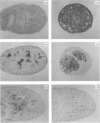
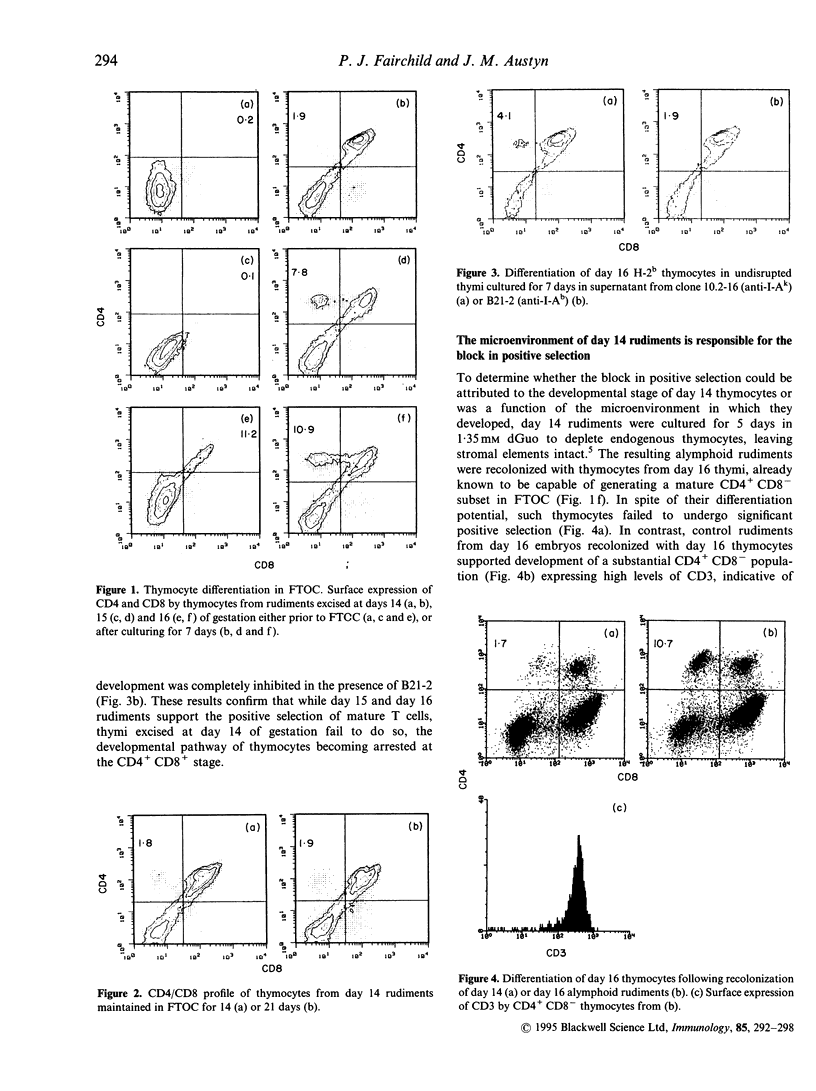
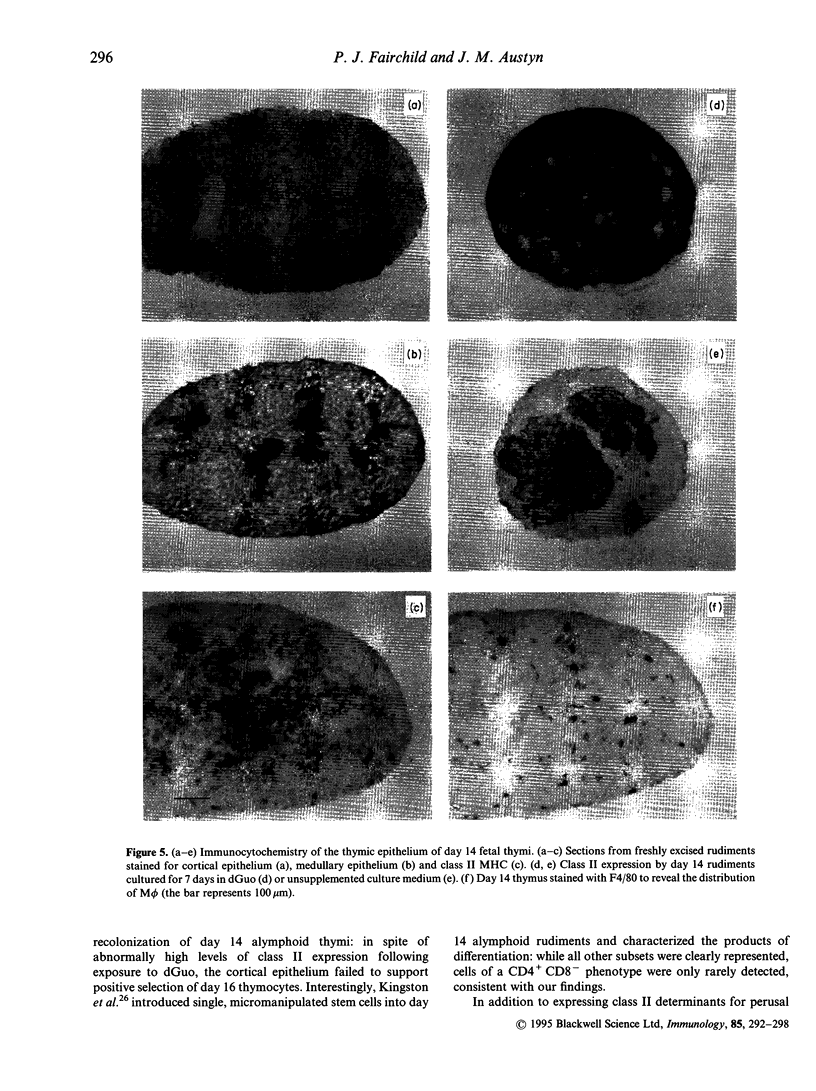
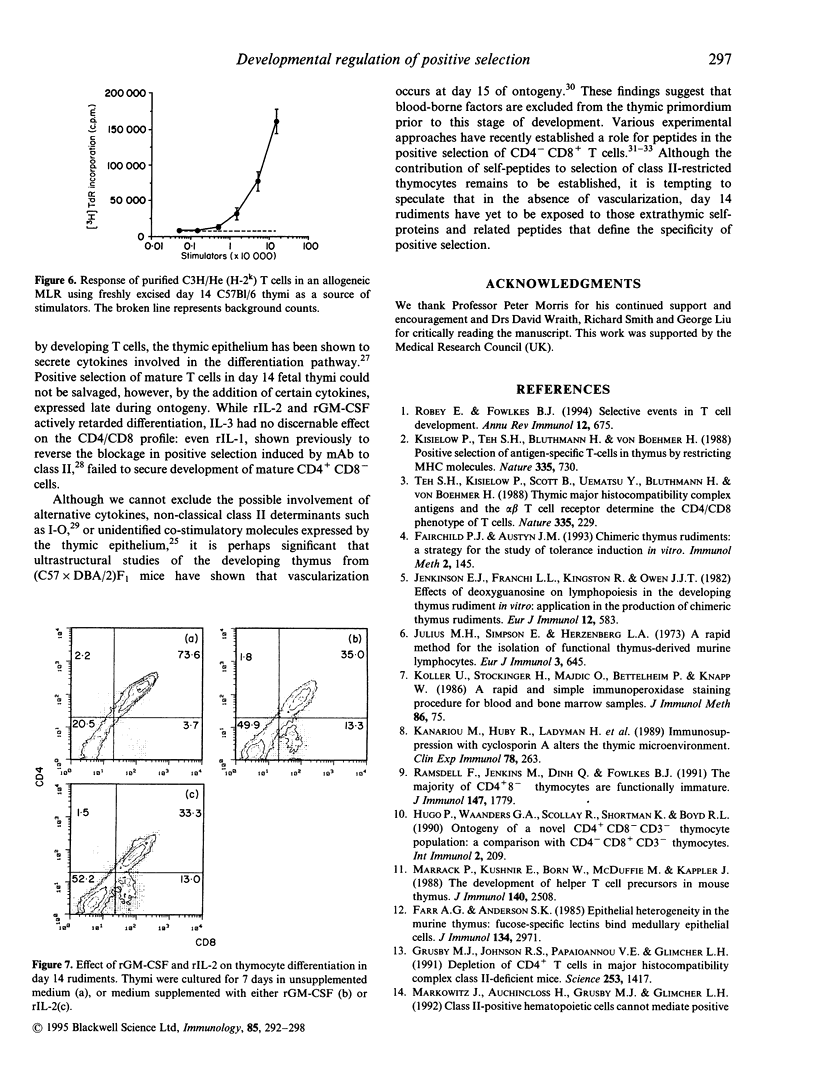
Abstract
Selection of a competent T-cell repertoire is dependent on complex interactions between immature thymocytes and components of the thymic stroma. These events may be preserved in vitro by excising developing thymus rudiments and maintaining them under carefully controlled conditions in fetal thymus organ cultures (FTOC). Using this approach, we have shown that the ability of C57B1/6 thymi to sustain positive selection of mature CD4+CD8- cells is profoundly influenced by the day of gestation on which they are excised: while thymocytes from day 14 rudiments fail to progress beyond the CD4+CD8+ stage of the developmental pathway, day 15 and day 16 thymi support the differentiation of CD4+CD8- thymocytes. Importantly, day 16 thymocytes transferred to day 14 deoxyguanosine-treated rudiments are likewise arrested at the CD4+CD8+ stage, suggesting that the thymic microenvironment of day 14 rudiments, rather than the state of differentiation of the thymocytes they contain, is responsible for the block in positive selection. Our studies of the stromal elements of day 14 rudiments have, however, revealed no obvious deficiencies in the cell types represented, or their expression of class II major histocompatibility complex (MHC) determinants. Furthermore, we have been unable to circumvent the blockage in positive selection by the addition of certain cytokines expressed late during gestation. These results suggest that subtle changes occurring at day 15 of ontogeny render the thymic microenvironment capable of positive selection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson G., Owen J. J., Moore N. C., Jenkinson E. J. Thymic epithelial cells provide unique signals for positive selection of CD4+CD8+ thymocytes in vitro. J Exp Med. 1994 Jun 1;179(6):2027–2031. doi: 10.1084/jem.179.6.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bix M., Raulet D. Inefficient positive selection of T cells directed by haematopoietic cells. Nature. 1992 Sep 24;359(6393):330–333. doi: 10.1038/359330a0. [DOI] [PubMed] [Google Scholar]
