Abstract
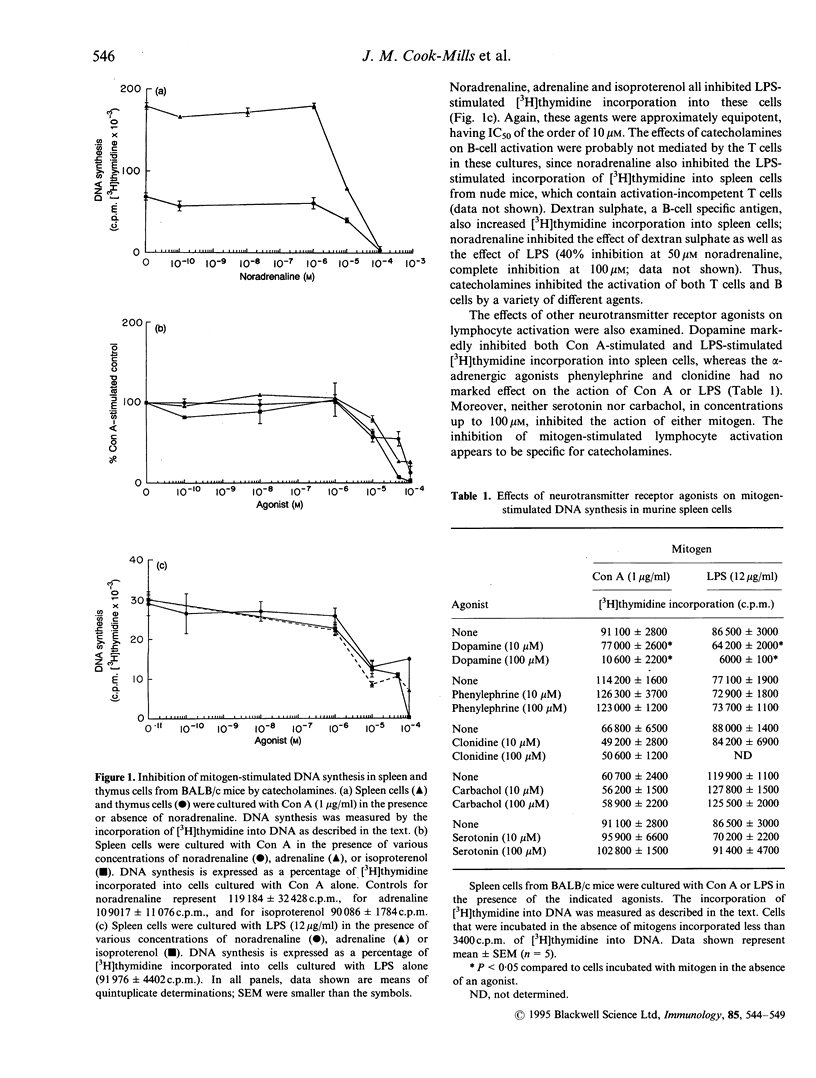
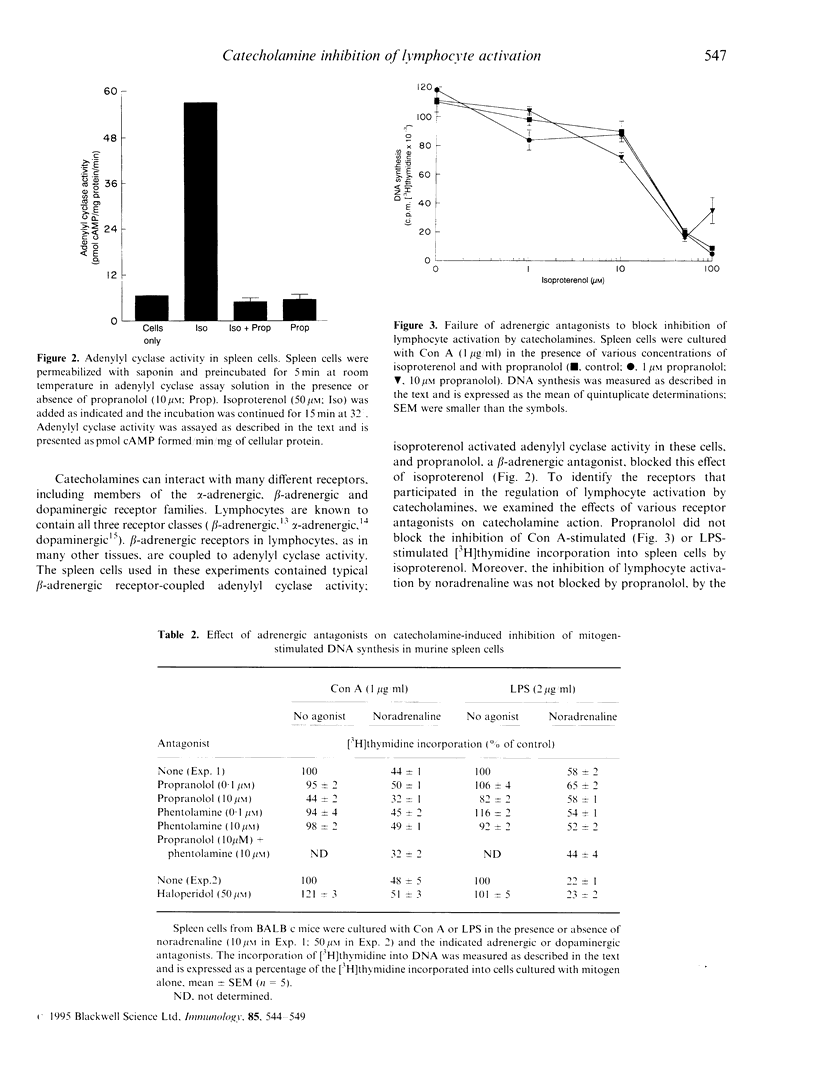
The effects of noradrenaline and other adrenergic agonists on lymphocyte activation were studied. Spleen and thymus cells from BALB/c mice were stimulated by mitogens and lymphocyte activation was monitored by measuring the incorporation of [methyl-3H]thymidine into DNA. Noradrenaline, adrenaline, isoproterenol and dopamine all inhibited the activation of spleen and thymus cells by concanavalin A, a T-cell specific mitogen, and the activation of spleen cells by lipopolysaccharide, a T-independent B-cell mitogen. The various catecholamines were approximately equipotent, having IC50 of approximately 10 microM. alpha-adrenergic agonists (phenylephrine, clonidine) did not inhibit lymphocyte activation. Noradrenaline, adrenaline and isoproterenol also inhibited DNA synthesis in S49 T lymphoma cells. The effects of adrenergic receptor antagonists on lymphocyte function were also studied. The inhibition of lymphocyte activation by catecholamines could not be reversed by antagonists to beta-adrenergic receptors (propranolol), alpha-adrenergic receptors (phentolamine), or dopaminergic receptors (haloperidol). Experiments with human peripheral blood leucocytes revealed that, as with murine cells, the beta-adrenergic antagonists propranolol and nadalol did not affect the catecholamine-mediated inhibition of lymphocyte activation. Although lymphocytes contain beta-adrenergic receptors that are coupled to adenylyl cyclase activity, catecholamines appear to inhibit murine lymphocyte activation by a mechanism that is independent of these or other classical adrenergic receptors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aune T. M., Kelley K. A., Ranges G. E., Bombara M. P. Serotonin-activated signal transduction via serotonin receptors on Jurkat cells. J Immunol. 1990 Sep 15;145(6):1826–1831. [PubMed] [Google Scholar]
