Abstract
Objective
To estimate medical costs associated with elevated fasting plasma glucose (FPG), and to determine whether costs differed for patients who met the 2003 (≥ 100 mg/dl) versus the 1997 (≥ 110mg/dl) ADA cut-point for impaired fasting glucose.
Research Design and Methods
We identified 28,335 patients with two or more FPG test results of at least 100mg/dl between 1 January 1994 and 31 December 2003. Those with evidence of diabetes before the second test were excluded. We categorized patients into two stages of abnormal glucose: 100-109 mg/dl and 110-125 mg/dl, and matched each of these subjects to a patient with a normal FPG test (<100mg/dl) on age, sex, and year FPG test. All subjects were followed until an FPG test qualified them for a higher stage, an anti-hyperglycemic drug dispense, health plan termination, or 31 December 2003.
Results
Adjusted annual costs were $4,357 among patients with normal FPG, $4,580 among stage 1 patients and $4,960 among stage 2 patients (p<.001, all comparisons). After removing normal FPG patients who progressed to a higher stage or diabetes, costs in the normal FPG stage were $3,799. Patients in both stages 1 and 2 had more cardiovascular comorbidities than normal FPG patients. In multivariate analysis, FPG was not significantly associated with costs after controlling for existing CVD.
Conclusions
Our results demonstrate that abnormal glucose metabolism is associated with higher medical care costs. However, much of the excess cost was attributable to concurrent CVD. The 2003 ADA cut-point identifies a group of patients with greater costs and comorbidity than normo-glycemic patients, but with lower costs and less comorbidity than patients above the 1997 cut-point.
Keywords: Costs, Impaired Fasting Glucose, cardiovascular disease
INTRODUCTION
The Diabetes Prevention Program (DPP),1 the Finnish Diabetes Prevention Study,2 and the STOP-NIDDM trial3 have now established that it is possible to prevent, or delay, type 2 diabetes onset in persons with abnormal glucose metabolism. Many hope that preventing diabetes will reduce future medical costs. We have demonstrated that excess costs for patients who ultimately develop diabetes begin at least eight years before diabetes diagnosis,4 and these pre-diagnostic costs are driven primarily by the treatment of cardiovascular risk factors and emerging cardiovascular disease (CVD). CVD is also the primary cost-driver after diabetes has been diagnosed.5 Therefore, while recognizing that delaying the hyperglycemia of diabetes might also delay CVD, the potential economic benefits of hyperglycemic screening and prevention must be examined. To our knowledge, no study has yet described the costs associated with elevated sub-diabetic levels of fasting plasma glucose.
In November 2003, the ADA reduced the cut-point for defining impaired fasting glucose (IFG) from 110 to 100 mg/dl, primarily to optimize the value of IFG in predicting future diabetes.6 Reducing the IFG cut point has generated considerable controversy partly because the new definition might place an added treatment burden on an already stressed health care system, and because the newly identified IFG patients may have different CVD risk profiles.7,8 To inform this debate, we examined the extent to which comorbidities and costs differ for persons at the new and old levels of IFG, especially in relation to those with normal FPG.
RESEARCH DESIGN AND METHODS
Study subjects were members of Kaiser Permanente Northwest (KPNW), a not-for-profit, group-model HMO. KPNW recommends lipid screening for men age 35 and older and women age 45 and older. FPG tests are routinely ordered with lipid panels. Between 1 January 1994 and 31 December 2003, the KPNW Regional Laboratory analyzed 603,486 FPG tests for 231,093 unique individuals. Of the 113,687 patients who had at least two tests, we identified 28,335 patients with two or more FPG test results of at least 100mg/dl and no evidence of diabetes (chart diagnosis of 250.xx, FPG > 125mg/dl, or use of an anti-hyperglycemic drug). We categorized these patients into two abnormal glucose levels: stage 1 (100-109 mg/dl) and stage 2 (110-125 mg/dl). The date of the earliest test in the stage was defined as the index date for that stage. Patients were followed until they recorded an FPG greater than the maximum of their current stage, an anti-hyperglycemic drug dispense, health plan membership termination, or 31 December 2003. Most patients remained in a single stage throughout the study, but 3,281 subjects progressed from stage 1 to stage 2.
