Abstract
Objectives: To examine whether the increased risk of cardiovascular events with rofecoxib represents a class effect of cyclooxygenase-2 (COX-2) specific inhibitors.
Design: Systematic review and meta-analysis of randomized double-blind clinical trials of celecoxib of at least 6 weeks' duration and presented data on serious cardiovascular thromboembolic events. Data sources included six bibliographic databases, the relevant files of the United States Food and Drug Administration, and pharmaceutical company websites.
Main outcome measures: Pooled fixed effects estimates of the odds ratios for risk of cardiovascular events with celecoxib compared with comparator treatment were calculated using the inverse variance weight method. The main outcome measure was myocardial infarction.
Results: Four placebo-controlled trials with 4422 patients were included in the primary meta-analysis comparing celecoxib with placebo. The odds ratio of myocardial infarction with celecoxib compared to placebo was 2.26 (95%confidence interval 1.0 to 5.1). For composite cardiovascular events [odd ratio 1.38 (95% CI 0.91 to 2.10)], cardiovascular deaths [OR 1.06 (95% CI 0.38 to 2.95)] and stroke [OR 1.0(95% CI 0.51 to 1.84)] there was no significant increase in risk with celecoxib. The secondary meta-analysis which included a total of six studies (with placebo, diclofenac, ibuprofen, and paracetamol as comparators) of 12 780 patients, showed similar findings with a significant increased risk with celecoxib for myocardial infarction [OR 1.88 (95% CI 1.15 to 3.08)] but not other outcome measures.
Conclusion: The available data indicate an increased risk of myocardial infarction with celecoxib therapy, consistent with a class effect for COX-2 specific inhibitors.
INTRODUCTION
The extent to which the increased risk of cardiovascular events associated with the use of the COX-2 specific inhibitor rofecoxib also applies to celecoxib is a matter of considerable debate.1-4 The uncertainty derives from the celecoxib research programme being primarily designed to determine its efficacy and the risk of gastrointestinal adverse effects, with clinical trials being neither powered nor primarily designed to determine the cardiovascular risk associated with its use. Furthermore, there is an apparent discordance in the findings of those studies which have reported information on cardiovascular events. For example, the Celecoxib Long-term Arthritis Safety Study (CLASS) reported no increase in major cardiovascular thromboembolic events, including myocardial infarction,5 whereas the recent Colorectal Adenoma Prevention Trial reported a 2.3- and 3.4-fold increased risk of cardiovascular events with the 400 mg and 800 mg daily doses of celecoxib, respectively.6 Alternative approaches have also reported conflicting results with both an increased risk and no risk being reported from studies of varied design.7-11
In an attempt to clarify this issue we have undertaken a systematic review and meta-analysis of double-blind, randomized, controlled studies of celecoxib that presented data on serious cardiovascular events. The primary meta-analysis included clinical trials that compared celecoxib with placebo, whereas in the secondary meta-analysis the placebo-controlled trials were analysed together with controlled trials that used non-steroidal anti-inflammatory drugs (NSAIDs) or paracetamol as comparator treatments.
METHODS
Search strategy
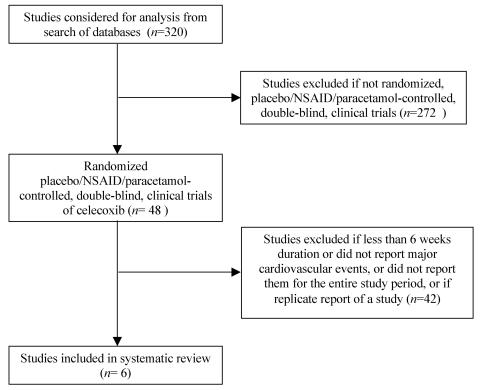
A search of studies containing the key words `celecoxib' or `COX-2 inhibitors and cardiovascular events' was conducted from Medline, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, ACP Journal Club, Database of Abstracts of Review of Effects, and EMBASE to April 2005. The United States Food and Drug Administration (FDA) website was scrutinized, including the proceedings of the relevant advisory committee meetings. Websites relating to celecoxib were reviewed, including those posted by Pfizer, the manufacturer of celecoxib. Pfizer was also approached for details of all relevant studies; no additional data were provided. Two researchers independently examined each paper for inclusion. The reference lists of relevant studies were also examined. The search strategy as recommended by the QUOROM statement is shown in Figure 1.
Figure 1.
Process of inclusion of studies in systematic review. NSAID, non-steroidal anti-inflammatory drug
Inclusion criteria
To be included in the systematic review, studies had to be randomized, double-blind, controlled clinical trials of at least 6 weeks' duration and report serious cardiovascular thromboembolic events.
