Abstract
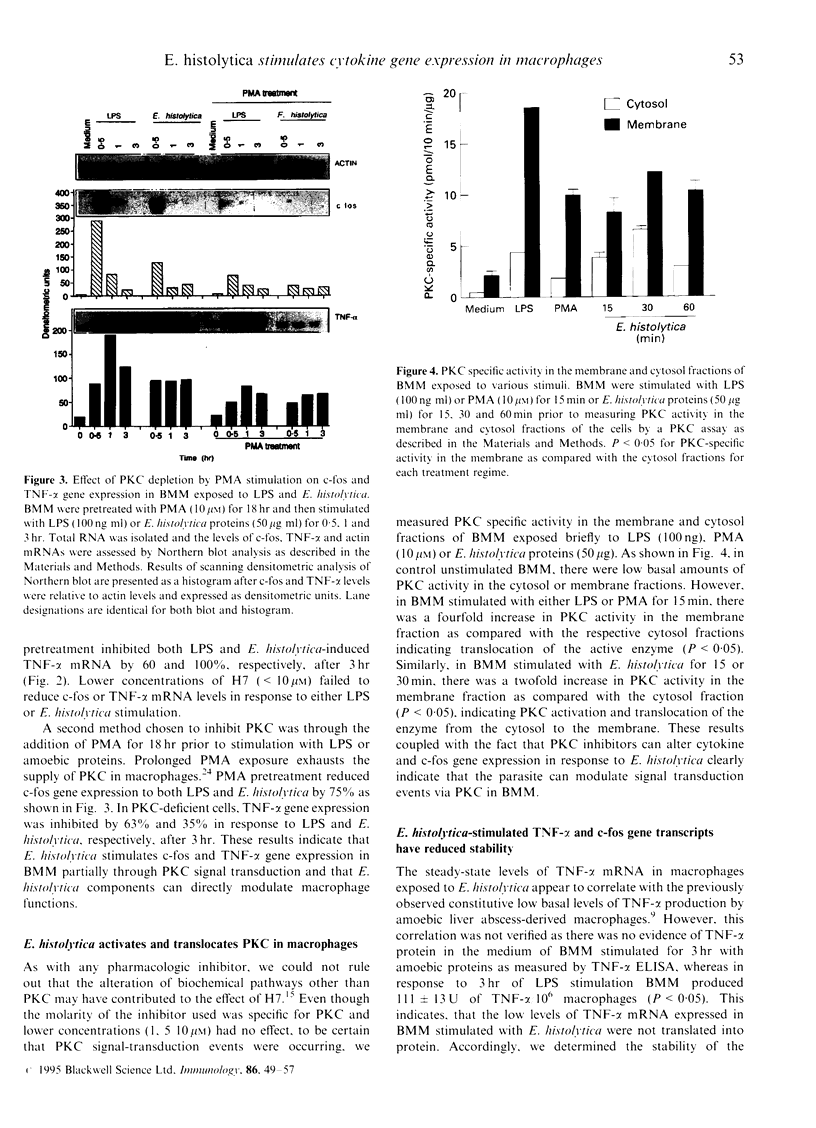
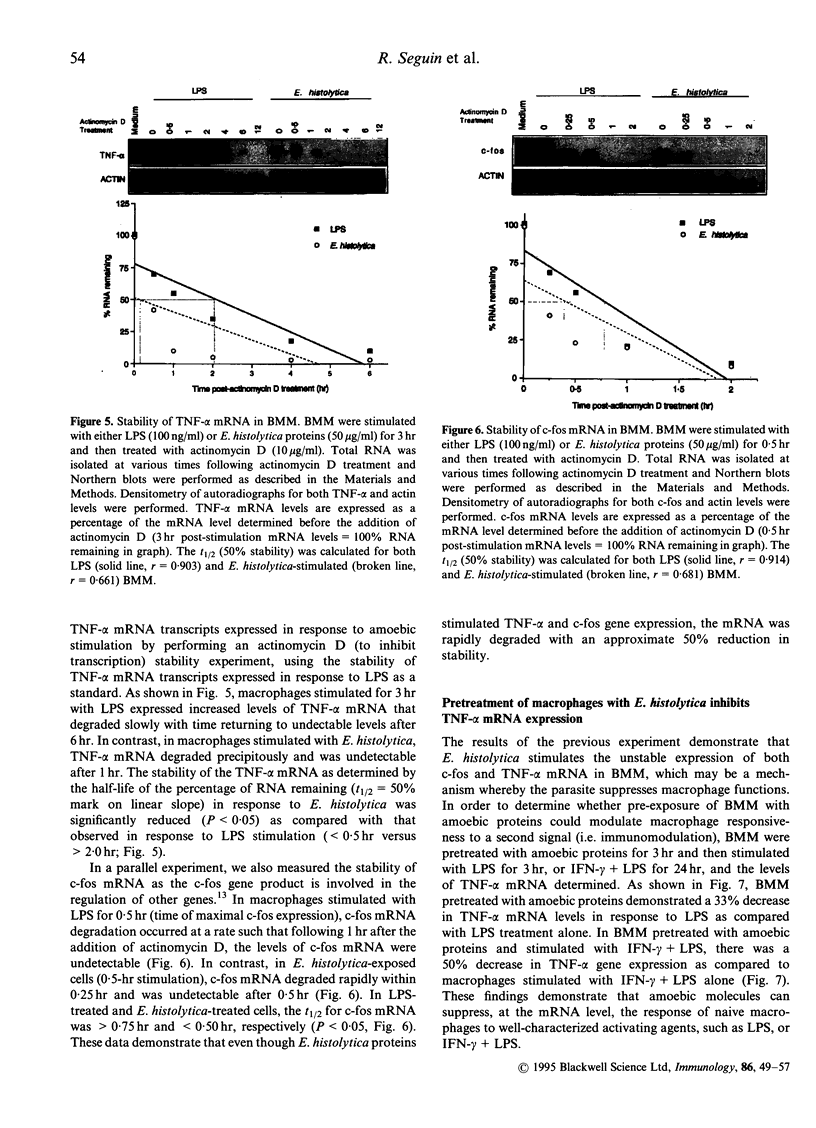
Macrophages play an important role in the control of and resistance to Entamoeba histolytica (E. histolytica). However, E. histolytica infections are characterized by suppression of cell-mediated immunity. To elucidate the molecular mechanisms whereby amoebae modulate host defences, we investigated whether the parasite elicits the 'immediate early' gene c-fos and cytokine tumour necrosis factor-alpha (TNF)-alpha mRNA and determined the signal transduction pathways involved in naive bone marrow-derived macrophages (BMM). E. histolytica stimulated a rapid and transient expression of c-fos and low levels of TNF-alpha mRNA, whereas the non-pathogenic Entamoeba moshkovshii (E. moshkovskii) did not. Inhibition of the protein kinase C (PKC) pathway with the pharmacological inhibitor H7 and by PKC depletion with phorbol myristate acetate showed that E. histolytica modulates TNF-alpha and c-fos gene expression through a PKC-dependent stimulus-response coupling event. E. histolytica activated and translocated PKC to the membrane fraction in BMM demonstrating a rapid and direct effect on PKC enzyme activity. Unlike lipopolysaccharide (LPS), BMM stimulated with E. histolytica had reduced stability of both c-fos and TNF-alpha mRNA transcripts (> 50%) and failed to secrete TNF-alpha protein. BMM treated with amoebic proteins and stimulated with LPS, or interferon-gamma (IFN-gamma)+LPS, resulted in a 33% and 50% reduction in TNF-alpha mRNA levels, respectively. These data argue that although E. histolytica stimulates c-fos and TNF-alpha gene expression through PKC signal transduction, the rapid degradation of the mRNAs, the lack of secreted TNF-alpha protein and the observed decreased responsiveness to a stimulatory signal may be a novel mechanism whereby the parasite modulates host defence mechanisms.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bravo R., Neuberg M., Burckhardt J., Almendral J., Wallich R., Müller R. Involvement of common and cell type-specific pathways in c-fos gene control: stable induction of cAMP in macrophages. Cell. 1987 Jan 30;48(2):251–260. doi: 10.1016/0092-8674(87)90428-4. [DOI] [PubMed] [Google Scholar]