- Bach M. A. Differences in Cyclic AMP Changes after Stimulation by Prostaglandins and Isoproterenol in Lymphocyte Subpopulations. J Clin Invest. 1975 May;55(5):1074–1081. doi: 10.1172/JCI108008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besedovsky H. O., del Rey A., Sorkin E., Da Prada M., Keller H. H. Immunoregulation mediated by the sympathetic nervous system. Cell Immunol. 1979 Dec;48(2):346–355. doi: 10.1016/0008-8749(79)90129-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carlson S. L., Brooks W. H., Roszman T. L. Neurotransmitter-lymphocyte interactions: dual receptor modulation of lymphocyte proliferation and cAMP production. J Neuroimmunol. 1989 Sep;24(1-2):155–162. doi: 10.1016/0165-5728(89)90109-4. [DOI] [PubMed] [Google Scholar]
- Chambers D. A., Cohen R. L., Perlman R. L. Neuroimmune modulation: signal transduction and catecholamines. Neurochem Int. 1993 Feb;22(2):95–110. doi: 10.1016/0197-0186(93)90002-m. [DOI] [PubMed] [Google Scholar]
- Chambers D. A., Martin D. W., Jr, Weinstein Y. The effect of cyclic nucleotides on purine biosynthesis and the induction of PRPP synthetase during lymphocyte activation. Cell. 1974 Dec;3(4):375–380. doi: 10.1016/0092-8674(74)90053-1. [DOI] [PubMed] [Google Scholar]
- Coffey R. G., Hadden J. W. Neurotransmitters, hormones, and cyclic nucleotides in lymphocyte regulation. Fed Proc. 1985 Jan;44(1 Pt 1):112–117. [PubMed] [Google Scholar]
- Emorine L. J., Marullo S., Briend-Sutren M. M., Patey G., Tate K., Delavier-Klutchko C., Strosberg A. D. Molecular characterization of the human beta 3-adrenergic receptor. Science. 1989 Sep 8;245(4922):1118–1121. doi: 10.1126/science.2570461. [DOI] [PubMed] [Google Scholar]
- Felten D. L., Felten S. Y., Bellinger D. L., Carlson S. L., Ackerman K. D., Madden K. S., Olschowki J. A., Livnat S. Noradrenergic sympathetic neural interactions with the immune system: structure and function. Immunol Rev. 1987 Dec;100:225–260. doi: 10.1111/j.1600-065x.1987.tb00534.x. [DOI] [PubMed] [Google Scholar]
- Graham D. G., Tiffany S. M., Bell W. R., Jr, Gutknecht W. F. Autoxidation versus covalent binding of quinones as the mechanism of toxicity of dopamine, 6-hydroxydopamine, and related compounds toward C1300 neuroblastoma cells in vitro. Mol Pharmacol. 1978 Jul;14(4):644–653. [PubMed] [Google Scholar]