We matched each of the unique 28,335 subjects to a KPNW member who had a normal FPG test (<100mg/dl) based on age, sex, and year of index FPG test. The resulting control subjects were followed until a recorded FPG ≥ 100mg/dl, an anti-hyperglycemic drug dispense, health plan termination, or 31 December 2003. We further divided the 26,309 control subjects who had not died by the end of 2003 into those who had no FPG test ≥ 100mg/dl (n=23,621); had progressed to stage 1 FPG (n=1,741); had progressed to stage 2 (n=462); or had progressed to diabetes (n=485).
KPNW maintains electronic databases containing information on all inpatient admissions, pharmacy dispenses, outpatient visits, and laboratory tests. These databases are linked through the unique health record number that each member receives when they first enroll in the health plan. We recently detailed our determination of unit costs based on these databases.9 Briefly, cost coefficients were applied uniformly in all years, inflated to 2003 dollars. To minimize the effects of censoring, we annualized costs by dividing by months of observation, and then multiplying by 12. We adjusted costs for age, sex and comorbidites (history of MI, stroke or depression, other CVD, congestive heart failure) and weighted them by months of observation (to further account for censoring) using SAS Proc GLM version 6.12 (Cary, North Carolina).
The electronic medical record (EMR) contains up to 20 physician-recorded ICD-9-CM diagnoses at each contact. Using these diagnoses, we identified comorbidities present at the time of the index FPG test. Smoking history, height, weight and blood pressure were obtained from the EMR. Lipid values were extracted from the laboratory database—for this study, we used the values nearest but prior to the index FPG test. We also counted the number of Adult Treatment Panel III (ATP III) criteria for the metabolic syndrome,10 however, because waist circumference was not available and FPG was the primary classification variable, the maximum number of criteria possible was three (triglycerides ≥ 150mg/dl; HDL < 40mg/dl in men or < 50mg/dl in women; systolic blood pressure ≥ 130mmHg or diastolic blood pressure ≥ 85mmHg).
To ascertain the independent contribution of FPG to annual total costs, we estimated a series of three regression models. The first included only FPG as the explanatory variable, the second added risk factors (age, sex, smoking history, BMI, systolic blood pressure, HDL cholesterol, triglycerides, LDL cholesterol), and the third added baseline diagnoses (history of MI, stroke or depression, other CVD, congestive heart failure).
RESULTS
Subjects averaged 58.6 years old and 54.0% were women (Table 1). Those with stage 2 FPG were about a year older on average than stage 1 subjects (59.5 vs. 58.3, p<0.001). CVD comorbidities of all types were much more common in the elevated FPG groups, and were slightly higher in stage 2 compared to stage 1. Use of anti-hypertensive, lipid lowering and other CVD drugs was also higher in the elevated FPG stages, and greatest in stage 2.
Table 1.