The primary meta-analysis included clinical trials that compared celecoxib with placebo. A secondary meta-analysis included trials that compared celecoxib with placebo, paracetamol or an NSAID.
Outcome measures
The primary outcome variable was myocardial infarction (fatal or non-fatal). The secondary outcome variables were fatal or non-fatal cerebrovascular events (thrombotic or haemorrhagic), cardiovascular mortality, and the composite outcome of serious cardiovascular thromboembolic events. Criteria for composite cardiovascular thromboembolic events were derived from the publication of the cardiovascular events from the CLASS trial.12 The criteria included myocardial infarction, cerebrovascular events, cardiovascular death, unstable angina or peripheral vascular event (arterial or venous). Two reviewers extracted the cardiovascular event data using a standardized form. Completed data forms were then checked by a third reviewer.
Analysis
The number of cardiovascular thromboembolic events in the celecoxib and control groups was stated. The pooled fixed effects estimates of the odds ratio for risk of cardiovascular events with the use of the active treatment were calculated by standard methods using the inverse variance weighting method.13 For zero cell counts, the standard method of adding 0.5 to each cell count was used. The I-squared inconsistency statistic was also calculated. The I-squared statistic is the percentage of total variation across studies due to heterogeneity.14 These methods assume a constant hazard ratio over time, that is the ratio of the myocardial infarction rate of one treatment compared with the other is the same at all time points. A secondary analysis was carried out by calculating the pooled absolute difference for the outcome variable of those studies which had at least one event.13
Study characteristics
The characteristics of the studies were reviewed to determine whether they were designed or powered to identify the risk of cardiovascular events with celecoxib therapy. With regard to design, the studies were reviewed to determine whether the assessment of cardiovascular risk was stated as one of the objectives, subjects with cardiovascular disease were included, criteria were defined for diagnosing a cardiovascular event, an electrocardiogram was routinely undertaken both pre- and post-treatment, and whether a blinded external board reviewed the cardiovascular events.
Power calculation
A power calculation was undertaken to determine whether the individual studies had an adequate power to identify an increased risk of myocardial infarction with celecoxib therapy. For a study to have 80% power, at a type I error rate of 5%, to detect the difference between an end point occurring 0.4% of the time and 0.8% of the time, there would need to be around 5850 subjects in each arm of a two arm trial. This calculation assumes a simple binomial test for a difference in proportions carried out once at study termination, rather than a more sophisticated survival analysis that might be used in such a trial. The choice of 0.4% for endpoint occurrence was based on this crude proportion from the four placebo controlled trials.
RESULTS
There were 48 randomized, double-blind clinical trials of celecoxib identified in the systematic review. Of these, 42 studies were excluded as the trial period was not of at least 6 weeks' duration, or major cardiovascular thromboembolic events (myocardial infarction, cerebrovascular event or cardiovascular death) were not reported or if they represented duplicate reports. Four placebo-controlled studies with a total of 4422 subjects were included in the primary meta-analysis.6,15-17 Six studies with a total of 12 780 subjects were included in the secondary analysis with comparator treatments including placebo, paracetamol, ibuprofen and diclofenac.6,12,15-18
The characteristics of the studies included in the meta-analyses are shown in Table 1. Treatment was for a wide range of medical conditions including rheumatoid arthritis, osteoarthritis, Alzheimer's disease, and the prevention of colorectal adenoma in high risk subjects. The duration of treatment ranged from 6-161 weeks. The doses of celecoxib studied were 200, 400 and 800 mg/day.
Table 1.
