Abstract
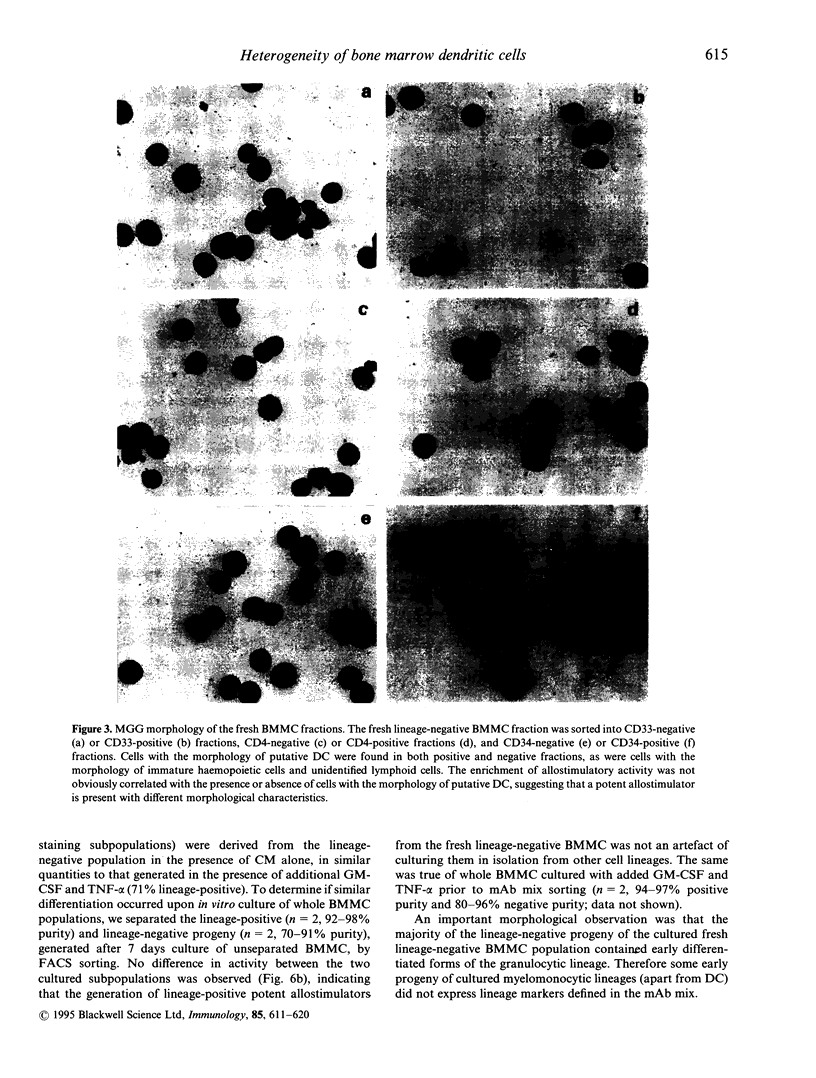
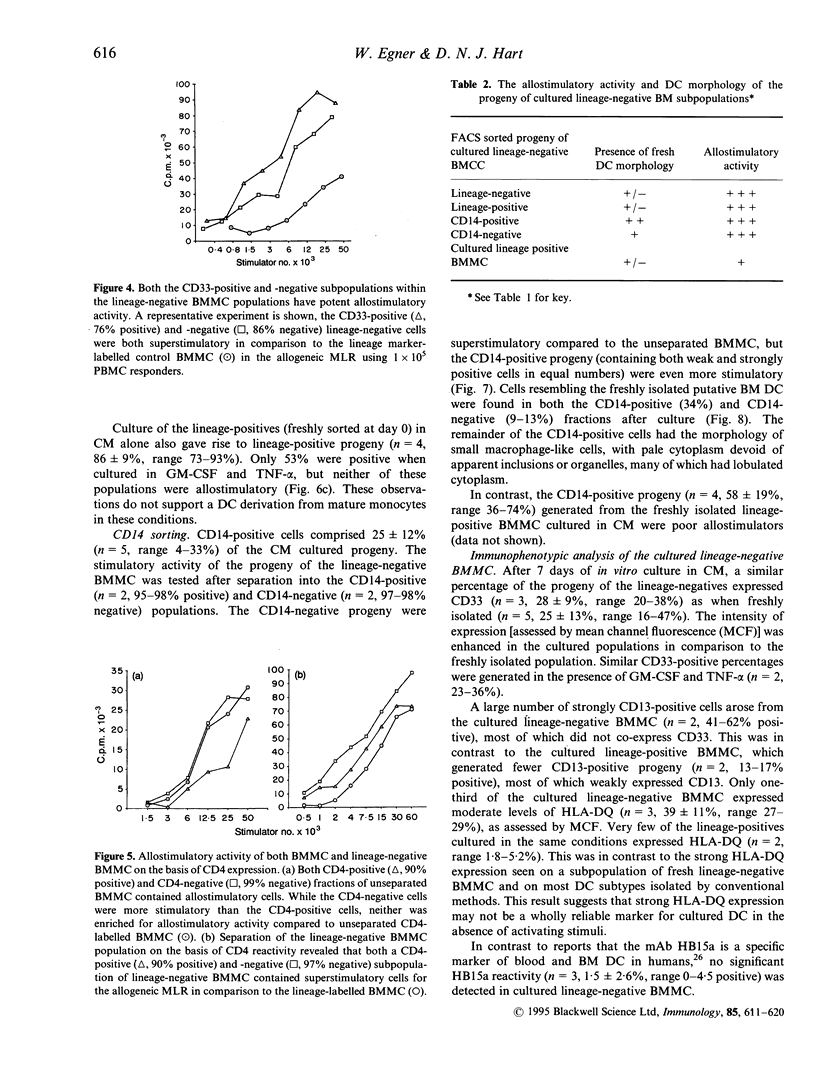
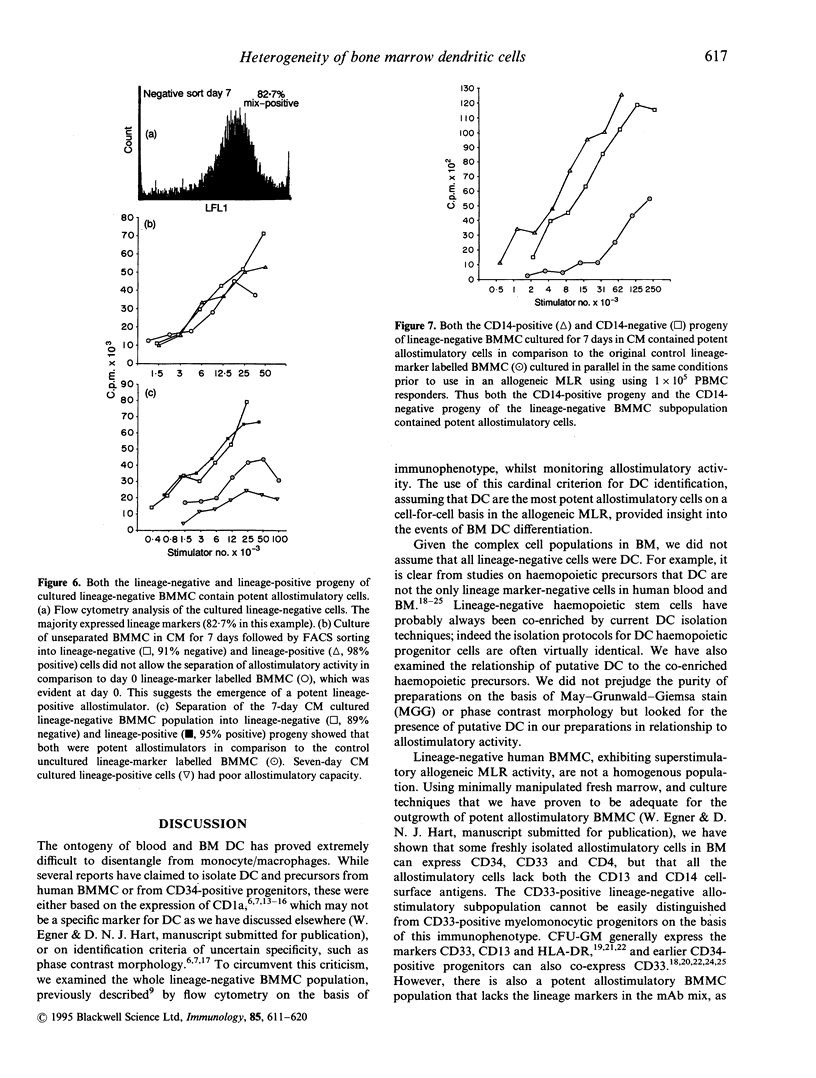
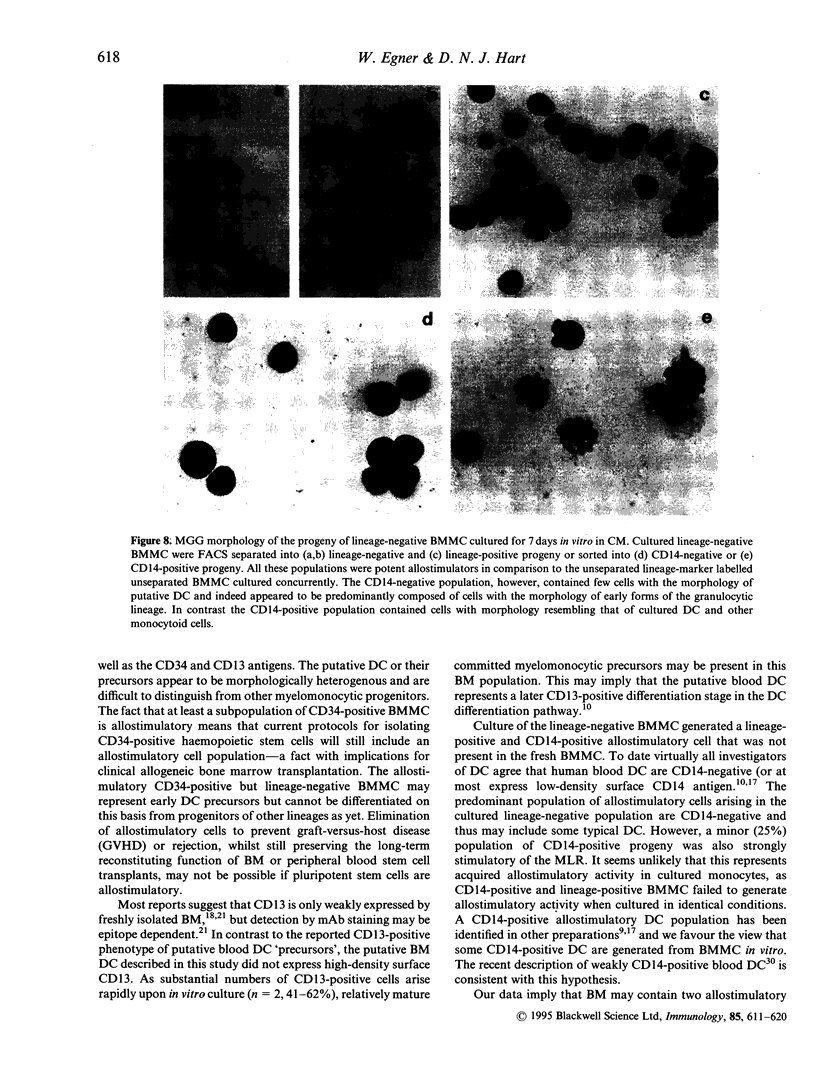
Putative dendritic cells (DC) and their precursors have been obtained from human bone marrow but their origin and relationship to other myeloid cells remains obscure. A minor bone marrow mononuclear cell (BMMC) population, which contains the most potent allostimulatory cells and lacks mature cell lineage markers (CD3, CD11b, CD14, CD15, CD16, CD19, CD57 and glycophorin A; lineage-negative) was enriched by immunoselection. These preparations, which contain cells with similar characteristics to freshly isolated human blood DC, were further subdivided by serial fluorescent-activated cell sorting (FACS). Potent allostimulatory cells were detected in the CD34, CD33 and CD4 positive and negative subpopulations. Cells with putative DC morphology were present in both the CD33 and CD4 positive and negative fractions. No significant CD13 or Thy-1 staining was seen in the lineage-negative population. In vitro culture of lineage-negative BMMC for 7 days in conditioned medium resulted in a up to fivefold expansion of cells and generated many lineage-positive progeny. This lineage-positive population was as allostimulatory as the negative progeny. Likewise, the CD14-positive and the CD14-negative cell progeny were equally allostimulatory. In contrast, the freshly isolated lineage-positive BMMC (containing CD14-positive monocytes) remained poor stimulators of the mixed lymphocyte reaction (MLR), even after culture in the presence of cytokines. These data suggest that there are at least two phenotypically diverse forms of potent allostimulatory cells in the lineage-negative fraction of human BM, at least some of which express the early haemopoietic precursor antigens CD34 