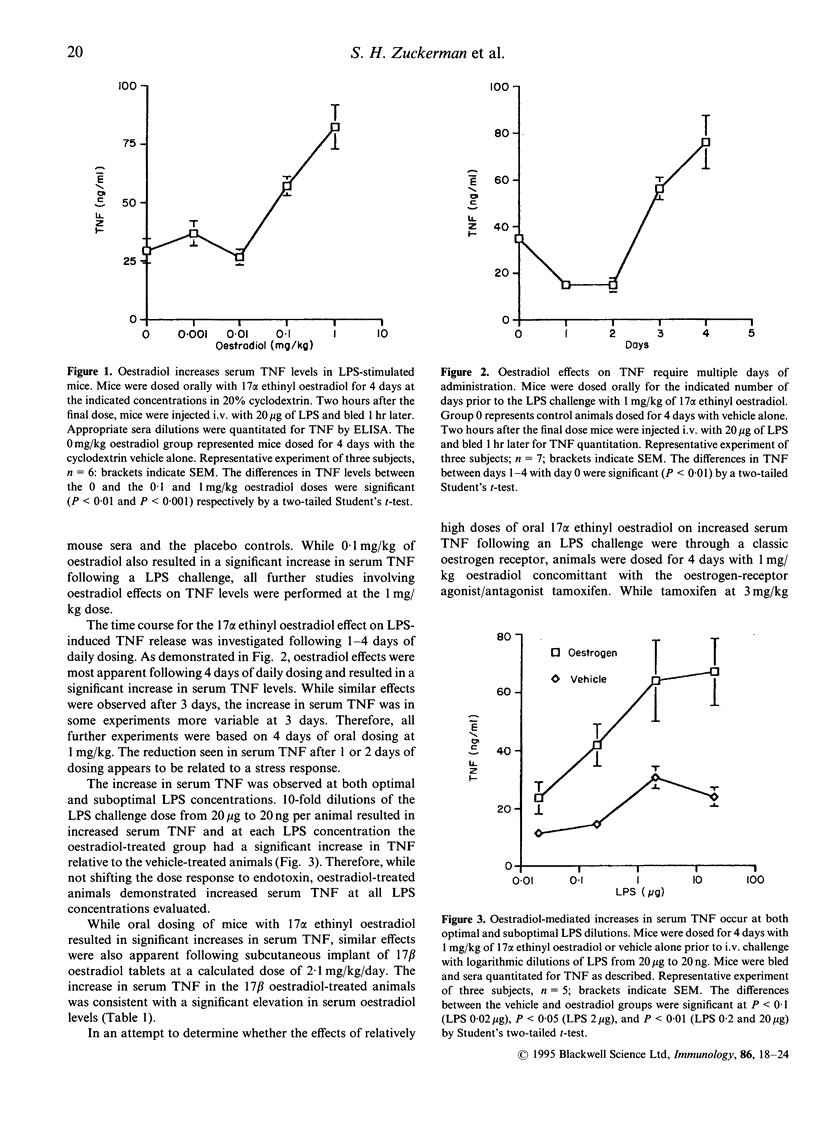
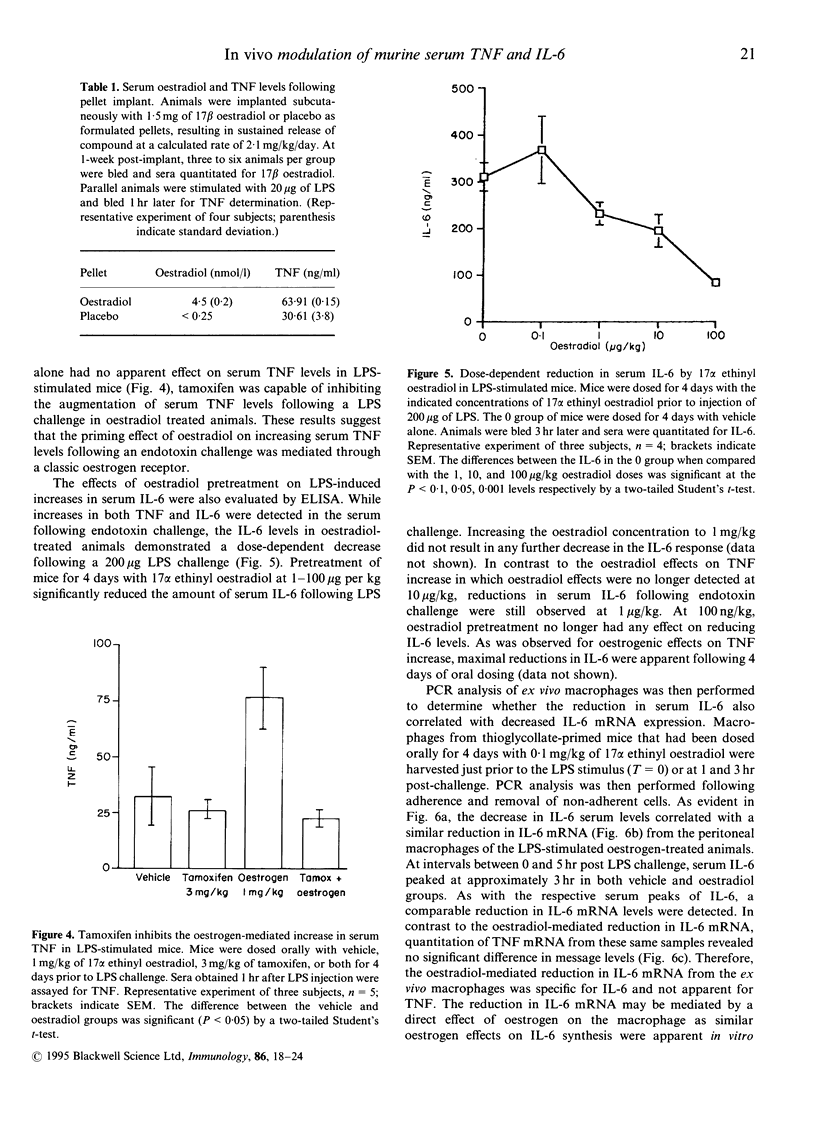
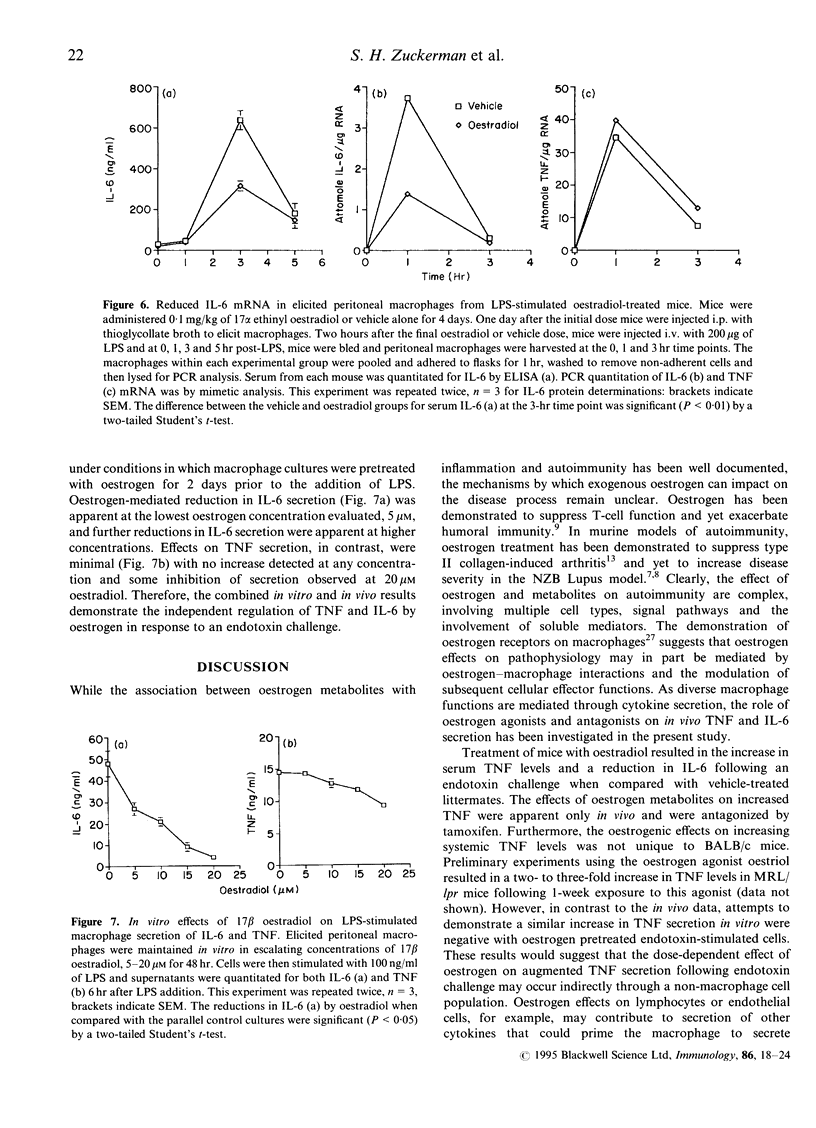
Abstract
Oestrogen has been reported to modulate tumour necrosis factor (TNF), interleukin (IL)-1 and IL-6 cytokine levels in human mononuclear cell cultures. In the present study, the effects of exogenous oestrogen administration on the cytokine response to an endotoxin challenge was investigated in a murine model of endotoxemia. Animals pretreated for 4 days with 17 alpha ethinyl oestradiol exhibited divergent regulation of TNF and IL-6 levels in sera from endotoxin-stimulated mice. Oestrogen treatment resulted in a significant increase in serum TNF while serum IL-6 levels, relative to the placebo group, decreased in response to an endotoxin challenge. These oestrogenic effects were dose dependent with maximal elevations observed in TNF at 1 mg/kg and maximal reduction in IL-6 at 0.1 mg/kg of 17 alpha ethinyl oestradiol. The increase in TNF levels by ethinyl oestradiol was blocked by co-administration of the oestrogen receptor antagonist tamoxifen. Oestrogen-mediated modulation of the TNF and IL-6 response to endotoxin was also apparent in animals implanted with 17 beta oestradiol pellets. The oestrogen-mediated effects on serum IL-6 were consistent with a reduction in IL-6 mRNA in peritoneal macrophages from oestrogen-treated mice. The effects of oestrogen on TNF and IL-6 production were also investigated in vitro. Oestradiol-treated macrophage cultures produced three- to fourfold lower amounts of IL-6 without any significant modulatory effects on TNF secretion. The combined in vivo and in vitro results demonstrate the modulation of IL-6 and TNF during endotoxemia by oestrogen analogues through an oestrogen receptor-dependent mechanism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Le J. M., Vilcek J. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J Immunol. 1989 Dec 1;143(11):3517–3523. [PubMed] [Google Scholar]
- Akira S., Taga T., Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Christiansen C., Christensen M. S., Larsen N. E., Transbøl I. B. Pathophysiological mechanisms of estrogen effect on bone metabolism. Dose-response relationships in early postmenopausal women. J Clin Endocrinol Metab. 1982 Dec;55(6):1124–1130. doi: 10.1210/jcem-55-6-1124. [DOI] [PubMed] [Google Scholar]
