Abstract
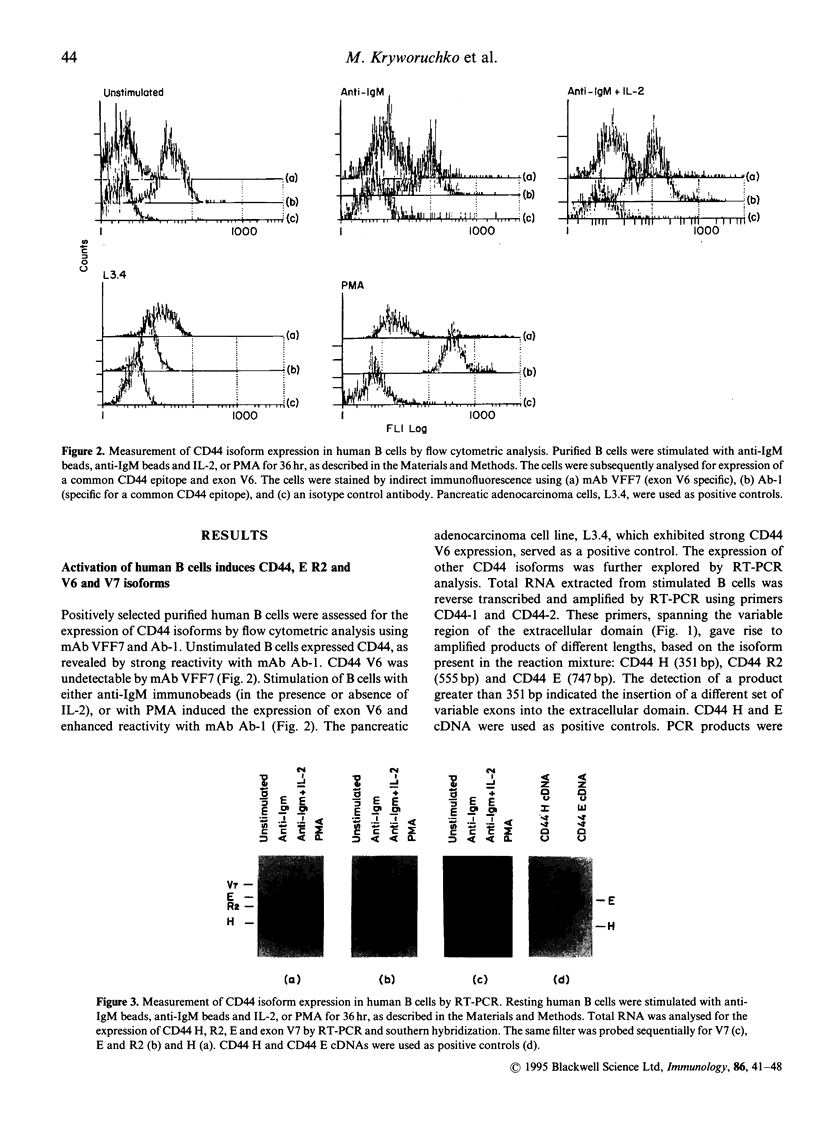
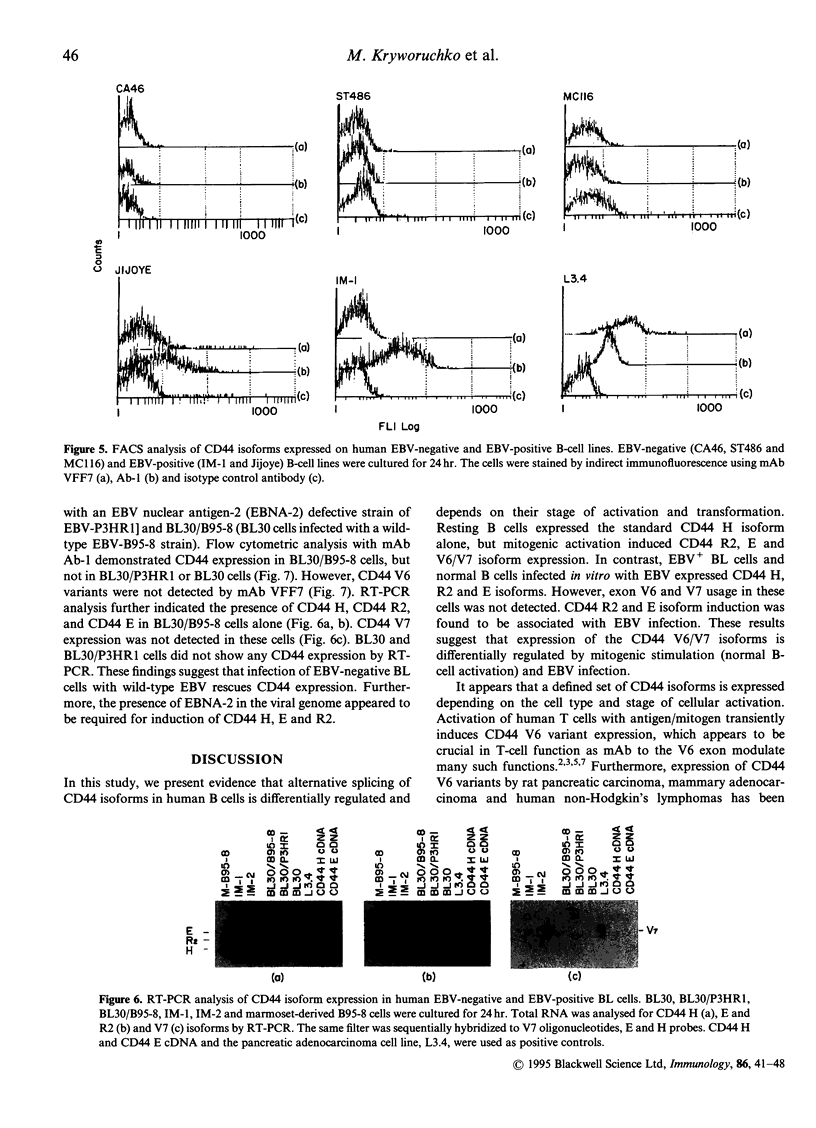
CD44, a cell adhesion molecule, exists in multiple isoforms that are generated by RNA alternative splicing. CD44 isoforms containing exon V6 (CD44 V6) have been associated with tumorigenesis and metastasis. We investigated the association between human B-cell activation and CD44 V6 isoform expression by analysing its expression in resting and mitogenically stimulated B cells. Results showed that resting B cells expressed the CD44 H (no variable exon) isoform alone. Activation of B cells [phorbol myristate acetate (PMA), surface immunoglobulin cross-linking alone or in the presence of interleukin-2 (IL-2)] induced CD44E (variable exon V8-10), R2 (VIO) and CD44 isoforms containing exons V6 and/or V7 (CD44 V6/V7). Epstein-Barr virus (EBV) infection of B cells, an alternative method of B-cell activation, induced the expression of CD44 E and R2 but not CD44 V6/V7. These results indicate that CD44 V6/V7 expression depends on the mode of activation. CD44 isoform expression was also investigated in a panel of EBV-negative and EBV-positive Burkitt's lymphoma (BL) B-cell lines. EBV-negative BL cells did not express CD44. In contrast, EBV-positive BL cells expressed CD44 H, R2 and E but not CD44 V6/V7 isoforms, suggesting an association between EBV infection and CD44 isoform induction. To determine directly the role of EBV in CD44 isoform induction, an EBV-negative BL cell line, BL30 (negative for all isoforms of CD44), BL30 infected in vitro with the EBNA-2-defective P3HR1 (BL30/P3HR1), and the wild-type B95-8 strain of EBV (BL30/B95-8) were examined. The parental BL30 cells infected with the wild-type EBV strain, but not with the P3HR-1 strain, expressed CD44 H, R2 and E isoforms, as seen in EBV-immortalized B cells. These studies suggest that (1) alternative splicing of CD44 isoforms is differentially regulated depending on the mode and state of cell activation, and that (2) the CD44 V6/V7 isoforms may represent B-cell activation antigens that are induced by mitogenic stimulation but not following EBV infection.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Bartolazzi A., Peach R., Aruffo A., Stamenkovic I. Interaction between CD44 and hyaluronate is directly implicated in the regulation of tumor development. J Exp Med. 1994 Jul 1;180(1):53–66. doi: 10.1084/jem.180.