or CD33. Some of these precursors generate CD14-positive allostimulatory cells upon in vitro culture, suggesting an intimate link between DC ontogeny and myeloid differentiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews R. G., Singer J. W., Bernstein I. D. Precursors of colony-forming cells in humans can be distinguished from colony-forming cells by expression of the CD33 and CD34 antigens and light scatter properties. J Exp Med. 1989 May 1;169(5):1721–1731. doi: 10.1084/jem.169.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender J. G., Unverzagt K. L., Walker D. E., Lee W., Van Epps D. E., Smith D. H., Stewart C. C., To L. B. Identification and comparison of CD34-positive cells and their subpopulations from normal peripheral blood and bone marrow using multicolor flow cytometry. Blood. 1991 Jun 15;77(12):2591–2596. [PubMed] [Google Scholar]
- Caux C., Dezutter-Dambuyant C., Schmitt D., Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992 Nov 19;360(6401):258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- Egner W., Andreesen R., Hart D. N. Allostimulatory cells in fresh human blood: heterogeneity in antigen-presenting cell populations. Transplantation. 1993 Oct;56(4):945–950. doi: 10.1097/00007890-199310000-00032. [DOI] [PubMed] [Google Scholar]
- Egner W., McKenzie J. L., Smith S. M., Beard M. E., Hart D. N. Identification of potent mixed leukocyte reaction-stimulatory cells in human bone marrow. Putative differentiation stage of human blood dendritic cells. J Immunol. 1993 Apr 1;150(7):3043–3053. [PubMed] [Google Scholar]
- Favaloro E. J., Bradstock K. F., Kabral A., Grimsley P., Zowtyj H., Zola H. Further characterization of human myeloid antigens (gp160,95; gp150; gp67): investigation of epitopic heterogeneity and non-haemopoietic distribution using panels of monoclonal antibodies belonging to CD-11b, CD-13 and CD-33. Br J Haematol. 1988 Jun;69(2):163–171. doi: 10.1111/j.1365-2141.1988.tb07618.x. [DOI] [PubMed] [Google Scholar]