Characteristics of studies
|
Study
|
Condition
|
Duration (weeks)
|
Celecoxib treatment
|
Comparator treatment#
|
|||
|---|---|---|---|---|---|---|---|
| No. of subjects | Total daily dose (mg) | Dosing regime | No. of subjects | Treatment | |||
| Solomon et al. (Ref 6) | P | 145-161 | 1356 | 400 800 | BD | 679 | Pl |
| CLASS (White et al., Ref 12)* | OA/RA | 52 | 3987 | 800 | BD | 3981 | lb, Di |
| Pfizer (Ref 15) | AD | 52 | 285 | 400 | BD | 140 | Pl |
| McKenna et al. (Ref 16) | OA | 6 | 201 | 200 | BD | 399 | Pl, Di |
| PreSAP (Levin, Ref 17) | P | 156 | 933 | 800 | OD | 628 | Pl |
| Geba et al. (Ref 18) | OA | 6 | 97 | 200 | OD | 94 | Para |
RA, rheumatoid arthritis; OA, osteoarthritis; AD, Alzheimer's disease; P, prevention of colorectal adenoma in high risk subjects; lb, ibuprofen; Di, diclofenac; Pl, placebo; Para, paracetamol; CV, cardiovascular; OD, once daily; BD, twice daily; PreSAP, Prevention of Colorectal Sporadic Adenomatous Polyps
For the CLASS (Celecoxib Long-term Arthritis Safety Study) trial, the findings documented in White et al. (Ref 12) were used in the meta-analysis
Two of the studies have not yet been published in peer-reviewed journals and the data for these trials were sourced from the internet.15,17,19,20 Data from the CLASS trial have been published in several papers and FDA internet files.5,12,21,22 For our meta-analysis, the findings as documented in the White et al. publication12 were used, as it specifically reported the cardiovascular events. Two web-based publications presented data from the Alzheimer's disease trial;15,20 for our meta-analysis, the findings from the Pfizer document were used as this report presented the most detailed database.15
Study quality
The six studies included in the meta-analyses were neither powered nor specifically designed to determine the risk of cardiovascular thromboembolic events associated with celecoxib therapy. Specifically, there were no studies that had an adequate sample size to determine a twofold increased risk of myocardial infarction with celecoxib therapy. In none of the studies was it documented that there was a systematic surveillance for cardiovascular events such as an ECG taken both pre- and post-treatment, and only one study defined the criteria for the diagnosis of cardiovascular events.6 A minority of studies reported that a blinded external board reviewed the cardiovascular data.
There were discrepancies in the reporting of cardiovascular events between the various publications of the CLASS trial. For example, no cardiovascular deaths were reported in the initial publication,5 whereas there were 13 cardiovascular deaths in the FDA report22 (five on celecoxib, eight on an NSAID) and 20 in the White et al. publication12 (10 in each treatment group). There were also variations in the number of adverse events and the way such events were classified in the two web-based reports of the Alzheimer's study.15,20 The document placed on the internet by Pfizer15 reported the incidence of cerebrovascular disorder as five events in three patients in the placebo group, compared to eight events in six patients in the celecoxib group. In contrast, on the FDA website20 the incidence of any cerebrovascular event was three in the placebo group and seven in the celecoxib group. In the Solomon et al. study6 and the PreSAP trial17,19 the causes of the cardiovascular deaths in the different treatment groups were not stated. As a result, it was not possible to determine the number of myocardial infarctions or cerebrovascular events that were fatal, and as a result, only the reported non-fatal events could be included in our meta-analysis for myocardial infarction and cerebrovascular event.
Primary meta-analysis
Myocardial infarction
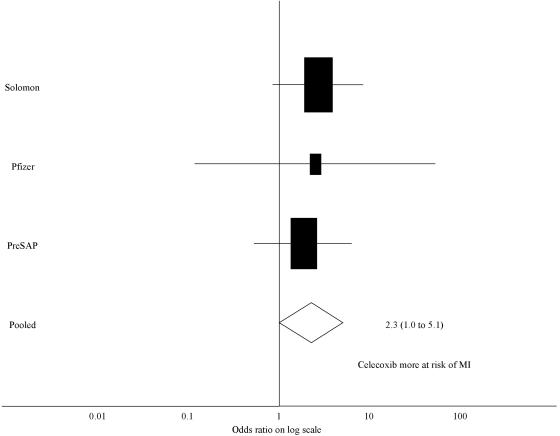
In the three studies which reported myocardial infarction data, there were 29 myocardial infarctions in the 2574 subjects on celecoxib (1.13%), and six myocardial infarctions in the 1447 subjects in the placebo group (0.41%). The odds ratio for a myocardial infarction with celecoxib was 2.26 (95% CI 1.0 to 5.1) (Figure 2).
Figure 2.
Forest plot of the log of the ratio for risk of myocardial infarction with the use of celecoxib compared to placebo, using the inverse variance weighting method (areas inversely proportional to the variance of the estimate). MI, myocardial infarction
Cerebrovascular events
There were 24 cerebrovascular events in the 2775 subjects (0.86%) of the celecoxib group compared to 13 cerebrovascular events in the placebo group of 1647 subjects (0.79%). The odds ratio was 1.0 (95% CI 0.51 to 1.84).
