Abstract
Previous work showed that C. elegans gon-14 is required for gonadogenesis. Here we report that gon-14 encodes a protein with similarity to LIN-15B, a class B synMuv protein. An extensive region of GON-14 contains blocks of sequence similarity to transposases of the hAT superfamily, but key residues are not conserved, suggesting a distant relationship. GON-14 also contains a putative THAP DNA-binding domain. A rescuing gon-14∷GON-14∷VENUS reporter is broadly expressed during development and localizes to the nucleus. Strong loss-of-function and predicted null gon-14 alleles have pleiotropic defects, including multivulval (Muv) defects and temperature-sensitive larval arrest. Although the gon-14 Muv defect is not enhanced by synMuv mutations, gon-14 interacts genetically with class B and class C synMuv genes, including lin-35/Rb, let-418/Mi-2β, and trr-1/TRRAP. The gon-14; synMuv double mutants arrest as larvae when grown under conditions supporting development to adulthood for the respective single mutants. The gon-14 larval arrest is suppressed by loss of mes-2/E(Z), mes-6/ESC, or mes-4, which encodes a SET domain protein. Additionally, gon-14 affects expression of pgl-1 and lag-2, two genes regulated by the synMuv genes. We suggest that gon-14 functions with class B and class C synMuv genes to promote larval growth, in part by antagonizing MES-2,3,6/ESC-E(z) and MES-4.
CHROMATIN structure can influence a broad range of biologically important processes, such as transcription, DNA replication, DNA damage repair, and homologous recombination. The structure of chromatin is modulated by post-translational modifications to the N-terminal tails of histones and by the activity of chromatin remodeling factors (reviewed in Jenuwein and Allis 2001; Becker and Hörz 2002). Although considerable progress has been made in identifying biochemical and genetic pathways that regulate chromatin structure, much remains unknown regarding how these pathways are utilized to control development.
Caenorhabditis elegans vulval development has emerged as a model for analyzing the chromatin regulation of specific cell fate decisions. Vulval development is positively regulated by an RTK/Ras signaling pathway and antagonized by the synthetic Multivulva (synMuv) genes, which encode homologs of transcriptional regulators and chromatin remodeling factors (reviewed in Fay and Han 2000; Lipsick 2004). The synMuv genes fall into at least three classes, A, B, and C, which act redundantly to control cell fate specification in six ectodermal blast cells called the vulval precursor cells (VPCs) (Ferguson and Horvitz 1989; Ceol and Horvitz 2004). In wild-type animals, three VPCs are induced to the vulval fate, while the three others assume a hypodermal fate (Sulston and Horvitz 1977). Single mutants of class A or class B synMuv genes typically exhibit normal VPC specification; however, in double mutants lacking one class A gene and one class B gene, all six VPCs adopt vulval fates, a defect called synMuv (Horvitz and Sulston 1980; Ferguson and Horvitz 1989). Class C synMuv genes function redundantly in VPC specification with both class A and class B genes (Ceol and Horvitz 2004).
Most relevant to this work are the class B synMuv genes, some of which encode nematode homologs of components integral to the vertebrate E2F–retinoblastoma (E2F–Rb) and NuRD (nucleosome remodeling and histone deacetylation) complexes (reviewed in Fay and Han 2000; Lipsick 2004). Studies in various organisms indicate that the E2F–Rb complex represses transcription of E2F target genes and cell cycle progression, in part by recruiting chromatin-modifying proteins such as histone deacetylases (HDACs) (reviewed in Frolov and Dyson 2004). Experiments with Drosophila embryos revealed that homologs of many class B synMuv proteins are physically associated in larger E2F- and Rb-containing complexes, known as Drosophila RBF, dE2F2, and dMyb-interacting proteins (dREAM) and Myb–synMuvB (Myb–MuvB); both complexes contain homologs of class B synMuv proteins LIN-35/Rb, EFL-1/E2F2, DPL-1/DP, LIN-9/Mip130/TWIT, LIN-37/Mip40, LIN-53/RbAp48, and LIN-54/Mip120, while the Myb–MuvB complex includes the additional class B homologs HDA-1/RPD3/HDAC, LIN-61/L(3)MBT, and LIN-52/dLIN-52 (Korenjak et al. 2004; Lewis et al. 2004). Although a dREAM or Myb–MuvB-related complex has not yet been purified in C. elegans, its existence is predicted on the basis of the common phenotypes of the class B synMuv mutants. In addition, some class B synMuv genes encode homologs of components of the NuRD complex, a transcriptional repressor complex that contains both histone deacetylase and ATP-dependent chromatin remodeling activities (reviewed in Becker and Hörz 2002); these include HDA-1 and LIN-53, as well as LET-418/Mi-2β (Lu and Horvitz 1998; Solari and Ahringer 2000; von Zelewsky et al. 2000; Thomas et al. 2003). Biochemically, C. elegans LET-418 and HDA-1 were co-immunoprecipitated with another class B protein, MEP-1, leading to the suggestion that these factors form an analogous C. elegans NuRD complex (Unhavaithaya et al. 2002). Other class B synMuv proteins include homologs of chromatin-associated proteins (e.g., HPL-2/HP1) and zinc finger proteins (e.g., LIN-13, LIN-15B, LIN-36, and TAM-1) (Clark et al. 1994; Huang et al. 1994; Hsieh et al. 1999; Thomas and Horvitz 1999; Meléndez and Greenwald 2000; Couteau et al. 2002; Reddy and Villeneuve 2004). The class C synMuv genes encode homologs of components of the Tip60/NuA4-like histone acetyltransferase complex, implicating an additional chromatin-remodeling complex in C. elegans vulval development (Ceol and Horvitz 2004). Therefore, both class B and class C synMuv proteins are likely to function as transcriptional or chromatin regulators.
Although the synMuv genes were identified by their synthetic vulval effects, some are also required for viability, larval growth, or development of other tissues, such as the gonad and male mating structures (Ferguson and Horvitz 1985, 1989; Lu and Horvitz 1998; Beitel et al. 2000; Meléndez and Greenwald 2000; von Zelewsky et al. 2000; Ceol and Horvitz 2001; Belfiore et al. 2002; Dufourcq et al. 2002; Unhavaithaya et al. 2002; Thomas et al. 2003; Ceol and Horvitz 2004). In addition, some synMuv genes interact synthetically with genes that regulate the cell cycle, pharyngeal morphogenesis, gonadogenesis, or larval growth (Boxem and van den Heuvel 2001; Fay et al. 2002, 2003, 2004; Bender et al. 2004a; Cui et al. 2004; Cardoso et al. 2005). Therefore, the synMuv genes have been implicated in the developmental control of many tissues.
Another group of transcriptional regulators critical to this work are the genes encoding the MES-2/MES-3/MES-6 complex, and MES-4, all of which are required for germline viability in C. elegans. The mes-2 and mes-6 genes encode orthologs of Drosophila Polycomb group proteins Enhancer of Zeste [E(Z)] and Extra Sex Combs (ESC), respectively (Holdeman et al. 1998; Korf et al. 1998). MES-2 and MES-6 associate with MES-3, a novel protein, to form the C. elegans analog of the Drosophila ESC-E(Z) and mammalian EED-EZH2 complexes (Paulsen et al. 1995; Xu et al. 2001; Cao and Zhang 2004). The MES-2,3,6/ESC-E(Z) complex has histone H3 Lysine27 (H3-K27) methyltransferase activity and represses transcription (Bender et al. 2004b; Cao and Zhang 2004). In C. elegans, the complex maintains repressive di- and trimethyl H3-K27 marks on the X chromosome in the adult germline and in early embryos (Fong et al. 2002; Bender et al. 2004b) and spatially restricts expression of Hox genes in the soma (Ross and Zarkower 2003). MES-4 is a SET domain protein that functions in a separate complex to promote germline viability; MES-4 localization is correlated with marks of active transcription and is excluded from the germline X chromosome by the MES-2,3,6/ESC-E(Z) complex (Fong et al. 2002). Importantly, disruption of the mes-2,3,6/ESC-E(Z) genes or of mes-4 can suppress certain class B synMuv mutant phenotypes (Unhavaithaya et al. 2002; Wang et al. 2005). One simple interpretation is that class B synMuv and MES activities act antagonistically to control transcription.
Here we describe the cloning and genetic analysis of gon-14, a gene identified in a genetic screen for mutations with early gonad defects (Siegfried et al. 2004). We find that gon-14 encodes a homolog of LIN-15B, which is a class B synMuv protein. Sequence analyses of GON-14 and other LIN-15B paralogs reveal several motifs suggestive of DNA regulation. We demonstrate that a rescuing GON-14 reporter protein is broadly expressed and localizes to the nucleus. The gon-14 locus does not act synthetically with class A or class B synMuv genes to control vulval development, but it does interact with class B and class C synMuv genes to control larval growth. Like some synMuv mutants, gon-14 mutant defects are suppressed by disrupting the mes genes. Furthermore, gon-14 mutants have defects in gene expression that are typical of class B synMuv mutants. Taken together, our results suggest that gon-14 functions in the nucleus to affect gene expression and likely acts in a lin-35/Rb- and let-418/Mi-2β-related process.
MATERIALS AND METHODS
Nematode strains and maintenance:
Animals were grown at 20° unless stated otherwise. All strains were derivative of Bristol strain N2 (Brenner 1974). The following mutations were described previously (Hodgkin 1997 and references therein): LG I: mes-3(bn35), dpy-5(e61), lin-35(n745), unc-13(e1091), xnp-1(tm678) (Bender et al. 2004a); LG II: rrf-3(pk1426) (Simmer et al. 2002), lin-38(n751), trr-1(n3630) (Ceol and Horvitz 2004); LG III: lin-37(n758), lin-36(n766), lin-9(n112), mut-7(pk204) (Ketting et al. 1999), rde-4(ne301) (Tabara et al. 1999); LG IV: eri-1(mg366) (Kennedy et al. 2004); LG V: tam-1(cc567) (Hsieh et al. 1999), let-418(ar114), dpy-11(e224), gon-14(q10, q12, q552, q631, q686) (Siegfried et al. 2004), snb-1(js124) (Nonet et al. 1998), mys-1(n4075) (Ceol and Horvitz 2004), unc-42(e270), him-5(e1490), psa-1(ku355) (Cui et al. 2004), nDf32 (Park and Horvitz 1986); and LG X: lin-15B(n744), lin-15A(n767). The following dominant GFP balancer chromosomes were used: mIn1[mIs14 dpy-10(e128)] II (Edgley and Riddle 2001), hT2[qIs48](I;III) (Miskowski et al. 2001), nT1[qIs51](IV;V) (Siegfried et al. 2004), and DnT1[qIs50](IV;V) (Belfiore et al. 2002). We also used the translocation eT1(III;V) (Hodgkin 1997) and molecular markers qIs57 [lag-2∷GFP, unc-119(+)] II and qIs56 [lag-2∷GFP, unc-119(+)] V (Siegfried et al. 2004).
