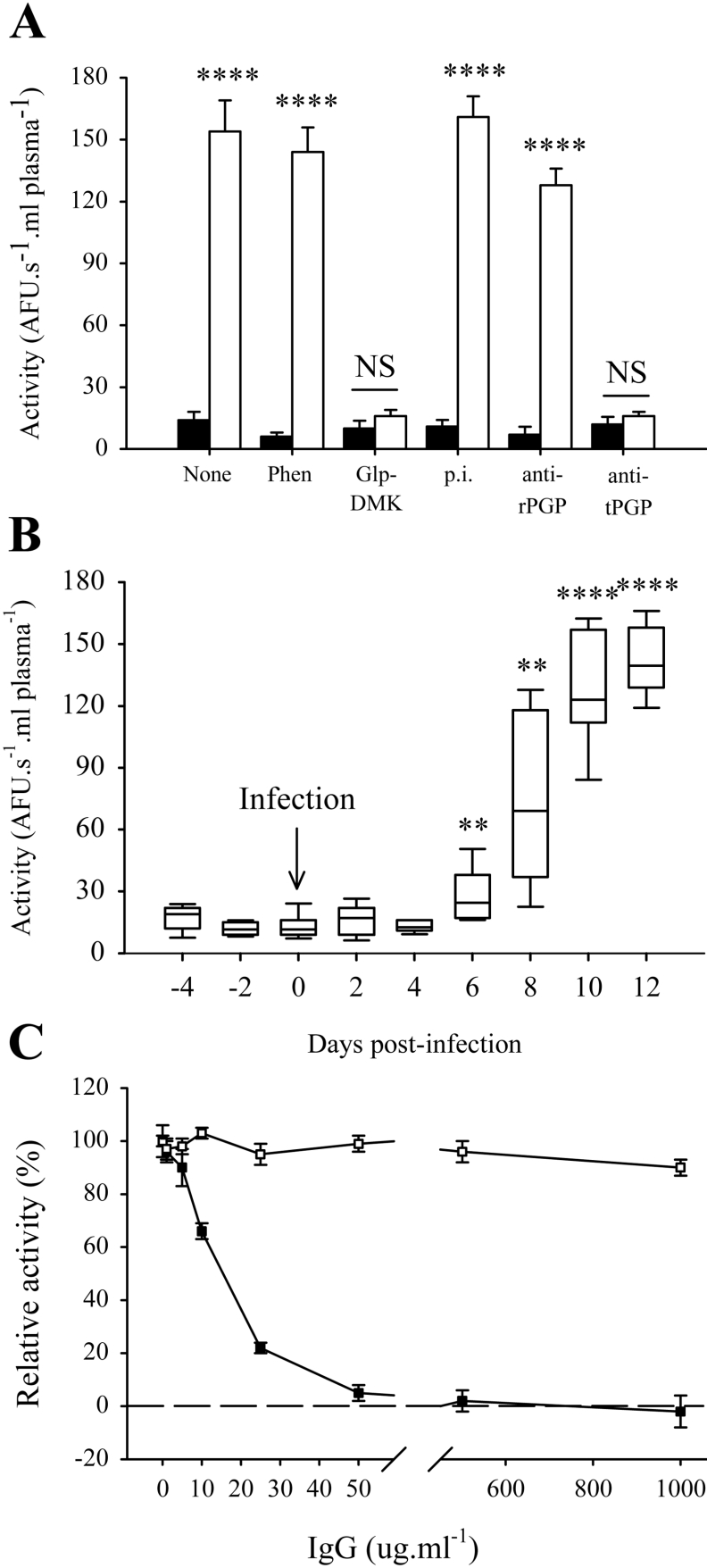
Potent Glp-AMC-hydrolysing activity was evident in the plasma of rats infected with T. brucei brucei (Figure 1A). This activity was minimal in healthy rats (Figure 1A), suggesting that it was either trypanosome-derived or elicited from host tissues during trypanosome infection. This activity was apparent in rat plasma starting at days 6–8 post-infection, and reached a plateau on day 12 post-infection (Figure 1B). Two forms of PGP exist [20]: type I PGP (EC 3.4.19.3), a soluble intracellular cysteine peptidase, and type II PGP (EC 3.4.19.6), which is both a soluble and a membrane-bound metallopeptidase. Glp-DMK is a potent and specific inhibitor of type I PGP [13], and completely abrogated Glp-AMC-hydrolysing activity in the plasma of trypanosome-infected rats (Figure 1A). In contrast, rabbit anti-rat PGP antibodies, which are specific inhibitors of rat type I PGP and which abrogate 100% of type I PGP activity in rat liver cytosol (although only 80% in human liver cytosol) [18] had marginal effect (Figure 1A). Additionally, the metal-ion chelator 1,10-phenanthroline, a documented inhibitor of the type II PGP [21], did not significantly alter the PGP activity in trypanosome-infected rat plasma (Figure 1A). Together, these results suggest that Glp-AMC-hydrolysing activity in trypanosome-infected rat plasma was due to a type I PGP, but not the endogenous rat type I PGP. Neither DCI nor pepstatin A inhibited the plasma PGP activity, ruling out serine or aspartic peptidases respectively. Since, in mammals, the type I PGPs are generally cytosolic enzymes that are not present in the plasma, this activity in the plasma of trypanosome-infected rats was most likely due to a trypanosome PGP. To conclusively identify this enzyme, we set out to purify it from trypanosome-infected rat plasma.
Figure 1. Identification of type I PGP activity in the plasma of trypanosome-infected rats.
(A) PGP activity in infected rat plasma is attributable to a type I PGP. Glp-AMC-hydrolysing activity in 100 μl aliquots of healthy rat plasma (closed bars) or infected rat plasma (open bars), preincubated (15 min, 37 °C) with 1,10-phenanthroline (Phen; 1 mM), Glp-DMK (100 μM), or preimmune (p.i.; 100 μg·ml−1), anti-rat PGP (rPGP; 100 μg·ml−1) or anti-trypanosome PGP (tPGP; 100 μg·ml−1) antibodies are shown. Results shown are the means±S.D. (n=3). Significance probabilities are indicated for infected plasma versus healthy plasma (open versus closed bars, within the same group). (B) Time course for the appearance of type I PGP activity in infected rat plasma and Glp-AMC-hydrolysing activity of plasma drawn from the tail vein of rats at 2 day intervals, starting 4 days prior to infection and ending at 12 days post-infection are shown. The bars represent the data range, while the boxes represent lower and upper quartiles. The line within the quartile box indicates the median (n=5). (C) PGP activity-neutralizing activity of week 9 rat anti-trypanosome PGP antibodies. Recombinant trypanosome PGP (1 nM active concentration, by mass) was preincubated with preimmune rat (open squares) or week 9 anti-trypanosome PGP (closed squares) antibodies (1–1000 μg·ml−1) for 15 min at 37 °C prior to assessment of activity against Glp-AMC. Results shown are the means±S.D. (n=3). AFU, arbitrary fluorescence units; NS, not significant; **P<0.05; ****P<0.001.