Patient Characteristics by Fasting Plasma Glucose Stage
| Normal Glucose (< 100mg/dL) |
Stage 1 IFG (100-109mg/dL) |
Stage 2 IFG (110-125mg/dL) |
|
|---|---|---|---|
| Number of Subjects | 28,335 | 18,738 | 12,878 |
| Age in Years1,2 | 58.6 (11.6) | 58.3 (11.5) | 59.5 (11.5) |
| Percent Female | 54.0% | 53.7% | 54.7% |
| Months of Observation1 | 59.3 (37.9) | 59.8 (36.2) | 50.7 (36.0) |
| History of Smoking1,3 | 18.8% | 21.7% | 22.1% |
| Comorbidities: | |||
| History of MI1,2,3 | 5.1% | 7.1% | 7.9% |
| History of Stroke1,2 | 7.5% | 8.1% | 9.1% |
| ASCVD1,2,3 | 12.1% | 17.3% | 19.8% |
| CHF1,2,3 | 5.6% | 7.0% | 9.2% |
| Depression1,3 | 19.1% | 21.0% | 21.0% |
| Died in Stage2,3 | 7.2% | 6.0% | 7.7% |
| Pharmaceutical Utilization: | |||
| Anti-Hypertensive Agents1,2,3 | 52.4% | 66.5% | 70.4% |
| Lipid Lowering Agents1,2,3 | 24.8% | 38.2% | 40.7% |
| Other CVD Medications1,3 | 28.6% | 34.6% | 35.3% |
| Anti-Depressants2,3 | 31.9% | 34.8% | 32.8% |
| Systolic Blood Pressure (mmHg)1,2,3 | 131 (14) | 135 (13) | 137 (13) |
| Diastolic Blood Pressure (mmHg)1,3 | 79 (7) | 80 (7) | 80 (8) |
| LDL Cholesterol (mg/dl)1,2 | 130 (32) | 130 (32) | 125 (31) |
| HDL Cholesterol (mg/dl)1,2,3 | 52 (16) | 49 (14) | 47 (13) |
| Triglycerides (mg/dl)1,2,3 | 156 (103) | 189 (156) | 208 (163) |
| Total Cholesterol (mg/dl)2,3 | 212 (36) | 215 (39) | 211 (39) |
| Body Mass Index (kg/m2)1,2,3 | 28.6 (5.4) | 31.3 (6.4) | 32.7 (6.9) |
| Number of Metabolic Syndrome Criteria (triglycerides, HDL, BP)1,2,3 | |||
| None | 23.7% | 12.8% | 8.1% |
| One | 38.7% | 34.3% | 29.6% |
| Two | 26.2% | 32.8% | 35.1% |
| All three | 11.5% | 20.2% | 27.2% |
| Annual Clinic Visits1,2,3 | 9.3 (11.3) | 9.7 (8.8) | 11.4 (17.2) |
| Annual Pharmaceutical Dispenses1,2,3 | 16.6 (19.2) | 19.8 (21.8) | 22.8 (46.3) |
Normal glucose group differs from Stage 2, p<.001
Stage 1 differs from Stage 2, p<.001
Normal glucose group differs from Stage 1, p<.001
Note: Numbers shown are means (standard deviations) or proportions (%).
We observed a graded difference in systolic blood pressure, HDL cholesterol, triglycerides, and body mass index across the three groups, with more favorable mean values for normal FPG subjects, and the poorest mean values for stage 2 subjects (p<0.001 for all comparisons). Of the three possible criteria for metabolic syndrome (blood pressure ≥ 130/85mmHg, HDL < 40 for men or 50 for women, triglycerides ≥ 150mg/dl), 23.7% of normal FPG subjects had none of them, compared to 12.8% of stage 1 subjects, and 8.1% of stage 2 (p<0.001). Conversely, 62.3% of stage 2 subjects had two or three criteria, compared to 37.2% of subjects with normal FPG.