Molecular and sequence analyses of gon-14:
gon-14 maps to approximate position +0.1 on LG V (Siegfried et al. 2004). We tested five cosmids (K04A8, EGAP9, ZK1055, F44C4, and T10H9) for rescue of gon-14 sterility by standard methods (Mello and Fire 1995). Two partially overlapping cosmids (ZK1055 and F44C4) rescued gon-14(q12) to fertility; rescue was also obtained by germline transformation of a PCR fragment containing F44C4.4, the only predicted locus common to both rescuing cosmids. RNA interference (RNAi) directed against this locus phenocopied the morphological defects of gon-14 mutants. To sequence the gon-14 alleles, the gon-14 open reading frame was PCR amplified from genomic DNA (Expand High Fidelity PCR System; Roche, Indianapolis), and three independent PCR products were sequenced for each allele.
Cell lineage and laser ablation:
Cell lineages were examined by standard methods (Sulston and Horvitz 1977). We identified gon-14 homozygotes by their lack of GFP fluorescence among progeny of gon-14(q12)/nT1[qIs51] heterozygotes. L1 divisions of the somatic gonad precursors, Z1 and Z4, and L3 divisions of P3.p–P8.p were followed at room temperature (22°–23.5°) in wild-type and gon-14(q12) hermaphrodites. In some animals, one germ cell precursor, Z2 or Z3, was ablated prior to analysis to reduce the number of cells in the gonad and facilitate identification of Z1 and Z4 descendants; this ablation does not affect the Z1/Z4 lineage in wild-type animals.
Laser ablations were done as described (Bargmann and Avery 1995), using a Micropoint Ablation Laser System (Photonics Instruments, Arlington, IL). Cell ablations were verified 1–8 hr postoperative, and their effect on development was assessed in adults.
RNAi:
RNAi feeding was done essentially as described (Fraser et al. 2000). Briefly, L3 or early L4 larvae were transferred to an “RNAi plate” seeded with bacteria expressing a gene-specific dsRNA and maintained for 24–36 hr. Adults were then transferred to a fresh RNAi plate and allowed to lay progeny for a period of 2–14 hr before removal. F1 progeny on the second plate were scored.
The vector used was pPD129.36 “L4440” (Timmons and Fire 1998) unless noted otherwise. The inserts were derived from cDNA clones or from PCR products amplified from either genomic or cDNA templates as follows: gon-14, BamHI/HindIII fragment from cDNA clone pYK569F11 (provided by Y. Kohara); lin-15B, PCR product from genomic template, 5′ primer GCACCAGCTCCGAAACCTATCACA, 3′ primer CCGACAATTTCTCCGTCTTCGAG; mes-2, PCR product from genomic template, 5′ primer TGCTTAAAGGCCACTTCAATGCTA, 3′ primer TTTGTTTGGCCATAGACGGTAGAG; lin-35, PCR product from cDNA cloned into pLitmus28i, 5′ primer AAACGAGCAGCCGATGAGCCT, 3′ primer AGTGCCGTGCATCAAGAACAC; and lin-15A, SacI/PstI fragment after PCR of cDNA template, 5′ primer AACGTTGATGCTATGCGAATG, 3′ primer CTGCGTTCTACAGTGTTCTGC. For other RNAi experiments, bacterial clones containing L4440 with inserts from the desired genes were obtained from MRC Geneservice (Kamath et al. 2003). In all cases, HT115 transformed with the “empty” L4440 vector, containing the multiple cloning site flanked by T7 sites, was used as a control.
Construction of double mutants:
Double mutants containing gon-14(q12) and a class A or class B synMuv allele or an RNAi-deficient allele were constructed by standard genetic crosses. To confirm presence of a class A synMuv allele, we used lin-35 or lin-15B RNAi; to confirm presence of a class B synMuv allele, we used lin-15A RNAi. In control experiments, gon-14 single mutants did not exhibit enhanced multivulva defects in these RNAi assays (see also results). For gon-14(q12); lin-36(n766) double mutants, the presence of n766 was confirmed by an allele-specific MaeIII restriction site. To confirm the presence of RNAi-deficient mutants (i.e., rde-4 or mut-7), we assayed for resistance to pos-1 RNAi, which caused highly penetrant embryonic lethality in wild-type animals and in gon-14 single mutants.
Scoring phenotypes:
VPC induction:
Induction of the VPCs, P3.p–P8.p, was scored in mid-to-late L4 stage larvae, essentially as described (Moghal and Sternberg 2003). Each VPC was assigned a score of 1 if both daughters had divided, 0.5 if only one daughter had divided, or 0 if neither one had divided.
Larval arrest:
Adults were identified by presence of embryos, by an everted vulva, or by adult alae. Worms were scored as arrested larvae if they failed to reach adulthood within a defined time interval after embryos were laid (1 week at 20° or 60–72 hr at 25°). Some additional gon-14(q12) homozygotes were examined after 8 days at 25°.
Construction of the gon-14∷GON-14∷VENUS transgene:
The gon-14 genomic region was PCR amplified from cosmid ZK1055 and cloned into pPD95.67 to make pJK1069, which includes the gon-14 coding region plus 3.8 kb of 5′- and 1.5 kb of 3′-flanking sequences. Site-directed mutagenesis was used to introduce AvrII and SphI sites at the 3′ end of gon-14a just before the stop codon. The venus coding region was PCR amplified from pPD95.79–venus (a gift from Takeshi Ishihara), using 5′ and 3′ primers containing AvrII and SphI sites, respectively, and cloned into the gon-14+AvrII/SphI vector, in frame with gon-14a, to make pJK1077. The DNA sequence was confirmed, and the plasmid was injected into adult hermaphrodites by standard methods (Mello and Fire 1995). We generated numerous gon-14∷GON-14∷VENUS transgenic lines that rescued gon-14(q12) to fertility (details available upon request). The expression pattern and subcellular localization of the GON-14∷VENUS fusion protein were similar in all lines. We generated the gon-14∷GON-14∷VENUS described here as follows: pJK1077 was linearized with FspI and mixed with S. cerevisiae genomic DNA (Novagen) that had been fragmented by sonication. A mixture of 6 ng/μl gon-14∷GON-14∷VENUS and 95 ng/μl S. cerevisiae DNA was injected into the germline of gon-14(q686 ts) adults maintained at permissive temperature (20°). F1 progeny of injected animals were shifted to 25° as L2–L4 larvae to select for fertile F2. One rescuing transgene was integrated by gamma irradiation to generate qIs89.
Immunohistochemistry:
Mixed-stage worms were fixed using a modified Finney-Ruvkun protocol (Finney and Ruvkun 1990). Homozygous gon-14(q12) mutants were isolated as non-GFP worms from the strain gon-14(q12)/nT1[qIs51], using a COPAS Biosort worm sorter (Union Biometrica, Somerville, MA). The primary antibody used was a rabbit polyclonal anti-PGL-1 (Kawasaki et al. 1998), a gift from Susan Strome, and the secondary antibody was Cy3-conjugated anti-rabbit (Jackson ImmunoResearch, West Grove, PA). Worms were costained with DAPI.
Sequence analysis:
Amino acid sequence alignments were constructed with MegAlign v5.53 (DNASTAR, Madison, WI) by using the Clustal W method (Chenna et al. 2003) and further modified by inspection. In all alignments, amino acid similarity was scored using the Blosum62 scoring matrix (Henikoff and Henikoff 1992). The genomic sequence of a putative C. remanei GON-14 ortholog was identified by using the C. elegans gon-14 genomic sequence in a BLAST search of the C. remanei sequence assembly database provided by the Washington University Genome Sequencing Center. One significant hit was identified, corresponding to a region within contig 8.2. A predicted exon–intron structure was then constructed by inspection.
RESULTS
Molecular identification of the gon-14 locus:
We identified the gon-14 locus, F44C4.4, by a combination of genetic mapping, germline transformation, and RNAi (see materials and methods). We then sequenced the F44C4.4 coding region of five gon-14 alleles and found a molecular lesion associated with each allele (Figure 1A). This locus has also been called iri-1, for inosine triphosphate (IP3) receptor interacting (Walker et al. 2004). Our analysis of gon-14/iri-1 extends this earlier work, but does not investigate the interaction between GON-14/IRI-1 and the C. elegans IP3 receptor, ITR-1. For simplicity, we refer to the locus as gon-14.
Figure 1.
The gon-14 gene structure and predicted protein motifs. (A) gon-14 gene structure, motifs, and mutations. The gon-14 mRNA is trans-spliced to SL1. Two isoforms, gon-14a and gon-14b, differ in the splice donor site in exon 13, resulting in differing frame usage in exon 14; these are predicted to encode proteins of 923 and 883 amino acids, respectively. Locations of molecular lesions in gon-14 alleles are indicated above the gene diagram; also see text. Solid bars below genes indicate motifs noted in the text. hAT transposase conserved blocks (A, B, C, and hATC) refer to regions typically conserved among hAT family proteins; the hATC domain contains blocks D, E, and F (Rubin et al. 2001). Block X, an additional conserved region (this work). See also supplemental Figure 1 at http://www.genetics.org/supplemental/. Bar, 500 bp. (B) hATC domain: amino acid alignment of GON-14 orthologs to the hATC domains of representative hAT transposases. Ce, C. elegans; Cr, C. remanei; Cb, C. briggsae; Ac, Activator ORFa (Zea mays); Hermes (Md, Musca domestica); Tfo1 (Fo, Fusarium oxysporum); Tramp (Hs, Homo sapiens). Solid boxes, amino acid residues identical to at least two hAT transposases. Shaded boxes, residues similar to at least two identical or three similar hAT transposases. Similarity was scored on the basis of the Blosum62 matrix. Open diamond, residue important for dimerization and transposase activity in Ac and/or Hermes (Essers et al. 2000; Michel et al. 2003); solid diamond, residue important for transposase activity in Ac and/or Hermes (Essers et al. 2000; Michel et al. 2003; Zhou et al. 2004). (C) THAP domain: alignment of THAP domains in GON-14 orthologs to THAP domains in related proteins. Ptrans, P-element transposase. Dm, Drosophila melanogaster. Other species abbreviations are as in Figure 1B. Asterisks, strictly conserved residues in THAP domains; solid boxes, identity to strictly conserved residues; shaded boxes, similarity to other conserved residues; boxed residues, potential similarity to the conserved proline (asterisk) and preceding small hydrophobic amino acid.
Two isoforms, gon-14a and gon-14b, have been identified by analysis of cDNAs (WS140; http://www.wormbase.org); these two isoforms differ in the splice donor site of intron 13 and generate alternate reading frames in the last exon (Figure 1A). RT–PCR analysis indicates that both isoforms are major transcripts (data not shown). The relative importance of the two transcripts is unknown. Putative C. briggsae and C. remanei gon-14 orthologs contain sequences corresponding to the last exon of gon-14a, but do not have a recognizable gon-14b (data not shown). The gon-14b transcript was described previously (Walker et al. 2004). For simplicity, we refer to both transcripts as gon-14, because our analyses focus on motifs in the common region. Using RT–PCR, we found that SL1 is trans-spliced to the gon-14 transcript at an AUG 48 nucleotides upstream of the previously predicted start AUG (data not shown). This upstream AUG is likely to be the actual translational start site, because the N-terminal region is part of a motif conserved in C. briggsae and C. remanei orthologs (see below).