- GOLDBERG A. F., BARKA T. Acid phosphatase activity in human blood cells. Nature. 1962 Jul 21;195:297–297. doi: 10.1038/195297a0. [DOI] [PubMed] [Google Scholar]
- Goordyal P., Isaacson P. G. Immunocytochemical characterization of monocyte colonies of human bone marrow: a clue to the origin of Langerhans cells and interdigitating reticulum cells. J Pathol. 1985 Jul;146(3):189–195. doi: 10.1002/path.1711460305. [DOI] [PubMed] [Google Scholar]
- Inaba K., Inaba M., Romani N., Aya H., Deguchi M., Ikehara S., Muramatsu S., Steinman R. M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992 Dec 1;176(6):1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M., Pack M. W., Aya H., Inaba M., Sudo T., Wolpe S., Schuler G. Identification of proliferating dendritic cell precursors in mouse blood. J Exp Med. 1992 May 1;175(5):1157–1167. doi: 10.1084/jem.175.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- Kabel P. J., de Haan-Meulman M., Voorbij H. A., Kleingeld M., Knol E. F., Drexhage H. A. Accessory cells with a morphology and marker pattern of dendritic cells can be obtained from elutriator-purified blood monocyte fractions. An enhancing effect of metrizamide in this differentiation. Immunobiology. 1989 Oct;179(4-5):395–341. doi: 10.1016/S0171-2985(89)80044-0. [DOI] [PubMed] [Google Scholar]
- Kato K., Radbruch A. Isolation and characterization of CD34+ hematopoietic stem cells from human peripheral blood by high-gradient magnetic cell sorting. Cytometry. 1993;14(4):384–392. doi: 10.1002/cyto.990140407. [DOI] [PubMed] [Google Scholar]
- Lu L., Walker D., Broxmeyer H. E., Hoffman R., Hu W., Walker E. Characterization of adult human marrow hematopoietic progenitors highly enriched by two-color cell sorting with My10 and major histocompatibility class II monoclonal antibodies. J Immunol. 1987 Sep 15;139(6):1823–1829. [PubMed] [Google Scholar]