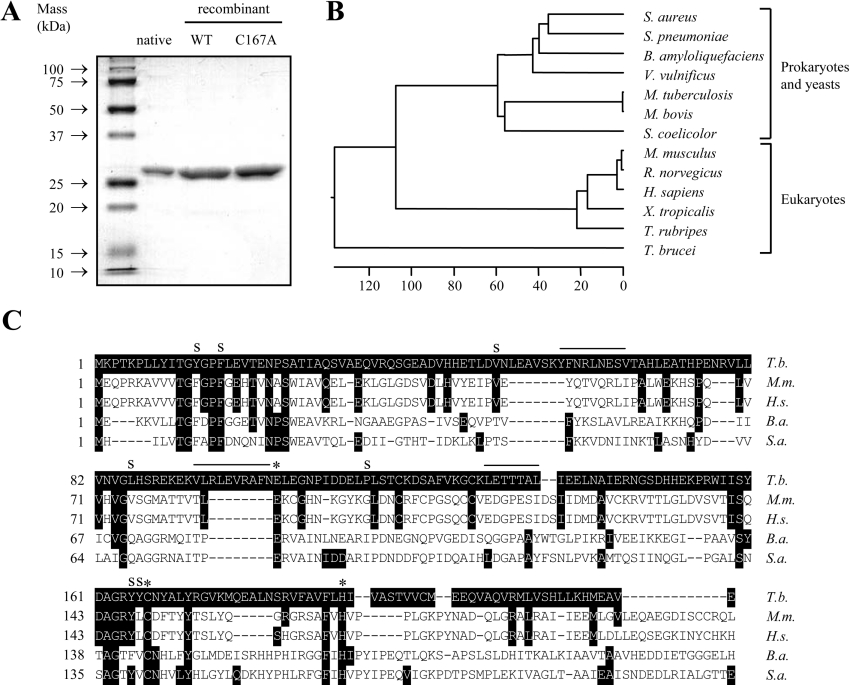
PGP was purified 9248-fold from T. brucei brucei-infected rat plasma, with a 27% yield (Table 1). The purified enzyme migrated at a molecular mass of 26 kDa on a reducing SDS/15% polyacrylamide gel (Figure 2A). This molecular mass is consistent with the molecular masses of other type I PGPs, including those from human [22] and rat [18] tissue. However, three peptides derived from endoproteinase Lys-C digests of the purified PGP (YFNRLNESV, LETTTAL and VLRLEVRAFN) did not display similarity to any protein sequences in the GenBank®/EBI database. Therefore, to conclusively identify the PGP that we had isolated, and to facilitate the generation of antibodies, we set out to clone the type I pgp gene from T. brucei brucei.
Table 1. Purification of PGP from T. brucei brucei-infected rat plasma.
The PGP activity of fractions was assayed against 10 μM Glp-AMC in 50 mM Tris/HCl/1 mM EDTA/1 mM TCEP (pH 7.4). AFU, arbitrary fluorescence units.
| Fraction | Activity (ΔAFU·s−1) | Specific activity (ΔAFU·s−1·mg−1) | Purification (fold change) | Yield (%) |
|---|---|---|---|---|
| Whole plasma | 11454 | 2.16 | 1.0 | 100 |
| TPP | 10538 | 3.25 | 1.5 | 92 |
| Cibacron Blue F3GA–Sepharose | 9007 | 5.35 | 2.4 | 79 |
| HiLoad™ 26/10 Q-Sepharose | 7112 | 65.25 | 30.2 | 62 |
| HiPrep™ 16/10 phenyl-Sepharose | 4023 | 1341 | 621 | 35 |
| RESOURCE™ Q-Sepharose | 3766 | 4570 | 2115 | 33 |
| Sephacryl S-100 HR | 3126 | 30057 | 9248 | 27 |
Figure 2. Type I PGP from T. brucei brucei.
(A) Purity of PGP preparations. Native PGP purified from infected rat plasma, and recombinant catalytically active wild-type (WT) or a catalytically inactive C167A variant (C167A) were resolved on a reducing SDS/15% polyacrylamide gel, and proteins were visualized by Coomassie Blue staining. (B) An unrooted dendrogram was prepared by comparing the full-length PGP amino acid sequences using the ClustalW alignment software of the MEGALIGN program (DNASTAR) with a PAM250 weight table set with: ktuple=1, gap penalty=3 and gap window=5. The scale at the bottom measures the evolutionary distance between sequences. The units indicate the number of substitution events. Sequences were obtained from the GenBank®/EBI database under the following accession numbers: Q6GDB4 (Staphylococcus aureus), P65678 (Streptococcus pneumoniae), P46107 (B. amyloliquefaciens), Q8D4N5 (Vibrio vulnificus), AAK44557 (Mycobacterium tuberculosis), AAB63524 (My. bovis), NP_624759 (Streptomyces coelicolor), CAC03615 (Mus musculus), BAD01533 (Rattus norvegicus), CAC03610 (Homo sapiens), AAH75524 (Xenopus tropicalis), CAC33026 (Takifugu rubripes) and AAY40294 (T. brucei). (C) Multiple sequence alignment of PGP from T. brucei (T.b.), Mus musculus (M.m.), H. sapiens (H.s.), B. amyloliquefaciens (B.a.) and Staph. aureus (S.a.). Sequence sources are as in (B). Substrate-binding residues are indicated by an ‘S’; catalytic residues are indicated with an asterisk (*). Overlined residues represent peptides generated by endoproteinase Lys-C digests.
Cloning and expression of the T. brucei brucei pgp gene
At the inception of the present study, the T. brucei pgp sequence was not available. Primers spanning the 5′- and 3′-ends of type I pgp genes from humans [22] and Bacillus amyloliquefaciens [23] (Supplementary Table S1 at http://www.BiochemJ.org/bj/394/bj3940635add.htm) did not yield any PCR product when T. brucei brucei genomic DNA was employed as a template. Neither did multiple primers spanning internal sequences of either gene (Supplementary Table S1). Therefore we prepared four sets of degenerate primers based on the human pgp sequence (Supplementary Table S1). One of these sets yielded a 275 bp amplicon, which we used to probe a T. brucei brucei genomic DNA library, and thereby isolated the T. brucei brucei type I pgp gene that contained an open reading frame of 669 bp, encoding a protein of 222 amino acids. This nucleotide sequence has been deposited in the GenBank®/EBI database under the accession number DQ017472. Interestingly, T. brucei brucei PGP (hereafter ‘trypanosome PGP’) is the most evolutionarily divergent member of this family of enzymes, including homologues from prokaryotic and eukaryotic organisms (Figure 2B), exhibiting 11 and 17% sequence identity respectively with the B. amyloliquefaciens and human PGPs. Subsequent sequencing of the T. brucei brucei genome places the pgp gene on chromosome 4 (GenBank®/EBI accession number AC079933). The sequence that we report here has three nucleotide base-pair differences when compared with the published genome sequence: A→G (position 55), G→A (position 276) and T→C (position 283), causing a single Ile19→Val amino acid substitution.
The PGP peptide sequence contained the conserved PGP active-site Glu104, Cys167 and His191 residues [15]. Structural elements that determine substrate specificity in the B. amyloliquefaciens PGP have been identified [24], and some of these elements are also conserved in the trypanosome PGP (Phe16 and Tyr165), while others are not (Tyr13, Val50, Leu86, Pro115 and Tyr166), although they generally exhibit hydrophobic properties, like their B. amyloliquefaciens PGP counterparts (Figure 2C). The three peptides derived from the PGP purified from infected rat plasma match exactly the deduced amino acid sequence of the trypanosome pgp gene (Figure 2C). These results conclusively demonstrate that the PGP isolated from infected rat plasma was trypanosome-derived.
Characterization of T. brucei brucei type I PGP activity
Recombinant catalytically active wild-type PGP and a catalytically inactive C167A PGP variant were expressed in E. coli with an average yield of 6 mg·l−1 of bacterial culture. Both recombinant enzymes migrated at a molecular mass comparable with that of the native enzyme purified from infected rat plasma (Figure 2A) after removal of the polyhistidine affinity tag with thrombin.
Antibodies raised in rats against recombinant wild-type trypanosome PGP exhibited potent PGP activity-neutralizing properties (Figure 1C). At 100 μg·ml−1, anti-trypanosome PGP antibodies abrogated 100% of the PGP activity of recombinant trypanosome PGP (10 nM active concentration, by mass), while half-maximal inhibition was observed at 17 μg·ml−1. These antibodies were more potent in neutralizing trypanosome PGP activity than were anti-rat PGP antibodies (Figure 1A), probably due to the poor similarity in the amino acid sequences of rat and trypanosome PGPs (17% similarity), and thus provided us with a potent tool with which to explore PGP activity in complex biological samples such as plasma. Indeed, when added to plasma from infected rats, the anti-trypanosome PGP antibodies brought total PGP activity down to levels comparable with those observed in healthy rat plasma (Figure 1A).