Sequence analysis of LIN-15B homology domain:
The GON-14 protein possesses a large domain with homology to LIN-15B, a class B synMuv protein (Figure 1A; also see Walker et al. 2004). Specifically, amino acids 251–723 of GON-14 align with amino acids 70–553 of LIN-15B (24% identity, 44% similarity); we refer to this region as the LIN-15B homology domain (Figure 1A, data not shown). A BLAST search using the GON-14 sequence revealed significant similarity to seven C. elegans predicted open reading frames, including LIN-15B and six uncharacterized proteins. The search also revealed similarity to six C. briggsae predicted open reading frames, including putative orthologs of GON-14 and LIN-15B. All of these homologs aligned to the LIN-15B homology domain (data not shown; also see Walker et al. 2004). No other BLAST hits were statistically significant (E-value <0.001).
Further analysis of the LIN-15B homology domain using the NCBI conserved domain database (Marchler-Bauer et al. 2003) revealed a motif with weak similarity to the hobo/Activator/Tam3 (hAT) transposase dimerization domain (hATC), pfam05699 (Figure 1, A and B). The hATC domain resides near the C terminus of DNA class II transposases of the hAT superfamily and is required for both in vivo transposase activity and in vitro dimerization (Essers et al. 2000; Michel et al. 2003). A multiple alignment of GON-14 to the hATC domains of representative hAT family proteins revealed conservation at several key residues required for hAT transposase activity and multimerization (Figure 1B; see also Essers et al. 2000; Michel et al. 2003). We next aligned the amino acid sequences of seven LIN-15B family members with representative hAT transposases and found additional regions of similarity outside the hATC domain; these regions corresponded to blocks of sequence that are typically conserved among hAT family members [Figure 1A (A, B, C, X); supplemental Figure 1 at http://www.genetics.org/supplemental/; Rubin et al. 2001]. The conserved blocks are small (10–26 amino acids), but they span ∼400 amino acids of the LIN-15B homology domain, and both order and approximate spacing of the blocks are conserved (supplemental Figure 1); the crystal structure of the Hermes hAT transposase revealed that blocks A, B, C, and hATC all contain residues situated in or near the active site (Hickman et al. 2005). However, the LIN-15B family proteins lack conservation at some key residues critical for transposase activity (Figure 1B; supplemental Figure 1).
Members of the hAT superfamily are mobile DNA elements (transposons) that each encode a transposase. The hAT transposase is necessary for catalyzing excision of the transposon from the genome and its reinsertion elsewhere in the genome (reviewed in Kempken and Windhofer 2001). Signature features of hAT transposable elements include terminal inverted repeats (TIRs) that are typically 10–20 nucleotides in length, as well as 8-bp target site duplications flanking the insertion site. We analyzed the sequences flanking lin-15B family genes and did not detect TIRs or target site duplications (M. Chesney, unpublished observations). Notably, the C. elegans genome contains other genes that are putative hAT family members; these have both TIRs and target site duplications and encode proteins with stronger similarity to hAT transposases (Bigot et al. 1996; Rubin et al. 2001; M. Chesney, unpublished observations). We conclude that the lin-15B family encodes distant relatives of hAT transposases, but suggest that lin-15B family genes do not have typical hAT transposon/transposase behavior themselves.
Other motifs in the GON-14 sequence:
To identify additional conserved motifs, we aligned the amino acid sequences of C. elegans GON-14 and the putative GON-14 orthologs from C. briggsae and C. remanei. C. elegans GON-14 is 44% identical and 60% similar to C. briggsae homolog CBG18977, and 46% identical and 66% similar to C. remanei GON-14, along the full lengths of the respective proteins. These alignments revealed a C-X2-C-X41-C-X2-H domain near the N terminus that resembles the Thanatos-associated protein (THAP) DNA-binding domain (Figure 1, A and C) (Roussigne et al. 2003); this motif was also identified in a recent database search for THAP domain-containing proteins (Clouaire et al. 2005). The N-terminal location of the GON-14 THAP domain is typical of THAP-containing proteins, but the GON-14 THAP domain lacks key phenylalanine and proline residues and is therefore divergent (Figure 1C; see also Clouaire et al. 2005). The GON-14 alignments also revealed a C-X2-C-X15-H-X4-H (C2H2) motif just N-terminal to the LIN-15B homology domain. Walker et al. (2004) noted a C-terminal proline-rich domain, which is also present in the C. briggsae and C. remanei orthologs (Figure 1A, data not shown).
GON-14 is a nuclear protein:
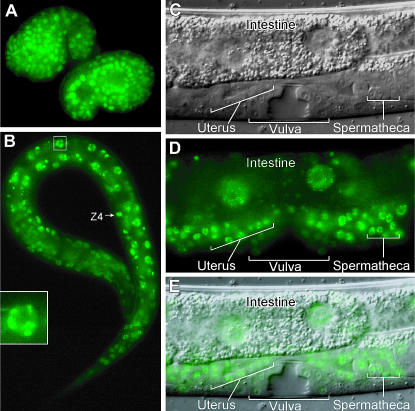
To determine the pattern of gon-14 expression and to learn the subcellular localization of GON-14 protein, we generated an integrated transgene, called gon-14∷GON-14∷VENUS, that rescued the defects of gon-14 mutants (see materials and methods). GON-14∷VENUS was broadly expressed in somatic cells, beginning in ∼50 cell embryos (data not shown). Expression persisted throughout embryogenesis (Figure 2A) and larval development (Figure 2, B–E), but became fainter in adults (data not shown). Of particular note, GON-14∷VENUS was present in the somatic gonadal precursors, Z1 and Z4, and the developing somatic gonad (Figure 2, B–E; supplemental Figure 2 at http://www.genetics.org/supplemental/; data not shown); the VPCs and developing vulva (Figure 2, C–E; supplemental Figure 2); and in the intestine (Figure 2, B–E; supplemental Figure 2). GON-14∷VENUS was predominantly nuclear (Figure 2, A–E) and often concentrated in nuclear puncta or speckles (Figure 2, B (inset) and C–E). The number of speckles per nucleus varied from a few to >30 (Figure 2 and data not shown). We did not detect expression in the germline; however, we cannot exclude the possibility that this was a result of transgene silencing, as germline-expressed genes are often silenced when present on transgenes (Kelly et al. 1997).
Figure 2.
GON-14∷VENUS is broadly expressed and predominantly nuclear. (A) Comma stage embryos: GON-14∷VENUS is present in most nuclei. (B) L1 larva: GON-14∷VENUS is present in most nuclei. Inset, nucleus magnified; GON-14∷VENUS localizes to nuclear speckles. (C–E) L4 larva. GON-14 is present in most nuclei, including the somatic gonad (anterior uterus and posterior spermatheca indicated), intestine, and vulva. GON-14∷VENUS is concentrated in nuclear speckles and appears to be largely excluded from nucleoli. (C) Nomarski. (D) Fluorescence. (E) Merge.
Identification of a gon-14 null allele:
To assess gon-14 function, we first identified a likely null mutant. Three gon-14 alleles were the best candidates: gon-14(q12) and gon-14(q10) each contain G to A nucleotide changes that result in premature termination codons at amino acids 598 and 656, respectively, while gon-14(q552) contains a seven-nucleotide deletion that alters the reading frame from amino acid 398 and results in a premature termination codon. By contrast, gon-14(q686) contains a C to T nucleotide change that results in a missense mutation, P289S, and gon-14(q631) has a single nucleotide change (G to A) in the splice acceptor site of the third intron, which impairs splicing of this intron (data not shown). Phenotypic analysis supports the conclusion that q10, q12, and q552 are all strong loss-of-function alleles and putative nulls. At 25°, q10, q12, and q552 mutants were phenotypically indistinguishable: they arrested as midstage larvae (see below), while q631 and q686 homozygotes became sterile adults (Siegfried et al. 2004 and this work). Furthermore, the q12 phenotype was not enhanced when placed in trans to nDf32, a deficiency that removes the entire gon-14 locus, but both q631 and q686 became more severe when placed in trans to nDf32 (Siegfried et al. 2004; data not shown). Therefore, all further characterization was conducted with gon-14(q12), which is referred to as gon-14(0).
gon-14 is a pleiotropic regulator of animal development:
Previous studies reported pleiotropic defects of hypomorphic gon-14 mutants (Siegfried et al. 2004) or animals treated with gon-14 RNAi (Kamath et al. 2003; Simmer et al. 2003; Walker et al. 2004). Our analysis of gon-14(0) mutants confirms and extends these previous reports. The gon-14(0) mutant exhibited defects in growth, organogenesis, and cell division (see below). These defects were more severe at 25° (Siegfried et al. 2004; this work), suggesting that removal of gon-14 reveals a temperature-sensitive process.
Gonad defects:
Wild-type adult hermaphrodites possess a gonad with two U-shaped gonadal arms and centrally located somatic gonadal structures that open into the vulva (Figure 3A). By contrast, gon-14(0) mutants grown at 20° developed into sterile adults that typically had short or abnormally shaped gonad arms and sometimes lacked one or both arms (Figure 3B, data not shown). The gon-14 gonad often lacked a recognizable uterus or spermatheca, and the vulva was typically protruding (89%, n = 126). The incidence of loss of gonadal arms correlated with loss of expression of the lag-2∷GFP distal tip cell marker, suggesting that in such cases either the distal tip cell was not made or its cell fate was not properly specified (data not shown). The gon-14(0) adult germline typically produced sperm (85%, n = 33) and abnormally shaped oocytes (91%, n = 33). Spermatocytes were also often observed in the body cavity, suggesting a defect in gonadal integrity. In some cases, gon-14(0) adults produced one or more fertilized embryos (18%, n = 33) that appeared to arrest early in embryogenesis (<100 cells, data not shown).
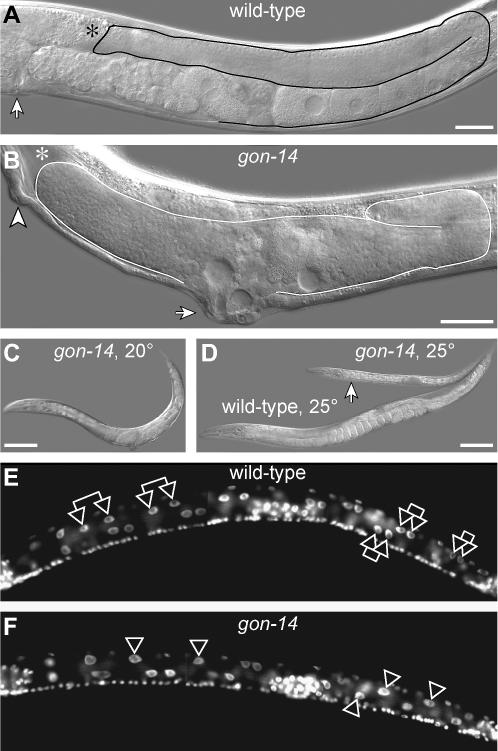
Figure 3.