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenbach M., Josefsen K., Yalamanchili R., Lenoir G., Kieff E. Epstein-Barr virus-induced genes: first lymphocyte-specific G protein-coupled peptide receptors. J Virol. 1993 Apr;67(4):2209–2220. doi: 10.1128/jvi.67.4.2209-2220.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenbach M., Liebowitz D., Wang F., Sample J., Kieff E. Epstein-Barr virus latent infection membrane protein increases vimentin expression in human B-cell lines. J Virol. 1989 Sep;63(9):4079–4084. doi: 10.1128/jvi.63.9.4079-4084.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calender A., Billaud M., Aubry J. P., Banchereau J., Vuillaume M., Lenoir G. M. Epstein-Barr virus (EBV) induces expression of B-cell activation markers on in vitro infection of EBV-negative B-lymphoma cells. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8060–8064. doi: 10.1073/pnas.84.22.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp R. L., Kraus T. A., Birkeland M. L., Puré E. High levels of CD44 expression distinguish virgin from antigen-primed B cells. J Exp Med. 1991 Mar 1;173(3):763–766. doi: 10.1084/jem.173.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado A., de Andres A., Cañadas E., Ruiz-Cabello F., Gomez O., Pedrinaci S., Garrido F. Characterization of CD44 antigen during lymphoid ontogeny. Immunobiology. 1991 Sep;183(1-2):1–11. doi: 10.1016/S0171-2985(11)80181-6. [DOI] [PubMed] [Google Scholar]
- Dougherty G. J., Cooper D. L., Memory J. F., Chiu R. K. Ligand binding specificity of alternatively spliced CD44 isoforms. Recognition and binding of hyaluronan by CD44R1. J Biol Chem. 1994 Mar 25;269(12):9074–9078. [PubMed] [Google Scholar]
- Dougherty G. J., Landorp P. M., Cooper D. L., Humphries R. K. Molecular cloning of CD44R1 and CD44R2, two novel isoforms of the human CD44 lymphocyte "homing" receptor expressed by hemopoietic cells. J Exp Med. 1991 Jul 1;174(1):1–5. doi: 10.1084/jem.174.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlin-Henriksson B., Manneborg-Sandlund A., Klein G. Expression of B-cell-specific markers in different Burkitt lymphoma subgroups. Int J Cancer. 1987 Feb 15;39(2):211–218. doi: 10.1002/ijc.2910390215. [DOI] [PubMed] [Google Scholar]
- Galandrini R., Albi N., Tripodi G., Zarcone D., Terenzi A., Moretta A., Grossi C. E., Velardi A. Antibodies to CD44 trigger effector functions of human T cell clones. J Immunol. 1993 May 15;150(10):4225–4235. [PubMed] [Google Scholar]