Cardiovascular death
In the three studies that reported the number of deaths from cardiovascular causes, there were 16 cardiovascular deaths in the 2574 subjects taking celecoxib (0.62%) and seven cardiovascular deaths in the 1447 subjects on placebo (0.48%). The odds ratio for cardiovascular death was 1.06 (95% CI 0.38 to 2.95).
Composite cardiovascular events
There were 79 composite cardiovascular events in the 2775 subjects in the celecoxib group (2.85%) and 30 composite cardiovascular events in the 1647 subjects taking placebo (1.82%). The odds ratio for a composite cardiovascular event was 1.38 (95% CI 0.91 to 2.10).
Table 2.
Raw data for the placebo controlled trials (primary analysis)
|
Study
|
Celecoxib
|
|
Placebo
|
|
OR (95% CI)
|
|---|---|---|---|---|---|
| Events | Total No. subjects | Events | Total No. subjects | ||
| Myocardial infarction* | |||||
| Solomon et al. (Ref 6) | 18 | 1356 | 3 | 679 | 2.67 (0.5 to 8.41) |
| Pfizer (Ref 15) | 2 | 285 | 0 | 140 | 2.48 (0.12 to 52.0) |
| PreSAP (Levin, Ref 17) | 9 | 933 | 3 | 628 | 1.84 (0.54 to 6.28) |
| Pooled | 2.26 (1.0 to 5.1) | ||||
| Absolute risk difference | 0.7% (0.2 to 1.2) | ||||
| I-squared statistic | 0 (0 to 0) | ||||
| Cerebrovascular event | |||||
| Solomon et al. (Ref 6) | 8 | 1356 | 3 | 679 | 1.22 (0.35 to 4.24) |
| Pfizer (Ref 15) | 8 | 285 | 5 | 140 | 0.75 (0.25 to 2.25) |
| McKenna et al. (Ref 16) | 0 | 201 | 0 | 200 | 1.0 (0.02 to 50.4) |
| PreSAP (Ref 17) | 8 | 933 | 5 | 628 | 1.04 (0.35 to 4.24) |
| Pooled | 1.0 (0.51 to 1.84) | ||||
| Absolute risk difference | 0.1% (−0.4 to 0.6) | ||||
| I-squared statistic | 0 (0 to 0) | ||||
| Cardiovascular deaths* | |||||
| Solomon et al. (Ref 6) | 9 | 1356 | 1 | 679 | 4.5 (0.57 to 35.8) |
| Pfizer (Ref 15) | 5 | 285 | 2 | 140 | 1.23 (0.24 to 6.4) |
| PreSAP (Levin, Ref 17) | 2 | 933 | 4 | 628 | 0.34 (0.06 to 1.84) |
| Pooled | 1.06 (0.38 to 2.95) | ||||
| Absolute risk difference | 0.9% (−0.1 to 1.8) | ||||
| I-squared statistic | 46 (0 to 84) | ||||
| Composite cardiovascular event: | |||||
| Solomon et al. (Ref 6) | 49 | 1356 | 13 | 679 | 1.87 (1.01 to 3.43) |
| Pfizer (Ref 15) | 11 | 285 | 5 | 140 | 1.03 (0.37 to 2.91) |
| McKenna et al. (Ref 16) | 0 | 201 | 0 | 200 | 1.0 (0.02 to 50.4) |
| PreSAP (Levin, Ref 17) | 19 | 933 | 12 | 628 | 1.05 (0.51 to 2.16) |
| Pooled | 1.38 (0.91 to 2.10) | ||||
| Absolute risk difference | 0.9% (−0.1 to 1.8) | ||||
| I-squared statistic | 0 (0 to 0) |
Secondary meta-analysis
Myocardial infarction
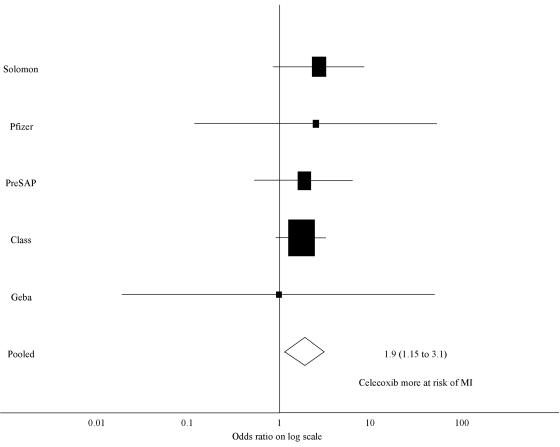
In the five studies that reported myocardial infarction data, there were 55 myocardial infarctions in the 6658 subjects (0.83%) on celecoxib compared to 21 myocardial infarctions in the 5522 subjects receiving control treatment (0.38%). The odds ratio for the risk of a myocardial infarction with celecoxib treatment was 1.88 (95% CI 1.15 to 3.08) (Figure 3).