Pleiotropic phenotype of gon-14(0) mutants. (A) Wild-type adult raised at 20°. Solid line, outline of posterior gonadal arm. Asterisk, distal end of gonadal arm is out of focal plane. Arrow, vulva. Bar, 25 μm. (B) gon-14(q12) adult raised at 20°. Solid line, outline of malformed gonad. Asterisk, anterior gonad arm reflexed out of the focal plane. Arrow, protruding vulva. Arrowhead, ectopic pseudovulva. Bar, 25 μm. (C) Size of gon-14(q12) mutant raised at 20°. Bar, 100 μm. (D) Sizes of wild-type adult (bottom) and arrested gon-14(q12) mutant (arrow), both raised at 25°. Bar, 100 μm. (E) Wild-type L2 raised at 25°, DAPI stained. Double arrows, selected intestinal sister nuclei. (F) gon-14(q12) L2 or L3 raised at 25°, DAPI stained. Single arrowheads, single intestinal nuclei that have failed to divide.
Growth defects:
When grown at 20°, gon-14(0) mutants reached adulthood ∼12–24 hr later than wild-type animals, and mutant adults were typically smaller than wild type (compare gon-14 worm in Figure 3C to wild-type worm in Figure 3D). When grown at 25°, mutants were about half as long as wild-type adults (Figure 3D), and after 1 week they usually showed no sign of vulval morphogenesis and did not make adult alae. Therefore, these gon-14(0) mutants appear to have arrested as midstage larvae. In addition, they were often constipated, consistent with previous reports of defecation defects (Walker et al. 2004).
Cell division defects:
The gon-14(0) mutants exhibited variably disrupted cell divisions in intestine, gonad, and vulva. Wild-type animals hatch with 20 intestinal nuclei, 14 of which divide during L1, to give rise to 34 intestinal nuclei by L2 (Sulston and Horvitz 1977). When grown at 25°, both wild-type and gon-14(0) L1s had 20 intestinal nuclei at hatching (n = 10); however, wild-type L2 larvae had 33 ± 1 nuclei (n = 10), whereas gon-14(0) L2s had only 24 ± 3 (n = 28) (Figure 3, E and F). In addition, somatic gonadal divisions were delayed in gon-14(0) mutants. In the wild type, the somatic gonad precursor cells (SGPs), Z1 and Z4, generated eight cells by L1 lethargus (n = 3), whereas in gon-14(0) mutants the SGPs either had not divided or had divided only once to generate only two to four cells by L1 lethargus (n = 4). Divisions of the VPCs were also variably delayed in gon-14(0) mutants grown at 25°. In some mutants, no VPC divisions were observed, whereas in others VPC divisions commenced 12–48 hr later than in the wild type. The delay in vulval development may have been related to the delay in gonadogenesis, as the extent of VPC divisions generally correlated with the size of the gonad in arrested larvae (data not shown).
Vulval defects:
When gon-14(0) mutants were grown at 20°, many had one or more ectopic vulvae (52%, n = 183) (Figure 3B). The gon-14 ectopic vulvae were smaller than those typically seen in synMuv class A; class B double mutants and often not visible in early adults. Ectopic vulvae were found in four different gon-14 mutants (q10, q12, q552, and q631), but not when gon-14 levels were reduced by RNAi in wild-type animals and only rarely when reduced by RNAi in rrf-3, an RNAi-hypersensitive mutant (data not shown). Therefore, gon-14 mutants have a Muv defect.
Characterization of the gon-14 Muv defect:
The gon-14 Muv defect was interesting in light of the molecular similarity between GON-14 and the LIN-15B synMuv protein. In wild-type animals, vulval fates are induced by signaling from the gonadal anchor cell, but in synMuv class A; class B double mutants, the VPCs can adopt vulval fates in the absence of the gonad (reviewed in Fay and Han 2000). To learn whether gon-14 ectopic vulvae are dependent on the gonadal signal, we used laser microsurgery to eliminate all four cells of the gonadal primordium in gon-14(0) L1 larvae, thereby removing the entire gonad before the anchor cell was born. Control wild-type worms were vulvaless after this ablation (n = 3), but half of the gonad-ablated gon-14(0) mutants had ectopic vulvae (n = 6), a fraction similar to that typical of untreated gon-14 mutants (see above). We conclude that the gon-14 Muv defect is independent of the gonad inductive signal.
Next, we examined the origin of the ectopic vulvae in gon-14(0) mutants. In wild-type hermaphrodites, three VPCs, P5.p, P6.p, and P7.p, adopt vulval fates and undergo two to three rounds of divisions to generate 22 vulval cells; the uninduced VPCs, P3.p, P4.p, and P8.p, typically divide once each before fusing with the hypodermis (Sulston and Horvitz 1977). By contrast, in synMuv class A; class B double mutants, all six VPCs can adopt vulval fates, resulting in the formation of ectopic vulval protrusions (Ferguson et al. 1987; Ferguson and Horvitz 1989). We examined the early VPC divisions of gon-14(0) and found that the daughters of P4.p and P8.p sometimes divided ectopically and their descendants failed to fuse with the hypodermis (n = 2). Consistent with these observations, examination of mid-L4 stage gon-14(0) larvae revealed frequent hyperproliferation of P3.p, P4.p, and P8.p (below and Table 1), indicating that gon-14 controls VPC fate specification.
TABLE 1.
gon-14 mutants exhibit multivulva defects but are not synMuv
| % inductiona
|
Average no. P(3–8).p vulval fates ± SEb
|
% animals with >3 vulval fates
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Genotype | P3.p | P4.p | P5.p | P6.p | P7.p | P8.p | n | ||
| Wild type | 0 | 0 | 100 | 100 | 100 | 0 | 3.0 ± 0.0 | 0 | 30 |
| lin-15A(n767) | 0 | 1 | 100 | 100 | 100 | 0 | 3.0 ± 0.0 | 2 | 50 |
| lin-38(n751) | 0 | 0 | 100 | 100 | 100 | 0 | 3.0 ± 0.0 | 0 | 40 |
| lin-36(n766) | 0 | 0 | 100 | 100 | 100 | 0 | 3.0 ± 0.0 | 0 | 30 |
| lin-36(n766); lin-15A(n767) | 83 | 98 | 100 | 100 | 100 | 88 | 5.7 ± 0.1 | 100 | 30 |
| gon-14(q12)c | 17 | 23 | 96 | 100 | 100 | 40 | 3.8 ± 0.1 | 69 | 54 |
| gon-14(q12); lin-15A(n767)c | 11 | 18 | 100 | 100 | 100 | 28 | 3.6 ± 0.1 | 46 | 52 |
| lin-38(n751); gon-14(q12)c | 14 | 17 | 97 | 100 | 94 | 33 | 3.6 ± 0.1 | 52 | 33 |
| lin-36(n766); gon-14(q12)c | 17 | 22 | 98 | 98 | 98 | 42 | 3.8 ± 0.1 | 60 | 30 |
Percentage of animals in which the given VPC was induced (see materials and methods).
Average number of P(3–8).p vulval fates per animal (see materials and methods).
gon-14 homozygotes were derived from heterozygous gon-14 parents.
Is gon-14 a synMuv gene?
We next asked whether gon-14 exhibits the synMuv defect. If gon-14 functions as a class A or class B synMuv gene, the penetrance of gon-14(0) Muv animals should be enhanced by a synMuv class B or class A mutation, respectively. For most synMuv loci, a Muv defect occurs only when both a class A gene and a class B gene are disrupted (Ferguson and Horvitz 1989). However, for at least three class B synMuv loci, hda-1, mep-1, and lin-13, single mutants display a Muv phenotype under certain conditions: hda-1 and mep-1 are Muv at low penetrance as single mutants (Belfiore et al. 2002; Dufourcq et al. 2002), but Muv at higher penetrance as double mutants that also remove a class A synMuv gene (Unhavaithaya et al. 2002; Thomas et al. 2003). In addition, lin-13 homozygotes are Muv at 25°, but behave as class B synMuv mutants at 15° (Meléndez and Greenwald 2000).
To assess whether gon-14 is a synMuv gene, we compared the number of VPCs induced in gon-14(0) single mutants to that in animals carrying both gon-14(0) and either a class A or a class B synMuv mutation (Table 1). Wild-type animals grown at 20° had 3.0 induced VPCs (n = 30), and gon-14(0) mutants had an average of 3.8 induced VPCs (n = 54). The gon-14 Muv defect was not enhanced in double mutants with lin-15A (class A), lin-38 (class A), or lin-36 (class B) synMuv mutations (Table 1). We conclude that gon-14 is not a class A or a class B synMuv gene.
gon-14 functions with class B and class C synMuv genes to control larval development:
While testing gon-14 for synMuv activity (see above), we discovered a synthetic larval arrest defect in certain gon-14; synMuv B double mutants. Whereas gon-14(0) single mutants developed into adults at 20°, gon-14; lin-15B and lin-35; gon-14 double mutants arrested as L1- or L2-sized larvae at the same temperature (Figure 4, B and C). The double mutants could undergo some postembryonic cell divisions in gonad, intestine, M, and P lineages, and in rare cases one or more VPCs divided; however, the arrested larvae never exhibited a mature vulva or adult alae (data not shown).
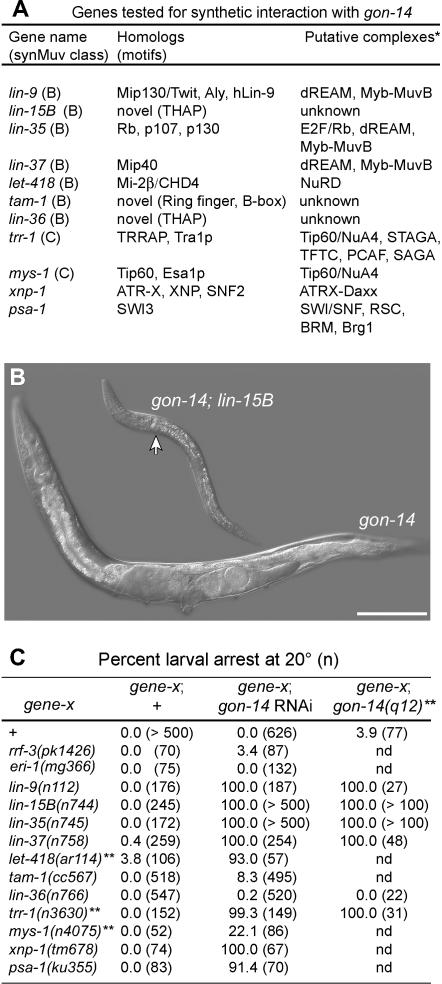
Figure 4.
gon-14 interacts genetically with the class B and class C synMuv genes to control larval growth. (A) Genes tested for gon-14 interactions, including members of the B and C synMuv classes as well as other genes that interact synthetically with the class B synMuv pathway. Asterisk, predicted protein complexes based on biochemical data from various organisms. See text for references. (B) gon-14(q12) single-mutant adult and gon-14(q12); lin-15B(n744) double-mutant arrested larva, both raised at 20°. Bar, 100 μm. (C) Frequency of larval arrest scored in gene-X single mutants, gon-14 animals, and gene-X; gon-14 animals, where gene-X is a synMuv mutant, a synMuv-interacting mutant, or an RNAi-hypersensitive mutant. gon-14 was disrupted by RNAi or by using a putative null allele, gon-14(q12), as noted. Double asterisk, progeny scored were descendants from parents heterozygous for the indicated allele. All were raised at 20°.