- Cid M. C., Kleinman H. K., Grant D. S., Schnaper H. W., Fauci A. S., Hoffman G. S. Estradiol enhances leukocyte binding to tumor necrosis factor (TNF)-stimulated endothelial cells via an increase in TNF-induced adhesion molecules E-selectin, intercellular adhesion molecule type 1, and vascular cell adhesion molecule type 1. J Clin Invest. 1994 Jan;93(1):17–25. doi: 10.1172/JCI116941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De M., Sanford T. R., Wood G. W. Interleukin-1, interleukin-6, and tumor necrosis factor alpha are produced in the mouse uterus during the estrous cycle and are induced by estrogen and progesterone. Dev Biol. 1992 May;151(1):297–305. doi: 10.1016/0012-1606(92)90234-8. [DOI] [PubMed] [Google Scholar]
- Evans G. F., Zuckerman S. H. Glucocorticoid-dependent and -independent mechanisms involved in lipopolysaccharide tolerance. Eur J Immunol. 1991 Sep;21(9):1973–1979. doi: 10.1002/eji.1830210902. [DOI] [PubMed] [Google Scholar]
- Farrar M. A., Schreiber R. D. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- Fattori E., Cappelletti M., Costa P., Sellitto C., Cantoni L., Carelli M., Faggioni R., Fantuzzi G., Ghezzi P., Poli V. Defective inflammatory response in interleukin 6-deficient mice. J Exp Med. 1994 Oct 1;180(4):1243–1250. doi: 10.1084/jem.180.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D., Netti F., Schreiber A. D. Effect of estradiol and steroid analogues on the clearance of immunoglobulin G-coated erythrocytes. J Clin Invest. 1985 Jan;75(1):162–167. doi: 10.1172/JCI111669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girasole G., Jilka R. L., Passeri G., Boswell S., Boder G., Williams D. C., Manolagas S. C. 17 beta-estradiol inhibits interleukin-6 production by bone marrow-derived stromal cells and osteoblasts in vitro: a potential mechanism for the antiosteoporotic effect of estrogens. J Clin Invest. 1992 Mar;89(3):883–891. doi: 10.1172/JCI115668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerne P. A., Carson D. A., Lotz M. IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J Immunol. 1990 Jan 15;144(2):499–505. [PubMed] [Google Scholar]