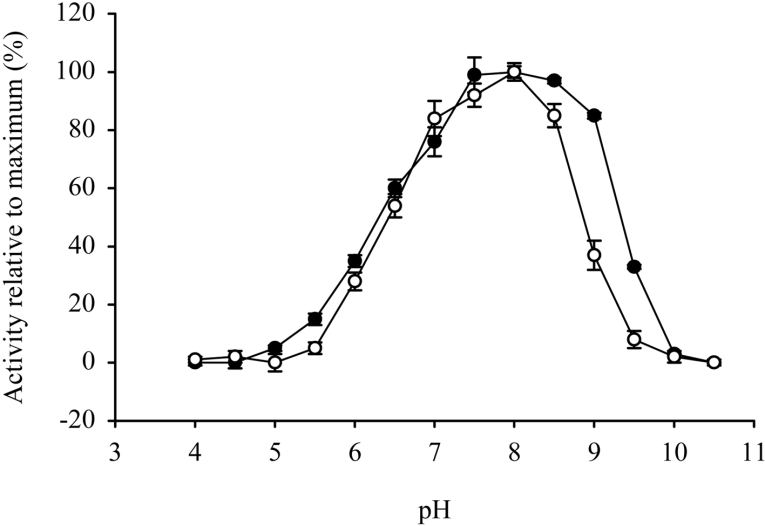
Trypanosome PGP exhibited a broad optimum pH of pH 7.5–8.5 (Figure 3). Furthermore, trypanosome PGP was optimally stable within the pH range 7.5–8.5 (Figure 3). These results are significant, since they indicate that trypanosome PGP is both maximally active and maximally stable at blood pH.
Figure 3. pH activity and stability optima for trypanosome PGP.
The activity (open circles) and stability (closed circles) of T. brucei brucei PGP was assessed in AMT buffer over the pH range 4–10.5 as detailed in the Experimental section. Values represent the means±S.D. (n=3).
Trypanosome PGP exclusively hydrolysed substrates with Glp in the P1 position (Table 2). Both Glp-AMC and Glp-pNA were efficiently hydrolysed (kcat/Km values between 1 and 4 s−1·μM−1). In contrast, the corresponding free amino acids, Glu-AMC and Glu-pNA, and the related amino acid Asp-AMC were not hydrolysed. Like Glp, proline also has a five-member ring structure; however, Pro-AMC was not cleaved by trypanosome PGP either (Table 2). Thus trypanosome PGP exhibits an absolute specificity for the Glp pyrrolidone ring in the P1 position. Trypanosome PGP displayed Km values for Glp-AMC and Glp-pNA as substrates that were comparable with those observed for bovine brain [25] and human recombinant [22] PGPs. Trypanosome PGP efficiently catalysed the removal of N-terminal Glp residues from two naturally occurring neuropeptides: TRH (Glp-His-Pro-NH2) and GnRH (Glp-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-NH2) (Table 2).
Table 2. Catalytic activity of PGP from T. brucei brucei.
The PGP activity was assayed in 50 mM Tris/HCl/1 mM EDTA/1 mM TCEP (pH 7.4). Values represent the means±S.D. (n=3).
| Native PGP | Recombinant PGP | |||||
|---|---|---|---|---|---|---|
| Substrate* | Km (μM) | kcat (s−1) | kcat/Km (s−1·μM−1) | Km (μM) | kcat (s−1) | kcat/Km (s−1·μM−1) |
| Glp-AMC | 18±6 | 58±12 | 3.2 | 28±6 | 47±9 | 1.7 |
| Glp-pNA | 28±9 | 52±7 | 1.9 | 44±8 | 64±8 | 1.5 |
| Glu-AMC | n.h. | n.h. | n.h. | n.h. | n.h. | n.h. |
| Glu-pNA | n.h. | n.h. | n.h. | n.h. | n.h. | n.h. |
| Asp-AMC | n.h. | n.h. | n.h. | n.h. | n.h. | n.h. |
| Pro-AMC | n.h. | n.h. | n.h. | n.h. | n.h. | n.h. |
| TRH† | 41±18 | 20±9 | 0.5 | 32±12 | 22±11 | 0.7 |
| GnRH† | 79±31 | 9±4 | 0.1 | 89±26 | 12±6 | 0.1 |
* No degradation of any substrate was observed by recombinant catalytically inactive (C167A) PGP. n.h., no hydrolysis detected.
† TRH and GnRH were hydrolysed exclusively at the Glp↓Xaa bond.
Consistent with trypanosome PGP being a cysteine peptidase, it was irreversibly inhibited by the thiol-reactive agents iodoacetamide and iodoacetic acid, and by the peptidyl diazomethane Glp-DMK (Table 3). However, trypanosome PGP was not inhibited by E-64, which is generally considered to be a class-specific inhibitor of cysteine peptidases. This has also been reported for human PGP [22]. The Glp analogue 2-pyrrolidone exhibited competitive inhibition of trypanosome PGP (Table 3). Trypanosome PGPs were not inhibited by cystatin C, high-molecular-mass kininogen or α2-macroglobulin (Table 3), which are three key cysteine peptidase inhibitors in mammalian plasma. Furthermore, these purified PGPs retained full catalytic activity in the presence of 50% (v/v) whole plasma (Table 3). Taken together, our kinetic data indicate that trypanosome PGPs (i) are maximally active and stable at blood pH, (ii) can hydrolyse mammalian regulatory peptides that are blocked at the N-terminus by Glp and (iii) host endogenous plasma peptidase inhibitors would not regulate trypanosome PGP activity.
Table 3. Inhibition of PGP from T. brucei brucei.
The PGP activity of fractions was assayed against 10 μM Glp-AMC in 50 mM Tris/HCl/1 mM EDTA/1 mM TCEP (pH 7.4). Values represent the means±S.D. (n=5).
| Native PGP | Recombinant PGP | |||
|---|---|---|---|---|
| Inhibitor* | Ki (μM) | kass (M−1·s−1) | Ki (μM) | kass (M−1·s−1) |
| Iodoacetamide† | 367±18 | 214±16 | ||
| Iodoacetic acid† | 318±33 | 200±17 | ||
| Glp-DMK† | (2.56±0.27)×105 | (2.02±0.44)×105 | ||
| 2-Pyrrolidone | 62±12 | 54±6 | ||
* No inhibition was observed in the presence of: EDTA (1 mM), DCI (1 mM), 4-(2-aminoethyl)benzenesulphonyl fluoride (1 mM), E-64 (10 μM), 1,10-phenanthroline (1 mM), bestatin (25 μM), soya-bean trypsin inhibitor (100 μg·ml−1), lima bean trypsin inhibitor (100 μg·ml−1), ovomucoid (100 μg·ml−1), Cystatin C (1 μg·ml−1), high-molecular-mass kininogen (1 μg·ml−1), pepstatin A (10 μM) or whole plasma (50%).
† Determinations were performed in the presence of 50 μM TCEP.
