Abstract
This article reviews the modulation of cognitive function by normal genetic variation. Although the heritability of “g” is well established, the genes that modulate specific cognitive functions are largely unidentified. Application of the allelic association approach to individual differences in cognition has begun to reveal the effects of single nucleotide polymorphisms on specific and general cognitive functions. This article proposes a framework for relating genotype to cognitive phenotype by considering the effect of genetic variation on the protein product of specific genes within the context of the neural basis of particular cognitive domains. Specificity of effects is considered, from genes controlling part of one receptor type to genes controlling agents of neuronal repair, and evidence is reviewed of cognitive modulation by polymorphisms in dopaminergic and cholinergic receptor genes, dopaminergic enzyme genes, and neurotrophic genes. Although allelic variation in certain genes can be reliably linked to cognition—specifically to components of attention, working memory, and executive function in healthy adults—the specificity, generality, and replicability of the effects are not fully known.
Keywords: genetics, cognition, SNPs, attention, memory, cholinergic, dopaminergic, neurotrophins
General cognitive ability is known to be highly heritable (Plomin, DeFries, McClearn, & McGuffin, 2001), although the particular genes that underlie the heritability have only recently been investigated. Individual genes can exert multiple effects on cognition. The neural pathways of such influences can be fairly discrete, as in the control of a subunit of one type of neuronal receptor, or relatively general, as in the modulation of the efficiency of neuronal repair. In this article, we review the nascent literature on the role of individual genes in modulating cognitive function with a view toward assessing the specificity of effects exerted by individual genes. We also examine the role of these genes in mediating age-related changes in cognition. Candidate genes selected for this review were largely single nucleotide polymorphisms (SNPs), for which there is growing evidence concerning both (a) cognitive and (b) physiological of the polymorphism.
Heritability Studies
A century ago, Spearman (1904) adopted the term “g” to capture the observation that individuals who do well on one test of intellectual functioning (e.g., verbal ability), tend to do well on others (e.g., memory and general reasoning). Operationally defined as what is common to different tests of cognitive ability, “g” has been found in multivariate genetic research on twins to account for much of the genetic variance in cognitive test performance. A sizeable literature from twin studies indicates heritabilities of about .5 for general cognitive ability. Moreover, this estimate of heritability increases with age, ranging from .2 in early childhood to .4 in adolescence to .8 in old age (McClearn et al., 1997; Plomin & Craig, 1997).
The heritability of “g” may have a substrate in cortical volume. Previous work has shown that cortical volume is correlated about .4 with “g,” such that individuals with greater brain volumes have higher “g” scores (Vernon, Wickett, Banzana, & Stelmack, 2000). This relation appears to be genetic. Individual differences in regional cortical volume of Broca's and Wernicke's areas, which were found to be linked to “g,” were also attributed to genetic factors (Thompson et al., 2001). Although this suggests that “g” varies with genetic effects on cortical volume, environmental influences cannot be ruled out, for regional brain volume itself is subject to change by experience (Maguire et al., 2000; Rosenzweig & Bennett, 1972, 1996).
Despite the compelling evidence amassed in twin studies in favor of the existence of “g,” the notion that a single factor controls a large range of cognitive abilities is controversial. Arguments have been made for the existence of “multiple intelligences,” including logical-mathematical, verbal, spatial, and musical (Gardner, 1983). This notion has even been extended to “emotional intelligence” (Goleman, 1995), although empirical support for this concept may be lacking (Matthews, Zeidner, & Roberts, 2003). However, recent physiological evidence suggests that “g” might arise, instead, from a more limited set of functions—namely working memory and executive functions. Performance on spatial and verbal tasks showing either high or low correlations with “g” were related to regional blood flow in specific brain regions. Tasks highly correlated with “g” were found to selectively activate lateral prefrontal cortex and a region in the medial frontal gyrus (Duncan et al., 2000). These findings suggest that “g” may be mediated by a limited set of cognitive abilities.
Specific cognitive functions also appear to be heritable but to differing degrees, suggesting the influence of different genes. McClearn et al. (1997) used a large range of tests, such as Thurstone's primary mental abilities (PMA) and subtests of the Wechsler Adult Intelligence Scale (WAIS; Wechsler, 1981) in elderly twins and found heritabilities of .62 for general cognitive ability, .55 for verbal ability, .32 for spatial ability, .62 for speed of processing, and .52 for memory. Similarly, measures of executive control (Digit Symbol Substitution, color-word interference, Trail Making B, and verbal fluency) were found to show a range of heritabilities from .34 to .68 (Swan & Carmelli, 2002). Posner and colleagues also obtained evidence of high heritability of .78 for a test of executive attention (Fan, Wu, Fossella, & Posner, 2001). A study comparing the heritability of varieties of memory found that working memory had the highest heritability (.49) whereas that of long-term memory was considerably lower, ranging from 47 for long-term memory (Thurstone's Picture Memory) to .28 for object recall (MIR) (Johansson et al., 1999). The high heritability of working memory (.43 to .49) has been independently confirmed (Ando, Ono, & Wright, 2001). Considered together, the twin literature on components of cognition indicates that cognitive systems that may contribute to “g” vary in their separate heritabilities.
This range of heritabilities could lead to the view that general cognitive ability is modular—that is, composed of separate, and separately controlled, processes. By this view, “g” could be a composite measure of a large number of genetic traits. A different approach claims that general cognitive ability emerges from the epistatic interaction of genes in addition to their additive effect—a view termed “emergenesis” (Lykken, McGue, Tellegen, & Bouchard, 1992). Alternatively, “g” could reflect the operation of a small set of traits that influence apparently diverse abilities (Plomin, 2001). For example, many aspects of cognitive ability depend on retention and manipulation of items in memory and on selective attention to one aspect of a task. Thus, “g” could reflect the operation of a small number of genes important in working memory and attention (Duncan et al., 2000; Swan & Carmelli, 2002).
These diverse views on the genetics of cognition share in common the conclusion that “g” is extremely unlikely to be controlled by a single gene. But if “g” is a function of multiple genes, what are the contributing genes? To date, the workhorse in the field of the genetics of cognition has been the twin paradigm in which identical and fraternal twins are compared to assess the heritability of a trait. This approach is powerful for showing the existence and degree of genetic influence, but it cannot speak to the particular genes involved. Recent advances in molecular genetics now allow a different, complementary approach to behavioral genetics known as allelic association. Most commonly used to associate disease with candidate genes, the association method can also relate specific genes to behavioral performance measures in healthy persons (Plomin & Crabbe, 2000). This approach has been recently applied to cognition, revealing evidence of modulation of cognitive task performance by specific genes. Allelic association requires identification of candidate genes—that is, genes deemed likely to influence a given cognitive ability or trait because of the functional role of each gene's protein product in the brain. The completion of the Human Genome Project and the attendant burgeoning literature on the function of individual genes and SNPs permits investigation into the genetics of cognition at the level of individual genes.
Overview
In this article, we examine the emerging literature on the role of normal genetic variation on cognition. We outline a framework for understanding the genetics of normal cognition, which involves combining the allelic association approach of behavioral genetics with the methods of modern cognitive neuroscience. Such an approach allows for theory-based, empirical analysis of the role of particular polymorphic genes in cognition. We recognize that no component of cognition is likely to be modified by only one gene and that the interpretation of individual differences in a particular cognitive function will ultimately involve specification of the role of many genes as well as of environmental factors (Plomin & Crabbe, 2000). Nevertheless, as will be discussed, certain genes appear to contribute in relatively specific ways to individual differences in aspects of cognition. By using this approach, the knowledge of cognitive neuroscience can be brought to bear on genetics to begin to specify the intermediate steps between genotype and phenotype.
The goal of this review is to consider what is known of the effect of single genes on cognition. We examine genes that control parts of neuronal receptors as well as those with broader effects on underlying mechanisms such as neuronal repair. For the most part, we discuss genes in relation to cognitive functioning in healthy adults across the life span. However, because much genetic work has been motivated by the need for understanding neuropsychiatric disorders, we also discuss studies of attention deficit disorder and hyperactivity (ADHD), schizophrenia, and Alzheimer's disease. We first examine dopaminergic (including DRD4, COMT, and DBH) and cholinergic (CHRNA4 and CHRNA7) genes because these have been the target of much research. These genes have been specifically linked to different components of attentional and memory processes. Subsequently, we discuss genes with a role in neuron health and plasticity. Neurotrophic genes (BDNF) and neuroprotection genes (apoE, estrogen receptor) are likely to become important in adulthood once brain aging processes start. Hence, another focus of our review is to examine the role of these genes in cognitive aging.
A FRAMEWORK FOR LINKING POLYMORPHIC GENES AND COGNITION
Greater than 99% of individual DNA sequences in the human genome do not differ between individuals and, hence, are of limited interest in investigating individual differences in normal cognition. However, a significant minority of DNA base pairs (bp) occur as different forms or alleles. Allelic variation is the result of slight differences in the chain of nucleic acids making up the gene—commonly the result of a substitution of one nucleotide for another. Consequently, the protein product of that gene is correspondingly altered. Such variations, or SNPs, occur at a rate of about 1 every 1000 bp in unrelated individuals. It now is estimated that there are about 1.8 million SNPs, but only about 5% to 10% of these are likely to be associated with disorders (Plomin et al., 2001). In some cases, allelic variation is related to disease. For example, one form of nocturnal frontal lobe epilepsy, which has an autosomal dominant form of inheritance, is characterized by an insertion into the gene controlling production of a cholinergic receptor—the alpha-4 nicotinic cholinergic receptor subunit (the gene is termed CHRNA4; Steinlein et al. (1997a). This insertion in the gene results in greater affinity of the receptor itself for acetylcholine, perhaps the result of lower permeability of the subunit to calcium (Steinlein et al., 1997a). Thus, a SNP can affect neurotransmission in the synapse in a way that has important consequences for cortical electrophysiology. Polymorphisms can influence brain function through effects with a range of specificities—from effects limited to one receptor type, as in the example above, to effects on neurotransmission systems, to whole-brain effects on neuron health.
The overall goal of this approach is to determine the role of specific genes in producing a cognitive phenotype. But even given that SNPs with functional significance for behavior represent only a small part of the human genome, there are still so many of them that forging valid links to cognition may prove difficult. However, the general problem of establishing a reliable association between genotype and phenotype may be mitigated by using the allelic association approach within a theoretical framework in which the intermediate steps between genotype and phenotype are specified (to a degree). Such a framework should capitalize on the breakthroughs in understanding the neural bases of cognition that have been made possible by modern cognitive neuroscience. As such, this approach combines the allelic association method of relating SNPs to behavior with knowledge gleaned from cognitive neuroscience. This approach allows theory-based, empirical analysis of the role of particular polymorphisms in cognition.
There are, however, limitations to the allelic association approach. This approach has been applied most often to the genetics of disease; in this context, it has been criticized for risk of Type I errors and failures to replicate (Ioannidis, Ntzani, Trikalinos, & Contopoulos-Ioannidis, 2001). The heritability of some diseases may derive from a number of genes, each with a small effect. Moreover, a single diagnostic category may actually encompass several different disorders. Both of these factors may play a role in the small (< 1%) effect sizes often seen in association studies of disease (e.g., Ioannidis et al., 2001). With regard to the genetics of cognition, these limitations can be partially ameliorated by marrying the association approach with cognitive neuroscience. This approach has a number of advantages. First, cognitive functions can be fractionated into component processes. It may be reasonable to assume that effects of single genes on component processes of cognitive functions will be larger than effects of single genes on a disease category. In contrast to the small (< 1%) effect sizes seen in association studies of disease, association studies of cognitive functions appear to yield larger effect sizes. Table 1 shows effect sizes from a sample of recent studies from this small literature. Secondly, for several functions, knowledge of brain networks and corresponding innervation can be used to guide selection of SNPs in neurotransmitter genes. Third, twin studies can be used to establish the heritability of a given cognitive function and/or component processes of that function (e.g., Fan, Wu, Fossella, & Posner, 2001). Fourth, the test-retest reliability of a given task aimed at a function or component process can be assessed. Fifth, knowledge of mechanisms of brain aging can also be used to guide selection of SNPs in genes controlling aspects of neuronal repair and synaptic plasticity.
Table 1.
Effect Sizes for Cognitive Allelic Association Studies
| Gene | Allele With Cognitive Effects | Task | fa | Source of Data |
|---|---|---|---|---|
| ApoE | ε4 | (a) cued discrimination (b) word pair recall, digit symbol | (a) .14 (b) .42 | (a) Greenwood, Sunderland, Friz, and Parasuraman, 2000 (b) Flory, Manuck, Ferrell, Ryan, and Muldoon, 2000 |
| BDNF Val66met | met | (a) WMSb delayed recall (b) scene recognition | (a) .26 (b) .46 | (a) Egan et al., 2003 (b) Hariri et al., 2003 |
| COMT val158met | met | (a) WCSTc (b) processing speed/attention | (a) .41 (b) .42 | (a) Egan et al., 2001 (b) Bilder et al., 2002 |
| DBH G444A | A | memory for location | .19 | Greenwood, Fossella, and Parasuraman, 2003 |
| DRD4 exon 3 VNTR | 7-repeat | (a)attentional orienting (b) RT | .29 | (a) Fossella et al., 2002 (b)Swanson et al., 2000 |
| DRD1 A-48G | G | novelty seeking questionnaire | .68 | Limosin, Loze, Rouillon, Ades, and Gorwood, 2003 |
| CHRNA4 C1545T | both? | cued discrimination | .30 | Greenwood et al., 2003 |
Cohen's method for unequal sample sizes (Cohen, 1988).
WMS = Wechsler Memory Scale.
WCST = Wisconsin Card Sort Test.
Although no broad framework for all of cognition currently exists, enough is known to begin to undertake such an approach for particular cognitive domains. For example, Posner has proposed an influential “attentional network” theory in which three separate attentional functions—orienting, alerting, and executive function—are linked to the activation of separate but overlapping cortical and subcortical networks (Posner & Petersen, 1990). Posner and colleagues have also argued that performance on psychological tests of these attentional functions can serve as phenotypes against which candidate genes can be tested (Fan et al., 2001; Fossella et al., 2002). We have also argued that specific information processing tests of particular attentional functions can serve as “behavioral assays” of the integrity of cortical networks, thereby allowing for an assessment of the effects of neurodegeneration on these networks (Parasuraman & Greenwood, 1998).
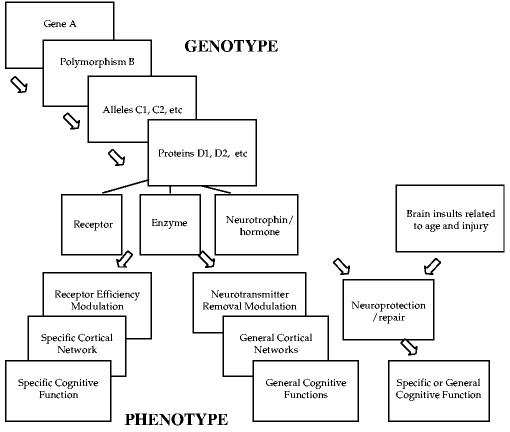
The neurochemical innervation of brain networks subserving particular cognitive functions are being increasingly well specified (Everitt & Robbins, 1997; Goldman-Rakic, 1998). For example, with respect to attentional function and as discussed in further detail in this article, a growing body of evidence from lesion and electrophysiological studies in animals and neuroimaging and pharmacological studies in humans points to the role of cholinergically mediated posterior brain networks in spatial attention and dopaminergically rich frontal networks in executive attention. Therefore, it follows that if progress is also made in delineating the genes and their protein products that influence these neurochemical pathways, then links can be established between genes and different aspects of cognitive function. Figure 1 provides a rough schematic of these links.
Figure 1.
Schematic of the Relation Between a Hypothetical Gene With a Behaviorally Relevant Polymorphism and Specific or General Effects on Cognition (the possible intermediate steps between genotype and phenotype are indicated).
As Figure 1 indicates, the protein products of particular genes can affect cognition through a number of pathways, including receptor modulation, neurotransmitter modulation, or neuroprotection. As will be discussed below, genes with a role in neuroprotection and neuronal repair may become particularly important in aging and following brain injury.
Receptors are neurotransmitter-gated ion channels, which, together with the neurotransmitters themselves, constitute the main signaling system in the brain. Chemical neurotransmitters, such as dopamine and acetylcholine, bind to specialized receptors on postsynaptic sites. This binding causes the ion channel in the receptor to open with the ensuing ion flow through the receptor channel producing the neuronal signal. Receptor structure and, hence, function are controlled by genes, some of which have functional polymorphisms.
Variations in receptor structure as a consequence of minor variations in the corresponding gene can affect efficiency of receptor transmission, as in the example above for the CHRNA4 gene. Even after a neurotransmitter is released into the synapse, its physiologic fate can be influenced by genes. There is a need for efficient removal of a neurotransmitter from the synaptic cleft because continued exposure of receptors to a neurotransmitter can desensitize the receptors. Neurotransmitters can be removed from the cleft by diffusion, by enzyme degradation, and by reuptake. The latter two mechanisms are controlled by genes.
Coding sequences of genes controlling receptors are highly conserved. Comparing amino acid sequences across species—for example, in ion channels—reveals regions with a high degree of similarity. Consequently, polymorphisms in neurotransmission genes are relatively rare (Kandel, Schwartz, & Jessell, 2000). The polymorphisms known to exist in brain dopaminergic and cholinergic systems generally involve receptor and transporter molecules. A polymorphism in a gene controlling part of a specific receptor type would at least initially only affect neurotransmission through that receptor type. In contrast, a polymorphism in a gene controlling an enzyme that degrades a family of neurotransmitters may have broader effects. A third category of genes plays a role in neuroprotection and thus can have either specific or broad effects. We will consider all three categories of genes.
DOPAMINERGIC GENES: MODULATION OF ATTENTION AND EXECUTIVE PROCESSES
Dopaminergic receptor genes are likely candidates for genetic effects on cognition because of the importance of dopaminergic innervation for functions of memory and attention. Dopamine agents have been shown to modulate working memory in monkeys (Sawaguchi & Goldman-Rakic, 1991) and humans (Muller, von Cramon, & Pollmann, 1998), and dopaminergic receptor genes have been linked to aspects of attention (Faraone, Doyle, Mick, & Biederman, 2001).
The DRD4 Gene: “Novelty Seeking” and Attention-Deficit/Hyperactivity Disorder
There are at least five known dopamine receptors (labeled D1 to D5), with the gene controlling the D4 dopamine receptor (DRD4 on chromosome 11) being very high in variability (Ebstein et al., 1996) because of the variation in a 48bp repeat. Alleles can contain a variable number of repeats of this sequence—typically 2, 4, or 7 repeats. The most common allele contains 4 repeats, and less common are the 2 and 7 repeats. The 7-repeat allele produces a receptor with reduced sensitivity to dopamine compared to 2- or 4-repeat alleles (Asghari et al., 1995).
The 7-repeat allele of the DRD4 gene has been associated with the personality trait of “novelty seeking,” measured as higher than average novelty-seeking scores on a personality inventory (Benjamin et al., 1996; Ebstein et al., 1996). Using a range of personality scales, Strobel, Wehr, Michel, & Brocke (1999) concluded that the 7-repeat allele was more associated with “exploratory” and “extraverted” traits rather than “impulsive” and “disorderly” traits. The same allele has been associated with decreased responsiveness of infants to novelty (Lakatos et al., 2003). However, others have failed to find such associations (Paterson, Sunohara, & Kennedy, 1999; Sullivan et al., 1998). A recent meta-analysis found that while 13 studies reported the association, 7 did not (Kluger, Siegfried, & Ebstein, 2002). Of interest in this context, a polymorphism in another dopamine receptor gene, DRD1, has been found to affect the same trait. The G allele of the DRD1 A48G polymorphism has been shown to increase in a gene-dose manner with novelty-seeking scores in male, but not female, alcoholics (Limosin, Loze, Rouillon, Ades, & Gorwood, 2003).
Evidence appears to be stronger for another association with the 7-repeat allele of the DRD4 gene—that is, with diagnosis of ADHD. ADHD, perhaps the most common childhood-onset behavioral disorder, is familial and heritable (Thapar, Holmes, Poulton, & Harrington, 1999). About 50% of ADHD cases are carriers of the DRD4 7-repeat allele, whereas only about 20% of controls carry that allele (LaHoste et al., 1996; Swanson et al., 1998). The reduced dopamine receptor sensitivity associated with 7-repeat alleles (Asghari et al., 1995) may underlie symptoms of ADHD. Although not all studies have observed the association of the 7-repeat allele and ADHD (Fisher et al., 2002), a recent meta-analysis has confirmed the association (Faraone et al., 2001).