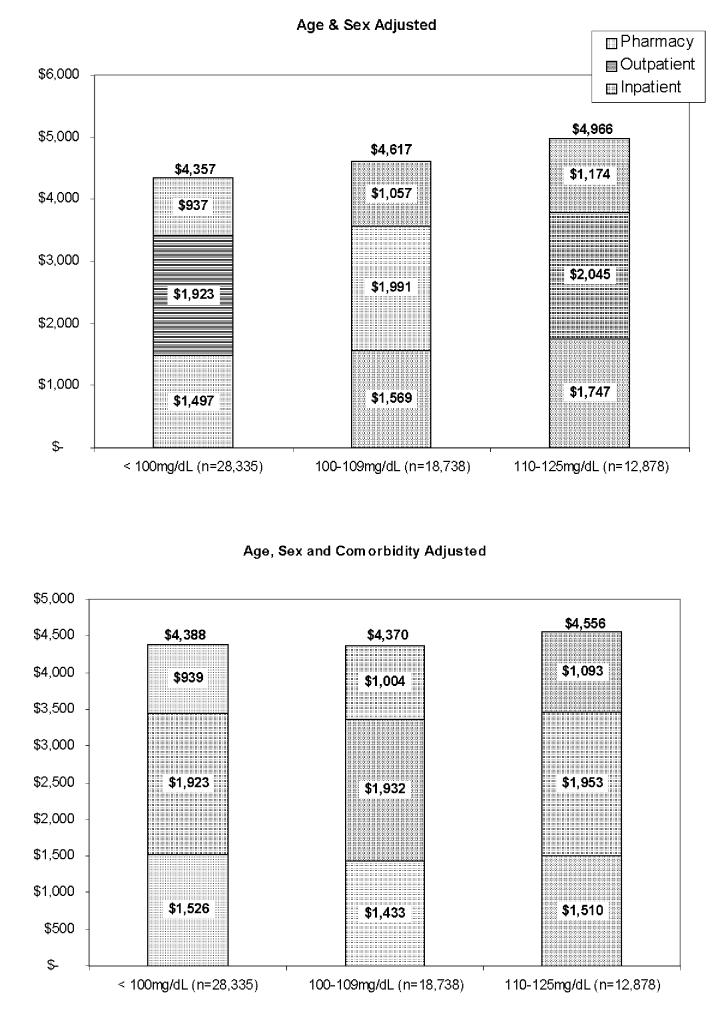
Age and sex-adjusted annualized total costs (Figure1, top half) were lowest among those with normal FPG ($4,357), about $260 higher in subjects with stage 1 IFG ($4,617), and over $600 higher in stage 2 IFG subjects ($4,966, p<0.001 for all comparisons). Differences between the inpatient, outpatient, and pharmacy components of total costs, displayed in the stacks of the bars, rose similarly with FPG stage, and were statistically significantly different in all between-group comparisons. After further adjustment for comorbidities (Figure 1, lower half), total costs for stage 2 subjects remained significantly greater than for normal or stage 1 subjects ($4,556 vs. $4,388 and $4,370, p<0.001), but except for the pharmaceutical component of costs, the difference between stage 1 and normal subjects disappeared.
Figure 1.
Annual Medical Care Costs by Fasting Plasma Glucose Stage
Table 2 describes the 26,309 patients who were initially assigned to the normal FPG control group and who survived through 2003, displayed by the highest FPG stage observed by 31 December 2003. Most subjects (89.8%) did not progress to a subsequent stage. Non-progressing patients were younger than those who progressed to either stage 1 or stage 2 (57.5 years vs. 60.2 and 60.7, respectively, p<0.001 for both comparisons), but of similar age to those who progressed to diabetes (57.0 years, not significant). Subjects who remained in normal FPG status were much more likely to be women (47.9% vs. 33.3%, 34.9%, and 39.0%, p<0.001), and much less likely to have CVD. Subjects who did not progress to a higher FPG stage had significantly lower systolic blood pressure, LDL cholesterol, triglycerides, total cholesterol and BMI observed while in the normal FPG category, and significantly higher HDL cholesterol. Nearly a third (32.1%) of subjects with normal FPG who ultimately progressed to diabetes already had three metabolic syndrome criteria, compared to just 10.5% of subjects who remained in normal FPG status (p<0.001). Finally, subjects who did not progress incurred significantly lower costs than those who progressed to an impaired fasting glucose stage or to diabetes ($3,785 vs. $4,459, $5,307, and $6,568, p<.001 for all comparisons). Inpatient, outpatient and pharmaceutical components of costs displayed a similar pattern. Adjustment for age, sex and comorbidities did not affect the comparisons (adjusted data not shown).
Table 2.