- Carding S. R., Hayday A. C., Bottomly K. Cytokines in T-cell development. Immunol Today. 1991 Jul;12(7):239–245. doi: 10.1016/0167-5699(91)90037-T. [DOI] [PubMed] [Google Scholar]
- DeLuca D., Mizel S. B. I-A-positive nonlymphoid cells and T cell development in murine fetal thymus organ cultures: interleukin 1 circumvents the block in T cell differentiation induced by monoclonal anti-I-A antibodies. J Immunol. 1986 Sep 1;137(5):1435–1441. [PubMed] [Google Scholar]
- Elliott J. I. The identity of the cells that positively select thymocytes. Immunol Rev. 1993 Oct;135:215–225. doi: 10.1111/j.1600-065x.1993.tb00650.x. [DOI] [PubMed] [Google Scholar]
- Fairchild P. J., Austyn J. M. Thymic dendritic cells: phenotype and function. Int Rev Immunol. 1990;6(2-3):187–196. doi: 10.3109/08830189009056629. [DOI] [PubMed] [Google Scholar]
- Farr A. G., Anderson S. K. Epithelial heterogeneity in the murine thymus: fucose-specific lectins bind medullary epithelial cells. J Immunol. 1985 May;134(5):2971–2977. [PubMed] [Google Scholar]
- Grusby M. J., Johnson R. S., Papaioannou V. E., Glimcher L. H. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science. 1991 Sep 20;253(5026):1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- Hogquist K. A., Gavin M. A., Bevan M. J. Positive selection of CD8+ T cells induced by major histocompatibility complex binding peptides in fetal thymic organ culture. J Exp Med. 1993 May 1;177(5):1469–1473. doi: 10.1084/jem.177.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo P., Waanders G. A., Scollay R., Shortman K., Boyd R. L. Ontogeny of a novel CD4+CD8-CD3- thymocyte subpopulation: a comparison with CD4- CD8+ CD3- thymocytes. Int Immunol. 1990;2(3):209–218. doi: 10.1093/intimm/2.3.209. [DOI] [PubMed] [Google Scholar]