Surprisingly, the specific cognitive deficits of ADHD are not fully understood. It has been claimed that sustained attention is deficient in ADHD and that neither selective attention (Manly et al., 2001) nor executive functioning (Wu, Anderson, & Castiello, 2002) is affected. This has been explicated somewhat by a finding that although children with ADHD are not deficient at shifting visuospatial attention, they are deficient at sustaining focused attention (Swanson et al., 1991). Working memory and planning also appear to be spared (Sonuga-Barke, Dalen, Daley, & Remington, 2002). That ADHD carriers of the 7-repeat allele do not appear to have processing deficits is consistent with the notion that the 7-repeat allele may be more important for the trait of novelty seeking than for deficiencies in processing.
The cognitive consequences of the 7-repeat allele have also been investigated in healthy individuals without ADHD. Posner and colleagues have developed a task—the attention network task (ANT)—designed to measure three aspects of attention simultaneously—alerting, orienting, and conflict (Fan, McCandliss, Sommer, Raz, & Posner, 2002). In Posner's terms, “alerting” refers to the achievement of an alert state, “orienting” refers to engagement and disengagement of visuospatial attention following a location cue, and “conflict” refers to the use of executive control to resolve conflicting location cues. The ANT task uses asterisk cues to the location of target arrows placed either above or below a centered fixation and requires discrimination of the direction of the arrows. To create the conflict condition, the target arrows are flanked by distractor arrows pointing in opposite directions. The cues are valid, invalid, or neutral (double cue) in indicating target location. Individuals carrying the 4-repeat allele showed slower reaction time (RT, time to respond) when flanker arrows conflicted with target arrows, compared to the 2-and 7-allele carriers (Fossella et al., 2002). There were no genotype differences in overall RT or alerting. This suggests that the 4-repeat, but not the 7-repeat, allele may be associated with subtle deficiencies in executive functioning.
Recently published evidence on the world-wide distribution of the 7-repeat allele suggests that the behavior associated with the allele may not be most accurately characterized as a disorder. Based on evidence of strong linkage disequilibrium (deviation from expected frequencies of alleles), it was argued that the 7-repeat allele is “younger” (in the evolutionary sense) than the other DRD4 alleles (Ding et al. 2002). This was interpreted to indicate that the 7-repeat allele was originally a rare mutation that subsequently increased in frequency through natural selection. It has been suggested that this allele conferred a reproductive advantage in competition for food in “local group male competition” (Harpending & Cochran, 2002) or from migration (Chen, Burton, Greenberger, & Dmitrieva, 1999). In light of evidence for the association of the 7-repeat allele with novelty seeking, this evidence suggests that the behavior associated with the 7-repeat allele may fall more into the category of normal individual differences than of disorders. Consistent with this is the finding of Fossella et al. (2002) of unimpaired executive attention in 7-repeat carriers. Also consistent is the finding that 7-repeat carriers diagnosed with ADHD showed normal RT speed and variability, whereas RT in noncarriers diagnosed with ADHD was slower, with more variable response patterns (Swanson et al., 2000). Thus, the 7-repeat allele may lead to novelty-seeking behavior consistent with a diagnosis of ADHD but without apparent processing deficits.
The COMT Gene
The dopamine system also appears to play a role in an aspect of cognition considered to be a component process of attention—that of executive control. The concept of executive control is one that is widely accepted, although there is not complete consensus about its characteristics. In general, executive control is thought to play a role in conflict resolution, difficult processing, and multitasking (Posner & DiGirolamo, 1998) as well as the process by which contents of memory storage are used in planning and execution of tasks (Smith & Jonides, 1999). Executive control appears to depend on prefrontal cortex (PFC). Lesion and electrophysiologic studies in the animal literature have shown the importance of PFC integrity for representation of the goals of planning (Fuster & Alexander, 1971; Goldman-Rakic, 1987). Several models have been advanced, most involving PFC and interactions among regions of PFC (Sylvester et al., 2003), that attempt to explain mediation of executive processes (MacPherson, Phillips, & Della Sala, 2002). For example, a division of labor has been proposed between dorsolateral prefrontal regions claimed to “monitor and select goal-relevant representations” and ventrolateral regions claimed to store such representations (Wagner, Maril, Bjork, & Schacter, 2001). These executive control processes appear to be dependent on dopaminergic innervation. Microinjections of D1 agonists and antagonists into PFC of behaving animals reveal that executive control performance depends on D1 receptor modulation of PFC and also on hippocampus inputs to PFC (Seamans, Floresco, & Phillips, 1998).
Several studies have found components of executive control to be highly heritable (Fan et al., 2001; Swan & Carmelli, 2002). The above-reviewed evidence showing dopaminergic innervation of the mediators of executive control in PFC suggests that one contributor to that heritability could be a gene with a role in the metabolism of dopamine. One candidate is the gene producing catechol-O-methyltransferase (COMT), an enzyme in the cytoplasm that is important in the metabolism of dopamine (and other catecholamines) following release into the synaptic cleft. A polymorphism in the COMT gene is known to affect activity of the enzyme controlled by the gene. This polymorphism is a G-to-A transition resulting in a valine-to-methionine substitution at codon 158 (val158met) on chromosome 22. Homozygosity for 158met leads to a 3- to 4-fold reduction in enzymatic activity in red blood cells and liver, compared with homozygosity for 158val (Lachman et al., 1996). This substitution in coding appears to affect brain function. The frequency of the low-activity met allele has been found to be markedly higher among alcoholics than controls (Tiihonen et al., 1999). The met allele is also more prevalent in individuals with panic disorder (Woo, Yoon, & Yu, 2002) and with reduced response to pain (Zubieta et al., 2003).
In light of the evidence that the COMT gene regulates dopamine metabolism, Egan et al. (2001) hypothesized that the high-activity val allele would result in faster degradation of dopamine at the synapse and thereby impair prefrontal functioning. This was assessed by a test sensitive to prefrontal processing—the Wisconsin Card Sort Test (WCST), a complex task requiring both working memory and executive control. Consistent with the hypothesis, increased gene dose of the low-activity met allele (0, 1, or 2 copies inherited) was associated with fewer perseverative errors on the WCST. Production of the low-activity enzyme would slow clearance of dopa-mine from the synapse. This result was seen in healthy controls, schizophrenics, and their unaffected siblings. Although schizophrenics and their siblings performed worse than controls, all groups showed an effect of geno-type. Thus, faster catabolism of prefrontal dopamine was found to impair prefrontal function (Egan et al., 2001). Importantly, this finding has been replicated in healthy individuals. Participants homozygous for the low-activity met allele made fewer perseverative errors on the WCST than did subjects carrying the high-activity val allele (Malhotra et al., 2002). However, the WCST is a complex task, and it is not clear which specific cognitive components of the task were affected by the COMT genetic variation. To clarify, a subsequent study by Weinberger and colleagues found a gene dose effect of the met allele on an “n-back” task (Goldberg et al., 2003). Such a task requires retention of memory for some aspect of a target item (e.g., color) during processing of nontarget items for some number of trials (“n”), typically 1 to 3. For example, under a 2-back condition, the color of the target 2 trials back has to be compared with the color of the current target. Thus, the information held in memory must be continually updated. Goldberg et al. found effects of gene dose of the met allele on n-back task performance but not on vigilance task performance. Another study of COMT genotype found the low-activity met allele was associated with improved processing speed and attention performance but had no effect on executive, memory, and motor functions (Bilder et al., 2002). Further work will be needed to determine whether effects of COMT genotype are selective for prefrontal functions.
The DBH Gene
Low plasma or cerebrospinal fluid (CSF) levels of dopamine beta-hydroxylase (DBH)—the enzyme converting dopamine to norepinephrine in adrenergic vesicles—are associated with psychotic symptoms in several psychiatric disorders. Plasma levels of DBH are under genetic control by the DBH gene, known to have several polymorphisms (Kohnke et al., 2002; Zabetian et al., 2001). One of these polymorphisms, a G-to-A substitution at 444, exon 2 (G444A) on chromosome 9, has been reported to predict smoking behavior (McKinney et al., 2000) and paranoia in cocaine users (Cubells et al., 2000) and in depressives (Wood, Joyce, Miller, Mulder, & Kennedy, 2002). DBH has also been associated with ADHD. Polymorphisms in both DBH and DAT1 genes were found to be associated with familial cases of ADHD (Daly, Hawi, Fitzgerald, & Gill, 1999).
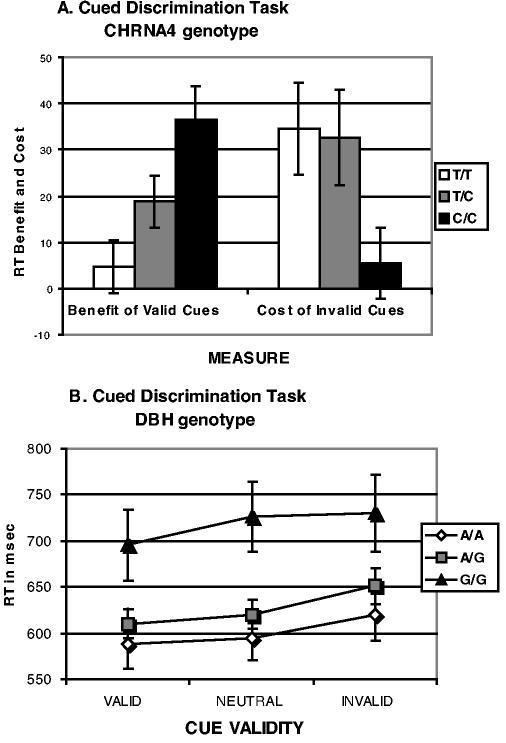
To determine the specificity of the effect of normal variation in the DBH gene, we compared the following two functions: working memory, which is strongly linked to dopaminergically innervated prefrontal regions (e.g., Abi-Dargham et al., 2002), and visuospatial attention, which is strongly linked to cholinergically innervated parietal regions (Davidson & Marrocco, 2000; Witte, Davidson, & Marrocco, 1997). Healthy young and old individuals were grouped by genotype of a C1545T mutation in the alpha-4 nicotinic acetylcholine receptor subunit (CHRNA4) gene (Steinlein et al., 1997b). This gene is expressed strongly in the parietal cortex, which is known to mediate visuospatial attention (Corbetta, 1998; Nobre et al., 1997). We also genotyped our sample for the G444A polymorphism of the DBH gene. This polymorphism has been shown to modulate plasma levels of the DBH enzyme (Cubells et al., 2000; Cubells et al., 1998), which is known to convert dopamine to norepinephrine. The DBH gene was chosen for its links to dopamine modulation, which is known to be important in working memory (Abi-Dargham et al., 2002; Sawaguchi & Goldman-Rakic, 1991). Visuospatial attention and working memory were measured (Greenwood, Fossella, & Parasuraman, 2003). Visuospatial attention was manipulated in a cued discrimination task in which arrow cues preceded a target letter and predicted its location with variable validity (Figure 2a). Response time (RT) to categorize the target as consonant or vowel was speeded by valid cues and slowed by invalid cues, both relative to a neutral cue (centered asterisk). These data were analyzed in a repeated-measures ANOVA with the Greenhouse-Geisser correction for sphericity. When analyzed by CHRNA4 genotype, this generated a significant main effect of cue validity (F[2,116] = 26.78, p < .0001) and stimulus onset asynchrony (SOA) (F[2,116] = 6.74, p < .002), which interacted (F[2,206] = 40.35, p < .0001). There was no main effect of Genotype (F[2,103] = .56), but there was a Cue Validity × Genotype interaction (F[4,206] = 2.45, p < .05). Cued discrimination RT can also be analyzed as RT “benefits” of valid cues (Neutral RT –Valid RT) and “costs” of invalid cues (Invalid RT – Neutral RT). Comparing benefits of valid cues with costs of invalid cues revealed a significant interaction of Measure (cost, benefit) × Genotype (F[2,82] = 4.54, p < .01). Figure 3a shows this is the result of greater costs associated with the CHRNA4 C allele and greater benefits associated with the T allele. Carrying out the same analysis for DBH shows only a main effect of genotype (F[2,61] = 5.31, p < .01) because of overall slower responding in 444G allele carriers (Figure 3b). Thus, CHRNA4 genotype, but not DBH genotype, modulated visuospatial attention performance.
Figure 2.
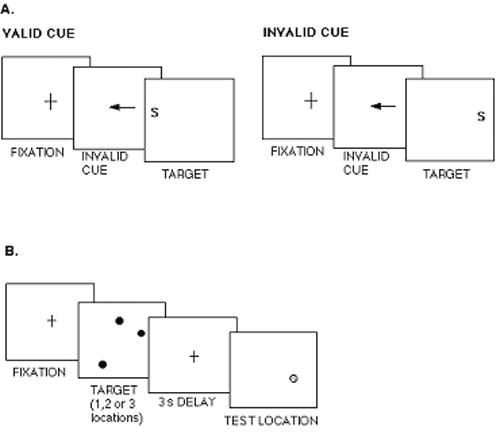
Schematic of a Directed-Attention Task and a Working-Memory Task. NOTE: (a) Schematic of a directed-attention task with central, symbolic arrow cues indicating the possible future location of a target letter. A speeded decision is required, indicating whether the target is consonant or vowel. (b) Schematic of a working-memory task in which the location of 1, 2, or 3 dots had to be remembered over a 3 sec delay. A decision is required about whether the test dot (shaded dot) appeared in the same location as one of the target dots (black dots).
Figure 3.
Response Times (RT) in a Cued Letter Discrimination Task. NOTE: (a) RT plotted as a function of CHRNA4 genotype (group sample sizes: T/T n= 63; T/C n= 25; C/C n= 18) for costs of invalid cues (Invalid – Neutral RT) and benefits of valid cues (Neutral – Valid RT); (b) RT plotted as a function of cue validity and DBH genotype (group sample sizes: G/G n= 18; G/A n= 33; A/A n= 13).
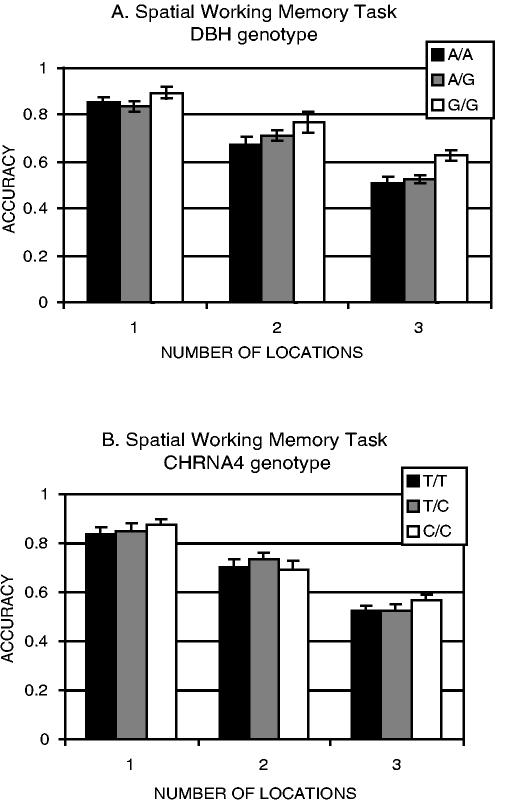
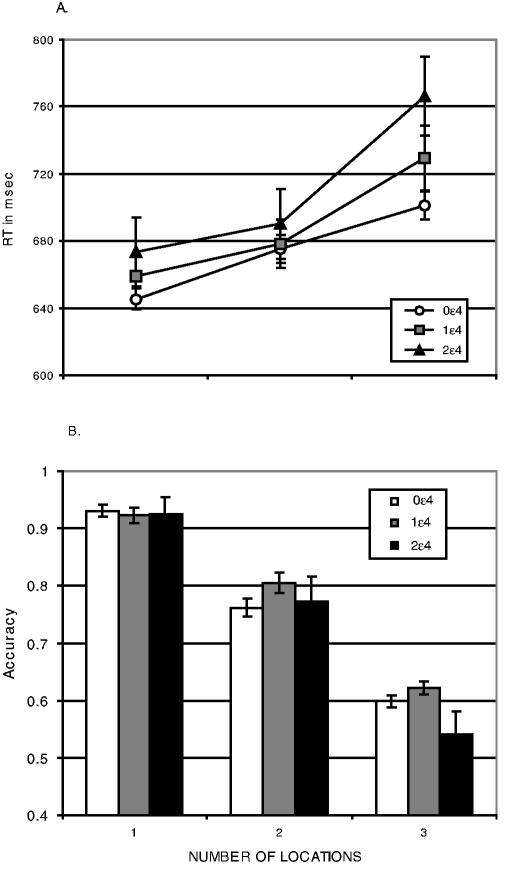
In addition, working memory was manipulated in a task that required retention of up to three locations over a 3 second delay (Figure 2b). Following the delay, memory for target location was measured as the accuracy of judging whether a probe appeared at one of the target locations. Memory accuracy declined as the number of targets increased (F[2,122] = 147.79, p < .0001). Memory was also modulated by DBH genotype, increasing in accuracy with the number of G alleles, particularly with multiple locations (Figure 4a; F(2,61) = 3.19, p < .05). However, memory was unaffected by CHRNA4 genotype (Figure 4b; F(2,53) = .25). These data suggest that the effect of the DBH gene is selective for working memory. Moreover, a double dissociation was observed with visuospatial attention modulated by a cholinergic but not a noradrenergic gene and spatial working memory modulated by a noradrenergic but not a cholinergic gene (Greenwood et al., 2003).
Figure 4.
Accuracy in a Spatial Working-Memory Task Plotted as a Function of Number of Locations to be Remembered Over a 3 Second Delay. NOTE: (a) DBH genotype (group sample sizes: A/A n= 17; A/G n= 35; G/G n= 12. (b) CHRNA4 genotype (group sample sizes: T/T n= 19; T/C n= 18; C/C n= 19).
Modulation of Executive Attention by Dopaminergic Genes: A Critical Evaluation
In the previous sections, we have reviewed evidence for the role of a number of dopaminergic genes—DRD4, COMT, and DBH—in the modulation of attentional processes underlying executive functioning. Can it be concluded that these polymorphisms affect executive attention per se? It could be hypothesized that receptor genes might exert more specific effects than enzyme genes. A receptor gene controls transmission only through a particular receptor type, whereas a gene controlling an enzyme degrading a neurotransmitter could exert effects more widely. Because they alter the rate of dopaminergic catabolism, enzyme genes could affect a number of cognitive systems and therefore might have less specific effects. In that regard, the DRD1 and DRD4 receptor genes show some specificity in that both have been linked to one dopamine-related disorder, ADHD, but not to another, schizophrenia (Georgieva et al., 2002; Hori, Ohmori, Shinkai, Kojima, & Nakamura, 2001). Moreover, the same DRD4 polymorphism appears to modulate RT speed and variability (Swanson et al., 2000) as well as executive attention (Fossella et al., 2002). This suggests that although the 7-repeat allele of the DRD4 gene may exert fairly specific effects on processing, those effects cannot as yet be characterized as limited to executive processes.
The COMT polymorphism might be predicted to have broader effects than the DRD4 polymorphism based on its control of an enzyme for dopamine. Variations in the COMT gene do not appear to be associated with ADHD (Hawi, Millar, Daly, Fitzgerald, & Gill, 2000) or bipolar disorder (Kirov, Jones, McCandless, Craddock, & Owen, 1999), although COMT may affect the risk of schizophrenia (Weinberger et al., 2001). The COMT val158met polymorphism has been related to certain aspects of cognition—namely, WCST performance both in schizophrenics (Egan et al., 2001) and in healthy individuals (Malhotra et al., 2002) as well as working-memory performance (Goldberg et al., 2003). However, this polymorphism is associated with effects that are not solely cognitive, as it appears to modulate pain perception (Zubieta et al., 2003) and risk of alcoholism (Tiihonen et al., 1999). Further, evidence from a range of neuropsychological tasks indicates that this COMT polymorphism may not modulate executive function (as has been claimed) but rather selective attention (reflected in Trails A and B and Digit Symbol tasks; Bilder et al., 2002).
Finally, we have obtained evidence for selective effects of a DBH polymorphism on working memory but not on visuospatial attention (Greenwood et al., 2003). However, as reviewed above, that same polymorphism has been linked to a number of disorders. Therefore, its specificity for memory needs to be confirmed.
Overall, although the DRD4, COMT, and DBH genes appear to modulate cognition, including executive attention and memory, in reliable ways, the extent of specificity of these effects is less clear. It is not yet possible to distinguish between dopaminergic genes that have relatively specific versus general effects.