Characteristics and Costs of Patients with Normal Fasting Glucose, by Progression Status
| Stayed Below 100mg/dl |
Progressed To Stage 1 (100 - 109 mg/dl) |
Progressed To Stage 2 (110 - 125 mg/dl) |
Progressed To Diabetes (> 125mg/dl) |
|
|---|---|---|---|---|
| Number of Subjects | 23,621 | 1,741 | 462 | 485 |
| Age in Years1,2,3,4 | 57.5 (11.5) | 60.2 (9.4) | 60.7 (9.3) | 57.0 (9.8) |
| Percent Female1,2,5 | 47.9% | 33.3% | 34.9% | 39.0% |
| History of Smoking3,5 | 18.2% | 18.7% | 18.0% | 25.0% |
| Baseline Comorbidities: | ||||
| History of MI1,2,5 | 2.2% | 6.2% | 7.1% | 6.0% |
| History of Stroke | 2.3% | 2.0% | 1.5% | 3.9% |
| ASCVD1,2,5 | 5.8% | 14.0% | 16.5% | 14.4% |
| CHF5 | 1.2% | 1.8% | 2.2% | 2.9% |
| Depression1,5 | 8.8% | 3.8% | 6.1% | 4.5% |
| Fasting Plasma Glucose (mg/dl)1,2,5 | 91 (6.0) | 94 (4.6) | 94 (4.8) | 93 (5.5) |
| Systolic Blood Pressure (mmHg)1,2,5,6 | 131 (14) | 135 (15) | 137 (15) | 136 (13) |
| Diastolic Blood Pressure (mmHg)1,2,5,6 | 79 (7) | 81 (8) | 82 (8) | 81 (8) |
| LDL Cholesterol (mg/dl)1,2,3,4 | 129 (31) | 144 (35) | 147 (37) | 132 (36) |
| HDL Cholesterol (mg.dl)1,2,3,5 | 53 (16) | 48 (14) | 46 (15) | 45 (13) |
| Triglycerides (mg/dl)1,2,3,4,5 | 150 (91) | 187 (144) | 200 (138) | 232 (265) |
| Total Cholesterol (mg/dl)1,2,3,4,5 | 211 (36) | 228 (40) | 231 (43) | 221 (50) |
| Body Mass Index (kg/m2)1,2,3,4,5,6 | 28.6 (5.3) | 29.8 (5.2) | 31.0 (5.6) | 33.6 (7.2) |
| Number of Metabolic Syndrome Criteria (triglycerides, HDL, BP)1,2,3,4,5 | ||||
| None | 25.1% | 13.1% | 6.7% | 7.6% |
| One | 39.1% | 37.1% | 29.0% | 23.6% |
| Two | 25.4% | 30.2% | 36.8% | 36.7% |
| All three | 10.5% | 19.6% | 27.5% | 32.1% |
| Pharmacy Costs2,3,4,5,6 | $843 ($1,505) | $803 ($1,094) | $1,040 ($2,155) | $1,356 ($2,201) |
| Outpatient Costs1,2,3,4,5 | $1,794 ($1,733) | $1,940 ($1,965) | $2,103 ($1,988) | $2,613 ($3,513) |
| Inpatient Costs1,2,3,5 | $1,148 ($4,168) | $1,716 ($8,335) | $2,164 ($6,993) | $2,599 ($6,834) |
| Total Costs1,2,3,4,5,6 | $3,785 ($5,640) | $4,459 ($9,402) | $5,307 ($8,423) | $6,568 ($9,607) |
Subjects who stayed below 100mg/dl differ from those who progressed to Stage 1, p<.001
Subjects who stayed below 100mg/dl differ from those who progressed to Stage 2, p<.001
Subjects who progressed to Stage 1 differ from those who progressed to Diabetes, p<.001
Subjects who progressed to Stage 2 differ from those who progressed to Diabetes, p<.001
Subjects who stayed below 100mg/dl differ from those who progressed to Diabetes, p<.001
Subjects who progressed to Stage 1 differ from those who progressed to Stage 2, p<.001
Numbers shown are means (standard deviations) or proportions (%).