We explored whether other synMuv or synMuv-related genes exhibited similar interactions with gon-14 by constructing additional double mutants or by using gon-14 RNAi. Figure 4A summarizes the genes tested in this work and their protein products. In control experiments, we saw little or no larval arrest after gon-14 RNAi of wild-type worms or of RNAi-hypersensitive mutants rrf-3 or eri-1 (Figure 4C). However, we observed highly penetrant larval arrest when testing gon-14 RNAi of several class B synMuv mutants, including lin-35, lin-15B, lin-9, lin-37, and the class C mutant trr-1. In each of these cases, the gon-14(RNAi); synMuv defects were phenocopied by gon-14(0); synMuv double mutants (Figure 4C). We also observed highly penetrant larval arrest defects in let-418 gon-14(RNAi) animals, whereas less penetrant larval arrest defects were seen in gon-14(RNAi) mys-1 animals, and little or no larval arrest was observed in lin-36; gon-14 double mutants or in lin-36; gon-14(RNAi) or tam-1 gon-14(RNAi) animals (Figure 4C). Additionally, no larval arrest was observed when gon-14 was disrupted in class A synMuv mutant backgrounds (data not shown). Therefore, gon-14 interacts with many, but not all, synMuv genes to control larval growth.
The class B synMuv genes interact genetically with some Swi/Snf family members to promote larval growth, including psa-1/SWI3 and xnp-1/ATR-X (Bender et al. 2004a; Cui et al. 2004; Cardoso et al. 2005). To ask whether gon-14 also interacts with these genes, we tested gon-14 RNAi in psa-1 and xnp-1 mutants. In both cases, gon-14 RNAi caused highly penetrant larval arrest defects that were not observed in the single mutants (Figure 4C), indicating that gon-14 functions redundantly with these Swi/Snf family genes to promote larval growth.
gon-14 larval arrest is suppressed by disrupting the mes genes:
The larval arrest of two class B synMuv mutants, mep-1 and let-418/Mi-2β, can be suppressed by disruption of mes-2, mes-3, mes-4, or mes-6 (Unhavaithaya et al. 2002), indicating that the synMuv genes antagonize the mes-2,3,6/ESC-E(Z) and mes-4 genes in control of larval development. To determine whether gon-14(0) mutants share this property, we disrupted the mes genes by RNAi in gon-14 mutants grown at 25°. All gon-14(0) mutants exhibited larval arrest in control experiments, but most developed into sterile adults when mes-2, mes-3, mes-4, or mes-6 was depleted by RNAi (Figure 5, A and C). Although the mes genes are required for RNAi, previous studies have demonstrated the feasibility of using RNAi to knock down genes required for RNAi (Dudley et al. 2002; Kim et al. 2005). A similar, but less penetrant, suppression of larval arrest was seen in mes-3(bn35); gon-14(0) double mutants lacking maternal and zygotic wild-type mes-3 (Figure 5C). Since the mes genes are required for RNAi (Dudley et al. 2002), we asked whether the gon-14(0) larval arrest could be suppressed by disrupting the RNAi pathway. However, rde-4; gon-14 and mut-7; gon-14 double mutants arrested as larvae at 25° (Figure 5C). Therefore, the suppression is not due to the disruption of RNAi.
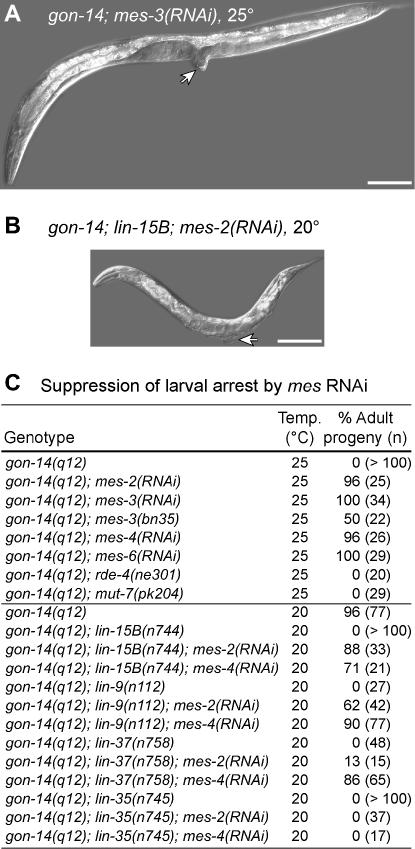
Figure 5.
gon-14 larval arrest is suppressed by MES-2,3,6/ESC-E(Z) or MES-4 depletion. (A) gon-14(q12); mes-3(RNAi) reaches adulthood when grown at 25°. Arrow, protruding vulva. Bar, 100 μm. (B) gon-14(q12); lin-15B(n744); mes-2(RNAi) reaches adulthood when grown at 20°. Arrow, protruding vulva. Bar, 100 μm. (C) Percentage of adult progeny of the specified genotype. For clarity, gene names are not listed by map position.
We next asked whether mes-2 or mes-4 RNAi could suppress the larval arrest of gon-14; synMuv B double mutants. Indeed, both mes-2 and mes-4 RNAi partially suppressed the larval arrest defects of several gon-14; synMuv B double mutants (Figure 5, B and C). These gon-14; synMuv B; mes(RNAi) animals were typically smaller than wild-type adults, but often had everted vulvae diagnostic of adulthood, whereas control gon-14; synMuv B double mutants did not reach adulthood (Figure 5, B and C). Furthermore, among animals that failed to reach adulthood, those treated with mes-2 or mes-4 RNAi typically exhibited more cell proliferation in the gonad and vulva than control RNAi treated animals (data not shown), suggesting partial suppression in these cases. In general, mes-4 RNAi caused more penetrant suppression of the gon-14; synMuv B larval arrest than did mes-2 RNAi (Figure 5C). The reason for this disparity is unclear: in parallel experiments, both treatments caused highly penetrant sterility in gon-14/+; synMuv B animals (data not shown), indicating that the respective mes gene was knocked down in each case. We conclude that the mes genes are required for the gon-14 single-mutant larval arrest at 25° and that both mes-2 and mes-4 promote the larval arrest of gon-14; synMuv B double mutants at 20°.
Altered gene expression in gon-14 mutants:
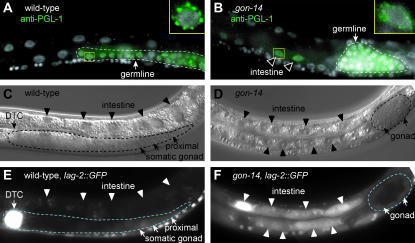
The class B synMuv genes also restrict the expression of certain germline-specific factors, such as the P-granule-associated protein, PGL-1. Whereas PGL-1 is normally expressed only in the germline, several synMuv mutants ectopically express PGL-1 in somatic tissues (Kawasaki et al. 1998; Unhavaithaya et al. 2002; Wang et al. 2005). To learn whether gon-14 shares this property, we examined PGL-1 protein in gon-14(0) mutants. PGL-1 was restricted to the germline in wild-type animals (Figure 6A), but it was observed ectopically in somatic cells of gon-14(0) mutants in addition to the normal germline staining (43%, n = 54) (Figure 6B). The effect was weaker than that reported for mep-1 and let-418, as ectopic PGL-1 was generally visible in only a few somatic cells per mutant, typically in intestinal cells or hypodermal cells. The ectopic PGL-1 in gon-14(0) somatic cells was localized to perinuclear structures typical of PGL-1 in the P-granules of wild-type germ cells (Figure 6, A and B, insets). Therefore, gon-14 affects PGL-1 expression.
Figure 6.
gon-14 represses PGL-1 and lag-2∷GFP expression. (A) Wild-type L3 larva, stained with DAPI (white) and antibodies to PGL-1 (green). PGL-1 is restricted to germ cells, outlined by a dashed line. Inset, nucleus is shown; PGL-1 is localized in a punctate perinuclear pattern. (B) Arrested gon-14(q12) larva, stained with DAPI (white) and antibodies to PGL-1 (green). Ectopic PGL-1 is observed in intestinal nuclei (arrowheads). PGL-1 is also observed in germ cells, outlined by a dashed line. Inset, nucleus is shown; somatic PGL-1 is localized in a punctate perinuclear pattern. (C and E) Wild-type L3 grown at 25°. lag-2∷GFP is expressed in the distal tip cell (DTC, arrow) and faintly in the proximal somatic gonad. The gonadal arm is outlined by a dashed line. lag-2∷GFP is not expressed in the intestine (arrowheads). (C) Nomarski. (E) Fluorescence. (D and F) Arrested gon-14(0) larva grown at 25°. lag-2∷GFP is ectopically expressed in the intestine (arrowheads) and faintly expressed in the somatic gonad (arrows). The gonad is outlined by a dashed line. (D) Nomarski. (F) Fluorescence.
In addition, most class B and class C synMuv genes repress expression of lag-2, such that loss of function results in ectopic lag-2∷GFP reporter expression in the intestine and sometimes in the hypodermis (Dufourcq et al. 2002; Poulin et al. 2005). We similarly found that lag-2∷GFP was ectopically expressed in gon-14(0) mutants. In wild-type larvae raised at 25°, lag-2∷GFP was expressed in the gonadal primordium, ventral nerve cord, distal tip cells, vulva, and proximal gonad, but not in the intestine (Figure 6, C and E, and data not shown). By contrast, most gon-14(0) larvae raised at 25° exhibited ectopic lag-2∷GFP expression in the intestine (Figure 6, D and F), indicating that gon-14 antagonizes lag-2 expression in the intestine.