- Gulshan S., McCruden A. B., Stimson W. H. Oestrogen receptors in macrophages. Scand J Immunol. 1990 Jun;31(6):691–697. doi: 10.1111/j.1365-3083.1990.tb02820.x. [DOI] [PubMed] [Google Scholar]
- Heaney R. P., Recker R. R., Saville P. D. Menopausal changes in bone remodeling. J Lab Clin Med. 1978 Dec;92(6):964–970. [PubMed] [Google Scholar]
- Henderson B. E., Ross R. K., Paganini-Hill A., Mack T. M. Estrogen use and cardiovascular disease. Am J Obstet Gynecol. 1986 Jun;154(6):1181–1186. doi: 10.1016/0002-9378(86)90696-4. [DOI] [PubMed] [Google Scholar]
- Heremans H., Van Damme J., Dillen C., Dijkmans R., Billiau A. Interferon gamma, a mediator of lethal lipopolysaccharide-induced Shwartzman-like shock reactions in mice. J Exp Med. 1990 Jun 1;171(6):1853–1869. doi: 10.1084/jem.171.6.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmdahl R. Estrogen exaggerates lupus but suppresses T-cell-dependent autoimmune disease. J Autoimmun. 1989 Oct;2(5):651–656. doi: 10.1016/s0896-8411(89)80004-6. [DOI] [PubMed] [Google Scholar]
- Holmdahl R., Jansson L., Meyerson B., Klareskog L. Oestrogen induced suppression of collagen arthritis: I. Long term oestradiol treatment of DBA/1 mice reduces severity and incidence of arthritis and decreases the anti type II collagen immune response. Clin Exp Immunol. 1987 Nov;70(2):372–378. [PMC free article] [PubMed] [Google Scholar]
- Hu S. K., Mitcho Y. L., Rath N. C. Effect of estradiol on interleukin 1 synthesis by macrophages. Int J Immunopharmacol. 1988;10(3):247–252. doi: 10.1016/0192-0561(88)90055-0. [DOI] [PubMed] [Google Scholar]
- Huber L. A., Scheffler E., Poll T., Ziegler R., Dresel H. A. 17 beta-estradiol inhibits LDL oxidation and cholesteryl ester formation in cultured macrophages. Free Radic Res Commun. 1990;8(3):167–173. doi: 10.3109/10715769009087990. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Taga T., Akira S. Cytokine signal transduction. Cell. 1994 Jan 28;76(2):253–262. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- Mazière C., Auclair M., Ronveaux M. F., Salmon S., Santus R., Mazière J. C. Estrogens inhibit copper and cell-mediated modification of low density lipoprotein. Atherosclerosis. 1991 Aug;89(2-3):175–182. doi: 10.1016/0021-9150(91)90058-b. [DOI] [PubMed] [Google Scholar]