Figure 3.
Forest plot of the log of the ratio for risk of myocardial infarction with the use of celecoxib compared to all comparator treatments (including placebo, diclofenac, ibuprofen, and paracetamol), using the inverse variance weighting method (areas inversely proportional to the variance of the estimate). MI, myocardial infarction
Cerebrovascular events
There were 28 cerebrovascular events in the 6859 subjects (0.41%) on celecoxib and 27 in 5921 subjects (0.46%) receiving the control treatment. The odds ratio for a cerebrovascular event with celecoxib treatment was 0.73 (95% CI 0.42 to 1.26).
Cardiovascular death
In the five studies that reported the number of deaths from cardiovascular causes, there were 26 cardiovascular deaths in 6561 subjects taking celecoxib (0.40%) and 17 cardiovascular deaths in the 5428 subjects on control treatment (0.31%). The odds ratio for the risk of a cardiovascular death with celecoxib therapy was 1.02 (95% CI 0.52 to 1.99).
Composite cardiovascular events
There were 134 composite cardiovascular events in 6859 subjects (1.95%) on celecoxib treatment and 81 in 5921 subjects (1.37%) on control treatments. The odds ratio for the risk of a composite cardiovascular event with celecoxib therapy was 1.22 (95% CI 0.92 to 1.62).
DISCUSSION
Principal findings
This meta-analysis provides evidence that the use of celecoxib, the most commonly prescribed COX-2 specific inhibitor, is associated with an increased risk of myocardial infarction. While this finding is limited by the small number of clinical trials of celecoxib that reported cardiovascular outcomes, it is consistent with a class effect of COX-2 specific inhibitors increasing the risk of myocardial infarction.
Strengths and weaknesses of the study
There are a number of methodological issues relevant to the interpretation of the findings. The first is whether all available studies were included in the meta-analysis. Despite the recognized difficulties encountered with publication bias, and the requirement to access multiple company and FDA websites,23 we are confident that we have identified all eligible studies of celecoxib. There was a requirement for studies to present data on at least one of the three major serious cardiovascular outcome measures, namely myocardial infarction, cardiovascular death or cerebrovascular event. We considered that an inability to report the presence or absence of these outcome measures indicated that the study was neither designed nor capable of identifying the occurrence of serious cardiovascular thromboembolic events.
Table 3.
Raw data for the placebo, diclofenac, ibuprofen and paracetamol controlled trials (secondary analysis)
|
Study
|
Celecoxib
|
|
Other
|
|
OR (95% CI)
|
|---|---|---|---|---|---|
| Events | Total No. subjects | Events | Total No. subjects | ||
| Myocardial infarction | |||||
| Solomon et al. (Ref 6) | 18 | 1356 | 3 | 679 | 2.67 (0.5 to 8.41) |
| CLASS (White et al., Ref 12) | 26 | 3987 | 15 | 3981 | 1.71 (0.91 to 3.21) |
| Pfizer (Ref 15) | 2 | 285 | 0 | 140 | 2.48 (0.12 to 52.0) |
| PreSAP (Levin, Ref 17) | 9 | 933 | 3 | 628 | 1.84 (0.54 to 6.28) |
| Geba et al. (Ref 18) | 0 | 97 | 0 | 94 | 0.97 (0.02 to 49.4) |
| Pooled | 1.88 (1.15 to 3.08) | ||||
| I-squared statistic | 0 (0 to 0) | ||||
| Cerebrovascular events | |||||
| Solomon et al. (Ref 6) | 8 | 1356 | 3 | 679 | 1.22 (0.35 to 4.24) |
| CLASS (White et al. Ref 12) | 4 | 3987 | 12 | 3981 | 0.36 (0.12 to 1.05) |
| Pfizer (Ref 15) | 8 | 285 | 5 | 140 | 0.75 (0.25 to 2.25) |
| McKenna et al. (Ref 16) | 0 | 201 | 2 | 399 | 0.39 (0.02 to 8.3) |
| PreSAP (Levin, Ref 17) | 8 | 933 | 5 | 628 | 1.04 (0.35 to 4.24) |
| Geba et al. (Ref 18) | 0 | 97 | 0 | 94 | 0.97 (0.02 to 49.4) |
| Pooled | 0.73 (0.42 to 1.26) | ||||
| I-squared statistic | 0 (0 to 56) | ||||
| Cardiovascular death | |||||
| Solomon et al. (Ref 6) | 9 | 1356 | 1 | 679 | 4.5 (0.57 to 35.8) |