CHOLINERGIC GENES: MODULATION OF VISUOSPATIAL ATTENTIONAL PROCESSES
Cholinergic genes are also good candidates for investigating genetic contributions to cognition—in particular, attention. Acetylcholine is a major neurotransmitter in the brain with cholinergic receptors modulating neuronal function in the hippocampus and parietal cortex (Xiang, Huguenard, & Prince, 1998). Although a majority of cholinergic receptors are muscarinic, nicotinic receptors are important in regulating fast synaptic transmission (Alkondon, Pereira, Eisenberg, & Albuquerque, 2000). This may underlie the important functional role that nicotinic receptors play in attention (Levin & Simon, 1998; Nordberg, 2001). Nicotinic acetylcholine receptors (nAChRs) are composed of subunits that assemble together to form the receptor itself. There are seven alpha-like subunits (alpha-2 to alpha-7 and alpha-9) and three beta-like subunits (beta-2, beta-3, and beta-4). The most widely distributed nicotinic receptor in the central nervous system is composed of alpha-4 and beta-2 nAChR subunits assembled together (Flores, DeCamp, Kilo, Rogers, & Hargreaves, 1996). Another major nicotine receptor consists of alpha-7 subunits assembled together.
The Cholinergic System and Visuospatial Attention: Nongenetic Evidence
Lesions of the basal forebrain cholinergic system reveal the importance of cholinergic system integrity for attention (Everitt & Robbins, 1997; Gallagher & Colombo, 1995; Voytko, 1996). Specifically, Voytko and colleagues tested monkeys with and without basal forebrain lesions in a task in which location cues preceded a detection target (Posner, 1980). Monkeys had to depress a button at the center of the screen until the target appeared. Cues to target location, either valid or invalid, appeared 200 ms before target onset. Both control and lesioned monkeys were faster to respond to the target when cues were valid (when the target appeared at the cued location) compared to when cues were invalid. However, the basal forebrain lesioned monkeys were slower than the controls to release the center button at target onset, particularly on invalid trials. The same monkeys showed no deficits on a delayed nonmatch to sample task learned preoperatively. This was interpreted as evidence that the cholinergic basal forebrain is more important for visuospatial attention than for working memory (Voytko et al., 1994).
Electrophysiological evidence also documents the modulation of attention by cholinergic neurotransmission. The P300 component of the event-related potential (ERP)—an attention-related component of the brain's electrical response to discrete stimulation—can be selectively eliminated from the ERP by administration of cholinergic antagonists such as scopolamine (Hammond, Meador, Aung-Din, & Wilder, 1987). Scopolamine also induces disproportionate slowing of responses to invalidly cued targets in rats (Phillips, McAlonan, Robb, & Brown, 2000). In addition, we have shown that scopolamine reduces the ability of Alzheimer's disease (AD) patients to scale visuospatial attention in response to cues of varying size. Individuals with mild to moderate AD were administered a cued visual search task in which a cue to target location varied in size and, hence, precision during a visual search task. A precise precue to target location speeded visual search in AD patients. This benefit of precue precision was largely eliminated by acute administration of scopolamine (Levy, Parasuraman, Greenwood, Dukoff, & Sunderland, 2000).
More direct evidence from monkeys indicates the importance of cholinergic mechanisms for processes of visuospatial attention specifically. The ability to shift visuospatial attention through space was impaired by direct infusion of scopolamine into the intraparietal sulcus of monkeys, where neurons had been identified by microelectrode recording as being responsive to location cuing (Davidson & Marrocco, 2000). Scopolamine infusion into other cortical regions did not affect attention-shifting behavior. Human cholinergic innervation in the parietal cortex appears to be nicotinic, not muscarinic (Mentis et al., 2001). Similar selective modulation has been seen in the rat (Phillips et al., 2000). This evidence points to selective dependence of the ability to shift visuospatial attention in space on cholinergic receptors in the intraparietal region.
Understanding the role of cholinergic genes on cognition is important in light of evidence that the cholinergic system may be particularly vulnerable to healthy aging. The “cholinergic hypothesis” of aging has attributed cognitive decline in aging to selective deterioration within the cholinergic system (Bartus, Dean, Beer, & Lippa, 1982). Although cholinergic neurons do not die in the course of healthy aging (Morrison & Hof, 1997), they undergo a reversible atrophy. Aged rats show a significant decrease in parietal synaptic sites, particularly in cholinergic neurons (Casu, Pan Wong, De Koninck, Ribeiro-Da-Silva, & Cuello, 2002) and exhibit atrophy of subcortical cholinergic neurons related to spatial learning deficits (Martinez-Serrano, Fischer, & Bjorklund, 1995). Aged monkeys show reductions in density of temporal and frontal cholinergic receptors (Tsukada et al., 2001) as well as loss of subcortical cholinergic markers and shrinkage of subcortical neurons (Smith, Roberts, Gage, & Tuszynski, 1999). Importantly, this age-related atrophy is reversible. It can be reversed by delivery of human nerve growth factor (NGF) to cholinergic cell bodies in basal forebrain of rats (Martinez-Serrano et al., 1995) and monkeys (Conner, Darracq, Roberts, & Tuszynski, 2001) and by administration of nicotine to mice (Rogers, Gahring, Collins, & Marks, 1998) and humans (Perry et al., 2000). That neurotrophins can reverse age-related atrophy suggests that genes controlling those substances could affect the course of aging. Finally, human cholinergic receptor subunits are particularly vulnerable to age-related decline (Picciotto & Zoli, 2002; Rogers et al., 1998; Whitehouse, 1997), suggesting that the genes controlling them may play a role in cognitive aging.
Nicotinic Genes and Aspects of Attention: The CHRNA4 Gene
A number of nAChR subunits are controlled by polymorphic genes which could contribute to individual differences in neuromodulation of attentional processes. At present, there is evidence for the modulation of attention by two cholinergic subunit genes, CHRNA4 and CHRNA7.
The alpha-4 nAChR subunit is a component of the most widely distributed nicotinic receptor in cortex, the alpha-4/beta-2 nAChR (Flores et al., 1996). Therefore, the loss of the alpha-4 subunit in normal and pathologic aging is significant. Aged mice have been reported to show almost total loss of alpha-4 nAChR subunits in the hippocampus (Rogers et al., 1998). In the human postmortem frontal cortex, there is an age-related decrease in both alpha-4 and beta-2 subunit expression (Tohgi, Utsugisawa, Yoshimura, Nagane, & Mihara, 1998). In AD, there is a selective loss of alpha-4 nAChRs in the cortex compared to alpha-3 and alpha-7 nAChRs (Martin-Ruiz et al., 1999). Moreover, the alpha-4 subunit was reduced significantly in the hippocampus (35%) and the temporal cortex (47%). In contrast, the alpha-7 subunit was reduced significantly (36%) in the hippocampus but not in the temporal cortex (Guan, Zhang, Ravid, & Nordberg, 2000).
The importance of these subunits to brain function and their vulnerability to age and disease suggests that variations in the genes controlling them could be a source of individual differences in cognition, perhaps most strongly in aging. Polymorphisms in both alpha-4 (Kent, Middle et al., 2001; Steinlein et al., 1999), and beta-2 (De Fusco et al., 2000; Rempel, Heyers, Engels, Sleegers, & Steinlein, 1998) genes are associated with nocturnal frontal lobe epilepsy. Another polymorphism of the human alpha-4 gene causing epilepsy in a Norwegian family increases the affinity of the receptor subunit for acetylcholine (Steinlein et al., 1997a). This increase in receptor sensitivity may underlie the neural hyperactivity of seizures.
Recently, we obtained evidence of a role for the alpha-4 nAChR gene (CHRNA4) in deploying visuospatial attention. As described in the preceding section, we compared effects of genotype of both a dopaminergic gene (DBH) and the CHRNA4 gene on both a memory (Figure 2b) and attention task (Figure 2a) performance. CHRNA4 genotype affected the shifting of visuospatial attention (Figure 3a) but not the accuracy of working memory (Figure 4b). DBH genotype did not affect attention performance (Figure 3b) but did affect accuracy of working memory (Figure 4a; Greenwood et al., 2003).
Modulation of Visuospatial Attention by Cholinergic Genes: A Critical Evaluation
This work shows that polymorphisms in cholinergic receptor subunit genes have functional consequences for behavior. The CHRNA4 polymorphism has been linked to cortical electrophysiology (Steinlein et al., 1997) and visuospatial attention (Greenwood et al., 2003). Moreover, the observed effect of CHRNA4 genotype on visuospatial attention appears to be selective for CHRNA4 genotype but not for DBH genotype. The CHRNA7 gene has also been linked to both electrophysiology and behavior—namely, cortical excitability in the P50 defect (Freedman et al., 1997) and sustained attention (Cullum et al., 1993). The lack of an association of the CHRNA7 gene with ADHD (Kent, Green et al., 2001) and the absence of the P50 defect in ADHD adults (Olincy et al., 2000) suggests that the effects of this polymorphism are not general. However, the number of studies in this area is still small, and further work is needed to determine the specificity of the observed cognitive effects of both of these nicotinic receptor polymorphisms.
NEUROTROPHIC GENES: MODULATION OF WORKING MEMORY
The molecular biology of memory formation involves expression of a number of genes. Explicit memory in mammals depends on long-term potentiation (LTP) in the hippocampus where enzyme and receptor genes are critical to the process (Kandel, 2001). In cortex, mRNA expression of the D1 dopamine receptor (DRD1) specifically appears to be important for LTP at the hippocampal-prefrontal cortex synapses (Gurden, Takita, & Jay, 2000). In the prefrontal cortex, working memory requires stimulation of the D1 dopamine receptors (DRD1) in the dorsolateral prefrontal cortex (DLPFC) in both monkeys (Sawaguchi & Goldman-Rakic, 1991) and humans (Abi-Dargham et al., 2002). This suggests that genes controlling both LTP and dopamine receptors could contribute to individual differences in memory formation ability.
Memory ability appears to be quite heritable. Estimates from twin studies range from .38 (Pedersen, Plomin, Nesselroade, & McClearn, 1992) to .43 (McGue & Bouchard, 1989). Estimates of working memory range from .43 to .49 (Ando et al., 2001; Johansson et al., 1999). Estimates of heritability of long-term memory range from .28 to .49 in a study of elderly twins (Johansson et al., 1999) and from .39 to .50 in a study with three adult age groups (Finkel, Pedersen, McGue, & McClearn, 1995). Working memory—the ability to maintain and manipulate information in a memory store (Baddeley & Hitch, 1974)—appears to depend on the integrity of two brain regions, the medial temporal lobe and DLPFC. Although polymorphisms in the DRD1 gene have been identified (Cichon, Nothen, Erdmann, & Propping, 1994; Cichon et al., 1996) and linked to psychiatric disorders (Ni et al., 2002; Sato, Soma, Nakayama, & Kanmatsuse, 2000) and novelty seeking (Limosin et al., 2003), they have not been linked to memory performance.
The BDNF Gene and Memory Formation
A gene controlling a neurotrophin appears to play a role in the other brain region critical for memory—the hippocampus. Neurotrophins are a family of polypeptides long known to be essential for the differentiation and survival of neurons during development (Levi-Montalcini, 1998). As reviewed above, age-related atrophy of cholinergic neurons can be reversed by neurotrophic factors. Recently, neurotrophins have been found to participate in synaptic modulation. Two reviews marshal evidence showing that neurotrophin activity can induce rapid changes in synaptic plasticity (Lu & Gottschalk, 2000; Poo, 2001). One neurotrophin, brain-derived neurotrophic factor (BDNF), appears to be important in plastic processes underlying memory formation.
Recognition memory formed in ewes for their lambs within 2 hours of birth is associated with increased BDNF mRNA expression in brain areas involved in the consolidation of olfactory memory—pyriform and entorhinal cortices (Broad, Mimmack, Keverne, & Kendrick, 2002). In hippocampal CA1 neuron cultures, BDNF induced a long-term enhancement of excitatory glutaminergic transmission (Schinder, Berninger, & Poo, 2000; Sherwood & Lo, 1999).
BDNF appears to regulate hippocampal LTP believed to underlie explicit memory in mammals (Chen, Kolbeck, Barde, Bonhoeffer, & Kossel, 1999; Kovalchuk, Hanse, Kafitz, & Konnerth, 2002; Patterson et al., 1996). A brief pulse of stimulation to any of the major synaptic pathways increases amplitude of excitatory postsynaptic potentials (EPSPs) in the target hippocampal neurons. Application of BDNF facilitates LTP (Figurov, Pozzo-Miller, Olafsson, Wang, & Lu, 1996), and its removal depresses LTP (Korte et al., 1995). Restoration of BDNF in BDNF knock-out mice reversed deficits in LTP (Patterson et al., 1996). LTP in rat dentate gyrus resulted in elevation of BDNF gene expression (Bramham, Southard, Sarvey, Herkenham, & Brady, 1996). LTP has a transient early phase and a longer late phase, and BDNF is important in both phases (Lu & Gottschalk, 2000; Poo, 2001). The mechanism underlying the synaptic effects of BDNF involves modulation of both synaptic density and the distribution of synaptic vesicles within presynaptic terminals of CA1 pyramidal neurons (Tyler & Pozzo-Miller, 2001). BDNF also enhances synaptic transmission by facilitating synaptic vesicle “docking” (Gottschalk, Pozzo-Miller, Figurov, & Lu, 1998; Xu, Gottschalk, et al., 2000).
With regard to learning, there is evidence for a direct role for BDNF gene expression in spatial memory in the hippocampus. Training in a radial arm maze, which requires formation of memories for spatial location, produced an increase in BDNF mRNA expression in the hippocampus but not in the frontal cortex. When the spatial learning was chemically inhibited, the increase in BDNF mRNA did not occur (Hall, Thomas, & Everitt, 2000). Finally, suppression of BDNF synthesis by antisense BDNF impaired spatial learning (Mizuno, Yamada, Olariu, Nawa, & Nabeshima, 2000).
BDNF also appears to play a role in human memory formation. Weinberger and colleagues hypothesized that normal variation in the BDNF gene might modulate memory performance. They assessed the effect of a previously described polymorphism arising from a guanine to adenine mutation producing a valine to methionine substitution at codon 66 (val66met) (Neves-Pereira et al., 2002) on the memory of normal individuals, schizophrenics, and their unaffected siblings (Egan et al., 2003). No relationship between BDNF genotype and schizophrenia incidence was seen; this was consistent with previous findings (Krebs et al., 2000; Wassink, Nelson, Crowe, & Andreasen, 1999). However, this group found that BDNF genotype did significantly affect performance on the Wechsler Memory Scale-R (WMS-R) Logical Memory Task, a task that requires immediate and delayed recall of a narrative. It was found that met/met homozygotes showed lower scores than val/val homozygotes or val/met heterozygotes. In contrast, BDNF genotype did not affect semantic memory—even with a delay—or performance on the Wisconsin Card Sort Task, a test of combined working memory and executive function.
In addition to behavioral measures, there is also physiological evidence of BDNF genetic modulation. Individuals with the low-activity met allele of the val66met polymorphism showed poorer episodic memory and lower levels of a marker of neuronal integrity in the hippocampus in vivo (Egan et al., 2003). Hippocampal blood flow was also measured during performance of a working-memory (n-back) task. The val/met group exhibited an “abnormal” pattern of increased activation of the hippo-campus, whereas the val/val group showed evidence of “hippocampal deactivation” (Egan et al., 2003). (There were insufficient numbers of met/met homozygotes to run in the imaging phase of the study.) The authors interpreted their results as showing that reduced hippocampal activation during a working-memory task represents “normal” hippocampal disengagement (Meyer-Lindenberg et al., 2001). Thus, they interpret increased hippocampal activation as abnormal and decreased hippocampal activation as normal.
Recent imaging work directly comparing episodic and working memory has shown activation of hippocampal and parahippocampal regions during both types of memory task (Cabeza, Dolcos, Graham, & Nyberg, 2002). Electrical activity has been recorded from depth electrodes in the perirhinal cortex and the hippocampus proper during memory formation (Fernandez et al., 1999). Imaging studies have also found activation in the parahippocampal gyrus to be greater during the presentation of items recalled compared to items not recalled on a subsequent memory task (Brewer, Zhao, Desmond, Glover, & Gabrieli, 1998; Wagner et al., 1998). Similarly, greater activation was seen in the left anterior hippocampus when objects had to be remembered along with their locations compared to when only the objects or only the locations had to be remembered (Mitchell, Johnson, Raye, & D'Esposito, 2000). Navigation through a virtual reality town was found to activate right hippocampus and right inferior parietal regions (Maguire et al., 1998). Thus, this literature is somewhat at odds with the finding of Egan et al. that increased hippocampal activation during a working-memory task suggests pathology. Nevertheless, the evidence of Egan et al. is important in showing that normal variation in BDNF genotype modulates memory performance.
Modulation of Working Memory by Neurotrophic Genes: A Critical Evaluation
The BDNF gene appears to exert its effects at several levels of hippocampal function, including neuronal, physiological, and behavioral. This elegant work from one laboratory (Egan et al., 2003; Hariri, Egan, Mattay, Kolachana, & Weinberger, 2002) provides much of the evidence for cognitive effects of variation in the BDNF gene. The cognitive effects will need to be replicated and also examined with a range of memory and nonmemory tasks to determine their specificity.
GENES MODULATING NEURON HEALTH AND PLASTICITY: EFFECTS ON COGNITION AND AGING
The genes discussed thus far are involved in controlling relatively discrete and specific aspects of neurotransmission. Those genes with protein products that are receptor subunits—such as alpha-4 and alpha-7 nAChR subunits—exert effects on the particular cholinergic receptor of which their subunit is a part. Effects of variation in subunit genes can be predicted to be greatest on networks mediated by the particular neurotransmitter. More general effects may be exerted by genes controlling the enzymes that degrade neurotransmitters following release, such as DBH and COMT. A third category of genetic effects on cognition involves genes that exert their effects on neuron health and plasticity; these are more likely to have a broad influence.
The best-known gene in this category is apolipoprotein E (apoE), involved in cholesterol transport and strongly linked to AD risk. The dominant amyloid prediction of AD (Hardy & Higgins, 1992) leads to the hypothesis that genes with a role in the degradation of beta-amyloid (Abeta), the substance thought to be the neurotoxic agent of AD, would be candidate genes for cognitive decline in old age. In addition, genes with a role in neuronal repair would likely be increasingly expressed in aging or subsequent to brain injury or pathology. One of these, the insulin-degrading enzyme (IDE) gene on chromosome 10 was prominently reported to be linked to AD (Bertram et al., 2000; Ertekin-Taner et al., 2000; Myers et al., 2000). Additional work suggests an AD-susceptibility gene on chromosome 10 with a weak effect on risk of AD (Blacker et al., 2003), but other laboratories have failed to replicate that association (Abraham et al., 2001; Boussaha et al., 2002). A recent haplotype analysis suggests a role for IDE variation only in the absence of the apoE-ε4 allele (Edland et al., 2003).
The ApoE Gene and Mechanisms of Neuronal Repair
The polymorphic apoE gene on chromosome 19 occurs as one of three alleles (ε2, ε3, and ε4), with mean frequencies in the general population of about 8%, 78%, and 14%, respectively (Utermann, Langenbeck, Beisiegel, & Weber, 1980). The ε4 allele modifies risk of AD in a “gene dose” manner, increasing with the number of ε4 alleles inherited from both parents, from 0 (noncarriers) to 1 (heterozygotes) to 2 (homozygotes) (Corder et al., 1993). Whereas 34% to 65% of individuals with AD carry the apoE-ε4 allele, it is present in only approximately 24% to 31% of the nonaffected adult population (Saunders et al., 1993). Investigators around the world have confirmed that carriers of the ε4 allele are at increased risk of developing AD (Farrer et al., 1997; Havlik et al., 2000; Henderson et al., 1995; Katzman et al., 1997; Perry, Collins, Harrell, Acton, & Go, 2001). In contrast, it has been claimed that carriers of the ε2 allele are at reduced risk of AD (Farrer et al., 1997; Talbot et al., 1994), although there is disagreement on this point (Sorbi et al., 1994; van Duijn et al., 1995).
The product of the apoE gene is a plasma protein involved in the transport of cholesterol and other hydro-phobic molecules. ApoE is the principal apolipoprotein in the brain and CSF, and its role as a lipid carrier may allow it to remove lipids from degenerating cells and supply lipids for growing neuronal processes (Rebeck et al., 1998). It has been postulated that glia recycle cholesterol from degenerating terminals, combine it with apoE, and supply it to neurons actively engaged in remodeling synapses and extending dendrites (Poirier, 1994). In support of this view, a complex of apoE and cholesterol has recently been identified as a strong promoter of synapse development (Mauch et al., 2001; Ullian, Sapperstein, Christopherson, & Barres, 2001).