Trypanosoma PGP is a ubiquitously expressed, cytosolic enzyme
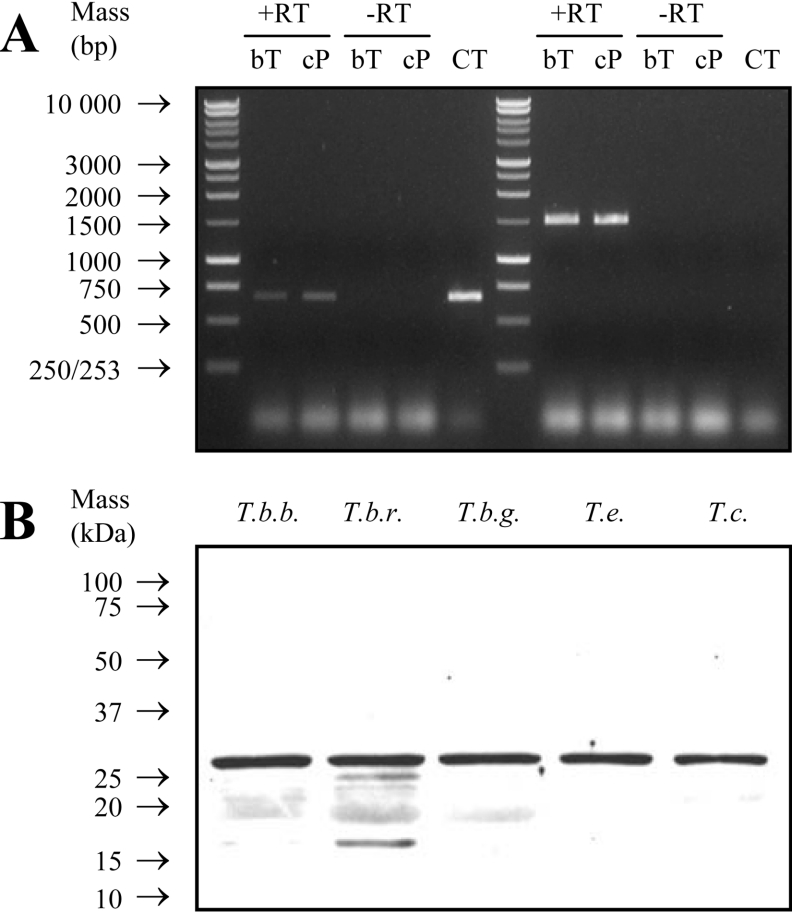
mRNA encoding PGP was detected in both bloodstream-form trypomastigote and insect-form promastigote trypanosomes (Figure 4A). Western blots probed with anti-trypanosome PGP antibodies specifically detected proteins of a molecular mass of approx. 25 kDa in lysates from human-infective T. brucei rhodesiense and T. brucei gambiense, as well as in the veterinary pathogens T. brucei brucei, T. evansi and T. congolense, which together span the ‘humoral’ and ‘haematic’ subgroups of African trypanosomes (Figure 4B). These results indicate that PGP is both expressed throughout the life cycle of the trypanosome, and is ubiquitously expressed amongst trypanosomatids.
Figure 4. Trypanosome PGP is ubiquitously expressed.
(A) Expression of the pgp gene in different life cycle stages of T. brucei brucei. The pgp gene expression was demonstrated by RT–PCR (+RT) from bloodstream-form trypomastigotes (bT) and cultured insect-form promastigotes (cP) mRNA. An RT reaction mixture to which no RT had been added (−RT) and plasmid pRM228 (containing the full-length T. brucei brucei pgp gene) served as negative and positive control templates respectively. RNA equivalence was demonstrated by amplifying a 1500 bp fragment of the opdb gene from the RT reaction mixture. CT, control. (B) Expression of the pgp gene in different African trypanosomes. Extracts (30 μg) of T. brucei brucei (T.b.b.), T. brucei rhodesiense (T.b.r.), T. brucei gambiense (T.b.g.), T. evansi (T.e.) and T. congolense (T.c.) were resolved on a reducing SDS/15% polyacrylamide gel, transferred on to a PVDF membrane and probed with anti-trypanosome PGP antibodies.
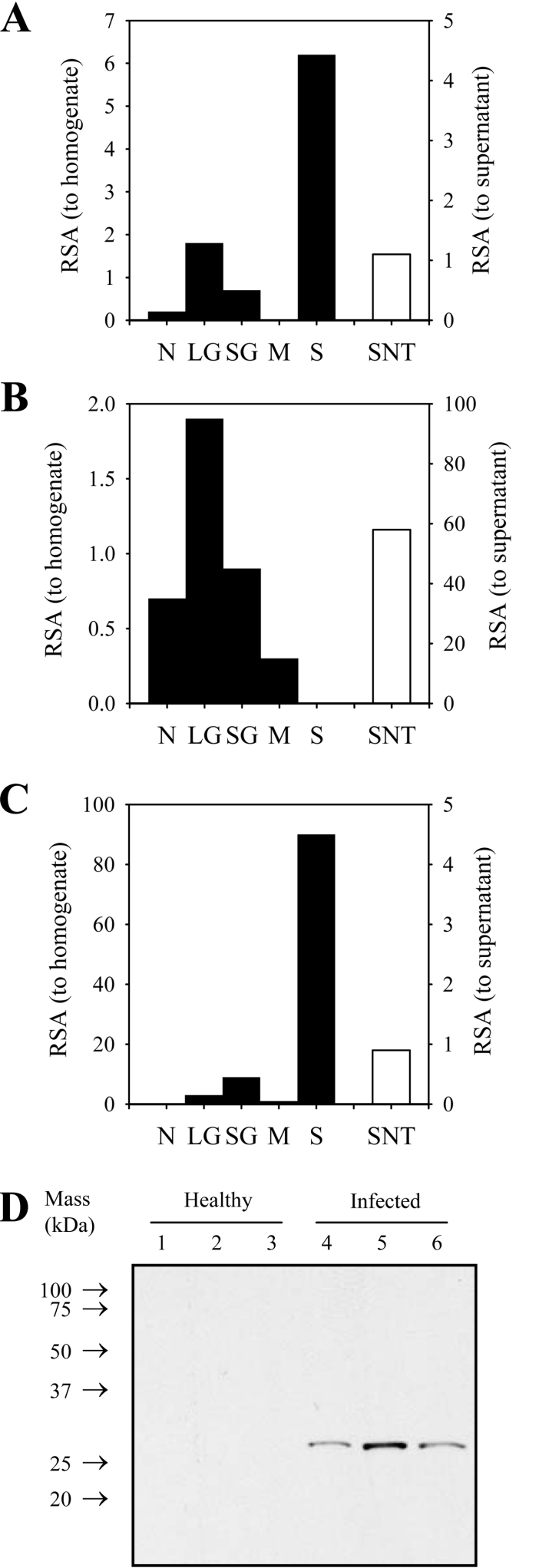
In subcellular fractions of trypomastigotes, PGP activity co- migrated with alanyl aminotransferase activity (Figures 5A and 5B), a cytosolic marker. Activity did not co-migrate with that of α-mannosidase, a large-granule marker (Figure 5C). The PGP activity was not released into the culture supernatant (Figure 5A); thus PGP is not a secreted enzyme, consistent with the lack of a secretion signal in the primary sequence (Figure 2C). These results suggest that PGP is liberated into the bloodstream during routine intravascular destruction of trypanosomes by immune defences of the mammalian host. This idea is supported by the observation that activity was apparent in the rat plasma starting at days 6–8 post-infection (Figure 1B), which is when the first wave of parasite destruction in the host circulation is usually observed [26].
Figure 5. Trypanosome PGP is a cytosolic enzyme that is released into the plasma of infected rats.
(A–C) Trypanosome PGP is a cytosolic enzyme. T. brucei brucei cell bodies were fractionated into crude nuclear (N), large granule (LG), small granule (SG), crude microsomal (M) and high-speed soluble supernatant (S) fractions. The specific activities of tyrosine aminotransferase (A), α-mannosidase (B) and PGP (C), in subcellular fractions, are expressed relative to the specific activity (RSA) of the homogenate (primary y-axis). Similarly, specific activities of these three enzymes in T. brucei brucei cell-culture supernatants (SNT) are expressed relative to the specific activity (RSA) in naïve media (secondary y-axis). (D) Immunoreactive PGP is present in the plasma of infected rats. Partially fractionated plasma, from three healthy rats (lanes 1–3) or three trypanosome-infected rats (lanes 4–6), was resolved by reducing Tris/Tricine SDS/PAGE on an SDS/15% polyacrylamide gel, transfer on to a PVDF membrane and probing with anti-trypanosome PGP antibodies.