| CLASS (White et al. Ref 12) | 10 | 3987 | 10 | 3981 | 0.99 (0.42 to 2.4) |
| Pfizer (Ref 15) | 5 | 285 | 2 | 140 | 1.23 (0.24 to 6.4) |
| PreSAP (Levin, Ref 17) | 2 | 933 | 4 | 628 | 0.34 (0.06 to 1.84) |
| Pooled | 1.02 (0.52 to 1.99) | ||||
| I-squared statistic | 18 (0 to 88) | ||||
| Composite cardiovascular events | |||||
| Solomon et al. (Ref 6) | 49 | 1356 | 13 | 679 | 1.87 (1.01 to 3.43) |
| CLASS (White et al., Ref 12) | 55 | 3987 | 49 | 3981 | 1.12 (0.76 to 1.65) |
| Pfizer (Ref 15) | 11 | 285 | 5 | 140 | 1.03 (0.37 to 2.91) |
| McKenna et al. (Ref 16) | 0 | 201 | 2 | 399 | 0.39 (0.02 to 8.3) |
| PreSAP (Levin, Ref 17) | 19 | 933 | 12 | 628 | 1.05 (0.51 to 2.16) |
| Geba et al. (Ref 18) | 0 | 97 | 0 | 94 | 0.97 (0.02 to 49.4) |
| Pooled | 1.22 (0.92 to 1.62) | ||||
| I-squared statistic | 0 (0 to 56) |
Another issue was the decision to undertake a primary meta-analysis restricted to studies which compared celecoxib with placebo. While this reduced the number of eligible studies, it did avoid the potential difficulty of interpreting studies in which comparisons were made with treatments such as NSAIDs which may influence cardiovascular risk.
It was not possible to investigate the potential effect that total daily dose, frequency of dosing and duration of treatment might have in determining cardiovascular risk due to the small number of trials that met the inclusion criteria. In terms of the duration of treatment all but two studies were of at least 1 year's duration. These two studies were of only 6 weeks' duration, were the smallest and of poorest quality, and added only two cardiovascular events to the combined meta-analyses. Inclusion of these studies reduced the magnitude of the risk of myocardial infarction associated with celecoxib therapy.
Trials over 6 weeks' duration were chosen to exclude trials where a single dose was administered or the duration of treatment was too short to expect there to be any long-term sequelae.
Strengths and weaknesses of included studies
The celecoxib clinical research programme was primarily designed to determine its efficacy and risk of gastrointestinal side effects. For these reasons we reviewed the characteristics of the studies to determine whether they were powered or designed to identify serious cardiovascular risks associated with medication use. This review identified that none of the studies was adequately powered to determine whether celecoxib increased the risk of myocardial infarction by twofold and that no clinical trial was primarily undertaken to assess cardiovascular risk. However, in contrast to the major clinical trials with rofecoxib24,25 and lumiracoxib,26 patients were not excluded due to previous cardiovascular disease, which allowed a more accurate determination of the risk in the population likely to receive this drug.
Our review identified inconsistencies in the reporting of the major cardiovascular events in the CLASS trial. For example, the original publication of the first six months of the CLASS trial did not report any deaths,5 whereas the two subsequent publications which reported the complete 12 months findings stated that there were 20 cardiovascular deaths12 and 13 cardiovascular deaths, respectively.22 It is difficult to conceive how such major differences in events could occur in reports from the same clinical trial, particularly with definite end points such as death. These inconsistencies add to the previous concerns about the reporting of gastrointestinal side-effects from the CLASS trial.27-29 Likewise, there were differences in the reporting of cerebrovascular events between the two web-based publications of the Alzheimer's trial.15,20 The difficulties in undertaking a meta-analysis when there is discordant data and differing classification of major outcomes from publications of the same clinical trial is evident.