There is other evidence of a role for apoE in neuronal repair and plasticity. ApoE genotype affects neurodegenerative diseases other than AD (Chapman, Korczyn, Karussis, & Michaelson, 2001). Possession of the ε4 compared to the ε3 allele is associated with a poorer prognosis in individuals with amyotrophic lateral sclerosis (Drory, Birnbaum, Korczyn, & Chapman, 2001) and with a faster rate of progression in multiple sclerosis (Fazekas et al., 2001). ApoE-ε4 carriers with traumatic brain injury show poorer recovery (Lichtman, Seliger, Tycko, & Marder, 2000) and worse memory performance compared to noncarriers, although they do not differ from each other on injury variables or measures of executive functioning (Crawford et al., 2002). Similarly, individuals with traumatic head injury show increased risk of AD if they are apoE-ε4 carriers (Mayeux et al., 1995). Thus, apoE genotype appears to modulate the brain response to insult generally, consistent with its role in neuronal repair.
The role of apoE in brain repair mechanisms has been studied at the neuronal level. Following lesions to the entorhinal cortex in mice, the apoE protein was observed to be upregulated in tandem with clearance of cholesterol and lipid debris from the site of the injury. This may reflect the redistribution of lipids in the service of neurite extension (White, Nicoll, & Horsburgh, 2001). Consistent with this finding, entorhinal cortex lesions were followed by persisting neurodegeneration products in the deafferented hippocampus in apoE knock-out mice but not in wild-type mice (Fagan et al., 1998).
Moreover, the apoE protein produced by the ε4 allele is less effective at moderating lesion damage than that produced by the ε3 allele. Transgenic mice with insertions of human apoE-ε3 or -ε4 alleles underwent lesions of the entorhinal cortex. Post-lesion neurodegeneration in the dentate gyrus was more pronounced in ε4 compared to ε3 mice. By 90 days post-lesion, compensatory sprouting in the dentate gyrus had increased 45% in the ε3 mice but only 20% in the ε4 mice (White, Nicoll, Roses, & Horsburgh, 2001). Similarly, in mouse hippocampal slice culture, greater apoE-ε3 gene expression was associated with increased sprouting of granule cell mossy fibers following injury, whereas greater ε4 expression was associated with decreased sprouting. This was interpreted to indicate actively suppressed regeneration by the lipoprotein produced by the apoE-ε4 gene (Teter et al., 2002). In human AD patients, apoE was found to be increased in the hippocampus in ε3 but not in ε4 carriers, also interpreted as reflecting suppressed neuronal repair mechanisms in ε4 carriers (Glockner, Meske, & Ohm, 2002). This literature documents a role for apoE in repair and recovery mechanisms that are reduced in effectiveness when the ε4 and not the ε3 allele directs apoE production.
Is there a cognitive phenotype of apoE ε4? Deficient processes of neuronal repair in ε4 carriers could lead to weaker recovery in the face of brain insult and, thus, eventually to impaired cognition. Consistent with that view, some studies have found that apoE-ε4 genotype is associated with cognitive decline in midlife or earlier, potentially decades before average age of onset of AD in those destined to develop the disease. Thus, the apoE gene may be a genetic source of individual differences in cognition in otherwise normal individuals experiencing the perhaps subtle brain insults of normal aging (Greenwood, Sunderland, Friz, & Parasuraman, 2000; Parasuraman, Greenwood, & Sunderland, 2002). If so, then there might be a cognitive phenotype characteristic of apoE-ε4 carriers that exists independently of AD (Smith et al., 1998; Yaffe, Cauley, Sands, & Browner, 1997) and that is the result of inefficient processes of neuronal repair.
Because apoE genotype is a risk factor for AD, any claim regarding the existence of an apoE-ε4 cognitive phenotype must distinguish cognitive deficits associated with a phenotype from those associated with a prodromal stage of AD. There is strong evidence that the ε4 allele is associated with poorer cognitive performance in nondemented individuals older than age 65 (Feskens et al., 1994; Reed, Carmelli, & Swan, 1994), even when such individuals are selected as “high functioning” (Bretsky, Guralnik, Launer, Albert, & Seeman, 2003). However, the most common type of AD—sporadic and nonfamilial—is primarily a disease of old age, and its prevalence accordingly increases with age (Evans et al., 1989). As a result, any large group of adults older than age 65 is likely to include some individuals who are in an early stage of AD (Sliwinski, Lipton, Buschke, & Stewart, 1996). Therefore, the presence of cognitive decline in a group of elderly ε4 carriers may simply reflect an increased proportion of persons with undiagnosed, “preclinical” AD in the group.
The existence of a long, prodromal stage of AD is one way to explain evidence that otherwise healthy elderly individuals possessing an ε4 allele are cognitively impaired relative to noncarriers (Daffner & Scinto, 2000). It has been estimated that cognitive dysfunction precedes diagnosis of AD by 5 to 6 years in those who later develop the disease (Fox, Warrington, Seiffer, Agnew, & Rossor, 1998; Linn et al., 1995). Consistent with this, a sizeable proportion of ε4 carriers with impaired cognition had converted to AD at follow-up (Bondi, Galaski, Salmon, & Thomas, 1999; Bondi et al., 1995).
However, not all ε4 carriers develop AD. Only about 50% of ε4 homozygotes—those at greatest statistical risk of the disease—have developed the disease by the 8th (Myers et al., 1996) or 9th (Henderson et al., 1995) decades. Therefore, weak neuronal repair processes apparently do not inexorably lead to AD, at least by age 90. Indeed, it has been argued that apoE influences when, rather than whether, an individual develops the disease (Meyer et al., 1998). AD may be hastened by weak repair mechanisms, but such fragility could also affect cognition in the absence of AD. If there were a cognitive phenotype of apoE ε4, at what age would deficient repair mechanisms in ε4 carriers be manifested in cognitive impairment?
Only a few studies have examined cognitive integrity as a function of apoE genotype prior to old age. ApoE genotype did not predict intelligence in a study of healthy children between the ages of 6 and 15 years old (Turic, Fisher, Plomin, & Owen, 2001). However, the apoE-ε4 allele was associated with greater decline in IQ from age 11 to age 80 (Deary et al., 2002). A group of individuals of mean age 46 (age range from 24 to 60) carrying at least one ε4 allele showed cognitive deficits relative to noncarriers on tasks of verbal learning, visual memory, and attention span (Flory, Manuck, Ferrell, Ryan, & Muldoon, 2000). But these data are certainly not extensive, and more studies need to be done in younger samples. Nevertheless, they do point to the possibility that cognitive effects, although not present in children, could possibly emerge in early adulthood once development is complete (Paus et al., 1999).
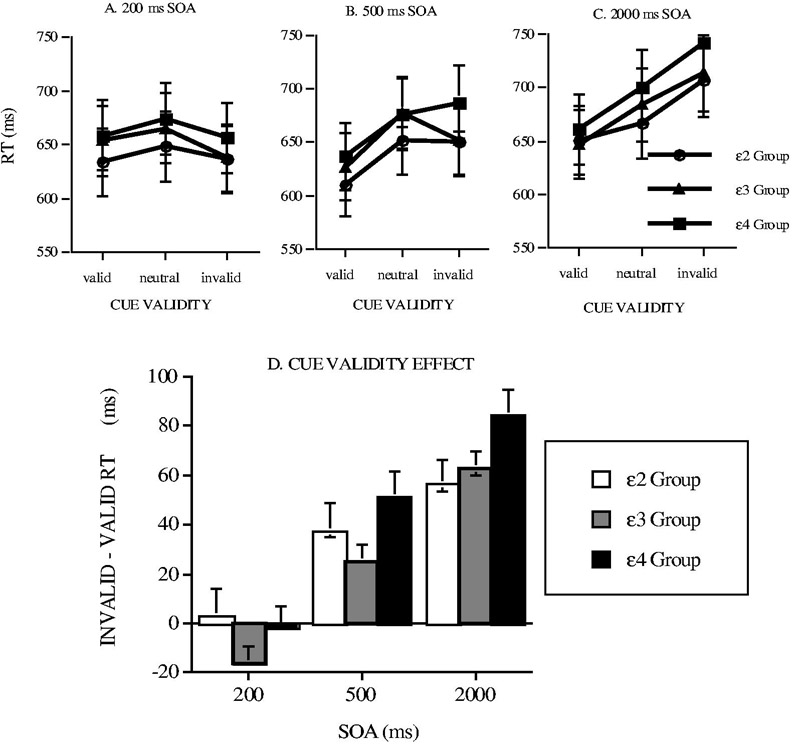
We have examined the relationship of apoE-ε4 geno-type to cognitive functioning prior to old age (Greenwood et al., 2000). We tested a group of medically screened nondemented, middle-aged, ε4 heterozygotes (mean age 58) who were unimpaired on standard neuropsychological tests. We found that these healthy individuals nevertheless showed deficiencies in components of tasks of spatial attention and visual search. On a cued discrimination task (adapted from Posner's cued detection paradigm), we found that carriers of the apoE-ε4 allele were particularly slow to respond when the location precue was invalid (Figure 5), an effect termed “dis-engagement” by Posner and colleagues (Posner, Walker, Friderich, & Rafal, 1984). On a cued visual search task in which search was required for a target letter whose location was precued with varying precision, we found that carriers of the apoE-ε4 allele showed reduced effects of precue precision compared to noncarriers (Figure 6).
Figure 5.
(a) Response Times (RT) in a Cued Letter Discrimination Task Plotted for the ε2, ε3, and ε4 ApoE Genotype Groups as a Function of Cue Validity and SOA, and (b) the Total Cue Validity Effect (Invalid – Valid RT) for Each ApoE Group and SOA. SOURCE: (d) From Greenwood, Sunderland, Friz, and Parasuraman, 2000. NOTE: ApoE = apolipoprotein E; SOA = stimulus onset asynchrony. (a) 200 ms, (b) 500 ms, and (c) 2000 ms.
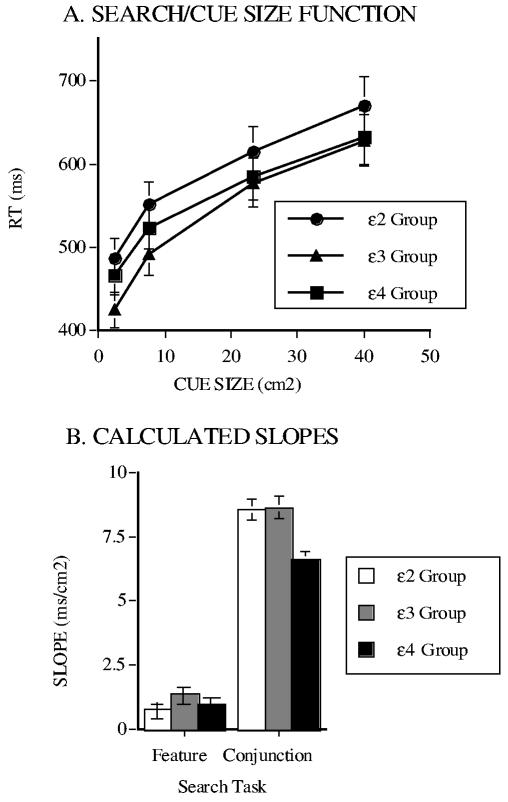
Figure 6.
(a) Visual Search Response Times (RT) Plotted as a Function of Cue Size for the ε2, ε3, and ε4 Genotype Groups, and (b) the Calculated Slope Measures of the Effects of Precue Precision for the Three Groups for the Feature and Conjunction Search Tasks. SOURCE = From Greenwood, Sunderland, Friz, and Parasuraman, 2000.
The results of the Greenwood et al. (2000) study are also of interest because the pattern of attentional impairment in the nondemented sample of apoE-ε4 carriers was qualitatively (but not quantitatively) the same as those observed in older, clinically diagnosed AD patients (Greenwood, Parasuraman, & Alexander, 1997; Parasuraman, Greenwood, Haxby, & Grady, 1992).
In a follow-up study, we confirmed and extended these results. We identified three cognitive processes—visuospatial attention, spatial working memory, and the effect of visuospatial attention on working memory—and devised tasks as behavioral assays of the integrity of components of these cognitive processes. The tasks were cued discrimination (Figure 2a), working memory (Figure 2b), and cued working memory (spatial location precued by means of cues varying in size and, hence, precision). We observed that ability to redirect visuospatial attention, retain memory for location, and benefit from precue precision in retention of memory for location were selectively affected by gene dose (0, 1, or 2) of the ε4 allele. Moreover, ε4 gene dose affected these cognitive processes differently. Attention was affected in a graded manner such that each additional ε4 allele incrementally slowed disengagement of visuospatial attention from invalidly cued space and reduced its effects on memory (Figure 7a). In contrast, apoE geno-type affected working memory performance only in homozygotes (two ε4 alleles; Figure 7b). In general, apoE genotype selectively affected component processes of cognition in healthy, middle-aged adults, only some of whom will go on to develop AD later in life (Henderson et al., 1995). This is consistent with the idea that a cognitive phenotype of the apoE-ε4 allele emerges in adulthood (Greenwood, Sunderland, Friz, Lambert, & Parasuraman, 2002).
Figure 7.
Results of the Follow-Up to the Study Shown in Figure 5. NOTE: (a) Response time (RT) from cued discrimination task plotted for each apoE group. (b) Accuracy from spatial working-memory task plotted for each apoE group (see text for explanation). Group sample sizes: 0 ε4: n = 113; 1 ε4: n = 52; 2 ε4: n = 12.
However, not all studies report cognitive deficits in middle-aged ε4 carriers. There are two possible reasons for this discrepancy. Studies which have observed ε4-related cognitive deficits in middle-age have either used assessment that is more comprehensive (Caselli et al., 2001; Flory et al., 2000) or aimed at more specific component processes of cognition (Greenwood et al., 2002; Greenwood et al., 2000) compared to studies not observing such effects (Plassman et al., 1997; Reiman, Armstrong, Matt, & Mattox, 1996). Moreover, even when middle-aged ε4 carriers do not show frank cognitive deficits, compared to noncarriers, they do show evidence of brain change—specifically, lower association cortex metabolism (Reiman, Caselli et al., 1996), smaller hippocampal volume (Plassman et al., 1997), and reduced inferotemporal blood flow related to task performance (Smith, Andersen, et al., 1999). Individuals diagnosed as having “mild cognitive impairment,” a possible prodromal stage of AD, show reductions in cholinergic basal forebrain receptors compared to age-matched controls. Moreover, in postmortem tissue, the number of receptors on cholinergic nucleus basalis neurons was correlated with performance in life on tests of working memory and attention (Mufson et al., 2002). This evidence does indicate some decline in brain integrity in ε carriers as early as middle age.
Modulation of cognition by apoE: A critical evaluation. Evidence that there are consequences for brain structure and cognition from apoE genotype is fairly convincing. However, the question remains, as posed above, to what extent these consequences are the result of a long prodromal phase of AD as opposed to a cognitive pheno-type. In fact, this point may be moot. As discussed above, to the extent that apoE is important in neuronal repair, differences in efficiency of repair related to apoE genotype could affect neuronal integrity at some point in adulthood once environmental insult and aging begin to exert their effects. If so, then a cognitive pheno-type associated with the apoE-ε4 allele would actually be a consequence of inefficient neuronal repair, whatever the cause of insult.
Estrogen Receptor Genes
Physiological and cognitive effects of estrogen. Estrogen appears to have broad excitatory and neuroprotective effects on neurons in the brain. In the basal forebrain cholinergic system, estrogen replacement in ovariectomized female rats leads rapidly (within 24 hours) to increased production of choline acetyltransferase (ChAT; Luine, 1985; Luine, Park, Joh, Reis, & McEwen, 1980), an enzyme important in the neurochemical pathway of acetylcholine. Over longer periods of administration (up to 28 weeks), a decline in choline uptake subsequent to ovariectomy was partially prevented by estrogen replacement. Estrogen has also been shown to enhance the strength of hippocampal LTP (Cordoba Montoya & Carrer, 1997), and, consequently, to enhance learning dependent on hippocampal integrity (Luine, Richards, Wu, & Beck, 1998).
Estrogen stimulates neuronal growth, exerting strong effects on synapse formation on dendritic spines in the hippocampus (Murphy, Cole, & Segal, 1998; Woolley & McEwen, 1992). Moreover, estrogen has recently been found to promote neurogenesis in the adult rat hippo-campus, both experimentally and during natural estrus (Tanapat, Hastings, Reeves, & Gould, 1999).
Estrogen also has a neuroprotective role in the brain. Pretreatment with estrogen protects against neuronal death following such brain insults as stroke and head injury (reviewed in Wise, Dubal, Wilson, Rau, & Bottner, 2001). Moreover, following neuronal insult, there is upregulation of both estrogen synthesis (Garcia-Segura, Azcoitia, & DonCarlos, 2001) and estrogen receptor expression at the site of injury (Dubal, Shughrue, Wilson, Merchenthaler, & Wise, 1999). Estrogen promotes hippocampal neuron survival (Diaz Brinton et al., 2000) and protects against ovariectomy-induced loss of BDNF expression in the hippocampus (Singh, Meyer, & Simpkins, 1995). In humans, such estrogenic neuroprotection is seen in better recovery by woman compared to men following brain injury; this is attributed to naturally produced estrogens (Groswasser, Cohen, & Keren, 1998).
The neuroprotective properties of estrogen may also underlie its role as one of the few substances that modify risk of AD. This has been studied in women undergoing what is termed hormone replacement therapy (HRT) in which estrogen, alone or combined with a progestin, is prescribed for the symptoms of menopause. There is evidence that HRT in postmenopausal women delays or prevents the onset of AD. Some of this evidence comes from observational studies that consistently report that among nondemented elderly women, HRT users show better cognition (Resnick, Metter, & Zonderman, 1997; Yaffe, Haan, Byers, Tangen, & Kuller, 2000) and lower risk of AD (Kawas, Resnick, & Morrison, 1997). Cross-sectional studies in healthy, nondemented postmenopausal women have found that verbal memory in particular is relatively preserved in HRT users compared to nonusers (Kampen & Sherwin, 1994; Sherwin, 1997). This finding has been confirmed by longitudinal studies (Maki & Resnick, 2000; Resnick et al., 1997). However, all these studies are limited by self-selection of the population to HRT use, by the different estrogen/progestin formulations used, and by the common use of nonspecific cognitive assessment.
Better evidence comes from randomized controlled trials in which women are randomly assigned to either HRT or placebo. A recent review of such studies has tentatively concluded that verbal memory is selectively preserved in postmenopausal women on estrogen replacement (Sherwin, 2002). A more rigorous meta-analysis of randomized controlled trials—in all of which the estrogen was unopposed by a progestin—concluded that mainly verbal memory improved only in those HRT users who were experiencing symptoms of menopause. A separate meta-analysis of the observational studies showed lower relative risk of AD in HRT users (LeBlanc, Janowsky, Chan, & Nelson, 2001). A study in which young women served as their own controls found deficits in verbal but not visual memory during ovarian suppression for uterine myomas. This verbal memory deficit was reversed in those randomly assigned to “add-back” estrogen after 3 months but not in those assigned to placebo (Sherwin & Tulandi, 1996). Similar results were observed in a study of young women undergoing surgical menopause (Phillips & Sherwin, 1992; Sherwin & Phillips, 1990).
As mentioned above, one problem with the literature assessing effects of estrogen on cognition using HRT arises from variation from study to study in the specific formulations of estrogen and progesterone used. Two new reports from the Women's Health Initiative Memory Study (WHIMS) examined cognitive change and dementia incidence in older women after 4 years of randomly assigned administration of a specific HRT formulation. In contrast to the animal literature documenting a neurotrophic effect of estrogen and the clinical literature showing a benefit of estrogen on cognition, this study found a cost associated with a combination of a conjugated equine estrogen and medroxyprogesterone acetate. This cost was seen both in lower Modified Mini-Mental State Exam performance (Rapp et al., 2003) and greater incidence of dementia, particularly vascular dementia (Shumaker et al., 2003), compared to women not assigned to HRT.
Although a sizeable literature documents the benefits of estrogen on the brain, there has been little work done on the cognitive effects of various clinically used progestins administered in combination with estrogen to inhibit uterine proliferation. Brinton and colleagues reported that whereas the progestin used in the WHIMS study (medroxyprogesterone acetate) completely counteracted the physiologic effects of estrogen on CA+ flow in hippocampal cell culture, a natural progesterone did not (Nilsen & Brinton, 2003). In rats, estrogen alone increased basal forebrain cholinergic function whereas estrogen administered with progesterone did not (Gibbs, 2000). Similarly, monkeys treated with conjugated equine estrogen combined with medroxyprogesterone acetate showed reduction in cholinergic enzymes (choline acetyltransferase and acetylcholinesterase). Treatment with estrogen alone did not have this effect (Gibbs, Nelson, Anthony, & Clarkson, 2002). In humans, an observational study found that users of unopposed estrogen experienced greater improvement in global cognition, abstract reasoning, and category fluency than never-users. In contrast, users of estrogen plus medroxyprogesterone acetate showed decline in global cognition and mental tracking (Rice et al., 2000).