- Chadee K., Meerovitch E., Moreau F. In vitro and in vivo interaction between trophozoites of Entamoeba histolytica and gerbil lymphoid cells. Infect Immun. 1985 Sep;49(3):828–832. doi: 10.1128/iai.49.3.828-832.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Denis M., Chadee K. Cytokine activation of murine macrophages for in vitro killing of Entamoeba histolytica trophozoites. Infect Immun. 1989 Jun;57(6):1750–1756. doi: 10.1128/iai.57.6.1750-1756.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M., Chadee K. In vitro and in vivo studies of macrophage functions in amebiasis. Infect Immun. 1988 Dec;56(12):3126–3131. doi: 10.1128/iai.56.12.3126-3131.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Bell R. M. Activation of protein kinase C by Triton X-100 mixed micelles containing diacylglycerol and phosphatidylserine. J Biol Chem. 1985 Aug 25;260(18):10039–10043. [PubMed] [Google Scholar]
- Higuchi Y., Setoguchi M., Yoshida S., Akizuki S., Yamamoto S. Enhancement of c-fos expression is associated with activated macrophages. Oncogene. 1988 May;2(5):515–521. [PubMed] [Google Scholar]
- Introna M., Hamilton T. A., Kaufman R. E., Adams D. O., Bast R. C., Jr Treatment of murine peritoneal macrophages with bacterial lipopolysaccharide alters expression of c-fos and c-myc oncogenes. J Immunol. 1986 Oct 15;137(8):2711–2715. [PubMed] [Google Scholar]
- Krupp I. M., Powell S. J. Antibody response to invasive amebiasis in Durban, South Africa. Am J Trop Med Hyg. 1971 May;20(3):414–420. doi: 10.4269/ajtmh.1971.20.414. [DOI] [PubMed] [Google Scholar]
- Kruys V., Marinx O., Shaw G., Deschamps J., Huez G. Translational blockade imposed by cytokine-derived UA-rich sequences. Science. 1989 Aug 25;245(4920):852–855. doi: 10.1126/science.2672333. [DOI] [PubMed] [Google Scholar]
- Liles W. C., Hunter D. D., Meier K. E., Nathanson N. M. Activation of protein kinase C induces rapid internalization and subsequent degradation of muscarinic acetylcholine receptors in neuroblastoma cells. J Biol Chem. 1986 Apr 25;261(12):5307–5313. [PubMed] [Google Scholar]
- Lin J. Y., Chadee K. Macrophage cytotoxicity against Entamoeba histolytica trophozoites is mediated by nitric oxide from L-arginine. J Immunol. 1992 Jun 15;148(12):3999–4005. [PubMed] [Google Scholar]
- Lin J. Y., Seguin R., Keller K., Chadee K. Tumor necrosis factor alpha augments nitric oxide-dependent macrophage cytotoxicity against Entamoeba histolytica by enhanced expression of the nitric oxide synthase gene. Infect Immun. 1994 May;62(5):1534–1541. doi: 10.1128/iai.62.5.1534-1541.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May W. S., Jr, Sahyoun N., Wolf M., Cuatrecasas P. Role of intracellular calcium mobilization in the regulation of protein kinase C-mediated membrane processes. Nature. 1985 Oct 10;317(6037):549–551. doi: 10.1038/317549a0. [DOI] [PubMed] [Google Scholar]