Comparison with other COX-2 inhibitors
The use of celecoxib was associated with a 2.26-fold increased risk of myocardial infarction when compared with placebo, and a 1.88-fold increased risk of myocardial infarction when compared with all comparator treatment groups. These risks are similar in magnitude to the 2.24-fold (95% CI 1.24 to 4.02) increased risk of myocardial infarction with rofecoxib reported in a comparable meta-analysis.24 Consistent with these meta-analyses, an increased risk of myocardial infarction has also been observed in studies of the COX-2 inhibitors parecoxib/valdecoxib.30,31
In contrast to the increased risk of myocardial infarction, our meta-analysis did not identify a corresponding increased risk of a cerebrovascular event. A similar pattern has been seen with rofecoxib,24 but not with parecoxib/valdecoxib.30,31 This finding suggests that the proposed mechanism whereby COX-2 inhibitors increase the cardiovascular risk, by shifting the functional balance of the vasoactive prostenoids,32-34 may preferentially apply to the pathogenesis of myocardial infarction rather than cerebrovascular events. This may be because myocardial infarction is predominantly due to thrombosis within the coronary arteries, whereas two-thirds of cerebrovascular events are due to thromboembolism from sources outside the brain.35
CONCLUSION
This systematic review and meta-analyses provide evidence of an increased risk of myocardial infarction associated with the use of celecoxib, consistent with a class effect of COX-2 inhibitors. This finding would suggest that the preferential risk/benefit assessment afforded celecoxib over other COX-2 inhibitors by the FDA36 may not be supported by the currently available evidence.
Contributors SA, PS, MW and RB were involved in the study design. BC, SA, PS and RB undertook the literature review, data collection and systematic review. MW undertook the statistical analysis. BC and RB wrote the manuscript with help from the other authors.
Competing interests No conflict of interest or competing interests are declared for any of the listed authors.
Funding Funding was received from the bequest of the Estate of Beverley Liddington. No pharmaceutical company had any role in the study design, data collection, analysis or interpretation, or in the writing of the report. The corresponding author had final responsibility for the decision to submit for publication.
References
- 1.Dieppe PA, Ebrahim S, Martin RM, Juni P. Lessons from the withdrawal of rofecoxib. BMJ 2005;329: 867-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drazen JM. COX-2 inhibitors—a lesson in unexpected problems. N Engl J Med 2005;352: 1131-2 [DOI] [PubMed] [Google Scholar]
- 3.Psaty BM, Furberg CD. COX-2 inhibitors—lessons in drug safety. N Engl J Med 2005;352: 1133-5 [DOI] [PubMed] [Google Scholar]
- 4.Maxwell SR, Webb DJ. COX-2 selective inhibitors - important lessons learned. Lancet 2005;365: 449-51 [DOI] [PubMed] [Google Scholar]
- 5.Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs non-steroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. JAMA 2000;284: 1247-55 [DOI] [PubMed] [Google Scholar]
- 6.Solomon SD, McMurray JJ, Pfeffer MA, et al. Adenoma Prevention with Celecoxib (APC) Study Investigators. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med 2005;352: 1071-80 [DOI] [PubMed] [Google Scholar]
- 7.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 2001;286: 954-9 [DOI] [PubMed] [Google Scholar]
- 8.Pitt B, Pepine C, Willerson JT. Cyclooxygenase-2 inhibition and cardiovascular events. Circulation 2002;106: 167-9 [DOI] [PubMed] [Google Scholar]
- 9.Graham DJ, Campen D, Hui R, et al. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclooxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet 2005;365: 475-81 [DOI] [PubMed] [Google Scholar]
- 10.Mamdani M, Rochon P, Juurlink DN, et al. Effect of selective cyclooxygenase-2 inhibitors and naproxen on short-term risk of acute myocardial infarction in the elderly. Arch Intern Med 2003;163: 481-6 [DOI] [PubMed] [Google Scholar]
- 11.Solomon DH, Schneeweiss S, Glynn RJ, et al. Relationship between selective cyclooxygenase-2 inhibitors and acute myocardial infarction in older adults. Circulation 2004;109: 2068-73 [DOI] [PubMed] [Google Scholar]