Medroxyprogesterone acetate has also been shown to antagonize estrogen's inhibitory effects on coronary artery atherosclerosis (Adams, Register, Golden, Wagner, & Williams, 1997; Register, Adams, Golden, & Clarkson, 1998). An accompanying editorial to the two WHIMS studies raised the possibility that medroxyprogesterone acetate could be a factor in the observation that vascular dementia largely explains the increased incidence of dementia in the HRT users (Yaffe, 2003). To fully understand the effect of HRT on cognitive aging, future work will need to assess effects of estrogen and progesterone independently. The large-scale WHIMS estrogen-alone trial that is currently ongoing (Shumaker et al., 1998) will provide important evidence on the effect of unopposed estrogen on cognitive aging.
Neuroprotection by estrogen may be partly mediated by changes in cerebral blood flow, which has been shown to increase over time in postmenopausal women on HRT (10 of whom were on estrogen, 6 on estrogen plus progesterone; (Maki & Resnick, 2000). In a rigorous randomized, double-blind, placebo-controlled, crossover trial in postmenopausal women, estrogen alone was shown to heighten blood flow changes during working-memory tasks (Shaywitz et al., 1999).
It has been hypothesized that an estrogen-signalling pathway may contribute to risk of AD. In cell culture, physiologic levels of estrogen reduce production of the putative AD neurotoxin A beta in cerebrocortical neurons (Xu et al., 1998). Moreover, the estrogen-induced increase in synaptic sprouting following lesions to dentate gyrus appears to be dependent on normal apoE gene expression (Stone, Rozovsky, Morgan, Anderson, & Finch, 1998). Estrogen attenuates the excitotoxicity and oxidative injury induced by Abeta on hippocampal neurons (Goodman, Bruce, Cheng, & Mattson, 1996). Women carrying one apoE-ε4 allele are at greater risk of developing AD compared with men with the same genotype (Breitner et al., 1999; Hendrie et al., 2001).
This literature documents both the cognitive effects of estrogen and the physiological bases of those effects. Although the behavioral data suggest that verbal memory shows the greatest benefit of estrogen, that finding could be the result of differences in task sensitivity. Although the physiologic evidence suggests hippocampal-dependent functions might show particular effects of estrogen (Murphy, Cole, Greenberger, & Segal, 1998; Murphy, Cole, & Segal, 1998; Tanapat et al., 1999), the blood flow data (Shaywitz et al., 1999) and the brain injury data (Dubal et al., 1999; Goodman et al., 1996; Groswasser et al., 1998) would predict more global effects. The neurophysiologic effects of estrogen in the brain make genes controlling its receptors attractive candidates for individual differences in cognition.
Estrogen receptor genes and cognition. Two types of estrogen receptors are known to reside in cell nuclei—ER-alpha and ER-beta—with overlapping distributions in the brain. The ER-alpha receptor is expressed in the hypothalamus and the amygdala. The ER-beta receptor is expressed mostly in the hippocampal formation, the entorhinal cortex, and the thalamus (Osterlund & Hurd, 2001), all regions important in memory formation. However, the neuroprotective properties of estrogen have been linked to ER-alpha (Wise et al., 2001). Although ER-alpha mRNA is not normally expressed in the cerebral cortex of adult rats (Shughrue, Scrimo, Lane, Askew, & Merchenthaler, 1997), Wise and colleagues have demonstrated that ER-alpha, but not ER-beta, is selectively and markedly upregulated following cerebral ischemia (Dubal et al., 1999).
Based on this strong evidence for estrogenic neuroprotection (reviewed in McEwen, 2001), particularly with regard to Abeta (Goodman et al., 1996; Xu et al., 1998), the ER receptor genes have been investigated as candidate genes for AD. Two restriction fragment length polymorphisms of the ER-alpha gene have been identified. The alleles of these genes are termed Pp (PvuII gene) and Xx (Xba1 gene) (Albagha, McGuigan, Reid, & Ralston, 2001; Maruyama et al., 2000). The PvuII restriction site results from a T-to-C substitution (T25C) and the XbaI site from an A-to-G substitution (A20G). The x allele is more active than the X allele (Maruyama et al., 2000). Using a case-control design, these polymorphisms were found to affect the risk of AD in both a Japanese (Isoe, Ji, Urakami, Adachi, 1997) and a Caucasian population (Brandi et al., 1999). This finding could not be replicated in another investigation using both types of populations (Maruyama et al., 2000). However, when the two ER-alpha polymorphisms were examined together with the apoE polymorphism, increased risk of AD was seen in individuals with at least one ε4 allele and the xpxp ER-alpha genotype (Brandi et al., 1999; Mattila, Rinne, Roytta, Laippala, & Lehtimaki, 2002). Lambert et al. (2001) found no effect of either ER-alpha or ER-beta polymorphisms alone, but they did find an effect of their interaction. Taking a different approach, Yaffe, Lui, Grady, Stone, and Morin (2002) investigated the effect of the ER-alpha polymorphism on cognition in nondemented older women. The Modified Mini-Mental State Exam (Teng & Chui, 1987) was administered initially and 6 to 8 years later. Elderly women who developed cognitive impairment over the 6 to 8 years were more likely to have a p allele or an x allele (Yaffe et al., 2002). This small literature suggests that ER receptor polymorphisms might have a modulating role in cognitive decline in healthy aging.
Modulation of cognition by estrogen receptor genes: A critical evaluation. There is convincing evidence of a modulating effect of estrogen on cognition and on neuronal protection, with the former perhaps mediated by the latter. The work on two known polymorphisms in the ER-alpha gene is preliminary, but the possible interaction with apoE suggests a role in cognitive aging.
DISCUSSION AND CONCLUSIONS
In this article, we have reviewed evidence that normal variation in single genes can modulate specific cognitive functions. It has been known for some time that SNPs can affect cognition but mainly in the context of devastating brain disease such as Huntington's and Alzheimer's diseases. The question we addressed here was whether, in the absence of frank disorder, some polymorphisms modulate cognitive functions within normal limits. The evidence clearly points to a positive answer to this question. Hence, one main conclusion is that allelic variation in specific single genes can be reliably linked to individual differences in particular components of cognition in healthy adults. However, much remains to be learned about the specificity, generality, and replicability of the effects reported to date.
It must be acknowledged that the question assumes a strict division between health and disease, whereas there may be a continuum between normal variation and disorder. The 7-repeat allele of the DRD4 polymorphism illustrates this point. This polymorphism is associated with a disorder (ADHD) but also with a normal personality trait (novelty seeking) (Benjamin et al., 1996) as well as the efficiency of executive attention in healthy adults (Fossella et al., 2002). Moreover, as discussed above, there is recent evidence that the 7-repeat allele confers an evolutionary advantage that may be related to the variation in its world-wide distribution (rare in Asia, common in South America; Ding et al., 2002). It has been speculated that this distribution is attributable to increased likelihood of migration in 7-repeat carriers (Chen, Burton, et al., 1999), which may be consistent with a trait of novelty seeking. Thus, for this gene, the difference between normal variation and cognitive disorder may be largely a matter of degree.
Although the field (one might call it cognitive neurogenetics) is in an early stage, there appear to be at least several genes that contribute to individual differences in specific cognitive functions. In this review, we considered gene products that vary in hypothesized effect specificity, from neurotransmitter receptors (e.g., DRD4) to agents with a role in neuroplasticity (e.g., apoE). This approach makes the assumption that a gene controlling one receptor will have effects limited to that receptor, whereas a gene controlling mechanisms of neuronal repair will have more widespread effects. If so, behavioral effects would follow a similar pattern of specificity and generality, respectively (see Figure 1). It might be argued that this approach can be overstated. A polymorphic gene affecting one receptor type could exert effects in brain regions with a distribution of that receptor type but could also exert effects downstream of that receptor. For example, nicotine influences dopamine release (reviewed in Levin & Simon, 1998), and BDNF mediates effects of estrogen on neuronal sprouting (Murphy, Cole, & Segal, 1998). However, given the newness of the field of the molecular genetics of cognition, the framework presented in Figure 1 can be used as a working hypothesis to drive future studies.
There is some evidence that genes controlling receptor functions can exert very specific effects. The CHRNA7 gene controls part of a cholinergic receptor, and a polymorphism in that gene has been associated in a number of studies with altered cortical electrophysiology in schizophrenics and their relatives (Freedman et al., 1997). However, the relation between the P50 defect and symptoms of schizophrenia remain obscure, and there has been only limited work on the cognitive consequences of that polymorphism (Cullum et al., 1993).
Similarly, the gene controlling the neurotrophin BDNF could be predicted to exert effects specific to hippocampal-dependent memory formation based on the gene's role in hippocampal LTP (Xu, Gottschalk, et al., 2000), neurotransmitter release (Kovalchuk et al., 2002; Tyler & Pozzo-Miller, 2001), and memory formation (Hall et al., 2000; Mizuno et al., 2000). Consistent with this, the BDNF gene appears to modulate olfactory-dependent memory in sheep (Broad et al., 2002), hippocampal blood flow (Hariri et al., 2002), and both episodic (Egan et al., 2003) and incidental (Hariri et al., 2002) memory in humans. Some specificity is suggested by findings that the BDNF gene does not modulate semantic memory on the WCST (Egan et al., 2003), which taxes both working memory and executive function. Further work will be needed to extend these findings to nonschizophrenics and to cognitive functions less strongly linked to hippocampal function. For example, as BDNF appears to have both a central and peripheral role in pain modulation (Bennett, 2001; Xu, Li, Li, Ruan, & Liu, 2000; Zhu, Friess, Wang, Zimmermann, & Buchler, 2001), it exerts neurotrophic effects outside the hippocampus. Moreover, the extant work has examined only a limited range of cognitive functions.
Somewhat broader effects could be hypothesized for polymorphisms in genes directing production of a neurotransmitter enzyme. The COMT gene controls an enzyme in the degradation path of dopamine. Although cognitive effects of the val158met COMT polymorphism have been reported, only one study has considered the breadth of that effect in healthy individuals. That study found effects on attention but not memory (Bilder et al., 2002), suggesting some specificity. However, a larger role in the brain is indicated by a report that the same COMT polymorphism modulating cognition also affects responsiveness to pain (Zubieta et al., 2003). Moreover, this COMT polymorphism affects risk of alcoholism (Tiihonen et al., 1999), schizophrenia (Egan et al., 2001; Li et al., 2000), and incidence of schizotypy (Avramopoulos et al., 2002), although not risk of mood disorders (Kirov et al., 1999) or ADHD (Hawi et al., 2000; Manor et al., 2000). This suggests wide effects on the dopamine system as might be expected of a gene that controls a dopamine-degrading enzyme.
Estrogen receptor genes might be expected to have both specific and broad effects, consistent with estrogen's dual roles in neuroprotection (Dubal et al., 1999; McEwen, 2001) and memory formation (Cordoba Montoya & Carrer, 1997; Luine et al., 1998). Consistent with a broad effect is evidence that a polymorphism in the ER-alpha gene modulates risk of AD (Brandi et al., 1999; Lambert et al., 2001; Mattila et al., 2002), although the evidence is inconsistent (Maruyama et al., 2000). We are aware of only one study examining cognition as a function of ER-alpha genotype. Consistent with a wide role for estrogen on neuronal health, age-related changes in cognitive integrity (broadly defined) appeared to be influenced by an ER-alpha polymorphism (Yaffe et al., 2002). These results suggest broad effects consistent with neuroprotection.
Polymorphisms may also be expressed unevenly over the lifespan. Genes with a role in neuronal support and repair functions—such as the AD-susceptibility gene apoE, the estrogen receptor gene ER-alpha, and the neurotrophic gene BDNF—might be expressed most strongly in response to demand for neuronal repair, such as in the presence of aging or following brain injury. Consistent with that view, although apoE genotype does not appear to influence cognition in youth (Turic et al., 2001), it has been consistently shown to affect cognitive integrity in old age (Bondi et al., 1995; Feskens et al., 1994; Reed et al., 1994), and there is some evidence that it affects cognition and brain structure within normal ranges in middle age as well (Flory et al., 2000; Greenwood et al., 2000; Plassman et al., 1997; Reiman, Caselli et al., 1996; Reiman et al., 1998). BDNF, another neurotrophic gene, might be expected to play a particular role in hippocampal integrity with aging. Polymorphisms in the BDNF gene have been associated with late-onset AD (Kunugi et al., 2001) and late-onset schizophrenia (Krebs et al., 2000). However, we are unaware of studies examining the role of this gene in cognitive aging. Finally, as mentioned above, the ER-alpha gene, also with neurotrophic functions, appears to modulate cognition in old age (Yaffe et al., 2002).
For each candidate gene, we have reviewed the evidence linking that gene to disease, noting the discrepancies in the literature, with some studies finding disease associations and others not. The DRD4 literature, reviewed briefly above, is a good example of this. A recent large-scale meta-analysis finds that such diversity of results is not unusual in the genetic epidemiology literature (Ioannidis et al., 2001). Applying meta-analytic methods to 370 studies examining associations with 36 genes, it was found that the strongest effects were usually seen by the first study reporting an association. The authors suggest both publication bias and population diversity as factors. Therefore, evidence from association studies should perhaps be considered only when findings are at least somewhat consistent or when meta-analyses have been conducted—for example, for DRD4 (Faraone et al., 2001).
The allelic association approach to genetic effects on cognition may have the same limits. If the apoE literature can be taken as an example, most studies of elderly apoE-ε4 carriers do find cognitive deficits, and studies of middle-aged apoE-ε4 carriers similarly find evidence either of cognitive deficits or brain change (reviewed above in subsection titled “Is there a cognitive phenotype of apoE ε4?”). It is acknowledged that apoE may exert an unusually strong effect. Nevertheless, other polymorphisms have shown genotype effects on cognition with relatively small samples. To be specific, Fossella et al., (2002) observed significant effects of DRD4 genotype on cognition with 220 participants. Whereas Egan et al. (2001) observed significant effects of COMT genotype on cognition with 274 participants, Malhotra et al. (2002) required only 73. This latter sample size is comparable to that of 97 reported by Greenwood et al. (2000) for apoE. As discussed above, the effect size obtained may depend on the sensitivity of the assessment. Egan et al. used the complex WCST, whereas Bilder et al. (2002) used more focused tests aimed at specific processes and obtained significance with an N of 58, only 7 of whom had the met/met genotype. These sample sizes can be contrasted with those used in studies of association with diagnosis, which typically number in the thousands (see Ioannidis et al., 2001). This indicates that specific aspects of cognition show genetic modulation with medium effect sizes (Table 1). However, the issue of replicability will not be resolved without both additional work and meta-analyses.
Individual differences in cognition have long been recognized to exist. The small but emergent and growing literature using the allelic association approach indicates that such individual differences may owe something to what is viewed as normal genetic variation. Future work assessing genetic effects on cognitive processes would benefit from selecting genes based on links to known brain networks, as in the genetic studies of visuospatial attention and working memory reviewed in this article. Such links can provide a reliable foundation for relating particular genetic polymorphisms to cognitive functions.