- Murray H. W. Pretreatment with phorbol myristate acetate inhibits macrophage activity against intracellular protozoa. J Reticuloendothel Soc. 1982 Jun;31(6):479–487. [PubMed] [Google Scholar]
- Radzioch D., Bottazzi B., Varesio L. Augmentation of c-fos mRNA expression by activators of protein kinase C in fresh, terminally differentiated resting macrophages. Mol Cell Biol. 1987 Feb;7(2):595–599. doi: 10.1128/mcb.7.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salata R. A., Pearson R. D., Ravdin J. I. Interaction of human leukocytes and Entamoeba histolytica. Killing of virulent amebae by the activated macrophage. J Clin Invest. 1985 Aug;76(2):491–499. doi: 10.1172/JCI111998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana K., Chen G. J., Huang D. S., Scuderi P., Watson R. R. Production of tumor necrosis factor alpha by resident and activated murine macrophages. J Leukoc Biol. 1992 Mar;51(3):251–255. doi: 10.1002/jlb.51.3.251. [DOI] [PubMed] [Google Scholar]
- Takasuka N., Tokunaga T., Akagawa K. S. Preexposure of macrophages to low doses of lipopolysaccharide inhibits the expression of tumor necrosis factor-alpha mRNA but not of IL-1 beta mRNA. J Immunol. 1991 Jun 1;146(11):3824–3830. [PubMed] [Google Scholar]
- Tannenbaum C. S., Hamilton T. A. Lipopolysaccharide-induced gene expression in murine peritoneal macrophages is selectively suppressed by agents that elevate intracellular cAMP. J Immunol. 1989 Feb 15;142(4):1274–1280. [PubMed] [Google Scholar]
- Wang W., Chadee K. Entamoeba histolytica alters arachidonic acid metabolism in macrophages in vitro and in vivo. Immunology. 1992 Jun;76(2):242–250. [PMC free article] [PubMed] [Google Scholar]
- Wang W., Chadee K. Entamoeba histolytica suppresses gamma interferon-induced macrophage class II major histocompatibility complex Ia molecule and I-A beta mRNA expression by a prostaglandin E2-dependent mechanism. Infect Immun. 1995 Mar;63(3):1089–1094. doi: 10.1128/iai.63.3.1089-1094.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Keller K., Chadee K. Entamoeba histolytica modulates the nitric oxide synthase gene and nitric oxide production by macrophages for cytotoxicity against amoebae and tumour cells. Immunology. 1994 Dec;83(4):601–610. [PMC free article] [PubMed] [Google Scholar]
- Wang W., Keller K., Chadee K. Modulation of tumor necrosis factor production by macrophages in Entamoeba histolytica infection. Infect Immun. 1992 Aug;60(8):3169–3174. doi: 10.1128/iai.60.8.3169-3174.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]