DISCUSSION
In this work we describe the cloning and genetic characterization of gon-14, which encodes a homolog of LIN-15B, a class B synMuv protein. Our work extends previous studies that characterized hypomorphic gon-14 alleles or gon-14/iri-1 RNAi (Kamath et al. 2003; Simmer et al. 2003; Siegfried et al. 2004; Walker et al. 2004) and supports three main conclusions. First, gon-14 regulates gene expression, perhaps by chromatin regulation. Second, members of the LIN-15B protein family are distantly related to hAT transposases, and most also contain THAP motifs. On the basis of these findings and the similar roles of gon-14 and lin-15B, we speculate that LIN-15B family members function in DNA regulation. Third, gon-14 functions redundantly with lin-35/Rb, let-418/Mi-2β, and other synMuv and synMuv-related genes, to promote larval growth and antagonize the mes-2,3,6/ESC-E(Z) and mes-4 genes.
gon-14 regulates gene expression:
Several lines of evidence suggest that gon-14 affects gene expression, perhaps by chromatin regulation. First, loss of gon-14 causes derepression of lag-2 in the intestine and of pgl-1 in somatic tissues. The class B synMuv genes, which are implicated in transcriptional repression and chromatin remodeling, are similarly required for repression of lag-2 in the intestine and of pgl-1 in the soma (Dufourcq et al. 2002; Unhavaithaya et al. 2002; Poulin et al. 2005; Wang et al. 2005). A regulatory role for gon-14 is further supported by the presence of two putative DNA binding motifs, THAP and C2H2, in the GON-14 sequence and by the nuclear localization of a rescuing GON-14∷VENUS fusion protein. In addition, GON-14 interacts genetically with various genes that control transcription and chromatin, including lin-35/Rb, let-418/Mi-2β, trr-1/TRRAP, xnp-1/ATR-X, psa-1/SWI3, and mes-2/E(z). Furthermore, loss-of-function mutations in the genes encoding the POP-1/TCF and SYS-1/β-catenin transcription factors are dominant enhancers of gon-14 homozygotes (Siegfried et al. 2004; Kidd et al. 2005). Therefore, GON-14 may establish a chromatin state critical for transcriptional activation by POP-1/TCF and SYS-1/β-catenin.
The gon-14 locus was previously implicated in signaling by the inosine triphosphate receptor, ITR-1 (Walker et al. 2004). GON-14/IRI-1 and ITR-1 fragments interacted in yeast two-hybrid and in vitro binding assays. Furthermore, depletion of gon-14/iri-1 by RNAi generated defects similar to those of itr-1 mutants and enhanced a hypomorphic itr-1 allele (Walker et al. 2004). These findings led to the suggestion that GON-14/IRI-1 interacts with ITR-1 in vivo to modulate its function (Walker et al. 2004). Our results suggest an alternative hypothesis, namely that gon-14 affects ITR-1-regulated processes by affecting gene expression. However, these two hypotheses are not mutually exclusive.
The LIN-15B family:
The LIN-15B protein family is defined by an ∼470-amino-acid LIN-15B homology domain that exhibits similarity to hAT family transposases. The most prominent conserved feature is the hATC domain, but four additional blocks of residues are also conserved with hAT transposases; these blocks span ∼400 amino acids of the LIN-15B homology domain, and both order and relative spacing of the blocks are conserved (this work; Rubin et al. 2001). The functional significance of this sequence similarity is unknown. Some residues critical for transposition are not conserved, and our analysis does not support the classification of lin-15B family genes as likely hAT transposons. Perhaps the LIN-15B family has retained some properties of hAT transposases, such as multimerization or DNA binding, but deploys them for chromatin regulation rather than for transposition.
Recent work suggests that gene regulation by hAT transposase-like proteins is a mechanism employed in diverse phyla to control development. An Arabadopsis hAT transposase-like protein, DAYSLEEPER, was found to be essential for plant development and to regulate global gene expression. Like GON-14, DAYSLEEPER lacks some conserved residues, and the locus is not flanked by TIRs and 8-bp target site duplications (Bundock and Hooykaas 2005). Thus, in at least two organisms, hAT-transposase-like proteins regulate gene expression and are required for development.
In addition to the LIN-15B homology domain, four C. elegans LIN-15B paralogs contain one or more THAP motifs (Reddy and Villeneuve 2004; Clouaire et al. 2005; this work; M. Chesney, unpublished data). The THAP motifs of human THAP1 and Drosophila P-element transposase exhibit sequence-specific DNA-binding activity in vitro (Lee et al. 1998; Clouaire et al. 2005). Therefore, GON-14 and its LIN-15B paralogs may bind to DNA. In addition, several THAP domain proteins have been implicated in chromatin regulation or DNA-associated functions. For instance, human THAP7 can recruit histone deacetylase to target sites (Macfarlan et al. 2005). THAP domains are also present in two other synMuv proteins, LIN-36 and LIN-15A, as well as in HIM-17, a chromatin-associated protein required for initiation of meiotic recombination (Reddy and Villeneuve 2004). The GON-14 THAP domain is divergent (Clouaire et al. 2005) and has not been tested for DNA-binding activity; however, this motif is conserved in putative orthologs from other nematodes, suggesting that it contributes to GON-14 function. The presence of THAP- and hAT-related domains in LIN-15B family proteins, together with the roles of both LIN-15B and GON-14 in gene expression (see above), suggests that LIN-15B family proteins have a common function in DNA regulation.
Redundancy between gon-14 and lin-15B:
Given the sequence similarity between GON-14 and LIN-15B, one prediction is that these proteins can substitute for each other to control processes critical for growth. Indeed, gon-14; lin-15B double mutants exhibit a synthetic early larval arrest. Although larval arrest can occur in gon-14 single mutants grown at 25°, this larval arrest occurs later than that seen in gon-14; lin-15B double mutants. Therefore, GON-14 and LIN-15B may be interchangeable for larval growth. Alternatively, GON-14 and LIN-15B may act in distinct complexes with partially redundant functions. This latter idea is suggested by the fact that lin-15B and gon-14 single mutants have different phenotypes: lin-15B mutants have no apparent defects on their own, while gon-14 mutants have severe pleiotropic defects, including larval arrest at restrictive temperatures and sterility at all temperatures; moreover, lin-15B is a synMuv gene, but gon-14 is not (Ferguson and Horvitz 1989; Clark et al. 1994; Huang et al. 1994; Siegfried et al. 2004; this work). These differences are not a consequence of differential expression, as both GON-14 and LIN-15B are nuclear proteins and broadly expressed throughout development (this work; L. Huang and P. Sternberg, personal communication). Therefore, the individual functions of GON-14 and LIN-15B have clearly become specialized within the same cell. Consistent with this idea, the two proteins differ in domain architecture outside of the LIN-15B homology domain. For instance, GON-14 harbors a C2H2 motif just N-terminal to its LIN-15B homology domain, but LIN-15B lacks this motif. In addition, GON-14 has one N-terminal THAP domain, whereas LIN-15B has two C-terminal THAP domains (this work; Reddy and Villeneuve 2004; Clouaire et al. 2005). These sequence differences may contribute to the distinct developmental roles of GON-14 and LIN-15B.
gon-14 functions with the synMuv genes and antagonizes the mes genes to promote larval growth:
The gon-14 locus is conditionally required for larval growth, and the larval growth defects are enhanced by mutations in several class B synMuv genes that encode homologs of components of the Rb and NuRD complexes. Additional enhancers include the class C genes mys-1/Tip60 and trr-1/TRRAP, whose homologs are components of the Tip60/NuA4 histone acetyltransferase complex; TRRAP is also found in a variety of chromatin-remodeling complexes (Timmers and Tora 2005). The gon-14 larval arrest is similarly enhanced by mutations in the Swi/Snf family members xnp-1/ATR-X and psa-1/SWI3, whose homologs are also implicated in chromatin remodeling (Martens and Winston 2003; Xue et al. 2003). Therefore, gon-14 genetically interacts with a variety of transcriptional regulators and chromatin remodeling factors in control of larval growth.
Further genetic analyses revealed that gon-14 mutants share several defects in common with certain class B synMuv mutants, most notably those affecting the putative C. elegans NuRD (CeNuRD) components LET-418/Mi-2β and MEP-1. Like both let-418 and mep-1, the gon-14 locus is essential for growth under certain conditions. The gon-14 single mutant exhibits temperature-dependent larval arrest defects, while let-418 and mep-1 mutants arrest as larvae when both maternal and zygotic gene products are eliminated but develop into adults when a functional maternal copy is present (von Zelewsky et al. 2000; Belfiore et al. 2002; Unhavaithaya et al. 2002; this work). In addition, the larval arrest defects of gon-14, let-418, and mep-1 single mutants are suppressed by maternal and zygotic loss of the mes-2,3,6/ESC-E(Z) or mes-4 genes (this work; Unhavaithaya et al. 2002). Thus, both GON-14 and CeNuRD function antagonistically with MES-2,3,6/ESC-E(Z) and MES-4 in control of larval growth. Other phenotypic similarities between gon-14, let-418, and mep-1 single mutants include sterility and gonadal and vulval morphological defects. (this work; von Zelewsky et al. 2000; Belfiore et al. 2002). In addition, like the CeNuRD components and most other class B synMuv genes, gon-14 negatively regulates lag-2 expression and represses somatic expression of PGL-1, a germline-specific protein (this work; Dufourcq et al. 2002; Unhavaithaya et al. 2002; Poulin et al. 2005; Wang et al. 2005). On the basis of these observations and of the genetic interaction between gon-14 and let-418/Mi-2β, we propose that gon-14 and CeNuRD function in a common process to promote larval growth.
The causes of the gon-14 and synMuv mutant larval arrest defects are unknown, but genetic analyses of gon-14, CeNuRD components let-418 and mep-1, and other class B synMuv genes have provided insights regarding a possible mechanism. One intriguing model is based on the apparently antagonistic activities of CeNuRD and the MES-2,3,6/ESC-E(Z) and MES-4 complexes. This model posits that the CeNuRD complex promotes a chromatin state that allows somatic developmental programs; in the absence of CeNuRD, the MES-2,3,6/ESC-E(Z) and MES-4 complexes promote a distinct chromatin state in somatic tissues that is typical of the germline and that partially represses somatic developmental programs (see Shin and Mello 2003; Wang et al. 2005). We propose that gon-14 functions in a common process with CeNuRD components and other synMuv B genes to promote somatic developmental programs. In support of this model, loss of gon-14, let-418/Mi-2β, or mep-1 causes ectopic expression in somatic tissues of germ cell-specific proteins, such as PGL-1, suggesting that the mutant soma has adopted a germ cell-like state (this work; Unhavaithaya et al. 2002). Furthermore, disruption of the mes genes suppresses the larval arrest defects of gon-14, let-418, or mep-1 single mutants and of gon-14; synMuv B double mutants and also partially suppresses the ectopic PGL-1 expression in mep-1 mutants (this work; Unhavaithaya et al. 2002). In the wild-type germline, the MES-2,3,6/ESC-E(Z) complex confers a repressive chromatin state on the X chromosome, while MES-4 binds to autosomes and colocalizes with marks of transcriptional activation (Fong et al. 2002; Bender et al. 2004b). In the absence of CeNuRD or GON-14 activities, the MES-2,3,6/ESC-E(Z) and MES-4 complexes might disrupt somatic development by suppressing the somatic X chromosome or other sites important for somatic development and promoting the expression of germline-specific factors that inhibit somatic development.