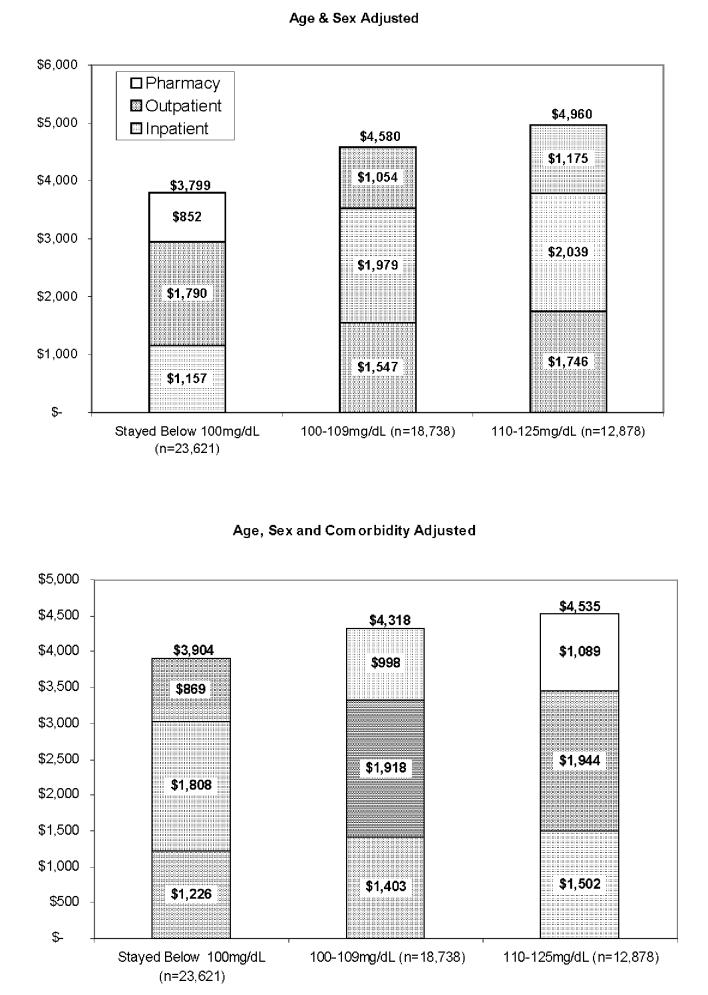
Figure 2 re-displays costs by FPG stage, but includes as controls only those subjects who survived to the end of 2003 without progressing to a higher FPG stage. In this comparison, age and sex-adjusted total costs and each component of costs differed significantly between stages (p<0.001 for all comparisons), being lowest among normal subjects and highest among stage 2 subjects. All these differences remained significant after further adjustment for comorbidities.
Figure 2.
Annual Medical Care Costs by Fasting Plasma Glucose Stage, Excluding Subjects with Normal Plasma Glucose Who Later Progressed to IFG or Diabetes
Analyzed continuously, each mg/dl of FPG added $25 (p<.001) to annual costs when not accounting for other factors (Table 3). Adjustment for age, sex and CVD risk factors reduced the independent contribution of FPG to $10 per mg/dl annually (p<.001). However, after further adjustment for presence of CVD, the independent contribution of FPG to annual costs was not statistically significantly different from zero.
Table 3.