- Hadden J. W., Hadden E. M., Middleton E., Jr Lymphocyte blast transformation. I. Demonstration of adrenergic receptors in human peripheral lymphocytes. Cell Immunol. 1970 Dec;1(6):583–595. doi: 10.1016/0008-8749(70)90024-9. [DOI] [PubMed] [Google Scholar]
- Horibata K., Harris A. W. Mouse myelomas and lymphomas in culture. Exp Cell Res. 1970 Apr;60(1):61–77. doi: 10.1016/0014-4827(70)90489-1. [DOI] [PubMed] [Google Scholar]
- Johnson D. L., Ashmore R. C., Gordon M. A. Effects of beta-adrenergic agents on the murine lymphocyte response to mitogen stimulation. J Immunopharmacol. 1981;3(2):205–219. doi: 10.3109/08923978109026427. [DOI] [PubMed] [Google Scholar]
- Munro S., Thomas K. L., Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993 Sep 2;365(6441):61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Rasenick M. M., Kaplan R. S. Guanine nucleotide activation of adenylate cyclase in saponin permeabilized glioma cells. FEBS Lett. 1986 Oct 27;207(2):296–301. doi: 10.1016/0014-5793(86)81508-3. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Titinchi S., Clark B. Alpha 2-adrenoceptors in human lymphocytes: direct characterisation by [3H]yohimbine binding. Biochem Biophys Res Commun. 1984 May 31;121(1):1–7. doi: 10.1016/0006-291x(84)90679-x. [DOI] [PubMed] [Google Scholar]
- Uzan A., Phan T., Le Fur G. Selective labelling of murine B lymphocytes by [3H]spiroperidol. J Pharm Pharmacol. 1981 Feb;33(2):102–103. doi: 10.1111/j.2042-7158.1981.tb13720.x. [DOI] [PubMed] [Google Scholar]
- Vischer T. L. The differential effect of cyclic AMP on lymphocyte stimulation by T- or B-cell mitogens. Immunology. 1976 May;30(5):735–739. [PMC free article] [PubMed] [Google Scholar]
- Williams L. T., Snyderman R., Lefkowitz R. J. Identification of beta-adrenergic receptors in human lymphocytes by (-) (3H) alprenolol binding. J Clin Invest. 1976 Jan;57(1):149–155. doi: 10.1172/JCI108254. [DOI] [PMC free article] [PubMed] [Google Scholar]