- Jenkinson E. J., Anderson G., Owen J. J. Studies on T cell maturation on defined thymic stromal cell populations in vitro. J Exp Med. 1992 Sep 1;176(3):845–853. doi: 10.1084/jem.176.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson E. J., Franchi L. L., Kingston R., Owen J. J. Effect of deoxyguanosine on lymphopoiesis in the developing thymus rudiment in vitro: application in the production of chimeric thymus rudiments. Eur J Immunol. 1982 Jul;12(7):583–587. doi: 10.1002/eji.1830120710. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kanariou M., Huby R., Ladyman H., Colic M., Sivolapenko G., Lampert I., Ritter M. Immunosuppression with cyclosporin A alters the thymic microenvironment. Clin Exp Immunol. 1989 Nov;78(2):263–270. [PMC free article] [PubMed] [Google Scholar]
- Kingston R., Jenkinson E. J., Owen J. J. A single stem cell can recolonize an embryonic thymus, producing phenotypically distinct T-cell populations. 1985 Oct 31-Nov 6Nature. 317(6040):811–813. doi: 10.1038/317811a0. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Teh H. S., Blüthmann H., von Boehmer H. Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature. 1988 Oct 20;335(6192):730–733. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- Köller U., Stockinger H., Majdic O., Bettelheim P., Knapp W. A rapid and simple immunoperoxidase staining procedure for blood and bone marrow samples. J Immunol Methods. 1986 Jan 22;86(1):75–81. doi: 10.1016/0022-1759(86)90267-x. [DOI] [PubMed] [Google Scholar]
- Mandel T. Differentiation of epithelial cells in the mouse thymus. Z Zellforsch Mikrosk Anat. 1970;106(4):498–515. doi: 10.1007/BF00340288. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kushnir E., Born W., McDuffie M., Kappler J. The development of helper T cell precursors in mouse thymus. J Immunol. 1988 Apr 15;140(8):2508–2514. [PubMed] [Google Scholar]
- Montgomery R. A., Dallman M. J. Analysis of cytokine gene expression during fetal thymic ontogeny using the polymerase chain reaction. J Immunol. 1991 Jul 15;147(2):554–560. [PubMed] [Google Scholar]