Acknowledgments
The authors thank Susan Strome, Andy Fire, Takeshi Ishihara, and Josh Kaplan for antibodies, strains, and reporter constructs. Additional strains were provided by the Caenorhabditis Genetics Center. We thank members of the Kimble lab for helpful discussions and Maureen Barr and Tristana von Will for critical reading of the manuscript. We are also grateful to Peggy Kroll-Conner and Jadwiga Forster for technical assistance and to Anne Helsley-Marchbanks and Laura Vanderploeg for assistance in preparation of the manuscript and figures. M.A.C. and A.R.K. were supported by National Institutes of Health (NIH) predoctoral training grants, T32 GM08349 (biotechnology) and T32 GM07215 (molecular biosciences), respectively. J.K. is an investigator with the Howard Hughes Medical Institute. This work was supported by NIH grant GM069454 to J.K.
References
- Bargmann, C. I., and L. Avery, 1995. Laser killing of cells in Caenorhabditis elegans, pp. 225–250 in Caenorhabditis elegans: Modern Biological Analysis of an Organism, edited by H. F. Epstein and D. C. Shakes. Academic Press, San Diego. [DOI] [PMC free article] [PubMed]
- Becker, P. B., and W. Hörz, 2002. ATP-dependent nucleosome remodeling. Annu. Rev. Biochem. 71: 247–273. [DOI] [PubMed] [Google Scholar]
- Beitel, G. J., E. J. Lambie and H. R. Horvitz, 2000. The C. elegans gene lin-9, which acts in an Rb-related pathway, is required for gonadal sheath cell development and encodes a novel protein. Gene 254: 253–263. [DOI] [PubMed] [Google Scholar]
- Belfiore, M., L. D. Mathies, P. Pugnale, G. Moulder, R. Barstead et al., 2002. The MEP-1 zinc-finger protein acts with MOG DEAH box proteins to control gene expression via the fem-3 3′ untranslated region in Caenorhabditis elegans. RNA 8: 725–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender, A. M., O. Wells and D. S. Fay, 2004. a lin-35/Rb and xnp-1/ATR-X function redundantly to control somatic gonad development in C. elegans. Dev. Biol. 273: 335–349. [DOI] [PubMed] [Google Scholar]
- Bender, L. B., R. Cao, Y. Zhang and S. Strome, 2004. b The MES-2/MES-3/MES-6 complex and regulation of histone H3 methylation in C. elegans. Curr. Biol. 14: 1639–1643. [DOI] [PubMed] [Google Scholar]
- Bigot, Y., C. Auge-Gouillou and G. Periquet, 1996. Computer analyses reveal a hobo-like element in the nematode Caenorhabditis elegans, which presents a conserved transposase domain common with the Tc1-Mariner transposon family. Gene 174: 265–271. [DOI] [PubMed] [Google Scholar]
- Boxem, M., and S. van den Heuvel, 2001. lin-35 Rb and cki-1 Cip/Kip cooperate in developmental regulation of G1 progression in C. elegans. Development 128: 4349–4359. [DOI] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundock, P., and P. Hooykaas, 2005. An Arabidopsis hAT-like transposase is essential for plant development. Nature 436: 282–284. [DOI] [PubMed] [Google Scholar]
- Cao, R., and Y. Zhang, 2004. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr. Opin. Genet. Dev. 14: 155–164. [DOI] [PubMed] [Google Scholar]
- Cardoso, C., C. Couillault, C. Mignon-Ravix, A. Millet, J. J. Ewbank et al., 2005. XNP-1/ATR-X acts with RB, HP1 and the NuRD complex during larval development in C. elegans. Dev. Biol. 278: 49–59. [DOI] [PubMed] [Google Scholar]
- Ceol, C. J., and H. R. Horvitz, 2001. dpl-1 DP and efl-1 E2F act with lin-35 Rb to antagonize Ras signaling in C. elegans vulval development. Mol. Cell 7: 461–473. [DOI] [PubMed] [Google Scholar]
- Ceol, C. J., and H. R. Horvitz, 2004. A new class of C. elegans synMuv genes implicates a Tip60/NuA4-like HAT complex as a negative regulator of Ras signaling. Dev. Cell 6: 563–576. [DOI] [PubMed] [Google Scholar]
- Chenna, R., H. Sugawara, T. Koike, R. Lopez, T. J. Gibson et al., 2003. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 31: 3497–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, S. G., X. Lu and H. R. Horvitz, 1994. The Caenorhabditis elegans locus lin-15, a negative regulator of a tyrosine kinase signaling pathway, encodes two different proteins. Genetics 137: 987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouaire, T., M. Roussigne, V. Ecochard, C. Mathe, F. Amalric et al., 2005. The THAP domain of THAP1 is a large C2CH module with zinc-dependent sequence-specific DNA-binding activity. Proc. Natl. Acad. Sci. USA 102: 6907–6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couteau, F., F. Guerry, F. Muller and F. Palladino, 2002. A heterochromatin protein 1 homologue in Caenorhabditis elegans acts in germline and vulval development. EMBO Rep. 3: 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, M., D. S. Fay and M. Han, 2004. lin-35/Rb cooperates with the SWI/SNF complex to control Caenorhabditis elegans larval development. Genetics 167: 1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley, N. R., J.-C. Labbé and B. Goldstein, 2002. Using RNA interference to identify genes required for RNA interference. Proc. Natl. Acad. Sci. USA 99: 4191–4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufourcq, P., M. Victor, F. Gay, D. Calvo, J. Hodgkin et al., 2002. Functional requirement for histone deacetylase 1 in Caenorhabditis elegans gonadogenesis. Mol. Cell. Biol. 22: 3024–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley, M. L., and D. L. Riddle, 2001. LG II balancer chromosomes in Caenorhabditis elegans: mT1(II;III) and the mIn1 set of dominantly and recessively marked inversions. Mol. Genet. Genomics 266: 385–395. [DOI] [PubMed] [Google Scholar]
- Essers, L., R. H. Adolphs and R. Kunze, 2000. A highly conserved domain of the maize Activator transposase is involved in dimerization. Plant Cell 12: 211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay, D. S., and M. Han, 2000. The synthetic multivulval genes of C. elegans: functional redundancy, Ras-antagonism, and cell fate determination. Genesis 26: 279–284. [DOI] [PubMed] [Google Scholar]
- Fay, D. S., S. Keenan and M. Han, 2002. fzr-1 and lin-35/Rb function redundantly to control cell proliferation in C. elegans as revealed by a nonbiased synthetic screen. Genes Dev. 16: 503–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay, D. S., E. Large, M. Han and M. Darland, 2003. lin-35/Rb and ubc-18, an E2 ubiquitin-conjugating enzyme, function redundantly to control pharyngeal morphogenesis in C. elegans. Development 130: 3319–3330. [DOI] [PubMed] [Google Scholar]
- Fay, D. S., X. Qiu, E. Large, C. P. Smith, S. Mango et al., 2004. The coordinate regulation of pharyngeal development in C. elegans by lin-35/Rb, pha-1, and ubc-18. Dev. Biol. 271: 11–25. [DOI] [PubMed] [Google Scholar]
- Ferguson, E. L., and H. R. Horvitz, 1985. Identification and characterization of 22 genes that affect the vulval cell lineages of Caenorhabditis elegans. Genetics 110: 17–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, E. L., and H. R. Horvitz, 1989. The multivulva phenotype of certain Caenorhabditis elegans mutants results from defects in two functionally redundant pathways. Genetics 123: 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, E. L., P. W. Sternberg and H. R. Horvitz, 1987. A genetic pathway for the specification of the vulval cell lineages of Caenorhabditis elegans. Nature 326: 259–267. (erratum: Nature 327: 82). [DOI] [PubMed] [Google Scholar]
- Finney, M., and G. Ruvkun, 1990. The unc-86 gene product couples cell lineage and cell identity in C. elegans. Cell 63: 895–905. [DOI] [PubMed] [Google Scholar]
- Fong, Y., L. Bender, W. Wang and S. Strome, 2002. Regulation of the different chromatin states of autosomes and X chromosomes in the germ line of C. elegans. Science 296: 2235–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser, A. G., R. S. Kamath, P. Zipperlen, M. Martinez-Campos, M. Sohrmann et al., 2000. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature 408: 325–330. [DOI] [PubMed] [Google Scholar]
- Frolov, M. V., and N. J. Dyson, 2004. Molecular mechanisms of E2F-dependent activation and pRB-mediated repression. J. Cell Sci. 117: 2173–2181. [DOI] [PubMed] [Google Scholar]
- Henikoff, S., and J. G. Henikoff, 1992. Amino acid substitution matrices from protein blocks. Proc. Natl. Acad. Sci. USA 89: 10915–10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman, A. B., Z. N. Perez, L. Zhou, P. Musingarimi, R. Ghirlando et al., 2005. Molecular architecture of a eukaryotic DNA transposase. Nat. Struct. Mol. Biol. 12: 715–721. [DOI] [PubMed] [Google Scholar]
- Hodgkin, J., 1997. Appendix 1: Genetics, pp. 881–1047 in C. elegans II, edited by D. L. Riddle, T. Blumenthal, B. J. Meyer and J. R. Priess. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Holdeman, R., S. Nehrt and S. Strome, 1998. MES-2, a maternal protein essential for viability of the germline in Caenorhabditis elegans, is homologous to a Drosophila Polycomb group protein. Development 125: 2457–2467. [DOI] [PubMed] [Google Scholar]
- Horvitz, H. R., and J. E. Sulston, 1980. Isolation and genetic characterization of cell-lineage mutants of the nematode Caenorhabditis elegans. Genetics 96: 435–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh, J., J. Liu, S. A. Kostas, C. Chang, P. W. Sternberg et al., 1999. The RING finger/B-box factor TAM-1 and a retinoblastoma-like protein LIN-35 modulate context-dependent gene silencing in Caenorhabditis elegans. Genes Dev. 13: 2958–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, L. S., P. Tzou and P. W. Sternberg, 1994. The lin-15 locus encodes two negative regulators of Caenorhabditis elegans vulval development. Mol. Biol. Cell 5: 395–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein, T., and C. D. Allis, 2001. Translating the histone code. Science 293: 1074–1080. [DOI] [PubMed] [Google Scholar]
- Kamath, R. S., A. G. Fraser, Y. Dong, G. Poulin, R. Durbin et al., 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421: 231–237. [DOI] [PubMed] [Google Scholar]