Multivariate Analyses of Annual Total Medical Care Costs
| Bivariate Model (R2=.002) |
With Risk Factors (R2=.06) |
Full Model (R2=.20) |
||||
|---|---|---|---|---|---|---|
| Parameter Estimate | p value | Parameter Estimate | p value | Parameter Estimate | p value | |
| Intercept | $2,259 | 0.001 | $2,046 | 0.001 | $1,514 | 0.001 |
| FPG (per mg/dl) | $25 | 0.001 | $9 | 0.001 | ($2) | 0.493 |
| Age (per year) | -- | -- | $110 | 0.001 | $58 | 0.001 |
| Female Sex | -- | -- | $423 | 0.001 | $194 | 0.001 |
| Smoking History | -- | -- | $861 | 0.001 | $383 | 0.001 |
| BMI (per kg/m2) | -- | -- | $33 | 0.001 | $17 | 0.001 |
| Systolic BP (per mmHg) | -- | -- | ($22) | 0.001 | ($4) | 0.063 |
| HDL Cholesterol (per mg/dl) | -- | -- | ($17) | 0.001 | ($7) | 0.001 |
| Triglycerides (per mg/dl) | -- | -- | $3 | 0.001 | $2 | 0.001 |
| LDL Cholesterol (per mg/dl) | -- | -- | ($22) | 0.001 | ($10) | 0.001 |
| ASCVD | -- | -- | -- | -- | $1,747 | 0.001 |
| CHF | -- | -- | -- | -- | $3,908 | 0.001 |
| Depression | -- | -- | -- | -- | $69 | 0.001 |
| History of MI | -- | -- | -- | -- | $1,504 | 0.001 |
| History of Stroke | -- | -- | -- | -- | $1,863 | 0.001 |
CONCLUSIONS
To our knowledge, our study is the first to estimate medical care costs for patients with sub-diabetic abnormal glucose metabolism. Consistent with other research,7 the CVD risk profiles for patients at the new ADA cut-point for IFG are not as severe as patients at the old cut-point. Our results indicate that while costs are greater for patients who meet the 2003 ADA cut-point for IFG, they are lower than costs for patients at the higher 1997 cut-point. Nonetheless, comparisons to patients with normal FPG suggest that the new cut-point identifies a group that has higher costs and greater CVD morbidity than normo-glycemic patients. Confounding with comorbidities such as CVD makes the isolation of the effect of elevated glucose difficult,11-13 but our results also indicate that for patients added by the new ADA definition, elevated FPG is not in itself associated with higher medical costs, primarily because these patients are more likely to have CVD. Adjustment for CVD comorbidity (Figure 1) removes an otherwise statistically significant difference in costs between normal FPG subjects and those in the 100-109mg/dl range. CVD-adjusted costs remained significantly higher for stage 2 IFG, suggesting that at higher levels, abnormal glucose metabolism does add independently to costs. However, when analyzed on a continuous basis, FPG does not independently explain any significant variance in annual costs after accounting for the presence of CVD.
Our analysis comparing those who did and did not progress to a higher FPG state demonstrates that patients with normal FPG are not all equivalent (Table 2). That is, some patients with apparently normal FPG may have a genetic predisposition to abnormal glucose metabolism that has not yet manifested, while most others are destined to remain within the normal range. Therefore, cost comparisons between normal and current IFG patients that include unmanifested IFG patients in the normal group, as in our initial analysis, understate the true costs of IFG. Our final analysis (Figure 2) illustrates this point. After removing patients from the normal group who later progressed to IFG or diabetes, we found substantial cost differences between normal and stage 1 IFG patients, even after adjustment for comorbidities.
Because of our observational design, assignment of patients to various stages of FPG was vulnerable to ascertainment bias. Although many FPG tests are conducted in the health plan due to routine screening, we suspect that many subjects were tested for clinical reasons we could not observe, particularly in the normal FPG group, in which the mean FPG exceeded 90mg/dl. Therefore, our cost estimates for control subjects might be overstated. We have attempted to address this limitation by subsequently excluding subjects who later progressed beyond normal FPG.
Although we weighted our cost estimates by months of observation, differential observation time is another potential source of bias if costs were incurred differentially at the end versus the beginning of a given stage. For example, costs for a subject identified in 2002 and followed only to the end of 2003 might well differ from a subject in the same stage identified in 1998 and followed until progression to a subsequent stage that occurred, say, in 2002. If costs were greater in the latter portion of the observed stage, our estimates probably understate the true cost of IFG. By the same logic, if costs are greatest at the beginning of a stage, as they are for diabetes,5 our estimates would overstate true IFG costs. This latter form of differential follow-up bias might be exacerbated by an interaction with ascertainment bias if an adverse health event that leads to FPG testing is driving early stage costs. Overall, however, average observation times of four to five years likely minimize the effects of differential follow-up.
In large sample sizes such as ours, even relatively small cost differences can appear statistically significant. For example, in our final analysis (Figure 2), the age, sex and comorbidity adjusted difference in costs between stage 1 and stage 2 IFG subjects was a statistically significant $217, an amount that represents about 5% of the total costs. Individually, this cost difference may be small, but it quickly reaches millions when multiplied by the large numbers of people to whom it applies. Our large sample size also minimizes the effect of large outliers on cost analyses. We re-analyzed our data excluding subjects with total costs in excess of three standard deviations beyond the mean (total costs > $31,985) with identical results.