- Owen J. J., Ritter M. A. Tissue interaction in the development of thymus lymphocytes. J Exp Med. 1969 Feb 1;129(2):431–442. doi: 10.1084/jem.129.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsdell F., Jenkins M., Dinh Q., Fowlkes B. J. The majority of CD4+8- thymocytes are functionally immature. J Immunol. 1991 Sep 15;147(6):1779–1785. [PubMed] [Google Scholar]
- Robey E., Fowlkes B. J. Selective events in T cell development. Annu Rev Immunol. 1994;12:675–705. doi: 10.1146/annurev.iy.12.040194.003331. [DOI] [PubMed] [Google Scholar]
- Robinson J. H. The ontogeny of antigen-presenting cells in fetal thymus evaluated by MLR stimulation. J Immunol. 1983 Apr;130(4):1592–1595. [PubMed] [Google Scholar]
- Ron Y., Lo D., Sprent J. T cell specificity in twice-irradiated F1----parent bone marrow chimeras: failure to detect a role for immigrant marrow-derived cells in imprinting intrathymic H-2 restriction. J Immunol. 1986 Sep 15;137(6):1764–1771. [PubMed] [Google Scholar]
- Sebzda E., Wallace V. A., Mayer J., Yeung R. S., Mak T. W., Ohashi P. S. Positive and negative thymocyte selection induced by different concentrations of a single peptide. Science. 1994 Mar 18;263(5153):1615–1618. doi: 10.1126/science.8128249. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Williams G. T., Kingston R., Jenkinson E. J., Owen J. J. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989 Jan 12;337(6203):181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- Spain L. M., Berg L. J. Developmental regulation of thymocyte susceptibility to deletion by "self"-peptide. J Exp Med. 1992 Jul 1;176(1):213–223. doi: 10.1084/jem.176.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh C. D., Gao E. K., Kosaka H., Lo D., Ahn C., Murphy D. B., Karlsson L., Peterson P., Sprent J. Two subsets of epithelial cells in the thymic medulla. J Exp Med. 1992 Aug 1;176(2):495–505. doi: 10.1084/jem.176.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh H. S., Kisielow P., Scott B., Kishi H., Uematsu Y., Blüthmann H., von Boehmer H. Thymic major histocompatibility complex antigens and the alpha beta T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature. 1988 Sep 15;335(6187):229–233. doi: 10.1038/335229a0. [DOI] [PubMed] [Google Scholar]