- Galandrini R., Galluzzo E., Albi N., Grossi C. E., Velardi A. Hyaluronate is costimulatory for human T cell effector functions and binds to CD44 on activated T cells. J Immunol. 1994 Jul 1;153(1):21–31. [PubMed] [Google Scholar]
- Günthert U., Hofmann M., Rudy W., Reber S., Zöller M., Haussmann I., Matzku S., Wenzel A., Ponta H., Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991 Apr 5;65(1):13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Telen M. J., Hale L. P., Denning S. M. CD44--a molecule involved in leukocyte adherence and T-cell activation. Immunol Today. 1989 Dec;10(12):423–428. doi: 10.1016/0167-5699(89)90040-6. [DOI] [PubMed] [Google Scholar]
- Herrlich P., Zöller M., Pals S. T., Ponta H. CD44 splice variants: metastases meet lymphocytes. Immunol Today. 1993 Aug;14(8):395–399. doi: 10.1016/0167-5699(93)90141-7. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jackson D. G., Buckley J., Bell J. I. Multiple variants of the human lymphocyte homing receptor CD44 generated by insertions at a single site in the extracellular domain. J Biol Chem. 1992 Mar 5;267(7):4732–4739. [PubMed] [Google Scholar]
- Jalkanen S., Jalkanen M., Bargatze R., Tammi M., Butcher E. C. Biochemical properties of glycoproteins involved in lymphocyte recognition of high endothelial venules in man. J Immunol. 1988 Sep 1;141(5):1615–1623. [PubMed] [Google Scholar]
- Jalkanen S., Joensuu H., Söderström K. O., Klemi P. Lymphocyte homing and clinical behavior of non-Hodgkin's lymphoma. J Clin Invest. 1991 May;87(5):1835–1840. doi: 10.1172/JCI115205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman G., Heider K. H., Horst E., Adolf G. R., van den Berg F., Ponta H., Herrlich P., Pals S. T. Activated human lymphocytes and aggressive non-Hodgkin's lymphomas express a homologue of the rat metastasis-associated variant of CD44. J Exp Med. 1993 Apr 1;177(4):897–904. doi: 10.1084/jem.177.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Rogers T., Maizel A., Sharma S. Loss of transforming growth factor beta 1 receptors and its effects on the growth of EBV-transformed human B cells. J Immunol. 1991 Aug 1;147(3):998–1006. [PubMed] [Google Scholar]
- Lesley J., Howes N., Perschl A., Hyman R. Hyaluronan binding function of CD44 is transiently activated on T cells during an in vivo immune response. J Exp Med. 1994 Jul 1;180(1):383–387. doi: 10.1084/jem.180.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S., Miyake K., June C. H., Kincade P. W., Hodes R. J. IL-5 induces a Pgp-1 (CD44) bright B cell subpopulation that is highly enriched in proliferative and Ig secretory activity and binds to hyaluronate. J Immunol. 1990 Dec 1;145(11):3618–3627. [PubMed] [Google Scholar]