REFERENCES
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. Journal of Neuroscience. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham R, Myers A, Wavrant-DeVrieze F, Hamshere ML, Thomas HV, Marshall H, et al. Substantial linkage dis-equilibrium across the insulin-degrading enzyme locus but no association with late-onset Alzheimer's disease. Human Genetics. 2001;109(6):646–652. doi: 10.1007/s00439-001-0614-1. [DOI] [PubMed] [Google Scholar]
- Adams MR, Register TC, Golden DL, Wagner JD, Williams JK. Medroxyprogesterone acetate antagonizes inhibitory effects of conjugated equine estrogens on coronary artery atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 1997;17(1):217–221. doi: 10.1161/01.atv.17.1.217. [DOI] [PubMed] [Google Scholar]
- Albagha OM, McGuigan FE, Reid DM, Ralston SH. Estrogen receptor alpha gene polymorphisms and bone mineral density: Haplotype analysis in women from the United Kingdom. Journal of Bone and Mineral Research. 2001;16(1):128–134. doi: 10.1359/jbmr.2001.16.1.128. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Nicotinic receptor activation in human cerebral cortical interneurons: A mechanism for inhibition and disinhibition of neuronal networks. Journal of Neuroscience. 2000;20(1):66–75. doi: 10.1523/JNEUROSCI.20-01-00066.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando J, Ono Y, Wright MJ. Genetic structure of spatial and verbal working memory. Behavior Genetics. 2001;31(6):615–624. doi: 10.1023/a:1013353613591. [DOI] [PubMed] [Google Scholar]
- Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. Journal of Neurochemistry. 1995;65(3):1157–1165. doi: 10.1046/j.1471-4159.1995.65031157.x. [DOI] [PubMed] [Google Scholar]
- Avramopoulos D, Stefanis NC, Hantoumi I, Smyrnis N, Evdokimidis I, Stefanis CN. Higher scores of self reported schizotypy in healthy young males carrying the COMT high activity allele. Molecular Psychiatry. 2002;7(7):706–711. doi: 10.1038/sj.mp.4001070. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch GJ. Working memory. In: Bower GH, editor. The psychology of learning and motivation. Vol. 8. Academic Press; New York: 1974. pp. 47–90. [Google Scholar]
- Bartus RT, Dean RL, III, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217(4558):408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Benjamin J, Patterson C, Greenberg BD, Murphy DL, Hamer DH. Population and familial association between the D4 dopamine receptor gene and measures of Novelty Seeking. Nature Genetics. 1996;12(1):81–84. doi: 10.1038/ng0196-81. [DOI] [PubMed] [Google Scholar]
- Bennett DL. Neurotrophic factors: Important regulators of nociceptive function. Neuroscientist. 2001;7(1):13–17. doi: 10.1177/107385840100700105. [DOI] [PubMed] [Google Scholar]
- Bertram L, Blacker D, Mullin K, Keeney D, Jones J, Basu S, et al. Evidence for genetic linkage of Alzheimer's disease to chromosome 10q. Science. 2000;290(5500):2302–2303. doi: 10.1126/science.290.5500.2302. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Czobor P, Malhotra AK, Kennedy JL, Ni X, et al. Neurocognitive correlates of the COMT Val(158)Met polymorphism in chronic schizophrenia. Biological Psychiatry. 2002;52(7):701–707. doi: 10.1016/s0006-3223(02)01416-6. [DOI] [PubMed] [Google Scholar]
- Blacker D, Bertram L, Saunders AJ, Moscarillo TJ, Albert MS, Wiener H, et al. Results of a high-resolution genome screen of 437 Alzheimer's disease families. Human Molecular Genetics. 2003;12(1):23–32. doi: 10.1093/hmg/ddg007. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Galaski D, Salmon DP, Thomas RG. Neuropsychological function and apolipoprotein E genotype in the preclinical detection of Alzheimer's disease. Psychology and Aging. 1999;14:295–303. doi: 10.1037//0882-7974.14.2.295. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Salmon DP, Monsch AU, Galasko D, Butters N, Klauber MR, et al. Episodic memory changes are associated with the APOE-epsilon 4 allele in nondemented older adults. Neurology. 1995;45(12):2203–2206. doi: 10.1212/wnl.45.12.2203. [DOI] [PubMed] [Google Scholar]
- Boussaha M, Hannequin D, Verpillat P, Brice A, Frebourg T, Campion D. Polymorphisms of insulin degrading enzyme gene are not associated with Alzheimer's disease. Neuroscience Letters. 2002;329(1):121–123. doi: 10.1016/s0304-3940(02)00586-4. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Southard T, Sarvey JM, Herkenham M, Brady LS. Unilateral LTP triggers bilateral increases in hippocampal neurotrophin and trk receptor mRNA expression in behaving rats: Evidence for interhemispheric communication. Journal of Comparative Neurology. 1996;368(3):371–382. doi: 10.1002/(SICI)1096-9861(19960506)368:3<371::AID-CNE4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Brandi ML, Becherini L, Gennari L, Racchi M, Bianchetti A, Nacmias B, et al. Association of the estrogen receptor alpha gene polymorphisms with sporadic Alzheimer's disease. Biochemical and Biophysical Research Communications. 1999;265(2):335–338. doi: 10.1006/bbrc.1999.1665. [DOI] [PubMed] [Google Scholar]
- Breitner JC, Wyse BW, Anthony JC, Welsh-Bohmer KA, Steffens DC, Norton MC, et al. APOE-epsilon 4 count predicts age when prevalence of AD increases, then declines: The Cache County Study. Neurology. 1999;53(2):321–331. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- Bretsky P, Guralnik JM, Launer L, Albert M, Seeman TE. The role of APOE-epsilon4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging. Neurology. 2003;60(7):1077–1081. doi: 10.1212/01.wnl.0000055875.26908.24. [DOI] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD. Making memories: Brain activity that predicts how well visual experience will be remembered. Science. 1998;281(5380):1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Broad KD, Mimmack ML, Keverne EB, Kendrick KM. Increased BDNF and trk-B mRNA expression in cortical and limbic regions following formation of a social recognition memory. European Journal of Neuroscience. 2002;16(11):2166–2174. doi: 10.1046/j.1460-9568.2002.02311.x. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, Graham R, Nyberg L. Similarities and differences in the neural correlates of episodic memory retrieval and working memory. Neuroimage. 2002;16(2):317–330. doi: 10.1006/nimg.2002.1063. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Osborne D, Reiman EM, Hentz JG, Barbieri CJ, Saunders AM, et al. Preclinical cognitive decline in late middle-aged asymptomatic apolipoprotein E-e4/4 homozygotes: A replication study. Journal of the Neurological Sciences. 2001;189(12):93–98. doi: 10.1016/s0022-510x(01)00577-9. [DOI] [PubMed] [Google Scholar]
- Casu MA, Pan Wong T, De Koninck Y, Ribeiro-Da-Silva A, Cuello AC. Aging causes a preferential loss of cholinergic innervation of characterized neocortical pyramidal neurons. Cerebral Cortex. 2002;12(3):329–337. doi: 10.1093/cercor/12.3.329. [DOI] [PubMed] [Google Scholar]
- Chapman J, Korczyn AD, Karussis DM, Michaelson DM. The effects of APOE genotype on age at onset and progression of neurodegenerative diseases. Neurology. 2001;57(8):1482–1485. doi: 10.1212/wnl.57.8.1482. [DOI] [PubMed] [Google Scholar]
- Chen C, Burton M, Greenberger E, Dmitrieva J. Population migration and the variation of dopamine D4 Receptor (DRD4) allele frequencies around the globe. Evolution and Human Behavior. 1999;20:309–324. [Google Scholar]
- Chen G, Kolbeck R, Barde YA, Bonhoeffer T, Kossel A. Relative contribution of endogenous neurotrophins in hippocampal long-term potentiation. Journal of Neuroscience. 1999;19(18):7983–7990. doi: 10.1523/JNEUROSCI.19-18-07983.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichon S, Nothen MM, Erdmann J, Propping P. Detection of four polymorphic sites in the human dopamine D1 receptor gene (DRD1) Human Molecular Genetics. 1994;3(1):209. doi: 10.1093/hmg/3.1.209. [DOI] [PubMed] [Google Scholar]
- Cichon S, Nothen MM, Stober G, Schroers R, Albus M, Maier W, et al. Systematic screening for mutations in the 5'-regulatory region of the human dopamine D1 receptor (DRD1) gene in patients with schizophrenia and bipolar affective disorder. American Journal of Medical Genetics. 1996;67(4):424–428. doi: 10.1002/(SICI)1096-8628(19960726)67:4<424::AID-AJMG21>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd Lawrence Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Conner JM, Darracq MA, Roberts J, Tuszynski MH. Nontropic actions of neurotrophins: Subcortical nerve growth factor gene delivery reverses age-related degeneration of primate cortical cholinergic innervation. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(4):1941–1946. doi: 10.1073/pnas.98.4.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: Identical, independent, or overlapping neural systems? Proceedings of the National Academy of Sciences of the United States of America. 1998;95:831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Cordoba Montoya DA, Carrer HF. Estrogen facilitates induction of long term potentiation in the hippocampus of awake rats. Brain Research. 1997;778(2):430–438. doi: 10.1016/s0006-8993(97)01206-7. [DOI] [PubMed] [Google Scholar]
- Crawford FC, Vanderploeg RD, Freeman MJ, Singh S, Waisman M, Michaels L, et al. APOE genotype influences acquisition and recall following traumatic brain injury. Neurology. 2002;58(7):1115–1118. doi: 10.1212/wnl.58.7.1115. [DOI] [PubMed] [Google Scholar]
- Cubells JF, Kranzler HR, McCance-Katz E, Anderson GM, Malison RT, Price LH, et al. A haplotype at the DBH locus, associated with low plasma dopamine beta-hydroxylase activity, also associates with cocaine-induced paranoia. Molecular Psychiatry. 2000;5(1):56–63. doi: 10.1038/sj.mp.4000657. [DOI] [PubMed] [Google Scholar]
- Cubells JF, van Kammen DP, Kelley ME, Anderson GM, O'Connor DT, Price LH, et al. Dopamine beta-hydroxylase: Two polymorphisms in linkage disequilibrium at the structural gene DBH associate with biochemical phenotypic variation. Human Genetics. 1998;102(5):533–540. doi: 10.1007/s004390050736. [DOI] [PubMed] [Google Scholar]
- Cullum CM, Harris JG, Waldo MC, Smernoff E, Madison A, Nagamoto HT, et al. Neurophysiological and neuropsychological evidence for attentional dysfunction in schizophrenia. Schizophrenia Research. 1993;10(2):131–141. doi: 10.1016/0920-9964(93)90048-n. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Scinto LFM. Early diagnosis of Alzheimer's disease. In: Scinto LFM, Daffner KR, editors. Early diagnosis of Alzeimer's disease. Humana; Totowa, NJ: 2000. [Google Scholar]
- Daly G, Hawi Z, Fitzgerald M, Gill M. Mapping susceptibility loci in attention deficit hyperactivity disorder: Preferential transmission of parental alleles at DAT1, DBH and DRD5 to affected children. Molecular Psychiatry. 1999;4(2):192–196. doi: 10.1038/sj.mp.4000510. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Marrocco RT. Local infusion of scopolamine into intraparietal cortex slows covert orienting in rhesus monkeys. Journal of Neurophysiology. 2000;83(3):1536–1549. doi: 10.1152/jn.2000.83.3.1536. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Whiteman MC, Pattie A, Starr JM, Hayward C, Wright AF, et al. Ageing: Cognitive change and the APOE varepsilon 4 allele. Nature. 2002;418(6901):932. doi: 10.1038/418932a. [DOI] [PubMed] [Google Scholar]
- De Fusco M, Becchetti A, Patrignani A, Annesi G, Gambardella A, Quattrone A, et al. The nicotinic receptor beta 2 subunit is mutant in nocturnal frontal lobe epilepsy. Nature Genetics. 2000;26(3):275–276. doi: 10.1038/81566. [DOI] [PubMed] [Google Scholar]
- Diaz Brinton R, Chen S, Montoya M, Hsieh D, Minaya J, Kim J, et al. The women's health initiative estrogen replacement therapy is neurotrophic and neuroprotective. Neurobiology and Aging. 2000;21(3):475–496. doi: 10.1016/s0197-4580(00)00109-3. [DOI] [PubMed] [Google Scholar]
- Ding YC, Chi HC, Grady DL, Morishima A, Kidd JR, Kidd KK, et al. Evidence of positive selection acting at the human dopamine receptor D4 gene locus. Proceedings of the National Academy of Sciences United States of America. 2002;99(1):309–314. doi: 10.1073/pnas.012464099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drory VE, Birnbaum M, Korczyn AD, Chapman J. Association of APOE epsilon4 allele with survival in amyotrophic lateral sclerosis. Journal of the Neurological Sciences. 2001;190(12):17–20. doi: 10.1016/s0022-510x(01)00569-x. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Shughrue PJ, Wilson ME, Merchenthaler I, Wise PM. Estradiol modulates bcl-2 in cerebral ischemia: A potential role for estrogen receptors. Journal of Neuroscience. 1999;19(15):6385–6393. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Seitz RJ, Kolodny J, Bor D, Herzog H, Ahmed A, et al. A neural basis for general intelligence. Science. 2000;289(5478):457–460. doi: 10.1126/science.289.5478.457. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Novick O, Umansky R, Priel B, Osher Y, Blaine D, et al. Dopamine D4 receptor (D4DR) exon III polymorphism associated with the human personality trait of Novelty Seeking. Nature Genetics. 1996;12(1):78–80. doi: 10.1038/ng0196-78. [DOI] [PubMed] [Google Scholar]
- Edland SD, Wavrant-De Vriese F, Compton D, Smith GE, Ivnik R, Boeve BF, et al. Insulin degrading enzyme (IDE) genetic variants and risk of Alzheimer's disease: Evidence of effect modification by apolipoprotein E (APOE) Neuroscience Letters. 2003;345(1):21–24. doi: 10.1016/s0304-3940(03)00488-9. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met Polymorphism Affects Activity-Dependent Secretion of BDNF and Human Memory and Hippocampal Function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Ertekin-Taner N, Graff-Radford N, Younkin LH, Eckman C, Baker M, Adamson J, et al. Linkage of plasma Abeta42 to a quantitative locus on chromosome 10 in late-onset Alzheimer's disease pedigrees. Science. 2000;290(5500):2303–2304. doi: 10.1126/science.290.5500.2303. [DOI] [PubMed] [Google Scholar]
- Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, et al. Prevalence of Alzheimer's disease in a community population of older persons. Higher than previously reported. Journal of the American Medical Association. 1989;262(18):2551–2556. [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annual Review of Psychology. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Murphy BA, Patel SN, Kilbridge JF, Mobley WC, Bu G, et al. Evidence for normal aging of the septohippocampal cholinergic system in apoE (−/−) mice but impaired clearance of axonal degeneration products following injury. Experimental Neurology. 1998;151(2):314–325. doi: 10.1006/exnr.1998.6818. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14(3):340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Fan J, Wu Y, Fossella JA, Posner MI. Assessing the heritability of attentional networks. BMC Neuroscience. 2001;2(1):14. doi: 10.1186/1471-2202-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Doyle AE, Mick E, Biederman J. Meta-analysis of the association between the 7-repeat allele of the dopa-mine D(4) receptor gene and attention deficit hyperactivity disorder. American Journal of Psychiatry. 2001;158(7):1052–1057. doi: 10.1176/appi.ajp.158.7.1052. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Journal of the American Medical Association. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- Fazekas F, Strasser-Fuchs S, Kollegger H, Berger T, Kristoferitsch W, Schmidt H, et al. Apolipoprotein E epsilon 4 is associated with rapid progression of multiple sclerosis. Neurology. 2001;57(5):853–857. doi: 10.1212/wnl.57.5.853. [DOI] [PubMed] [Google Scholar]
- Fernandez G, Effern A, Grunwald T, Pezer N, Lehnertz K, Dumpelmann M, et al. Real-time tracking of memory formation in the human rhinal cortex and hippocampus. Science. 1999;285(5433):1582–1585. doi: 10.1126/science.285.5433.1582. [DOI] [PubMed] [Google Scholar]
- Feskens EJM, Havekes LM, Kalmijn S, Knijff P. d., Launer LJ, Kromhout D. Apolipoprotein e4 allele and cognitive decline in elderly men. British Journal of Medicine. 1994;309:1202–1206. doi: 10.1136/bmj.309.6963.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381(6584):706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Finkel D, Pedersen NL, McGue M, McClearn GE. Heritability of cognitive abilities in adult twins: Comparison of Minnesota and Swedish data. Behavior Genetics. 1995;25(5):421–431. doi: 10.1007/BF02253371. [DOI] [PubMed] [Google Scholar]
- Fisher SE, Francks C, McCracken JT, McGough JJ, Marlow AJ, MacPhie IL, et al. A genomewide scan for loci involved in attention-deficit/hyperactivity disorder. American Journal of Human Genetics. 2002;70(5):1183–1196. doi: 10.1086/340112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CM, DeCamp RM, Kilo S, Rogers SW, Hargreaves KM. Neuronal nicotinic receptor expression in sensory neurons of the rat trigeminal ganglion: Demonstration of alpha3beta4, a novel subtype in the mammalian nervous system. Journal of Neuroscience. 1996;16(24):7892–7901. doi: 10.1523/JNEUROSCI.16-24-07892.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory JD, Manuck SB, Ferrell RE, Ryan CM, Muldoon MF. Memory performance and the apolipoprotein E polymorphism in a community sample of middle-aged adults. American Journal of Medical Genetics. 2000;96(6):707–711. doi: 10.1002/1096-8628(20001204)96:6<707::aid-ajmg1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Fossella J, Sommer T, Fan J, Wu Y, Swanson JM, Pfaff DW, et al. Assessing the molecular genetics of attention networks. BMC Neuroscience. 2002;3(1):14. doi: 10.1186/1471-2202-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NC, Warrington EK, Seiffer AL, Agnew SK, Rossor MN. Presymptomatic cognitive deficits in individuals at risk of familial Alzheimer's disease. A longitudinal prospective study. Brain. 1998;121(9):1631–1639. doi: 10.1093/brain/121.9.1631. [DOI] [PubMed] [Google Scholar]
- Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(2):587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173:652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Colombo PJ. Ageing: The cholinergic hypothesis of cognitive decline. Current Opinion in Neurobiology. 1995;5(2):161–168. doi: 10.1016/0959-4388(95)80022-0. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Progress in Neurobiology. 2001;63(1):29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Georgieva L, Dimitrova A, Nikolov I, Koleva S, Tsvetkova R, Owen MJ, et al. Dopamine transporter gene (DAT1) VNTR polymorphism in major psychiatric disorders: Family-based association study in the Bulgarian population. Acta Psychiatrica Scandinavica. 2002;105(5):396–399. doi: 10.1034/j.1600-0447.2002.1o174.x. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Effects of gonadal hormone replacement on measures of basal forebrain cholinergic function. Neuroscience. 2000;101(4):931–938. doi: 10.1016/s0306-4522(00)00433-4. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Nelson D, Anthony MS, Clarkson TB. Effects of long-term hormone replacement and of tibolone on choline acetyltransferase and acetylcholinesterase activities in the brains of ovariectomized, cynomologus monkeys. Neuroscience. 2002;113(4):907–914. doi: 10.1016/s0306-4522(02)00239-7. [DOI] [PubMed] [Google Scholar]
- Glockner F, Meske V, Ohm TG. Genotype-related differences of hippocampal apolipoprotein E levels only in early stages of neuropathological changes in Alzheimer's disease. Neuroscience. 2002;114(4):1103–1114. doi: 10.1016/s0306-4522(02)00178-1. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, et al. Executive subprocesses in working memory: Relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Archives of General Psychiatry. 2003;60(9):889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Mountcastle VB, editor. Handbook of Physiology. Vol. 5. American Physiological Society; Washington, DC: 1987. pp. 373–417. [Google Scholar]
- Goldman-Rakic PS. The cortical dopamine system: Role in memory and cognition. Advances in Pharmacology. 1998;42:707–711. doi: 10.1016/s1054-3589(08)60846-7. [DOI] [PubMed] [Google Scholar]
- Goleman D. Emotional intelligence. Bantam; New York: 1995. [Google Scholar]
- Goodman Y, Bruce AJ, Cheng B, Mattson MP. Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid beta-peptide toxicity in hippocampal neurons. Journal of Neurochemistry. 1996;66(5):1836–1844. doi: 10.1046/j.1471-4159.1996.66051836.x. [DOI] [PubMed] [Google Scholar]
- Gottschalk W, Pozzo-Miller LD, Figurov A, Lu B. Presynaptic modulation of synaptic transmission and plasticity by brain-derived neurotrophic factor in the developing hippocampus. Journal of Neuroscience. 1998;18(17):6830–6839. doi: 10.1523/JNEUROSCI.18-17-06830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood P, Sunderland T, Friz J, Lambert JC, Parasuraman R. Can a “cognitive” phenotype of APOE epsilon 4 be detected in middle age? Paper presented at the Society for Neuroscience; Orlando, FL: 2002. [Google Scholar]
- Greenwood PM, Fossella JF, Parasuraman R. Double dissociation of modulation of visuospatial attention and working memory by normal allelic variation in cholinergic and dopaminergic genes. Paper presented at the Cognitive Neuroscience Society; New York: 2003. [Google Scholar]
- Greenwood PM, Parasuraman R, Alexander GE. Changes in the ability to dynamically adjust the attentional focus from youth to old age to Alzheimer Disease. Paper presented at the Society for Neuroscience; New Orleans, LA: 1997. [Google Scholar]
- Greenwood PM, Sunderland T, Friz JL, Parasuraman R. Genetics and visual attention: Selective deficits in healthy adult carriers of the varepsilon 4 allele of the apolipoprotein E gene. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(21):11661–11666. doi: 10.1073/pnas.97.21.11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groswasser Z, Cohen M, Keren O. Female TBI patients recover better than males. Brain Injury. 1998;12(9):805–808. doi: 10.1080/026990598122197. [DOI] [PubMed] [Google Scholar]
- Guan ZZ, Zhang X, Ravid R, Nordberg A. Decreased protein levels of nicotinic receptor subunits in the hippocampus and temporal cortex of patients with Alzheimer's disease. Journal of Neurochemistry. 2000;74(1):237–243. doi: 10.1046/j.1471-4159.2000.0740237.x. [DOI] [PubMed] [Google Scholar]