Trypanosome PGP dramatically decreases TRH plasma half-life
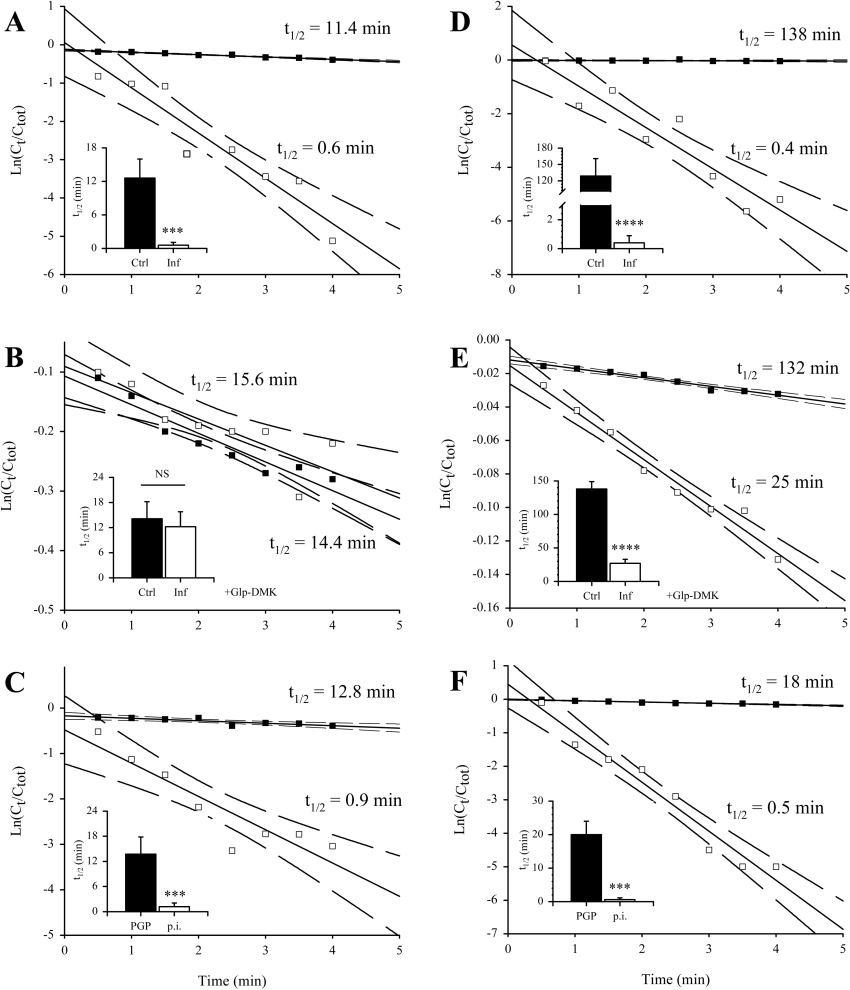
Our observations that trypanosome PGP is released into the host circulation, where it retains catalytic activity, suggested to us that PGP might influence peptide hormone dynamics in the plasma of infected hosts. To explore this possibility, we employed the neuropeptide TRH that is blocked at the N-terminus with Glp, and is a substrate for PGP in vitro (Table 2). We determined that the half-life for the disappearance of the intact peptide (t1/2) in plasma harvested from healthy rats was 12.6±3.4 min (Figure 6A), which closely approximates to the t1/2 for TRH in human plasma in vitro (9.4 min) [17]. This t1/2 was dramatically (21-fold) reduced to 0.59±0.50 min in plasma from infected rats (Figure 6A). Since this elevated degradation rate could be due to type I or type II PGP, we employed two specific inhibitors of type I PGP to identify the class of enzyme responsible. When Glp-DMK (a specific inhibitor of type I PGP; 100 μM) was added to plasma from infected rats, the t1/2 was elevated to 12.2±3.6 min (Figure 6A), which is comparable with the t1/2 observed in plasma from healthy rats. These results implicate a type I PGP in the abnormal turnover of TRH in plasma from infected rats. When anti-trypanosome PGP antibodies were added to plasma from infected rats (100 μg·ml−1), the TRH t1/2 was elevated to 13.7±4.1 min (Figure 6C), which again is comparable with the t1/2 observed in plasma from healthy rats, while preimmune antibodies at the same concentration were without effect. These results confirm that a trypanosome-derived type I PGP is responsible for the degradation of TRH in the plasma from infected rats.
Figure 6. Trypanosome PGP reduces the plasma half-life TRH and GnRH in vitro.
A single representative experiment is illustrated in a scatter plot that shows the loss of radioactive tracer from the plasma, while the insets give the averaged values of three independently determined half-lives for each experimental group. Half-life of [L-His-4-3H(n),L-Pro-3,4-3H(n)]TRH in (A) untreated plasma or (B) plasma treated with Glp-DMK (100 μM; 37 °C, 15 min), from healthy (Ctrl; ■) or infected rats (Inf; □). (C) Half-life of [L-His-4-3H(n),L-Pro-3,4-3H(n)]TRH in plasma from infected rats preincubated with rat antitrypanosome PGP antibodies (PGP; ■) or preimmune rat antibodies (p.i.; □) (100 μg·ml−1; 37 °C, 15 min). (D) Half-life of 125I-Tyr5-GnRH in (D) untreated plasma or (E) plasma treated with Glp-DMK (100 μM; 37 °C, 15 min), from healthy (Ctrl; ■) or infected rats (Inf; □). (F) Half-life of 125I-Tyr5-GnRH in plasma from infected rats preincubated with rat anti-trypanosome PGP antibodies (PGP; ■) or preimmune rat antibodies (p.i.; □) (100 μg·ml−1; 37 °C, 15 min). In the scatter plots, solid lines indicate the regression analysis of the plots, while dashed lines indicate the 95% confidence intervals. In the insets, bars represent the means±S.D. (n=3). NS, not significant; ***P<0.05; ****P<0.001.
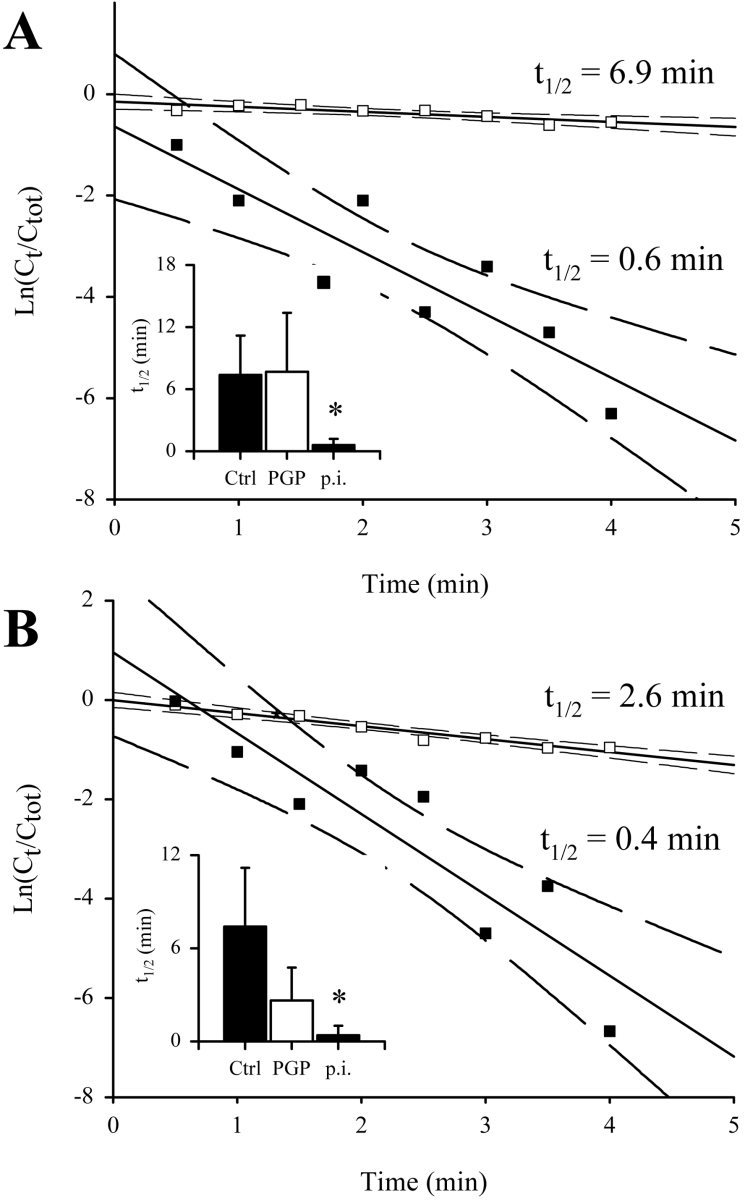
In live healthy rats, we measured an in vivo TRH t1/2 of 7.4±3.8 min (Figure 7A) that compares well with a t1/2 of 6.2 min in humans [27]. In infected rats passively immunized with rat anti-trypanosome PGP antibodies, a similar value of 7.7±5.7 was obtained (Figure 7A). In contrast, in rats passively immunized with preimmune antibodies, the in vivo t1/2 for TRH was reduced 13-fold to 0.60±0.62 min (Figure 7A). Together, our results indicate that trypanosome PGP is responsible for the abnormally rapid degradation of TRH in the circulation of infected rats.