- Markowicz S., Engleman E. G. Granulocyte-macrophage colony-stimulating factor promotes differentiation and survival of human peripheral blood dendritic cells in vitro. J Clin Invest. 1990 Mar;85(3):955–961. doi: 10.1172/JCI114525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty U., Steinman R. M., Peng M., Cameron P. U., Gezelter S., Kopeloff I., Swiggard W. J., Pope M., Bhardwaj N. Dendritic cells freshly isolated from human blood express CD4 and mature into typical immunostimulatory dendritic cells after culture in monocyte-conditioned medium. J Exp Med. 1993 Sep 1;178(3):1067–1076. doi: 10.1084/jem.178.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. H., Ruppert J., Gieseler R. K., Najar H. M., Xu H. Differentiation of human monocytes into CD14 negative accessory cells: do dendritic cells derive from the monocytic lineage? Pathobiology. 1991;59(3):122–126. doi: 10.1159/000163628. [DOI] [PubMed] [Google Scholar]
- Reid C. D., Fryer P. R., Clifford C., Kirk A., Tikerpae J., Knight S. C. Identification of hematopoietic progenitors of macrophages and dendritic Langerhans cells (DL-CFU) in human bone marrow and peripheral blood. Blood. 1990 Sep 15;76(6):1139–1149. [PubMed] [Google Scholar]
- Reid C. D., Stackpoole A., Meager A., Tikerpae J. Interactions of tumor necrosis factor with granulocyte-macrophage colony-stimulating factor and other cytokines in the regulation of dendritic cell growth in vitro from early bipotent CD34+ progenitors in human bone marrow. J Immunol. 1992 Oct 15;149(8):2681–2688. [PubMed] [Google Scholar]
- Rossi G., Heveker N., Thiele B., Gelderblom H., Steinbach F. Development of a Langerhans cell phenotype from peripheral blood monocytes. Immunol Lett. 1992 Feb;31(2):189–197. doi: 10.1016/0165-2478(92)90145-e. [DOI] [PubMed] [Google Scholar]