- 12.White WB, Faich G, Whelton A, Maurath C, et al. Comparison of thromboembolic events in patients treated with celecoxib, a cyclooxygenase-2 specific inhibitor, versus ibuprofen or diclofenac. Am J Cardiol 2002;89: 425-30 [DOI] [PubMed] [Google Scholar]
- 13.Whitehead A. Meta-analysis of Controlled Clinical Trials. Chichester: J Wiley, 2002: 352
- 14.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21: 1539-58 [DOI] [PubMed] [Google Scholar]
- 15.Pfizer Inc. A double-blind, randomized, placebo-controlled, comparative study of celecoxib (SC-58635) for the inhibition of progression of Alzheimer's disease. [www.clinicalstudyresults.org/documents/company-study_75_0.pdf] Accessed February 2005
- 16.McKenna F, Borenstein D, Wendt H, Wallemark C, Lefkowith JB, Geis GS. Celecoxib versus diclofenac in the management of osteoarthritis of the knee. Scand J Rheumatol 2001;30: 11-18 [DOI] [PubMed] [Google Scholar]
- 17.Levin B. Prevention of Spontaneous Adenomatous Polyps Trial [www.fda.gov/ohrms/dockets/ac/05/slides/2005-4090S1_09_FDA-Levin_files/frame.htm#slide0714.htm] Accessed May 2005
- 18.Geba GP, Weaver AL, Polis AB, Dixon ME, Schnitzer TJ. Vioxx, Acetaminophen, Celecoxib Trial (VACT) Group. Efficacy of rofecoxib, celecoxib, and acetaminophen in osteoarthritis of the knee: a randomized trial. JAMA 2002;287: 64-71 [DOI] [PubMed] [Google Scholar]
- 19.Levin B. The PreSAP Trial (Prevention of Colorectal Sporadic Adenomatous Polyps) [www.masterdocs.com/PDF/C7%20The%20PreSAP%20Trial%20(Prevention%20of%20Colorectal%20Sporadic%20Adenomatous%20Polyps)%20-%20Bernard%20Levin%20MD.pdf] Accessed May 2005
- 20.Advisory Committee Briefing Document. Celecoxib and valdecoxib cardiovascular safety. [www.fda.gov/ohrms/dockets/ac/05/briefing/2005-4090B1_03_Pfizer-Celebrex-Bextra.pdf] Accessed May 2005
- 21.Witter J. Celecoxib Medical Officer Review. [www.fda.gov/ohrms/dockets/ac/05/briefing/2005-4090B1_19_T-FDA-Tab-L-1.pdf] Accessed May 2005
- 22.CLASS Advisory Committee Briefing Document. [www.fda.gov/ohrms/dockets/ac/01/briefing/3677b1_01_searle.pdf] Accessed May 2005
- 23.Cleland LG, James MJ. COX-2 inhibition and thrombotic tendency. Med J Aust 2002;176: 89. [DOI] [PubMed] [Google Scholar]
- 24.Jüni P, Nartey L, Reichenbach S, Sterchi R, Dieppe PA, Egger M. Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. Lancet 2004;364: 2021-9 [DOI] [PubMed] [Google Scholar]
- 25.Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med 2000;343: 1520-8 [DOI] [PubMed] [Google Scholar]
- 26.Farkouh ME, Kirshner H, Harrington RA, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: randomised controlled trial. Lancet 2004;364: 675-84 [DOI] [PubMed] [Google Scholar]
- 27.Jüni P, Rutjes AW, Dieppe PA. Are selective COX-2 inhibitors superior to traditional non-steroidal anti-inflammatory drugs? BMJ 2002;324: 1287-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metcalfe S, Dougherty S, McNee W. Systematic review of celecoxib for osteoarthritis and rheumatoid arthritis: celecoxib's relative gastrointestinal safety is overstated. BMJ 2003;326: 334. [PubMed] [Google Scholar]
- 29.Hrachovec JB, Mora M. Reporting of 6-month vs 12-month data in a clinical trial of celecoxib. JAMA 2001;285: 2398. [PubMed] [Google Scholar]
- 30.Ott E, Nussmeier NA, Duke PC, et al. Efficacy and safety of the cyclooxygenase 2 inhibitors parecoxib and valdecoxib in patients undergoing coronary artery bypass surgery. J Thorac Cardiovasc Surg 2003;125: 1481-92 [DOI] [PubMed] [Google Scholar]
- 31.Nussmeier NA, Whelton AA, Brown MT, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med 2005;352: 1081-91 [DOI] [PubMed] [Google Scholar]
- 32.Fitzgerald GA. COX-2 and beyond: approaches to prostaglandin inhibition in human disease. Nat Rev Drug Discov 2003;2: 879-90 [DOI] [PubMed] [Google Scholar]
- 33.Fitzgerald GA. Coxibs and cardiovascular disease. N Engl J Med 2004;351: 1709-11 [DOI] [PubMed] [Google Scholar]
- 34.McAdam BF, Catella-Lawson F, Mardini IA, Kapoor S, Lawson JA, FitzGerald GA. Systematic biosynthesis of prostacylin to cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci USA 1999;96: 272-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar P, Clark M, eds. Clinical Medicine 5th edn. London; New York: WB Saunders, 2002: 1446.
- 36.FDA. Joint Meeting of the Arthritis Advisory Committee and the Drug Safety and Risk Management Advisory Committee, February 16-18 2005. [www.fda.gov/ohrms/dockets/ac/05/minutes/2005-4090M1_Final.pdf] Accessed May 2005