- Gurden H, Takita M, Jay TM. Essential role of D1 but not D2 receptors in the NMDA receptor-dependent long-term potentiation at hippocampal-prefrontal cortex synapses in vivo. Journal of Neuroscience. 2000;20(22):RC106. doi: 10.1523/JNEUROSCI.20-22-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nature Neuroscience. 2000;3(6):533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Hammond EJ, Meador KJ, Aung-Din R, Wilder BJ. Cholinergic modulation of human P3 event-related potentials. Neurology. 1987;37(2):346–350. doi: 10.1212/wnl.37.2.346. [DOI] [PubMed] [Google Scholar]
- Hardy J, Higgins GA. Alzheimer's disease: The amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Egan MF, Mattay VS, Kolachana B, Weinberger DR. BDNF VAL66MET genetic variation and the response of the human hippocampus. Paper presented at the Society for Neuroscience; Orlando, FL: 2002. [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, et al. Brain-derived neurotrophic facto val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. Journal of Neuroscience. 2003;23(17):6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpending H, Cochran G. In our genes. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(1):10–12. doi: 10.1073/pnas.012612799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlik RJ, Izmirlian G, Petrovitch H, Ross GW, Masaki K, Curb JD, et al. APOE-epsilon4 predicts incident AD in Japanese-American men: The Honolulu-Asia aging study. Neurology. 2000;54(7):1526–1529. doi: 10.1212/wnl.54.7.1526. [DOI] [PubMed] [Google Scholar]
- Hawi Z, Millar N, Daly G, Fitzgerald M, Gill M. No association between catechol-O-methyltransferase (COMT) gene polymorphism and attention deficit hyperactivity disorder (ADHD) in an Irish sample. American Journal of Medical Genetics. 2000;96(3):282–284. doi: 10.1002/1096-8628(20000612)96:3<282::aid-ajmg9>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Henderson AS, Easteal S, Jorm AF, Mackinnon AJ, Korten AE, Christensen H, et al. Apolipoprotein E allele epsilon 4, dementia, and cognitive decline in a population sample. Lancet. 1995;346(8987):1387–1390. doi: 10.1016/s0140-6736(95)92405-1. [DOI] [PubMed] [Google Scholar]
- Hendrie HC, Ogunniyi A, Hall KS, Baiyewu O, Unverzagt FW, Gureje O, et al. Incidence of dementia and Alzheimer disease in 2 communities: Yoruba residing in Ibadan, Nigeria, and African Americans residing in Indianapolis, Indiana. Journal of the American Medical Association. 2001;285(6):739–747. doi: 10.1001/jama.285.6.739. [DOI] [PubMed] [Google Scholar]
- Hori H, Ohmori O, Shinkai T, Kojima H, Nakamura J. Association between three functional polymorphisms of dopa-mine D2 receptor gene and tardive dyskinesia in schizophrenia. American Journal of Medical Genetics. 2001;105(8):774–778. doi: 10.1002/ajmg.10045. [DOI] [PubMed] [Google Scholar]
- Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nature Genetics. 2001;29(3):306–309. doi: 10.1038/ng749. [DOI] [PubMed] [Google Scholar]
- Isoe K, Ji Y, Urakami K, Adachi Y. Genetic association of estrogen receptor gene polymorphisms with Alzheimer's disease. Alzheimer's Research. 1997;3:195–197. [Google Scholar]
- Johansson B, Whitfield K, Pedersen NL, Hofer SM, Ahern F, McClearn GE. Origins of individual differences in episodic memory in the oldest-old: A population-based study of identical and same-sex fraternal twins aged 80 and older. Journal of Ger-ontology. Series B, Psychological Sciences and Social Sciences. 1999;54(3):173–179. doi: 10.1093/geronb/54b.3.p173. [DOI] [PubMed] [Google Scholar]
- Kampen DL, Sherwin BB. Estrogen use and verbal memory in healthy postmenopausal women. Obstetrics & Gynecology. 1994;83:979–983. doi: 10.1097/00006250-199406000-00017. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: A dialogue between genes and synapses. Science. 2001;294(5544):1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM, editors. Principles of neural science. 4th McGraw-Hill; New York: 2000. [Google Scholar]
- Katzman R, Zhang MY, Chen PJ, Gu N, Jiang S, Saitoh T, et al. Effects of apolipoprotein E on dementia and aging in the Shanghai Survey of Dementia. Neurology. 1997;49(3):779–785. doi: 10.1212/wnl.49.3.779. [DOI] [PubMed] [Google Scholar]
- Kawas C, Resnick S, Morrison A. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer's disease: The Baltimore Longitudinal Study of Aging. Neurology. 1997;48:1517–1521. doi: 10.1212/wnl.48.6.1517. [DOI] [PubMed] [Google Scholar]
- Kent L, Green E, Holmes J, Thapar A, Gill M, Hawi Z, et al. No association between CHRNA7 microsatellite markers and attention-deficit hyperactivity disorder. American Journal of Medical Genetics. 2001;105(8):686–689. doi: 10.1002/ajmg.1553. [DOI] [PubMed] [Google Scholar]
- Kent L, Middle F, Hawi Z, Fitzgerald M, Gill M, Feehan C, et al. Nicotinic acetylcholine receptor alpha4 subunit gene polymorphism and attention deficit hyperactivity disorder. Psychiatric Genetics. 2001;11(1):37–40. doi: 10.1097/00041444-200103000-00007. [DOI] [PubMed] [Google Scholar]
- Kirov G, Jones I, McCandless F, Craddock N, Owen MJ. Family-based association studies of bipolar disorder with candidate genes involved in dopamine neurotransmission: DBH, DAT1, COMT, DRD2, DRD3 and DRD5. Molecular Psychiatry. 1999;4(6):558–565. doi: 10.1038/sj.mp.4000565. [DOI] [PubMed] [Google Scholar]
- Kluger AN, Siegfried Z, Ebstein RP. A meta-analysis of the association between DRD4 polymorphism and novelty seeking. Molecular Psychiatry. 2002;7(7):712–717. doi: 10.1038/sj.mp.4001082. [DOI] [PubMed] [Google Scholar]
- Kohnke MD, Zabetian CP, Anderson GM, Kolb W, Gaertner I, Buchkremer G, et al. A genotype-controlled analysis of plasma dopamine beta-hydroxylase in healthy and alcoholic subjects: Evidence for alcohol-related differences in noradrenergic function. Biological Psychiatry. 2002;52(12):1151–1158. doi: 10.1016/s0006-3223(02)01427-0. [DOI] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(19):8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk Y, Hanse E, Kafitz KW, Konnerth A. Postsynaptic induction of BDNF-mediated long-term potentiation. Science. 2002;295(5560):1729–1734. doi: 10.1126/science.1067766. [DOI] [PubMed] [Google Scholar]
- Krebs MO, Guillin O, Bourdell MC, Schwartz JC, Olie JP, Poirier MF, et al. Brain derived neurotrophic factor (BDNF) gene variants association with age at onset and therapeutic response in schizophrenia. Molecular Psychiatry. 2000;5(5):558–562. doi: 10.1038/sj.mp.4000749. [DOI] [PubMed] [Google Scholar]
- Kunugi H, Ueki A, Otsuka M, Isse K, Hirasawa H, Kato N, et al. A novel polymorphism of the brain-derived neurotrophic factor (BDNF) gene associated with late-onset Alzheimer's disease. Molecular Psychiatry. 2001;6(1):83–86. doi: 10.1038/sj.mp.4000792. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Morrow B, Shprintzen R, Veit S, Parsia SS, Faedda G, et al. Association of codon 108/158 catechol-O-methyltransferase gene polymorphism with the psychiatric manifestations of velo-cardio-facial syndrome. American Journal of Medical Genetics. 1996;67(5):468–472. doi: 10.1002/(SICI)1096-8628(19960920)67:5<468::AID-AJMG5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- LaHoste GJ, Swanson JM, Wigal SB, Glabe C, Wigal T, King N, et al. Dopamine D4 receptor gene polymorphism is associated with attention deficit hyperactivity disorder [see comments] Molecular Psychiatry. 1996;1(2):121–124. [PubMed] [Google Scholar]
- Lakatos K, Nemoda Z, Birkas E, Ronai Z, Kovacs E, Ney K, et al. Association of D4 dopamine receptor gene and serotonin transporter promoter polymorphisms with infants' response to novelty. Molecular Psychiatry. 2003;8(1):90–97. doi: 10.1038/sj.mp.4001212. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Harris JM, Mann D, Lemmon H, Coates J, Cumming A, et al. Are the estrogen receptors involved in Alzheimer's disease? Neuroscience Letters. 2001;306(3):193–197. doi: 10.1016/s0304-3940(01)01806-7. [DOI] [PubMed] [Google Scholar]
- LeBlanc ES, Janowsky J, Chan BK, Nelson HD. Hormone replacement therapy and cognition: Systematic review and meta- analysis. Journal of the American Medical Association. 2001;285(11):1489–1499. doi: 10.1001/jama.285.11.1489. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The saga of the nerve growth factor. Neuroreport. 1998;9(16):R71–83. [PubMed] [Google Scholar]
- Levin ED, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology (Berlin) 1998;138(34):217–230. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- Levy JA, Parasuraman R, Greenwood PM, Dukoff R, Sunderland T. Acetylcholine affects the spatial scale of attention: Evidence from Alzheimer's disease. Neuropsychology. 2000;14(2):288–298. doi: 10.1037//0894-4105.14.2.288. [DOI] [PubMed] [Google Scholar]
- Li T, Ball D, Zhao J, Murray RM, Liu X, Sham PC, et al. Family-based linkage disequilibrium mapping using SNP marker haplotypes: Application to a potential locus for schizophrenia at chromosome 22q11. Molecular Psychiatry. 2000;5(1):77–84. doi: 10.1038/sj.mp.4000638. [DOI] [PubMed] [Google Scholar]
- Lichtman SW, Seliger G, Tycko B, Marder K. Apolipoprotein E and functional recovery from brain injury following postacute rehabilitation. Neurology. 2000;55(10):1536–1539. doi: 10.1212/wnl.55.10.1536. [DOI] [PubMed] [Google Scholar]
- Limosin F, Loze JY, Rouillon F, Ades J, Gorwood P. Association between dopamine receptor D1 gene DdeI polymorphism and sensation seeking in alcohol-dependent men. Alcoholism, Clinical and Experimental Research. 2003;27(8):1226–1228. doi: 10.1097/01.ALC.0000081624.57507.87. [DOI] [PubMed] [Google Scholar]
- Linn RT, Wolf PA, Bachman DL, Knoefel JE, Cobb JL, Belanger AJ, et al. The “preclinical phase” of probable Alzheimer's disease. A 13-year prospective study of the Framingham cohort. Archives of Neurology. 1995;52(5):485–490. doi: 10.1001/archneur.1995.00540290075020. [DOI] [PubMed] [Google Scholar]
- Lu B, Gottschalk W. Modulation of hippocampal synaptic transmission and plasticity by neurotrophins. Progress in Brain Research. 2000;128:231–241. doi: 10.1016/S0079-6123(00)28020-5. [DOI] [PubMed] [Google Scholar]
- Luine V, Park D, Joh T, Reis D, McEwen B. Immuno-chemical demonstration of increased choline acetyltransferase concentration in rat preoptic area after estradiol administration. Brain Research. 1980;191(1):273–277. doi: 10.1016/0006-8993(80)90332-7. [DOI] [PubMed] [Google Scholar]
- Luine VN. Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Experimental Neurology. 1985;89(2):484–490. doi: 10.1016/0014-4886(85)90108-6. [DOI] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Hormones and Behavior. 1998;34(2):149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Lykken DT, McGue M, Tellegen A, Bouchard TJ., Jr. Emergenesis. Genetic traits that may not run in families. The American Psychologist. 1992;47(12):1565–1577. doi: 10.1037//0003-066x.47.12.1565. [DOI] [PubMed] [Google Scholar]
- MacPherson SE, Phillips LH, Della Sala S. Age, executive function, and social decision making: A dorsolateral prefrontal theory of cognitive aging. Psychology and Aging. 2002;17(4):598–609. [PubMed] [Google Scholar]
- Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, O'Keefe J. Knowing where and getting there: A human navigation network. Science. 1998;280(5365):921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(8):4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Resnick SM. Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiology and Aging. 2000;21(2):373–383. doi: 10.1016/s0197-4580(00)00123-8. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. American Journal of Psychiatry. 2002;159(4):652–654. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]
- Manly T, Anderson V, Nimmo-Smith I, Turner A, Watson P, Robertson IH. The differential assessment of children's attention: The Test of Everyday Attention for Children (TEA-Ch), normative sample and ADHD performance. Journal of Child Psychology and Psychiatry. 2001;42(8):1065–1081. doi: 10.1111/1469-7610.00806. [DOI] [PubMed] [Google Scholar]
- Manor I, Kotler M, Sever Y, Eisenberg J, Cohen H, Ebstein RP, et al. Failure to replicate an association between the catechol-O-methyltransferase polymorphism and attention deficit hyperactivity disorder in a second, independently recruited Israeli cohort. American Journal of Medical Genetics. 2000;96(6):858–860. doi: 10.1002/1096-8628(20001204)96:6<858::aid-ajmg33>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz CM, Court JA, Molnar E, Lee M, Gotti C, Mamalaki A, et al. Alpha4 but not alpha3 and alpha7 nicotinic acetylcholine receptor subunits are lost from the temporal cortex in Alzheimer's disease. Journal of Neurochemistry. 1999;73(4):1635–1640. doi: 10.1046/j.1471-4159.1999.0731635.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Serrano A, Fischer W, Bjorklund A. Reversal of age-dependent cognitive impairments and cholinergic neuron atrophy by NGF-secreting neural progenitors grafted to the basal forebrain. Neuron. 1995;15(2):473–484. doi: 10.1016/0896-6273(95)90051-9. [DOI] [PubMed] [Google Scholar]
- Maruyama H, Toji H, Harrington CR, Sasaki K, Izumi Y, Ohnuma T, et al. Lack of an association of estrogen receptor alpha gene polymorphisms and transcriptional activity with Alzheimer disease. Archives of Neurology. 2000;57(2):236–240. doi: 10.1001/archneur.57.2.236. [DOI] [PubMed] [Google Scholar]
- Matthews G, Zeidner M, Roberts R. Emotional intelligence: Science and myth. MIT Press; Cambridge, MA: 2003. [Google Scholar]
- Mattila KM, Rinne JO, Roytta M, Laippala P, Lehtimaki T. Lack of association between an estrogen receptor 1 gene polymorphism and Parkinson's disease with dementia. Acta Neurologica Scandinavica. 2002;106(3):128–130. doi: 10.1034/j.1600-0404.2002.01331.x. [DOI] [PubMed] [Google Scholar]
- Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294(5545):1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- Mayeux R, Ottman R, Maestre G, Ngai C, Tang MX, Ginsberg H, et al. Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with Alzheimer's disease. Neurology. 1995;45(31):555–557. doi: 10.1212/wnl.45.3.555. [DOI] [PubMed] [Google Scholar]
- McClearn GE, Johansson B, Berg S, Pedersen NL, Ahern F, Petrill SA, et al. Substantial genetic influence on cognitive abilities in twins 80 or more years old. Science. 1997;276(5318):1560–1563. doi: 10.1126/science.276.5318.1560. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Invited review: Estrogens effects on the brain: Multiple sites and molecular mechanisms. Journal of Applied Physiology. 2001;91(6):2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- McGue M, Bouchard TJ., Jr. Genetic and environmental influences on human behavioral differences. Annual Review of Neuroscience. 1998;21:1–24. doi: 10.1146/annurev.neuro.21.1.1. [DOI] [PubMed] [Google Scholar]
- McKinney EF, Walton RT, Yudkin P, Fuller A, Haldar NA, Mant D, et al. E. Association between polymorphisms in dopamine metabolic enzymes and tobacco consumption in smokers. Pharmacogenetics. 2000;10(6):483–491. doi: 10.1097/00008571-200008000-00001. [DOI] [PubMed] [Google Scholar]
- Mentis MJ, Sunderland T, Lai J, Connolly C, Krasuski J, Levine B, et al. Muscarinic versus nicotinic modulation of a visual task. a pet study using drug probes. Neuropsychopharmacology. 2001;25(4):555–564. doi: 10.1016/S0893-133X(01)00264-0. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Tschanz JT, Norton MC, Welsh-Bohmer KA, Steffens DC, Wyse BW, et al. APOE genotype predicts when—not whether—one is predisposed to develop Alzheimer disease. Nature Genetics. 1998;19:321–322. doi: 10.1038/1206. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan, Weinberger DR, et al. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. American Journal of Psychiatry. 2001;158(11):1809–1817. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, D'Esposito M. fMRI evidence of age-related hippocampal dysfunction in feature binding in working memory. Brain research. Cognitive brain research. 2000;10(12):197–206. doi: 10.1016/s0926-6410(00)00029-x. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. Journal of Neuroscience. 2000;20(18):7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Ma SY, Dills J, Cochran EJ, Leurgans S, Wuu J, et al. Loss of basal forebrain P75(NTR) immunoreactivity in subjects with mild cognitive impairment and Alzheimer's disease. Journal of Comparative Neurology. 2002;443(2):136–153. doi: 10.1002/cne.10122. [DOI] [PubMed] [Google Scholar]
- Muller U, von Cramon DY, Pollmann S. D1- versus D2- receptor modulation of visuospatial working memory in humans. Journal of Neuroscience. 1998;18(7):2720–2728. doi: 10.1523/JNEUROSCI.18-07-02720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. Journal of Neuroscience. 1998;18(7):2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Segal M. Brain-derived neurotrophic factor mediates estradiol-induced dendritic spine formation in hippocampal neurons. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(19):11412–11417. doi: 10.1073/pnas.95.19.11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers A, Holmans P, Marshall H, Kwon J, Meyer D, Ramic D, et al. Susceptibility locus for Alzheimer's disease on chromosome 10. Science. 2000;290(5500):2304–2305. doi: 10.1126/science.290.5500.2304. [DOI] [PubMed] [Google Scholar]
- Myers RH, Schaefer EJ, Wilson PW, D'Agostino R, Ordovas JM, Espino A, et al. Apolipoprotein E epsilon4 association with dementia in a population-based study: The Framingham study. Neurology. 1996;46(3):673–677. doi: 10.1212/wnl.46.3.673. [DOI] [PubMed] [Google Scholar]
- Neves-Pereira M, Mundo E, Muglia P, King N, Macciardi F, Kennedy JL. The brain-derived neurotrophic factor gene confers susceptibility to bipolar disorder: Evidence from a family-based association study. American Journal of Human Genetics. 2002;71(3):651–655. doi: 10.1086/342288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni X, Trakalo JM, Mundo E, Macciardi FM, Parikh S, Lee L, et al. Linkage disequilibrium between dopamine D1 receptor gene (DRD1) and bipolar disorder. Biological Psychiatry. 2002;52(12):1144–1150. doi: 10.1016/s0006-3223(02)01433-6. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Mechanism of estrogen-mediated neuroprotection: Regulation of mitochondrial calcium and Bcl-2 expression. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(5):2842–2847. doi: 10.1073/pnas.0438041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre AC, Sebestyen GN, Gitelman DR, Mesulam MM, Frackowiak RS, Frith CD. Functional localization of the system for visuospatial attention using positron emission tomography. Brain. 1997;120:515–533. doi: 10.1093/brain/120.3.515. [DOI] [PubMed] [Google Scholar]
- Nordberg A. Nicotinic receptor abnormalities of Alzheimer's disease: Therapeutic implications. Biological Psychiatry. 2001;49(3):200–210. doi: 10.1016/s0006-3223(00)01125-2. [DOI] [PubMed] [Google Scholar]
- Olincy A, Ross RG, Harris JG, Young DA, McAndrews MA, Cawthra E, et al. The P50 auditory event-evoked potential in adult attention-deficit disorder: Comparison with schizophrenia. Biological Psychiatry. 2000;47(11):969–977. doi: 10.1016/s0006-3223(00)00239-0. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Hurd YL. Estrogen receptors in the human forebrain and the relation to neuropsychiatric disorders. Progress in Neurobiology. 2001;64(3):251–267. doi: 10.1016/s0301-0082(00)00059-9. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Greenwood PM. Selective attention in aging and dementia. In: Parasuraman R, editor. The attentive brain. MIT Press; Cambridge: 1998. pp. 461–487. [Google Scholar]
- Parasuraman R, Greenwood PM, Haxby JV, Grady CL. Visuospatial attention in dementia of the Alzheimer type. Brain. 1992;115:711–733. doi: 10.1093/brain/115.3.711. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Greenwood PM, Sunderland T. The apolipoprotein E gene, attention, and brain function. Neuropsychology. 2002;16:254–274. doi: 10.1037//0894-4105.16.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson AD, Sunohara GA, Kennedy JL. Dopamine D4 receptor gene: Novelty or nonsense? Neuropsychopharmacology. 1999;21(1):3–16. doi: 10.1016/S0893-133X(98)00115-8. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16(6):1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, et al. Structural maturation of neural pathways in children and adolescents: In vivo study. Science. 1999;283(5409):1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Pedersen NL, Plomin R, Nesselroade JR, McClearn GE. A quantitative genetic analysis of cognitive abilities during the second half of the life span. Psychological Science. 1992;3:346–353. [Google Scholar]
- Perry E, Martin-Ruiz C, Lee M, Griffiths M, Johnson M, Piggott M, et al. Nicotinic receptor subtypes in human brain ageing, Alzheimer and Lewy body diseases. European Journal of Pharmacology. 2000;393(13):215–222. doi: 10.1016/s0014-2999(00)00064-9. [DOI] [PubMed] [Google Scholar]
- Perry RT, Collins JS, Harrell LE, Acton RT, Go RC. Investigation of association of 13 polymorphisms in eight genes in southeastern African American Alzheimer disease patients as compared to age-matched controls. American Journal of Medical Genetics. 2001;105(4):332–342. doi: 10.1002/ajmg.1371. [DOI] [PubMed] [Google Scholar]
- Phillips JM, McAlonan K, Robb WG, Brown VJ. Cholinergic neurotransmission influences covert orientation of visuospatial attention in the rat. Psychopharmacology (Berlin) 2000;150(1):112–116. doi: 10.1007/s002130000437. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17(5):485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M. Nicotinic receptors in aging and dementia. Journal of Neurobiology. 2002;53(4):641–655. doi: 10.1002/neu.10102. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Welsh-Bohmer KA, Bigler ED, Johnson SC, Anderson CV, Helms MJ, et al. Apolipoprotein E epsilon 4 allele and hippocampal volume in twins with normal cognition. Neurology. 1997;48(4):985–989. doi: 10.1212/wnl.48.4.985. [DOI] [PubMed] [Google Scholar]
- Plomin R. The genetics of g in human and mouse. Nature Reviews. Neuroscience. 2001;2(2):136–141. doi: 10.1038/35053584. [DOI] [PubMed] [Google Scholar]