Figure 7. Trypanosome PGP reduces the in vivo plasma half-life of TRH and GnRH.
(A) Plasma half-life of [L-His-4-3H(n),L-Pro-3,4-3H(n)]TRH injected into the jugular vein of infected rats preinfused for 2 h (25 μg·min−1) with rat anti-trypanosome PGP (PGP; □) or preimmune rat antibodies (p.i.; ■). The inset also describes the t1/2 for [L-His-4-3H(n),L-Pro-3,4-3H(n)]TRH in healthy rats (Ctrl; original results not shown). (B) Plasma half-life of 125I-Tyr5-GnRH injected into the jugular vein of infected rats preinfused for 2 h (25 μg·min−1) with rat anti-trypanosome PGP (PGP; □) or preimmune rat antibodies (p.i.; ■). The inset also describes the t1/2 for 125I-Tyr5-GnRH in healthy rats (Ctrl; original results not shown). In the scatter plots, solid lines indicate the regression analysis of the plots, while broken lines indicate 95% confidence intervals. In the insets, the bars reflect the means±S.D. (n=3). *P<0.5.
Interestingly, type I PGPs are not credited with a role in normal TRH metabolism. Rather, a unique membrane-bound type II PGP, highly specific for TRH [28], called TRH-degrading enzyme, inactivates TRH at the cell surface [29]. A soluble form of this enzyme is shed from the cell surface which regulates TRH levels in the plasma [30], and is the primary TRH-degrading activity in healthy serum and plasma [31]. Type I PGPs do not play a significant role in the normal degradation of TRH in vivo [32]. Our results indicate not only that a trypanosome-derived type I PGP promotes abnormal degradation of TRH in vitro and in vivo, but also that this trypanosome-derived PGP is exclusively responsible for this abnormal activity, since Glp-DMK and anti-trypanosome PGP antibodies alone were able to restore both the in vitro and in vivo plasma t1/2 to values observed for healthy animals. Thus other peptidases liberated by trypanosomes, or induced in the host, did not contribute to this abnormal TRH degradation.
Trypanosome PGP moderately decreases GnRH plasma half-life
GnRH is another neuropeptide blocked at the N-terminus with Glp and is also a substrate for trypanosome PGP (Table 2). The GnRH t1/2 was dramatically reduced 323-fold, from 129±32 min in plasma from healthy rats (GnRH is very stable in plasma in vitro, with published in vitro t1/2 values of up to 9 h [33]) to 0.4±0.5 min (Figure 6D) in plasma from infected rats. Thus peptidases liberated by trypanosomes into the plasma, or activated in the host, promote abnormally fast degradation of GnRH. In contrast with that observed for TRH, the addition of Glp-DMK to infected plasma increased the t1/2 of GnRH to 27±6 min (Figure 6E), which is 21% of the value observed in healthy plasma. Similarly, addition of anti-trypanosome PGP antibodies to plasma from infected rats raised the GnRH t1/2 to 20±4 min (Figure 6F), which is 16% of the value observed in plasma from healthy rats. We observed a similar effect in vivo in live rats, where a t1/2 of 7.4±3.8 min was observed in healthy rats (Figure 7B), comparing well with a published value of 10 min [34]. In infected rats passively immunized with preimmune antibodies, the in vivo t1/2 was reduced to 0.4±0.6 min (Figure 7B). This value was elevated to 2.6±2.1 min (35% of the t1/2 observed in healthy rats) in infected rats passively immunized with rat anti-trypanosome PGP antibodies (Figure 7B). Therefore, in contrast with that observed for TRH, multiple peptidases, including PGP, are responsible for the abnormal degradation of GnRH in the plasma of infected hosts. Many peptidases are accredited with a role in degrading GnRH in vivo, including angiotensin-converting enzyme [35], thimet oligopeptidase and prolyl endopeptidase [34]. Since peptidases similar to thimet oligopeptidase [14] and prolyl endopeptidase [36] from trypanosomatids have been described, it is quite possible that upon intravascular destruction of trypanosomes, they are also liberated into the bloodstream and may influence GnRH metabolism in the host.
DISCUSSION
Our results, which constitute the first report of trypanosome-derived cysteine peptidase activity in the plasma of infected hosts and the first description of PGP from a protozoan, demonstrate that trypanosomes contain a type I PGP. During intravascular destruction of trypanosomes in the host circulation, trypanosome PGP is released into the bloodstream and retains catalytic activity, as it is not inhibited by host plasma cysteine peptidase inhibitors: cystatin C, kininogen and α2-macroglobulin. While other trypanosome cysteine peptidases like trypanopain are also released into the host bloodstream [37], they are rapidly (kass≈5×107 M−1·s−1) and tightly (Ki≈5 pM) complexed with cystatins and kininogen, rendering them catalytically inactive; therefore no trypanopain activity is evident in plasma from infected rats [8].
We demonstrated that PGP released by trypanosomes could modulate plasma neuropeptide levels of infected hosts, in vitro and in vivo. If 100% of the Glp-AMC-hydrolysing activity in infected rat plasma was attributable to trypanosome PGP, the plasma PGP concentration is approx. 178 nM, while TRH and GnRH are present at 28 fM [38] and 2 fM [39] respectively. PGP is in vast molar excess. Both TRH and GnRH are secreted by the hypothalamus and are transported to the anterior pituitary in the hypophysial portal blood. Degradation of TRH by type II PGP during TRH transport in hypophysial portal blood is a functional control element regulating TRH activity [20], and it is likely that GnRH is similarly regulated. Both TRH and GnRH would encounter high levels of trypanosome PGP in the hypophysial portal blood, which we speculate would decrease circulating levels of these two neuropeptides, with potentially important implications for host endocrine function. Indeed, African trypanosomiasis is characterized by extensive endocrine lesions [40,41]. We were unable to compare plasma TRH and GnRH levels in infected versus healthy rats in the present study. However, trypanosome-infected hosts exhibit pathology that is consistent with reduced circulating levels of TRH (manifested by a significant decline in plasma 3,5,3′-tri-iodothyronine, thyroxine and thyroid-stimulating hormone [42,43]) and GnRH {manifested by decreased secretion of follicle-stimulating hormone, LH (luteinizing hormone) and testosterone [43]}. Supporting this idea, abnormal GnRH degradation products are detected in the plasma of infected hosts, suggesting activity of a trypanosome peptidase on GnRH [5]. Furthermore, the LH response to GnRH agonists in infected hosts is normal [6], leading other investigators to speculate that “insufficient amounts [of GnRH] could have reached the pituitary gland due…to degradation by proteases released by the trypanosome” [6]. We provide evidence that trypanosome PGP is one of those proteases.
We propose in the present study that decreased circulating levels of TRH and GnRH, as a consequence of their hydrolysis by trypanosome PGP, could underlie perturbations in the hypothalamic–pituitary–adrenal and hypothalamic–pituitary–gonadal axes in trypanosome infections. This idea remains entirely speculative, however, since definitive proof for a role of trypanosome PGP in the pathogenesis of African trypanosomiasis could only be obtained with a pgp-knockout trypanosome. That fact notwithstanding, our hypothesis is supported by the dramatic reductions observed in plasma levels of testosterone and LH that coincide with the trypanolytic phase in rats [7], which is exactly when we would expect PGP to be liberated by trypanosomes during an infection. The activity of trypanosome PGP in host tissues would not be restricted to hydrolysing TRH and GnRH, but would also extend to other regulatory peptides N-terminally blocked with a pyroglutamyl moiety, for example neurotensin and gastrin. The idea that peptidases released by trypanosomes can influence host neuropeptide dynamics has important implications for our understanding of the endocrine lesions observed in African trypanosomiasis.