- Nilsson K., Klein G. Phenotypic and cytogenetic characteristics of human B-lymphoid cell lines and their relevance for the etiology of Burkitt's lymphoma. Adv Cancer Res. 1982;37:319–380. doi: 10.1016/s0065-230x(08)60886-6. [DOI] [PubMed] [Google Scholar]
- Screaton G. R., Bell M. V., Bell J. I., Jackson D. G. The identification of a new alternative exon with highly restricted tissue expression in transcripts encoding the mouse Pgp-1 (CD44) homing receptor. Comparison of all 10 variable exons between mouse, human, and rat. J Biol Chem. 1993 Jun 15;268(17):12235–12238. [PubMed] [Google Scholar]
- Seiter S., Arch R., Reber S., Komitowski D., Hofmann M., Ponta H., Herrlich P., Matzku S., Zöller M. Prevention of tumor metastasis formation by anti-variant CD44. J Exp Med. 1993 Feb 1;177(2):443–455. doi: 10.1084/jem.177.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Van Seventer G. A., Siraganian R., Wahl L., Shaw S. Dual role of the CD44 molecule in T cell adhesion and activation. J Immunol. 1989 Oct 15;143(8):2457–2463. [PubMed] [Google Scholar]
- Stamenkovic I., Aruffo A., Amiot M., Seed B. The hematopoietic and epithelial forms of CD44 are distinct polypeptides with different adhesion potentials for hyaluronate-bearing cells. EMBO J. 1991 Feb;10(2):343–348. doi: 10.1002/j.1460-2075.1991.tb07955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sy M. S., Guo Y. J., Stamenkovic I. Distinct effects of two CD44 isoforms on tumor growth in vivo. J Exp Med. 1991 Oct 1;174(4):859–866. doi: 10.1084/jem.174.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan P. H., Santos E. B., Rossbach H. C., Sandmaier B. M. Enhancement of natural killer activity by an antibody to CD44. J Immunol. 1993 Feb 1;150(3):812–820. [PubMed] [Google Scholar]
- Wang F., Gregory C. D., Rowe M., Rickinson A. B., Wang D., Birkenbach M., Kikutani H., Kishimoto T., Kieff E. Epstein-Barr virus nuclear antigen 2 specifically induces expression of the B-cell activation antigen CD23. Proc Natl Acad Sci U S A. 1987 May;84(10):3452–3456. doi: 10.1073/pnas.84.10.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Gregory C., Sample C., Rowe M., Liebowitz D., Murray R., Rickinson A., Kieff E. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J Virol. 1990 May;64(5):2309–2318. doi: 10.1128/jvi.64.5.2309-2318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]