- Santiago-Schwarz F., Belilos E., Diamond B., Carsons S. E. TNF in combination with GM-CSF enhances the differentiation of neonatal cord blood stem cells into dendritic cells and macrophages. J Leukoc Biol. 1992 Sep;52(3):274–281. [PubMed] [Google Scholar]
- Thomas R., Davis L. S., Lipsky P. E. Isolation and characterization of human peripheral blood dendritic cells. J Immunol. 1993 Feb 1;150(3):821–834. [PubMed] [Google Scholar]
- Thomas R., Lipsky P. E. Human peripheral blood dendritic cell subsets. Isolation and characterization of precursor and mature antigen-presenting cells. J Immunol. 1994 Nov 1;153(9):4016–4028. [PubMed] [Google Scholar]
- Verfaillie C., Blakolmer K., McGlave P. Purified primitive human hematopoietic progenitor cells with long-term in vitro repopulating capacity adhere selectively to irradiated bone marrow stroma. J Exp Med. 1990 Aug 1;172(2):509–502. doi: 10.1084/jem.172.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Gunji Y., Nakamura M., Hayakawa K., Maeda M., Osawa H., Nagayoshi K., Kasahara T., Suda T. Expression of c-kit mRNA and protein during the differentiation of human hematopoietic progenitor cells. Exp Hematol. 1993 Aug;21(9):1233–1238. [PubMed] [Google Scholar]
- Zhou L. J., Schwarting R., Smith H. M., Tedder T. F. A novel cell-surface molecule expressed by human interdigitating reticulum cells, Langerhans cells, and activated lymphocytes is a new member of the Ig superfamily. J Immunol. 1992 Jul 15;149(2):735–742. [PubMed] [Google Scholar]
- de Fraissinette A., Schmitt D., Dezutter-Dambuyant C., Guyotat D., Zabot M. T., Thivolet J. Culture of putative Langerhans cell bone marrow precursors: characterization of their phenotype. Exp Hematol. 1988 Oct;16(9):764–768. [PubMed] [Google Scholar]