Our multivariate analyses yielded an apparently anomalous result—better LDL cholesterol and systolic blood pressure appeared to be associated with higher costs. However, further analysis (not shown) revealed that patients existing CVD had lower values than those without CVD. For example, patients with a history of MI had an average LDL of 111 mg/dl, compared to 127 mg/dl for patients without an MI history (p<.001). Systolic blood pressure followed a similar but less extreme pattern (132 vs. 134 mmHg, p<.001). Thus, in our data, lower values on these important risk factors served as markers for costly disease.
We identified a subset of patients with apparently normal FPG who progressed to IFG or diabetes. These patients were substantially costlier while their FPG was normal than their counterparts who did not progress to defined levels of abnormal glucose (IFG or diabetes). Further research should focus on whether costs provide a valid method of identifying persons at greatest risk of progressing to type 2 diabetes.
Footnotes
Sponsored by the National Institute of Diabetes, Digestive and Kidney Diseases Grant No. 1 R21 DK063961-01A1
This is an author-created, uncopyedited electronic version of an article accepted for publication in Diabetes Care (http://care.diabetesjournals.org). The American Diabetes Association (ADA), publisher of Diabetes Care, is not responsible for any errors or omissions in this version of the manuscript or any version derived from it by third parties. The definitive version is available online at http://care.diabetesjournals.org/cgi/contents/full/28/9/2223
REFERENCES
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with life-style intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomilehto J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, et al. Acarbose for prevention of type 2 diabetes: the STOP-NIDDM randomized trial. Lancet. 2002;359:2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- Nichols GA, Glauber HS, Brown JB. Type 2 diabetes: Incremental medical care costs during the first eight years preceding diagnosis. Diabetes Care. 2000;23:1654–1659. doi: 10.2337/diacare.23.11.1654. [DOI] [PubMed] [Google Scholar]
- Brown JB, Nichols GA, Glauber HS, Bakst AW. Type 2 diabetes: Incremental medical care costs during the first eight years after diagnosis. Diabetes Care. 1999;22:1116–1124. doi: 10.2337/diacare.22.7.1116. [DOI] [PubMed] [Google Scholar]
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Follow-up Report on the Diagnosis of Diabetes Mellitus. Diabetes Care. 2003;26(11):3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- Borch-Johnsen Colagiuri S, Balkan B, Glumer C, Carstensen B, Ramachandran R, et al. Creating a pandemic of prediabetes: the proposed new diagnostic criteria for impaired fasting glucose. Diabetologia. 2004;47(8):1396–1402. doi: 10.1007/s00125-004-1468-6. [DOI] [PubMed] [Google Scholar]
- Davidson MB, Landsman PB, Alexander CM. Lowering the criterion for impaired fasting glucose will not provide clinical benefit. Diabetes Care. 2003;26(12):3329–3330. doi: 10.2337/diacare.26.12.3329. [DOI] [PubMed] [Google Scholar]
- Brown JB, Pedula KL, Bakst AW. The progressive cost of complications in type 2 diabetes mellitus. Arch Intern Med. 1999;159(16):1873–1880. doi: 10.1001/archinte.159.16.1873. [DOI] [PubMed] [Google Scholar]
- Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Meigs JB, Nathan DM, D’Agostino RB, Sr., Wilson PW. Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care. 2002;25(10):1845–1850. doi: 10.2337/diacare.25.10.1845. [DOI] [PubMed] [Google Scholar]
- Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events: a meta-regression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–240. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- Alexander CM, Landsman PB, Teutsch SM. Diabetes mellitus, impaired fasting glucose, atherosclerotic risk factors, and prevalence of coronary heart disease. Am J Cardiol. 2000;86:897–902. doi: 10.1016/s0002-9149(00)01118-8. [DOI] [PubMed] [Google Scholar]