- Plomin R, Crabbe J. DNA. Psychological Bulletin. 2000;126(6):806–828. doi: 10.1037/0033-2909.126.6.806. [DOI] [PubMed] [Google Scholar]
- Plomin R, Craig I. Human behavioural genetics of cognitive abilities and disabilities. Bioessays. 1997;19(12):1117–1124. doi: 10.1002/bies.950191211. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral Genetics. 4th Worth; New York: 2001. [Google Scholar]
- Poirier J. Apolipoprotein E in animal models of CNS injury and in Alzheimer's disease. Trends in Neurosciences. 1994;17(12):525–530. doi: 10.1016/0166-2236(94)90156-2. [DOI] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nature Reviews. Neuroscience. 2001;2(1):24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, DiGirolamo GJ. Executive attention: Conflict, target detection, and cognitive control. In: Parasuraman R, editor. The Attentive Brain. MIT Press; Cambridge, MA: 1998. pp. 401–423. [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friderich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. Journal of Neuroscience. 1984;4:1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: The Women's Health Initiative Memory Study: A randomized controlled trial. Journal of the American Medical Association. 2003;289(20):2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- Rebeck GW, Alonzo NC, Berezovska O, Harr SD, Knowles RB, Growdon JH, et al. Structure and functions of human cerebrospinal fluid lipoproteins from individuals of different APOE genotypes. Experimental Neurology. 1998;149(1):175–182. doi: 10.1006/exnr.1997.6710. [DOI] [PubMed] [Google Scholar]
- Reed T, Carmelli D, Swan GE. Lower cognitive performance in normal older adult male twins carrying the apolipoprotein E e4 allelle. Archives of Neurology. 1994;52:1189–1192. doi: 10.1001/archneur.1994.00540240033012. [DOI] [PubMed] [Google Scholar]
- Register TC, Adams MR, Golden DL, Clarkson TB. Conjugated equine estrogens alone, but not in combination with medroxyprogesterone acetate, inhibit aortic connective tissue remodeling after plasma lipid lowering in female monkeys. Arteriosclerosis, Thrombosis, and Vascular Biology. 1998;18(7):1164–1171. doi: 10.1161/01.atv.18.7.1164. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Armstrong SM, Matt KS, Mattox JH. The application of positron emission tomography to the study of the normal menstrual cycle. Human Reproduction. 1996;11(12):2799–2805. doi: 10.1093/oxfordjournals.humrep.a019214. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, et al. Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. New England Journal of Medicine. 1996;334(12):752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Uecker A, Caselli RJ, Lewis S, Bandy D, de Leon MJ, et al. Hippocampal volumes in cognitively normal persons at genetic risk for Alzheimer's disease. Annals of Neurology. 1998;44:288–291. doi: 10.1002/ana.410440226. [DOI] [PubMed] [Google Scholar]
- Rempel N, Heyers S, Engels H, Sleegers E, Steinlein OK. The structures of the human neuronal nicotinic acetylcholine receptor beta2- and alpha3-subunit genes (CHRNB2 and CHRNA3) Human Genetics. 1998;103(6):645–653. doi: 10.1007/s004390050885. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Metter EJ, Zonderman AB. Estrogen replacement therapy and longitudinal decline in visual memory. Neurology. 1997;49:1491–1497. doi: 10.1212/wnl.49.6.1491. [DOI] [PubMed] [Google Scholar]
- Rice MM, Graves AB, McCurry SM, Gibbons LE, Bowen JD, McCormick WC, et al. Postmenopausal estrogen and estrogen-progestin use and 2-year rate of cognitive change in a cohort of older Japanese American women: The Kame Project. Archives Internal Medicine. 2000;160(11):1641–1649. doi: 10.1001/archinte.160.11.1641. [DOI] [PubMed] [Google Scholar]
- Rogers SW, Gahring LC, Collins AC, Marks M. Age-related changes in neuronal nicotinic acetylcholine receptor subunit alpha4 expression are modified by long-term nicotine administration. Journal of Neuroscience. 1998;18(13):4825–4832. doi: 10.1523/JNEUROSCI.18-13-04825.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL. Cerebral changes in rats exposed individually to an enriched environment. Journal of Comparative and Physiological Psychology. 1972;80(2):304–313. doi: 10.1037/h0032978. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL. Psychobiology of plasticity: Effects of training and experience on brain and behavior. Behavioural Brain Research. 1996;78(1):57–65. doi: 10.1016/0166-4328(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Sato M, Soma M, Nakayama T, Kanmatsuse K. Dopamine D1 receptor gene polymorphism is associated with essential hypertension. Hypertension. 2000;36(2):183–186. doi: 10.1161/01.hyp.36.2.183. [DOI] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, St. George-Hyslop PH, Pericak-Vance MA, Joo SH, et al. Association of apolipoprotein E allele e4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: Involvement in working memory. Science. 1991;251(4996):947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Schinder AF, Berninger B, Poo M. Postsynaptic target specificity of neurotrophin-induced presynaptic potentiation. Neuron. 2000;25(1):151–163. doi: 10.1016/s0896-6273(00)80879-x. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. Journal of Neuroscience. 1998;18(4):1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, et al. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. Journal of the American Medical Association. 1999;281(13):1197–1202. doi: 10.1001/jama.281.13.1197. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen effects on cognition in menopausal women. Neurology. 1997;48(57):S21–26. doi: 10.1212/wnl.48.5_suppl_7.21s. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive aging in women. Trends in Pharmacological Sciences. 2002;23(11):527–534. doi: 10.1016/s0165-6147(02)02093-x. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Phillips SM. Estrogen and cognitive functioning in surgically menopausal women. Annals of the New York Academy of Sciences. 1990;592:474–476. [Google Scholar]
- Sherwin BB, Tulandi T. “Add-back” estrogen reverses cognitive deficits induced by a gonadotropin-releasing hormone agonist in women with leiomyomata uteri. Journal of Clinical Endocrinology and Metabolism. 1996;81:2545–2549. doi: 10.1210/jcem.81.7.8675575. [DOI] [PubMed] [Google Scholar]
- Sherwood NT, Lo DC. Long-term enhancement of central synaptic transmission by chronic brain-derived neurotrophic factor treatment. Journal of Neuroscience. 1999;19(16):7025–7036. doi: 10.1523/JNEUROSCI.19-16-07025.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue P, Scrimo P, Lane M, Askew R, Merchenthaler I. The distribution of estrogen receptor-beta mRNA in forebrain regions of the estrogen receptor-alpha knockout mouse. Endocrinology. 1997;138(12):5649–5652. doi: 10.1210/endo.138.12.5712. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Thal L, Wallace RB, Ockene JK, Hendrix SL, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: The Women's Health Initiative Memory Study: A randomized controlled trial. Journal of the American Medical Association. 2003;289(20):2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Reboussin BA, Espeland MA, Rapp SR, McBee WL, Dailey M, et al. The Women's Health Initiative Memory Study (WHIMS): A trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Controlled Clinical Trials. 1998;19(6):604–621. doi: 10.1016/s0197-2456(98)00038-5. [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Simpkins JW. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology. 1995;136(5):2320–2324. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- Sliwinski M, Lipton RB, Buschke H, Stewart W. The effects of preclinical dementia on estimates of normal cognitive functioning in aging. Journal of Gerontology. Series B, Psychological Sciences and Social Sciences. 1996;51:P217–225. doi: 10.1093/geronb/51b.4.p217. [DOI] [PubMed] [Google Scholar]
- Smith CD, Andersen AH, Kryscio RJ, Schmitt FA, Kindy MS, Blonder LX, et al. Altered brain activation in cognitively intact individuals at high risk for Alzheimer's disease. Neurology. 1999;53(7):1391–1396. doi: 10.1212/wnl.53.7.1391. [DOI] [PubMed] [Google Scholar]
- Smith DE, Roberts J, Gage FH, Tuszynski MH. Age-associated neuronal atrophy occurs in the primate brain and is reversible by growth factor gene therapy. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(19):10893–10898. doi: 10.1073/pnas.96.19.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Smith GE, Bohac DL, Waring SC, Kokmen E, Tangalos EG, Ivnik RJ, et al. Apolipoprotein E genotype influences cognitive “phenotype” in patients with Alzheimer's disease but not in healthy control subjects. Neurology. 1998;50(2):355–362. doi: 10.1212/wnl.50.2.355. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Dalen L, Daley D, Remington B. Are planning, working memory, and inhibition associated with individual differences in preschool ADHD symptoms? Developmental Neuropsychology. 2002;21(3):255–272. doi: 10.1207/S15326942DN2103_3. [DOI] [PubMed] [Google Scholar]
- Sorbi S, Nacmias B, Forleo P, Latorraca S, Gobbini I, Bracco L, et al. ApoE allele frequencies in Italian sporadic and familial Alzheimer's disease. Neuroscience Letters. 1994;177(12):100–102. doi: 10.1016/0304-3940(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Spearman C. “General intelligence” objectively determined and measured. American Journal of Psychology. 1904;15:201–293. [Google Scholar]
- Steinlein OK, Magnusson A, Stoodt J, Bertrand S, Weiland S, Berkovic SF, et al. An insertion mutation of the CHRNA4 gene in a family with autosomal dominant nocturnal frontal lobe epilepsy. Human Molecular Genetics. 1997a;6(6):943–947. doi: 10.1093/hmg/6.6.943. [DOI] [PubMed] [Google Scholar]
- Steinlein OK, Deckert J, Nothen MM, Franke P, Maier W, Beckmann H, et al. Neuronal nicotinic acetylcholine receptor alpha 4 subunit (CHRNA4) and panic disorder: An association study. American Journal of Medical Genetics. 1997b;74(2):199–201. doi: 10.1002/(sici)1096-8628(19970418)74:2<199::aid-ajmg17>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Steinlein OK, Stoodt J, de Vos RA, Steur EN, Wevers A, Schutz U, et al. Mutation screening of the CHRNA4 and CHRNB2 nicotinic cholinergic receptor genes in Alzheimer's disease. Neuroreport. 1999;10(14):2919–2922. doi: 10.1097/00001756-199909290-00008. [DOI] [PubMed] [Google Scholar]
- Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Finch CE. Increased synaptic sprouting in response to estrogen via an apolipoprotein E-dependent mechanism: Implications for Alzheimer's disease. Journal of Neuroscience. 1998;18(9):3180–3185. doi: 10.1523/JNEUROSCI.18-09-03180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel A, Wehr A, Michel A, Brocke B. Association between the dopamine D4 receptor (DRD4) exon III polymorphism and measures of Novelty Seeking in a German population. Molecular Psychiatry. 1999;4(4):378–384. doi: 10.1038/sj.mp.4000535. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Fifield WJ, Kennedy MA, Mulder RT, Sellman JD, Joyce PR. No association between novelty seeking and the type 4 dopamine receptor gene (DRD4) in two New Zealand samples. American Journal of Psychiatry. 1998;155(1):98–101. doi: 10.1176/ajp.155.1.98. [DOI] [PubMed] [Google Scholar]
- Swan GE, Carmelli D. Evidence for genetic mediation of executive control: A study of aging male twins. Journal of Gerontology. Series B, Psychological Sciences and Social Sciences. 2002;57(2):P133–143. doi: 10.1093/geronb/57.2.p133. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Oosterlaan J, Murias M, Schuck S, Flodman P, Spence MA, et al. Attention deficit/hyperactivity disorder children with a 7-repeat allele of the dopamine receptor D4 gene have extreme behavior but normal performance on critical neuropsychological tests of attention. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(9):4754–4759. doi: 10.1073/pnas.080070897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM, Posner M, Potkin S, Bonforte S, Youpa D, Fiore C, et al. Activating tasks for the study of visual-spatial attention in ADHD children: A cognitive anatomic approach. Journal of Child Neurology. 1991;6(Supplement):S119–127. doi: 10.1177/0883073891006001s12. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Sunohara GA, Kennedy JL, Regino R, Fineberg E, Wigal T, et al. Association of the dopamine receptor D4 (DRD4) gene with a refined phenotype of attention deficit hyperactivity disorder (ADHD): A family-based approach. Molecular Psychiatry. 1998;3(1):38–41. doi: 10.1038/sj.mp.4000354. [DOI] [PubMed] [Google Scholar]
- Sylvester CY, Wager TD, Lacey SC, Hernandez L, Nichols TE, Smith EE, et al. Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia. 2003;41(3):357–370. doi: 10.1016/s0028-3932(02)00167-7. [DOI] [PubMed] [Google Scholar]
- Talbot C, Lendon C, Craddock N, Shears S, Morris JC, Goate A. Protection against Alzheimer's disease with apoE e2. Lancet. 1994;343:1432–1433. doi: 10.1016/s0140-6736(94)92557-7. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. Journal of Neuroscience. 1999;19(14):5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. Journal of Clinical Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- Teter B, Xu PT, Gilbert JR, Roses AD, Galasko D, Cole GM. Defective neuronal sprouting by human apolipoprotein E4 is a gain-of-negative function. Journal of Neuroscience Research. 2002;68(3):331–336. doi: 10.1002/jnr.10221. [DOI] [PubMed] [Google Scholar]
- Thapar A, Holmes J, Poulton K, Harrington R. Genetic basis of attention deficit and hyperactivity. British Journal of Psychiatry. 1999;174:105–111. doi: 10.1192/bjp.174.2.105. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, vanErp T, Poutanen VP, Huttunen M, et al. Genetic influences on brain structure. Nature Neuroscience. 2001;4(12):1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Hallikainen T, Lachman H, Saito T, Volavka J, Kauhanen J, et al. Association between the functional variant of the catechol-O-methyltransferase (COMT) gene and type 1 alcoholism. Molecular Psychiatry. 1999;4(3):286–289. doi: 10.1038/sj.mp.4000509. [DOI] [PubMed] [Google Scholar]
- Tohgi H, Utsugisawa K, Yoshimura M, Nagane Y, Mihara M. Age-related changes in nicotinic acetylcholine receptor subunits alpha4 and beta2 messenger RNA expression in postmortem human frontal cortex and hippocampus. Neuroscience Letters. 1998;245(3):139–142. doi: 10.1016/s0304-3940(98)00205-5. [DOI] [PubMed] [Google Scholar]
- Tsukada H, Kakiuchi T, Nishiyama S, Ohba H, Sato K, Harada N, et al. Age differences in muscarinic cholinergic receptors assayed with (+)N- [(11)C]methyl-3-piperidyl benzilate in the brains of conscious monkeys. Synapse. 2001;41(3):248–257. doi: 10.1002/syn.1082. [DOI] [PubMed] [Google Scholar]
- Turic D, Fisher PJ, Plomin R, Owen MJ. No association between apolipoprotein E polymorphisms and general cognitive ability in children. Neuroscience Letters. 2001;299(12):97–100. doi: 10.1016/s0304-3940(00)01789-4. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. Journal of Neuroscience. 2001;21(12):4249–4258. doi: 10.1523/JNEUROSCI.21-12-04249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291(5504):657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Utermann G, Langenbeck U, Beisiegel U, Weber W. Genetics of the apolipoprotein E system in man. American Journal of Human Genetics. 1980;32(3):339–347. [PMC free article] [PubMed] [Google Scholar]
- vanDuijn CM, deKnijff P, Wehnert A, DeVoecht J, Bronzova JB, Havekes LM, et al. The apolipoprotein E epsilon 2 allele is associated with an increased risk of early-onset Alzheimer's disease and a reduced survival. Annals of Neurology. 1995;37(5):605–610. doi: 10.1002/ana.410370510. [DOI] [PubMed] [Google Scholar]
- Vernon PA, Wickett JC, Banzana PG, Stelmack RM. In: Handbook of intelligence. Sternberg RJ, editor. Cambridge University Press; Cambridge, UK: 2000. [Google Scholar]
- Voytko ML. Cognitive functions of the basal forebrain cholinergic system in monkeys: Memory or attention? Behavioural Brain Research. 1996;75(12):13–25. doi: 10.1016/0166-4328(95)00143-3. [DOI] [PubMed] [Google Scholar]
- Voytko ML, Olton DS, Richardson RT, Gorman LK, Tobin JR, Price DL. Basal forebrain lesions in monkeys disrupt attention but not learning and memory. Journal of Neuroscience. 1994;14(1):167–186. doi: 10.1523/JNEUROSCI.14-01-00167.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Bjork RA, Schacter DL. Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral prefrontal cortex. Neuroimage. 2001;14(6):1337–1347. doi: 10.1006/nimg.2001.0936. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, et al. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281(5380):1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Wassink TH, Nelson JJ, Crowe RR, Andreasen NC. Heritability of BDNF alleles and their effect on brain morphology in schizophrenia. American Journal of Medical Genetics. 1999;88(6):724–728. doi: 10.1002/(sici)1096-8628(19991215)88:6<724::aid-ajmg25>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. Psychological Corporation; New York: 1981. [Google Scholar]
- Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, et al. Prefrontal neurons and the genetics of schizophrenia. Biological Psychiatry. 2001;50(11):825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- White F, Nicoll JA, Horsburgh K. Alterations in ApoE and ApoJ in relation to degeneration and regeneration in a mouse model of entorhinal cortex lesion. Experimental Neurology. 2001;169(2):307–318. doi: 10.1006/exnr.2001.7655. [DOI] [PubMed] [Google Scholar]
- White F, Nicoll JA, Roses AD, Horsburgh K. Impaired neuronal plasticity in transgenic mice expressing human apolipoprotein E4 compared to E3 in a model of entorhinal cortex lesion. Neurobiology of Disease. 2001;8(4):611–625. doi: 10.1006/nbdi.2001.0401. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ. Genesis of Alzheimer's disease. Neurology. 1997;48:S2–S7. doi: 10.1212/wnl.48.5_suppl_7.2s. [DOI] [PubMed] [Google Scholar]
- Wise PM, Dubal DB, Wilson ME, Rau SW, Bottner M. Minireview: Neuroprotective effects of estrogen-new insights into mechanisms of action. Endocrinology. 2001;142(3):969–973. doi: 10.1210/endo.142.3.8033. [DOI] [PubMed] [Google Scholar]
- Witte EA, Davidson MC, Marrocco RT. Effects of altering brain cholinergic activity on covert orienting of attention: Comparison of monkey and human performance. Psychopharmacology (Berlin) 1997;132(4):324–334. doi: 10.1007/s002130050352. [DOI] [PubMed] [Google Scholar]
- Woo JM, Yoon KS, Yu BH. Catechol Omethyltransferase genetic polymorphism in panic disorder. American Journal of Psychiatry. 2002;159(10):1785–1787. doi: 10.1176/appi.ajp.159.10.1785. [DOI] [PubMed] [Google Scholar]
- Wood JG, Joyce PR, Miller AL, Mulder RT, Kennedy MA. A polymorphism in the dopamine beta-hydroxylase gene is associated with “paranoid ideation” in patients with major depression. Biological Psychiatry. 2002;51(5):365–369. doi: 10.1016/s0006-3223(01)01367-1. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. Journal of Neuroscience. 1992;12(7):2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KK, Anderson V, Castiello U. Neuropsychological evaluation of deficits in executive functioning for ADHD children with or without learning disabilities. Developmental Neuropsychology. 2002;22(2):501–531. doi: 10.1207/S15326942DN2202_5. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. Cholinergic switching within neocortical inhibitory networks. Science. 1998;281(5379):985–988. doi: 10.1126/science.281.5379.985. [DOI] [PubMed] [Google Scholar]
- Xu B, Gottschalk W, Chow A, Wilson RI, Schnell E, Zang K, et al. The role of brain-derived neurotrophic factor receptors in the mature hippocampus: Modulation of long-term potentiation through a presynaptic mechanism involving TrkB. Journal of Neuroscience. 2000;20(18):6888–6897. doi: 10.1523/JNEUROSCI.20-18-06888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Gouras GK, Greenfield JP, Vincent B, Naslund J, Mazzarelli L, et al. Estrogen reduces neuronal generation of Alzheimer beta-amyloid peptides. Nature Medicine. 1998;4(4):447–451. doi: 10.1038/nm0498-447. [DOI] [PubMed] [Google Scholar]
- Xu HW, Li XC, Li HD, Ruan HZ, Liu ZZ. Effects of corticotrophin on pain behavior and BDNF, CRF levels in frontal cortex of rats suffering from chronic pain. Acta Pharmacologica Sinica. 2000;21(7):600–604. [PubMed] [Google Scholar]
- Yaffe K. Hormone therapy and the brain: Deja vu all over again? Journal of the American Medical Association. 2003;289(20):2717–2719. doi: 10.1001/jama.289.20.2717. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Cauley J, Sands L, Browner W. Apolipoprotein E phenotype and cognitive decline in a prospective study of elderly community women. Archives of Neurology. 1997;54(9):1110–1114. doi: 10.1001/archneur.1997.00550210044011. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Haan M, Byers A, Tangen C, Kuller L. Estrogen use, APOE, and cognitive decline: Evidence of gene-environment interaction. Neurology. 2000;54(10):1949–1954. doi: 10.1212/wnl.54.10.1949. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lui LY, Grady D, Stone K, Morin P. Estrogen receptor 1 polymorphisms and risk of cognitive impairment in older women. Biological Psychiatry. 2002;51(8):677–682. doi: 10.1016/s0006-3223(01)01289-6. [DOI] [PubMed] [Google Scholar]
- Zabetian CP, Anderson GM, Buxbaum SG, Elston RC, Ichinose H, Nagatsu T, et al. A quantitative-trait analysis of human plasma-dopamine beta-hydroxylase activity: Evidence for a major functional polymorphism at the DBH locus. American Journal of Human Genetics. 2001;68(2):515–522. doi: 10.1086/318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu ZW, Friess H, Wang L, Zimmermann A, Buchler MW. Brain-derived neurotrophic factor (BDNF) is upregulated and associated with pain in chronic pancreatitis. Digestive Diseases and Sciences. 2001;46(8):1633–1639. doi: 10.1023/a:1010684916863. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, et al. COMT val158met genotype affects mu-opioid neuro-transmitter responses to a pain stressor. Science. 2003;299(5610):1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]