Online data
Acknowledgments
We thank Dr Janine Robert-Baudouy (formerly of the Laboratoire de Genetique Moleculaire des Microorganismes, CNRS-UMR 5577, Villeurbanne, France) for advice, Dr István Vadász (Division of Pulmonary and Critical Care Medicine, Northwestern University School of Medicine, Chicago, IL, U.S.A.) for expert advice and a critical reading of this paper, and Dr Isabel Roditi (Institut fur Zellbiologie, Universität Bern, Bern, Switzerland) and Dr Oliver Eickelberg (Uniklinikum Giessen und Marburg, Giessen, Germany) for outstanding support. This study was supported by grants from the Deutsche Forschungsgemeinschaft (SFB547) to R. E. M. and P. B., the International Livestock Research Institute (to R. P.) and Sankyo Company (to K. A.).
References
- 1.Barrett M. P., Burchmore R. J., Stich A., Lazzari J. O., Frasch A. C., Cazzulo J. J., Krishna S. The trypanosomiases. Lancet. 2003;362:1469–1480. doi: 10.1016/S0140-6736(03)14694-6. [DOI] [PubMed] [Google Scholar]
- 2.Tizard I., Nielsen K. H., Seed J. R., Hall J. E. Biologically active products from African trypanosomes. Microbiol. Rev. 1978;42:664–681. doi: 10.1128/mr.42.4.664-681.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boutignon F., Huet-Duvillier G., Demeyer D., Richet C., Degand P. Study of proteolytic activities released by incubation of trypanosomes (Trypanosoma brucei brucei) in pH 5.5 and pH 7.0 phosphate/glucose buffers. Biochim. Biophys. Acta. 1990;1035:369–377. doi: 10.1016/0304-4165(90)90102-3. [DOI] [PubMed] [Google Scholar]
- 4.Knowles G., Black S. J., Whitelaw D. D. Peptidase in the plasma of mice infected with Trypanosoma brucei brucei. Parasitology. 1987;95:291–300. doi: 10.1017/s0031182000057747. [DOI] [PubMed] [Google Scholar]
- 5.Tetaert D., Soudan B., Huet-Duvillier G., Degand P., Boersma A. Unusual cleavage of peptidic hormones generated by trypanosome enzymes released in infested rat serum. Int. J. Pept. Protein Res. 1993;41:147–152. doi: 10.1111/j.1399-3011.1993.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 6.Ng'wena A. G., Patel N. B., Wango E. O. Plasma luteinizing hormone levels in response to gonadotropin-releasing hormone agonist and clonidine in Trypanosoma congolense-infected female goats. Brain Res. Bull. 1997;44:591–595. doi: 10.1016/s0361-9230(97)00305-5. [DOI] [PubMed] [Google Scholar]
- 7.Soudan B., Tetaert D., Hublart M., Racadot A., Croix D., Boersma A. Experimental ‘chronic’ African trypanosomiasis: endocrine dysfunctions generated by parasitic components released during the tryptanolytic phase in rats. Exp. Clin. Endocrinol. 1993;101:166–172. doi: 10.1055/s-0029-1211225. [DOI] [PubMed] [Google Scholar]
- 8.Troeberg L., Pike R. N., Morty R. E., Berry R. K., Coetzer T. H., Lonsdale-Eccles J. D. Proteases from Trypanosoma brucei brucei. Purification, characterisation and interactions with host regulatory molecules. Eur. J. Biochem. 1996;238:728–736. doi: 10.1111/j.1432-1033.1996.0728w.x. [DOI] [PubMed] [Google Scholar]
- 9.Morty R. E., Lonsdale-Eccles J. D., Morehead J., Caler E. V., Mentele R., Auerswald E. A., Coetzer T. H., Andrews N. W., Burleigh B. A. Oligopeptidase B from Trypanosoma brucei, a new member of an emerging subgroup of serine oligopeptidases. J. Biol. Chem. 1999;274:26149–26156. doi: 10.1074/jbc.274.37.26149. [DOI] [PubMed] [Google Scholar]
- 10.Bastos I. M., Grellier P., Martins N. F., Cadavid-Restrepo G., de Souza-Ault M. R., Augustyns K., Teixeira A. R., Schrével J., Maigret B., da Silveira J. F., et al. Molecular, functional and structural properties of the prolyl oligopeptidase of Trypanosoma cruzi (POP Tc80), which is required for parasite entry into mammalian cells. Biochem. J. 2005;388:29–38. doi: 10.1042/BJ20041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morty R. E., Pellé R., Vadász I., Uzcanga G. L., Seeger W., Bubis J. Oligopeptidase B from Trypanosoma evansi: a parasite peptidase that inactivates atrial natriuretic factor in the bloodstream of infected hosts. J. Biol. Chem. 2005;280:10925–10937. doi: 10.1074/jbc.M410066200. [DOI] [PubMed] [Google Scholar]
- 12.Morty R. E., Lonsdale-Eccles J. D., Mentele R., Auerswald E. A., Coetzer T. H. Trypanosome-derived oligopeptidase B is released into the plasma of infected rodents, where it persists and retains full catalytic activity. Infect. Immun. 2001;69:2757–2761. doi: 10.1128/IAI.69.4.2757-2761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilk S., Friedman T. C., Kline T. B. Pyroglutamyl diazomethyl ketone: potent inhibitor of mammalian pyroglutamyl peptide hydrolase. Biochem. Biophys. Res. Commun. 1985;130:662–668. doi: 10.1016/0006-291x(85)90468-1. [DOI] [PubMed] [Google Scholar]
- 14.Morty R. E., Vadász I., Bulau P., Dive V., Oliveira V., Seeger W., Juliano L. Tropolysin: a new oligopeptidase from African trypanosomes. Biochemistry. 2005;44:14658–14669. doi: 10.1021/bi051035k. [DOI] [PubMed] [Google Scholar]
- 15.Le Saux O., Gonzales T., Robert-Baudouy J. Mutational analysis of the active site of Pseudomonas fluorescens pyrrolidone carboxyl peptidase. J. Bacteriol. 1996;178:3308–3313. doi: 10.1128/jb.178.11.3308-3313.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morty R. E., Morehead J. Cloning and characterization of a leucyl aminopeptidase from three pathogenic Leishmania species. J. Biol. Chem. 2002;277:26057–26065. doi: 10.1074/jbc.M202779200. [DOI] [PubMed] [Google Scholar]
- 17.Møss J., Bundgaard H. Kinetics and pattern of degradation of thyrotropin-releasing hormone (TRH) in human plasma. Pharm. Res. 1990;7:751–755. doi: 10.1023/a:1015875824238. [DOI] [PubMed] [Google Scholar]
- 18.Abe K., Watanabe N., Kosaka T., Yamada M., Tokui T., Ikeda T. Hydrolysis of synthetic substrate, L-pyroglutamyl p-nitroanilide is catalyzed solely by pyroglutamyl aminopeptidase I in rat liver cytosol. Biol. Pharm. Bull. 2003;26:1528–1533. doi: 10.1248/bpb.26.1528. [DOI] [PubMed] [Google Scholar]
- 19.Polson A., Ruiz-Bravo C. Fractionation of plasma with polyethylene glycol. Vox Sang. 1972;23:107–118. [PubMed] [Google Scholar]
- 20.Cummins P. M., O'Connor B. Pyroglutamyl peptidase: an overview of the three known enzymatic forms. Biochim. Biophys. Acta. 1998;1429:1–17. doi: 10.1016/s0167-4838(98)00248-9. [DOI] [PubMed] [Google Scholar]