- Mori H., Sawairi M., Itoh N., Hanabayashi T., Tamaya T. Effects of sex steroids on cell differentiation and interleukin-1 beta production in the human promyelocytic leukemia cell line HL-60. J Reprod Med. 1992 Oct;37(10):871–878. [PubMed] [Google Scholar]
- Oka M., Vainio U. Effect of pregnancy on the prognosis and serology of rheumatoid arthritis. Acta Rheumatol Scand. 1966;12(1):47–52. doi: 10.3109/rhe1.1966.12.issue-1-4.06. [DOI] [PubMed] [Google Scholar]
- Pacifici R., Rifas L., McCracken R., Vered I., McMurtry C., Avioli L. V., Peck W. A. Ovarian steroid treatment blocks a postmenopausal increase in blood monocyte interleukin 1 release. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2398–2402. doi: 10.1073/pnas.86.7.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A., Prefontaine K. E., Ray P. Down-modulation of interleukin-6 gene expression by 17 beta-estradiol in the absence of high affinity DNA binding by the estrogen receptor. J Biol Chem. 1994 Apr 29;269(17):12940–12946. [PubMed] [Google Scholar]
- Recker R. R., Saville P. D., Heaney R. P. Effect of estrogens and calcium carbonate on bone loss in postmenopausal women. Ann Intern Med. 1977 Dec;87(6):649–655. doi: 10.7326/0003-4819-87-6-649. [DOI] [PubMed] [Google Scholar]
- Reduction in incidence of rheumatoid arthritis associated with oral contraceptives. Royal College of General Practitioners' Oral Contraception Study. Lancet. 1978 Mar 18;1(8064):569–571. [PubMed] [Google Scholar]
- Roubinian J. R., Talal N., Greenspan J. S., Goodman J. R., Siiteri P. K. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. J Exp Med. 1978 Jun 1;147(6):1568–1583. doi: 10.1084/jem.147.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato F., Ouchi Y., Masuyama A., Nakamura T., Hosoi T., Okamoto Y., Sasaki N., Shiraki M., Orimo H. Effects of estrogen replacement on insulin-like growth factor I concentrations in serum and bone tissue and on interleukin 1 secretion from spleen macrophages in oophorectomized rats. Calcif Tissue Int. 1993 Aug;53(2):111–116. doi: 10.1007/BF01321888. [DOI] [PubMed] [Google Scholar]