- Kawasaki, I., Y.-H. Shim, J. Kirchner, J. Kaminker, W. B. Wood et al., 1998. PGL-1, a predicted RNA-binding component of germ granules, is essential for fertility in C. elegans. Cell 94: 635–645. [DOI] [PubMed] [Google Scholar]
- Kelly, W. G., S. Xu, M. K. Montgomery and A. Fire, 1997. Distinct requirements for somatic and germline expression of a generally expressed Caernorhabditis elegans gene. Genetics 146: 227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempken, F., and F. Windhofer, 2001. The hAT family: a versatile transposon group common to plants, fungi, animals, and man. Chromosoma 110: 1–9. [DOI] [PubMed] [Google Scholar]
- Kennedy, S., D. Wang and G. Ruvkun, 2004. A conserved siRNA-degrading RNase negatively regulates RNA interference in C. elegans. Nature 427: 645–649. [DOI] [PubMed] [Google Scholar]
- Ketting, R. F., T. H. A. Haverkamp, H. G. A. M. van Luenen and R. H. A. Plasterk, 1999. mut-7 of C. elegans, required for transposon silencing and RNA interference, is a homolog of Werner syndrome helicase and RNaseD. Cell 99: 133–141. [DOI] [PubMed] [Google Scholar]
- Kidd, III, A. R., J. A. Miskowski, K. R. Siegfried, H. Sawa and J. Kimble, 2005. A β-catenin identified by functional rather than sequence criteria and its role in Wnt/MAPK signaling. Cell 121: 761–772. [DOI] [PubMed] [Google Scholar]
- Kim, J. K., H. W. Gabel, R. S. Kamath, M. Tewari, A. Pasquinelli et al., 2005. Functional genomic analysis of RNA interference in C. elegans. Science 308: 1164–1167. [DOI] [PubMed] [Google Scholar]
- Korenjak, M., B. Taylor-Harding, U. K. Binne, J. S. Satterlee, O. Stevaux et al., 2004. Native E2F/RBF complexes contain Myb-interacting proteins and repress transcription of developmentally controlled E2F target genes. Cell 119: 181–193. [DOI] [PubMed] [Google Scholar]
- Korf, I., Y. Fan and S. Strome, 1998. The Polycomb group in Caenorhabditis elegans and maternal control of germline development. Development 125: 2469–2478. [DOI] [PubMed] [Google Scholar]
- Lee, C. C., E. L. Beall and D. C. Rio, 1998. DNA binding by the KP repressor protein inhibits P-element transposase activity in vitro. EMBO J. 17: 4166–4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, P. W., E. L. Beall, T. C. Fleischer, D. Georlette, A. J. Link et al., 2004. Identification of a Drosophila Myb-E2F2/RBF transcriptional repressor complex. Genes Dev. 18: 2929–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsick, J. S., 2004. synMuv verite–Myb comes into focus. Genes Dev. 18: 2837–2844. [DOI] [PubMed] [Google Scholar]
- Lu, X., and H. R. Horvitz, 1998. lin-35 and lin-53, two genes that antagonize a C. elegans Ras pathway, encode proteins similar to Rb and its binding protein RbAp48. Cell 95: 981–991. [DOI] [PubMed] [Google Scholar]
- Macfarlan, T., S. Kutney, B. Altman, R. Montross, J. Yu et al., 2005. Human THAP7 is a chromatin-associated, histone tail-binding protein that represses transcription via recruitment of HDAC3 and nuclear hormone receptor corepressor. J. Biol. Chem. 280: 7346–7358. [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer, A., J. B. Anderson, C. DeWeese-Scott, N. D. Fedorova, L. Y. Geer et al., 2003. CDD: a curated Entrez database of conserved domain alignments. Nucleic Acids Res. 31: 383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens, J. A., and F. Winston, 2003. Recent advances in understanding chromatin remodeling by Swi/Snf complexes. Curr. Opin. Genet. Dev. 13: 136–142. [DOI] [PubMed] [Google Scholar]
- Mello, C., and A. Fire, 1995. DNA transformation, pp. 451–482 in Caenorhabditis elegans: Modern Biological Analysis of an Organism, edited by H. F. Epstein and D. C. Shakes. Academic Press, San Diego.
- Meléndez, A., and I. Greenwald, 2000. Caenorhabditis elegans lin-13, a member of the LIN-35 Rb class of genes involved in vulval development, encodes a protein with zinc fingers and an LXCXE motif. Genetics 155: 1127–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel, K., D. A. O'Brochta and P. W. Atkinson, 2003. The C-terminus of the Hermes transposase contains a protein multimerization domain. Insect Biochem. Mol. Biol. 33: 959–970. [DOI] [PubMed] [Google Scholar]
- Miskowski, J., Y. Li and J. Kimble, 2001. The sys-1 gene and sexual dimorphism during gonadogenesis in Caenorhabditis elegans. Dev. Biol. 230: 61–73. [DOI] [PubMed] [Google Scholar]
- Moghal, N., and P. W. Sternberg, 2003. A component of the transcriptional mediator complex inhibits RAS-dependent vulval fate specification in C. elegans. Development 130: 57–69. [DOI] [PubMed] [Google Scholar]
- Nonet, M. L., O. Saifee, H. Zhao, J. B. Rand and L. Wei, 1998. Synaptic transmission deficits in Caenorhabditis elegans synaptobrevin mutants. J. Neurosci. 18: 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, E. C., and H. R. Horvitz, 1986. Mutations with dominant effects on the behavior and morphology of the nematode Caenorhabditis elegans. Genetics 113: 821–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen, J. E., E. E. Capowski and S. Strome, 1995. Phenotypic and molecular analysis of mes-3, a maternal-effect gene required for proliferation and viability of the germ line in C. elegans. Genetics 141: 1383–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin, G., Y. Dong, A. G. Fraser, N. A. Hopper and J. Ahringer, 2005. Chromatin regulation and sumoylation in the inhibition of Ras-induced vulval development in Caenorhabditis elegans. EMBO J. 24: 2613–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, K. C., and A. M. Villeneuve, 2004. C. elegans HIM-17 links chromatin modification and competence for initiation of meiotic recombination. Cell 118: 439–452. [DOI] [PubMed] [Google Scholar]
- Ross, J. M., and D. Zarkower, 2003. Polycomb group regulation of Hox gene expression in C. elegans. Dev. Cell 4: 891–901. [DOI] [PubMed] [Google Scholar]
- Roussigne, M., S. Kossida, A.-C. Lavigne, T. Clouaire, V. Ecochard et al., 2003. The THAP domain: a novel protein motif with similarity to the DNA-binding domain of P element transposase. Trends Biochem. Sci. 28: 66–69. [DOI] [PubMed] [Google Scholar]
- Rubin, E., G. Lithwick and A. A. Levy, 2001. Structure and evolution of the hAT transposon superfamily. Genetics 158: 949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, T. H., and C. C. Mello, 2003. Chromatin regulation during C. elegans germline development. Curr. Opin. Genet. Dev. 13: 455–462. [DOI] [PubMed] [Google Scholar]
- Siegfried, K. R., A. R. Kidd, III, M. A. Chesney and J. Kimble, 2004. The sys-1 and sys-3 genes cooperate with Wnt signaling to establish the proximal-distal axis of the C. elegans gonad. Genetics 166: 171–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmer, F., M. Tijsterman, S. Parrish, S. P. Koushika, M. L. Nonet et al., 2002. Loss of the putative RNA-directed RNA polymerase RRF-3 makes C. elegans hypersensitive to RNAi. Curr. Biol. 12: 1317–1319. [DOI] [PubMed] [Google Scholar]
- Simmer, F., C. Moorman, A. M. van der Linden, E. Kuijk, P. V. E. van den Berghe et al., 2003. Genome-wide RNAi of C. elegans using the hypersensitive rrf-3 strain reveals novel gene functions. PLoS Biol. 1: E12. [DOI] [PMC free article] [PubMed]
- Solari, F., and J. Ahringer, 2000. NURD-complex genes antagonise Ras-induced vulval development in Caenorhabditis elegans. Curr. Biol. 10: 223–226. [DOI] [PubMed] [Google Scholar]
- Sulston, J. E., and H. R. Horvitz, 1977. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev. Biol. 56: 110–156. [DOI] [PubMed] [Google Scholar]
- Tabara, H., M. Sarkissian, W. G. Kelly, J. Fleenor, A. Grishok et al., 1999. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell 99: 123–132. [DOI] [PubMed] [Google Scholar]
- Thomas, J. H., and H. R. Horvitz, 1999. The C. elegans gene lin-36 acts cell autonomously in the lin-35 Rb pathway. Development 126: 3449–3459. [DOI] [PubMed] [Google Scholar]
- Thomas, J. H., C. J. Ceol, H. T. Schwartz and H. R. Horvitz, 2003. New genes that interact with lin-35 Rb to negatively regulate the let-60 ras pathway in Caenorhabditis elegans. Genetics 164: 135–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers, H. T. M., and L. Tora, 2005. SAGA unveiled. Trends Biochem. Sci. 30: 7–10. [DOI] [PubMed] [Google Scholar]
- Timmons, L., and A. Fire, 1998. Specific interference by ingested dsRNA. Nature 395: 854. [DOI] [PubMed]
- Unhavaithaya, Y., T. H. Shin, N. Miliaras, J. Lee, T. Oyama et al., 2002. MEP-1 and a homolog of the NURD complex component Mi-2 act together to maintain germline-soma distinctions in C. elegans. Cell 111: 991–1002. [DOI] [PubMed] [Google Scholar]
- von Zelewsky, T., F. Palladino, K. Brunschwig, H. Tobler, A. Hajnal et al., 2000. The C. elegans Mi-2 chromatin-remodelling proteins function in vulval cell fate determination. Development 127: 5277–5284. [DOI] [PubMed] [Google Scholar]
- Walker, D. S., S. Ly, N. J. D. Gower and H. A. Baylis, 2004. IRI-1, a LIN-15B homologue, interacts with inositol-1,4,5-triphosphate receptors and regulates gonadogenesis, defecation, and pharyngeal pumping in Caenorhabditis elegans. Mol. Biol. Cell 15: 3073–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D., S. Kennedy, D. Conte, Jr., J. K. Kim, H. W. Gabel et al., 2005. Somatic misexpression of germline P granules and enhanced RNA interference in retinoblastoma pathway mutants. Nature 436: 593–597. [DOI] [PubMed] [Google Scholar]
- Xu, L., J. Paulsen, Y. Yoo, E. B. Goodwin and S. Strome, 2001. Caenorhabditis elegans MES-3 is a target of GLD-1 and functions epigenetically in germline development. Genetics 159: 1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, Y., R. Gibbons, Z. Yan, D. Yang, T. L. McDowell et al., 2003. The ATRX syndrome protein forms a chromatin-remodeling complex with Daxx and localizes in promyelocytic leukemia nuclear bodies. Proc. Natl. Acad. Sci. USA 100: 10635–10640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, L., R. Mitra, P. W. Atkinson, A. B. Hickman, F. Dyda et al., 2004. Transposition of hAT elements links transposable elements and V(D)J recombination. Nature 432: 995–1001. [DOI] [PubMed] [Google Scholar]