- 21.Czekay G., Bauer K. Identification of the thyrotropin-releasing-hormone-degrading ectoenzyme as a metallopeptidase. Biochem. J. 1993;290:921–926. doi: 10.1042/bj2900921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dando P. M., Fortunato M., Strand G. B., Smith T. S., Barrett A. J. Pyroglutamyl-peptidase I: cloning, sequencing, and characterisation of the recombinant human enzyme. Protein Expr. Purif. 2003;28:111–119. doi: 10.1016/s1046-5928(02)00632-0. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimoto T., Shimoda T., Kitazono A., Kabashima T., Ito K., Tsuru D. Pyroglutamyl peptidase gene from Bacillus amyloliquefaciens: cloning, sequencing, expression, and crystallization of the expressed enzyme. J. Biochem. (Tokyo) 1993;113:67–73. doi: 10.1093/oxfordjournals.jbchem.a124005. [DOI] [PubMed] [Google Scholar]
- 24.Ito K., Inoue T., Takahashi T., Huang H. S., Esumi T., Hatakeyama S., Tanaka N., Nakamura K. T., Yoshimoto T. The mechanism of substrate recognition of pyroglutamyl-peptidase I from Bacillus amyloliquefaciens as determined by X-ray crystallography and site-directed mutagenesis. J. Biol. Chem. 2001;276:18557–18562. doi: 10.1074/jbc.M011724200. [DOI] [PubMed] [Google Scholar]
- 25.Cummins P. M., O'Connor B. Bovine brain pyroglutamyl aminopeptidase (type-1): purification and characterisation of a neuropeptide-inactivating peptidase. Int. J. Biochem. Cell Biol. 1996;28:883–893. doi: 10.1016/1357-2725(96)00034-9. [DOI] [PubMed] [Google Scholar]
- 26.Darsaud A., Bourdon L., Chevrier C., Keita M., Bouteille B., Queyroy A., Canini F., Cespuglio R., Dumas M., Buguet A. Clinical follow-up in the rat experimental model of African trypanosomiasis. Exp. Biol. Med. (Maywood) 2003;228:1355–1362. doi: 10.1177/153537020322801114. [DOI] [PubMed] [Google Scholar]
- 27.Morley J. E., Garvin T. J., Pekary A. E., Utiger R. D., Nair M. G., Baugh C. M., Hershman J. M. Plasma clearance and plasma half-disappearance time of exogenous thyrotropin-releasing hormone and pyroglutamyl-N3im-methyl-histidyl prolineamide. J. Clin. Endocrinol. Metab. 1979;48:377–380. doi: 10.1210/jcem-48-3-377. [DOI] [PubMed] [Google Scholar]
- 28.Kelly J. A., Slator G. R., Tipton K. F., Williams C. H., Bauer K. Kinetic investigation of the specificity of porcine brain thyrotropin-releasing hormone-degrading ectoenzyme for thyrotropin-releasing hormone-like peptides. J. Biol. Chem. 2000;275:16746–16751. doi: 10.1074/jbc.M910386199. [DOI] [PubMed] [Google Scholar]
- 29.Schomburg L., Turwitt S., Prescher G., Lohmann D., Horsthemke B., Bauer K. Human TRH-degrading ectoenzyme cDNA cloning, functional expression, genomic structure and chromosomal assignment. Eur. J. Biochem. 1999;265:415–422. doi: 10.1046/j.1432-1327.1999.00753.x. [DOI] [PubMed] [Google Scholar]
- 30.Schmitmeier S., Thole H., Bader A., Bauer K. Purification and characterization of the thyrotropin-releasing hormone (TRH)-degrading serum enzyme and its identification as a product of liver origin. Eur. J. Biochem. 2002;269:1278–1286. doi: 10.1046/j.1432-1033.2002.02768.x. [DOI] [PubMed] [Google Scholar]
- 31.Friedman T. C., Wilk S. The effect of inhibitors of prolyl endopeptidase and pyroglutamyl peptide hydrolase on TRH degradation in rat serum. Biochem. Biophys. Res. Commun. 1985;132:787–794. doi: 10.1016/0006-291x(85)91201-x. [DOI] [PubMed] [Google Scholar]
- 32.Friedman T. C., Wilk S. Delineation of a particulate thyrotropin-releasing hormone-degrading enzyme in rat brain by the use of specific inhibitors of prolyl endopeptidase and pyroglutamyl peptide hydrolase. J. Neurochem. 1986;46:1231–1239. doi: 10.1111/j.1471-4159.1986.tb00643.x. [DOI] [PubMed] [Google Scholar]
- 33.McDermott J. R., Smith A. I., Biggins J. A., Hardy J. A., Dodd P. R., Edwardson J. A. Degradation of luteinizing hormone-releasing hormone by serum and plasma in vitro. Regul. Pept. 1981;2:69–79. doi: 10.1016/0167-0115(81)90001-x. [DOI] [PubMed] [Google Scholar]
- 34.Lasdun A., Reznik S., Molineaux C. J., Orlowski M. Inhibition of endopeptidase 24.15 slows the in vivo degradation of luteinizing hormone-releasing hormone. J. Pharmacol. Exp. Ther. 1989;251:439–447. [PubMed] [Google Scholar]
- 35.Williams T. A., Barnes K., Kenny A. J., Turner A. J., Hooper N. M. A comparison of the zinc contents and substrate specificities of the endothelial and testicular forms of porcine angiotensin converting enzyme and the preparation of isoenzyme-specific antisera. Biochem. J. 1992;288:875–881. doi: 10.1042/bj2880875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grellier P., Vendeville S., Joyeau R., Bastos I. M., Drobecq H., Frappier F., Teixeira A. R., Schrével J., Davioud-Charvet E., Sergheraert C., et al. Trypanosoma cruzi prolyl oligopeptidase Tc80 is involved in nonphagocytic mammalian cell invasion by trypomastigotes. J. Biol. Chem. 2001;276:47078–47086. doi: 10.1074/jbc.M106017200. [DOI] [PubMed] [Google Scholar]
- 37.Authié E., Muteti D. K., Mbawa Z. R., Lonsdale-Eccles J. D., Webster P., Wells C. W. Identification of a 33-kilodalton immunodominant antigen of Trypanosoma congolense as a cysteine protease. Mol. Biochem. Parasitol. 1992;56:103–116. doi: 10.1016/0166-6851(92)90158-g. [DOI] [PubMed] [Google Scholar]
- 38.Mortimer C. H., McNeilly A. S., Rees L. H., Lowry P. J., Gilmore D., Dobbie H. G. Radioimmunoassay and chromatographic similarity of circulating endogenous gonadotropin releasing hormone and hypothalamic extracts in man. J. Clin. Endocrinol. Metab. 1976;43:882–888. doi: 10.1210/jcem-43-4-882. [DOI] [PubMed] [Google Scholar]
- 39.Azukizawa M., Mitsuma T., Ota M., Miki T., Ichihara K., Kawashima M., Miyai K., Kumahara Y. Fluctuation of the plasma TRH level in normal subjects in a 4-hour observation period. Endocrinol. Jpn. 1980;27:371–374. doi: 10.1507/endocrj1954.27.371. [DOI] [PubMed] [Google Scholar]
- 40.Ikede B. O., Elhassan E., Akpavie S. O. Reproductive disorders in African trypanosomiasis: a review. Acta Trop. 1988;45:5–10. [PubMed] [Google Scholar]
- 41.Reincke M., Arlt W., Heppner C., Petzke F., Chrousos G. P., Allolio B. Neuroendocrine dysfunction in African trypanosomiasis. The role of cytokines. Ann. N. Y. Acad. Sci. 1998;840:809–821. doi: 10.1111/j.1749-6632.1998.tb09619.x. [DOI] [PubMed] [Google Scholar]
- 42.al-Qarawi A. A., Abdel-Rahman H., Elmougy S. A. Impairment in the pituitary-thyroid axis of the Camelus dromedarius infected with Trypanosoma evansi. Dtsch. Tierarztl. Wochenschr. 2001;108:172–174. [PubMed] [Google Scholar]
- 43.Soudan B., Tetaert D., Racadot A., Degand P., Boersma A. Decrease of testosterone level during an experimental African trypanosomiasis: involvement of a testicular LH receptor desensitization. Acta Endocrinol. (Copenhagen) 1992;127:86–92. doi: 10.1530/acta.0.1270086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.