- Stampfer M. J., Colditz G. A., Willett W. C., Manson J. E., Rosner B., Speizer F. E., Hennekens C. H. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses' health study. N Engl J Med. 1991 Sep 12;325(11):756–762. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- Starnes H. F., Jr, Pearce M. K., Tewari A., Yim J. H., Zou J. C., Abrams J. S. Anti-IL-6 monoclonal antibodies protect against lethal Escherichia coli infection and lethal tumor necrosis factor-alpha challenge in mice. J Immunol. 1990 Dec 15;145(12):4185–4191. [PubMed] [Google Scholar]
- Steinberg A. D., Melez K. A., Raveche E. S., Reeves J. P., Boegel W. A., Smathers P. A., Taurog J. D., Weinlein L., Duvic M. Approach to the study of the role of sex hormones in autoimmunity. Arthritis Rheum. 1979 Nov;22(11):1170–1176. doi: 10.1002/art.1780221103. [DOI] [PubMed] [Google Scholar]
- Stock J. L., Coderre J. A., McDonald B., Rosenwasser L. J. Effects of estrogen in vivo and in vitro on spontaneous interleukin-1 release by monocytes from postmenopausal women. J Clin Endocrinol Metab. 1989 Feb;68(2):364–368. doi: 10.1210/jcem-68-2-364. [DOI] [PubMed] [Google Scholar]
- Tabibzadeh S. S., Santhanam U., Sehgal P. B., May L. T. Cytokine-induced production of IFN-beta 2/IL-6 by freshly explanted human endometrial stromal cells. Modulation by estradiol-17 beta. J Immunol. 1989 May 1;142(9):3134–3139. [PubMed] [Google Scholar]
- Vandenbroucke J. P., Valkenburg H. A., Boersma J. W., Cats A., Festen J. J., Huber-Bruning O., Rasker J. J. Oral contraceptives and rheumatoid arthritis: further evidence for a preventive effect. Lancet. 1982 Oct 16;2(8303):839–842. doi: 10.1016/s0140-6736(82)90809-1. [DOI] [PubMed] [Google Scholar]
- Walsh B. W., Schiff I., Rosner B., Greenberg L., Ravnikar V., Sacks F. M. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. N Engl J Med. 1991 Oct 24;325(17):1196–1204. doi: 10.1056/NEJM199110243251702. [DOI] [PubMed] [Google Scholar]
- Zuckerman S. H., Evans G. F., Butler L. D. Endotoxin tolerance: independent regulation of interleukin-1 and tumor necrosis factor expression. Infect Immun. 1991 Aug;59(8):2774–2780. doi: 10.1128/iai.59.8.2774-2780.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman S. H., Shellhaas J., Butler L. D. Differential regulation of lipopolysaccharide-induced interleukin 1 and tumor necrosis factor synthesis: effects of endogenous and exogenous glucocorticoids and the role of the pituitary-adrenal axis. Eur J Immunol. 1989 Feb;19(2):301–305. doi: 10.1002/eji.1830190213. [DOI] [PubMed] [Google Scholar]


