Abstract
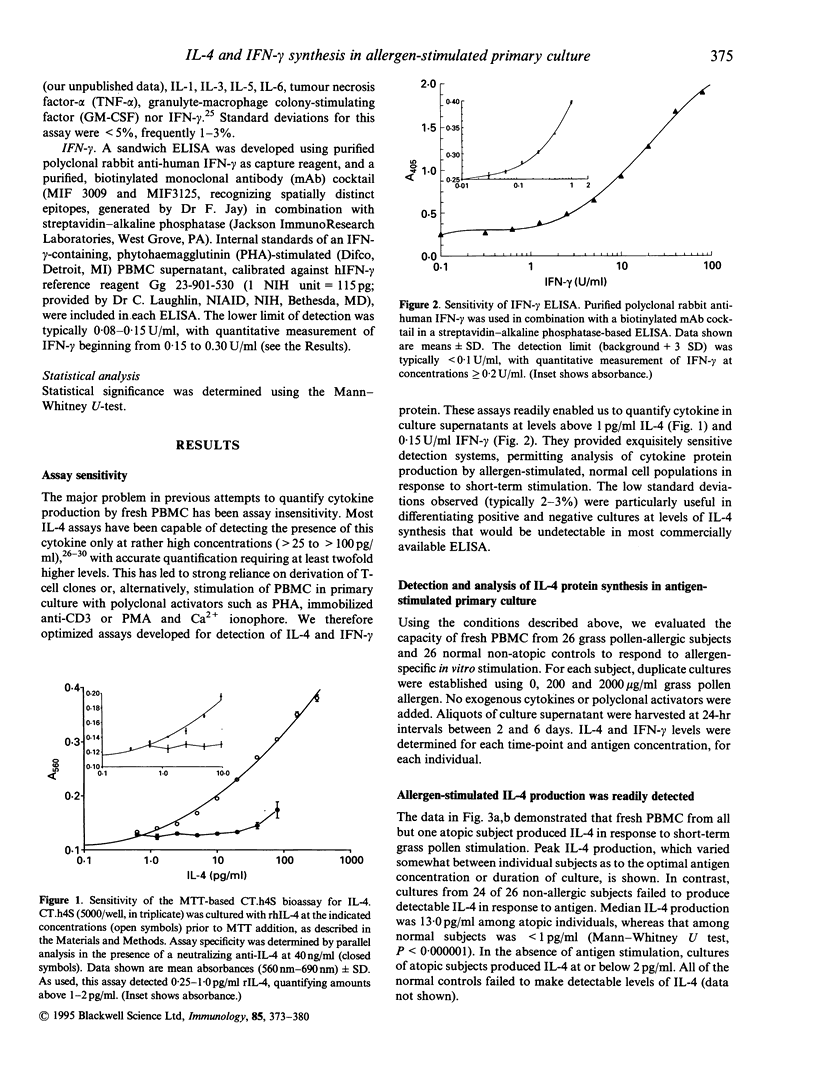
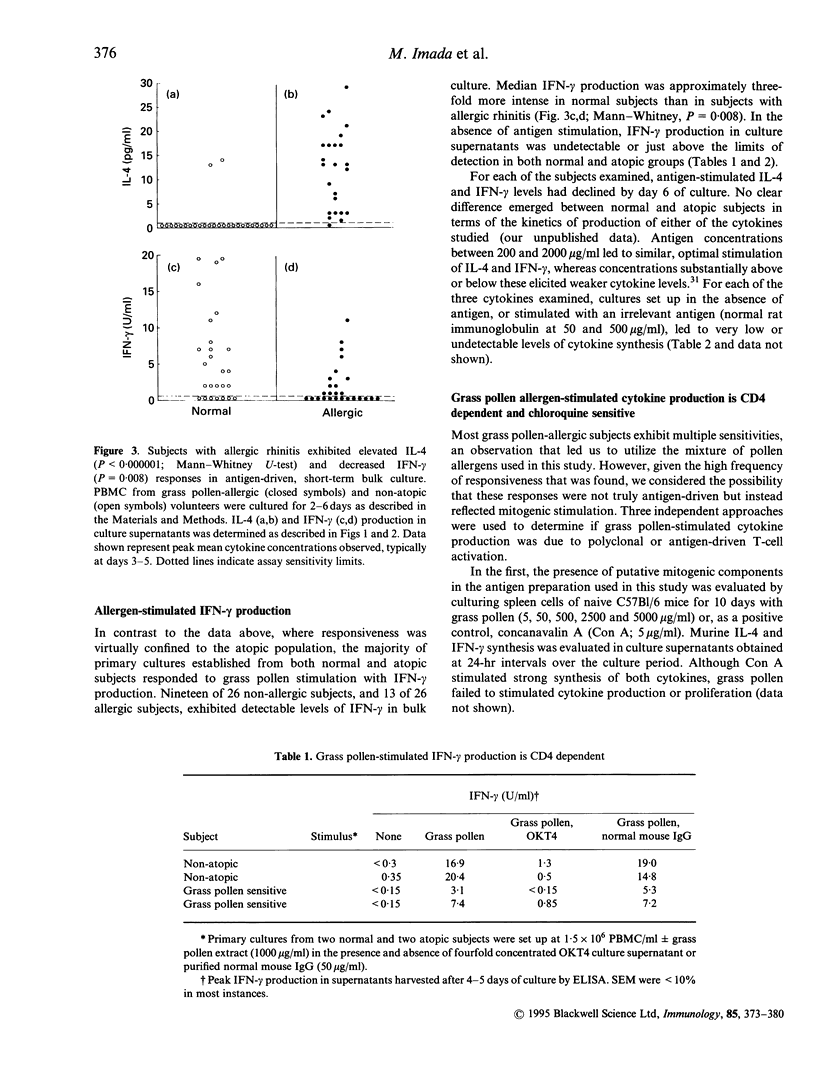
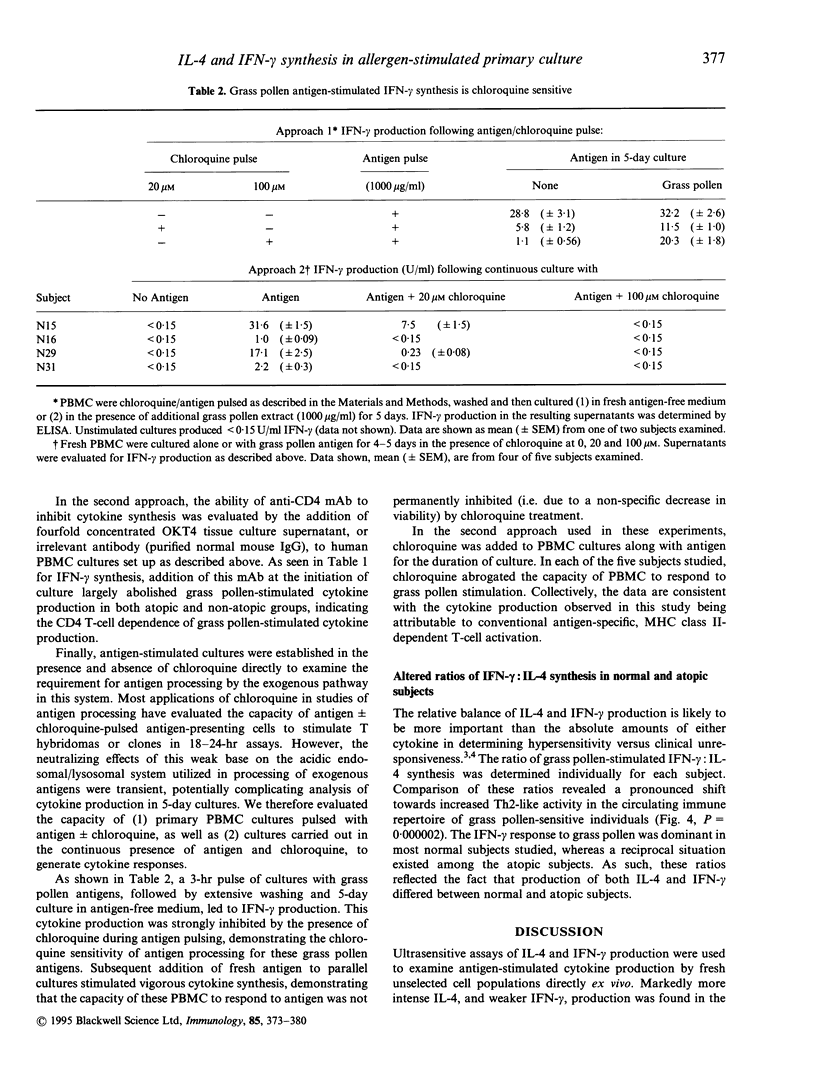
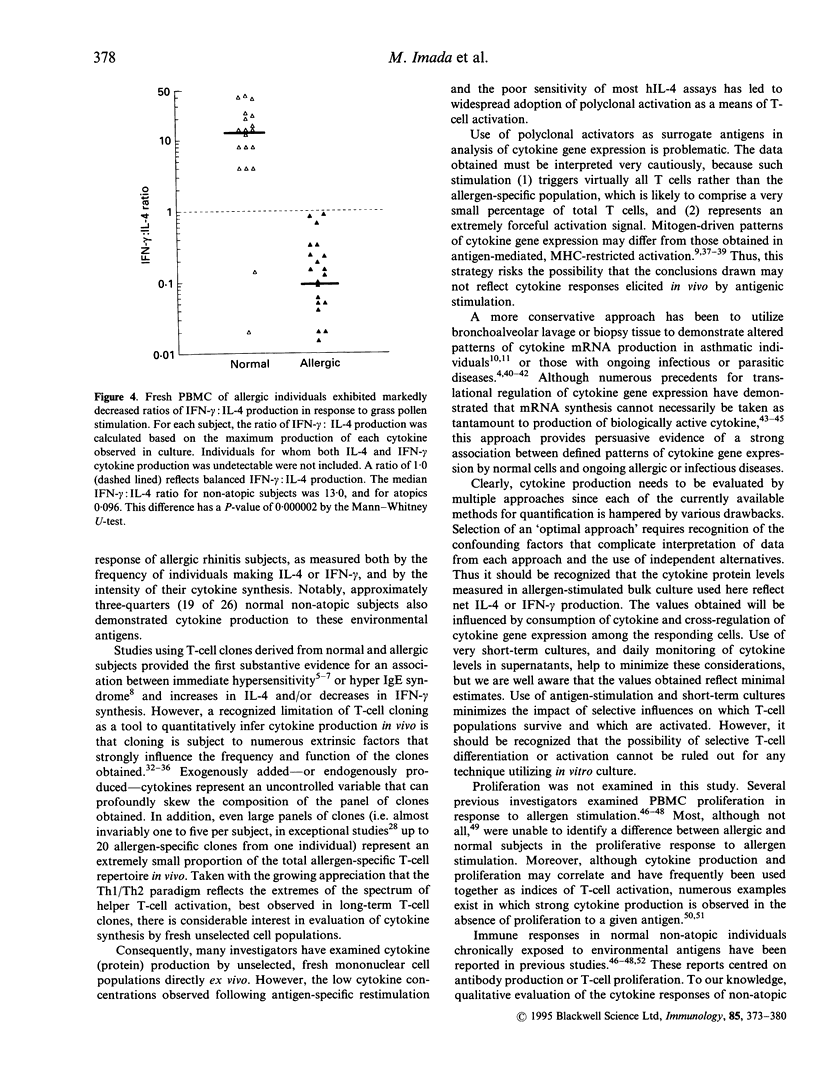
The balance of interleukin-4 (IL-4) to interferon-gamma (IFN-gamma) production that is induced following exposure to common environmental antigens is believed to be instrumental in determining whether hypersensitivity or clinical unresponsiveness results to that antigen. To date, evaluation of cytokine (protein) production has been based predominantly on allergen-reactive CD4 T-cell clones or activation of fresh unselected peripheral blood mononuclear cell (PBMC) populations with non-physiological stimuli such as phorbal myristate acetate (PMA) and calcium ionophore, phytohaemagglutinin (PHA), anti-CD3 or anti-CD2/anti-CD28 monoclonal antibodies (mAb). Here, ultrasensitive IL-4 and IFN-gamma assays were optimized to allow direct analysis of antigen-stimulated cytokine production by fresh human PBMC. Primary cultures of cells from grass pollen-sensitive allergic rhinitis subjects and non-atopic controls were stimulated using a range of grass pollen allergen concentrations in the absence of exogenous cytokines or polyclonal activators. The majority of subjects (45 of 52) exhibited chloroquine-sensitive, CD4-dependent cytokine production in allergen-stimulated, short-term primary culture. Median IL-4 production was substantially greater among atopics (13.0 pg/ml versus < 1 pg/ml, Mann-Whitney U test, P < 0.0000001) and IFN-gamma was lower (P = 0.008), providing direct evidence for an imbalance in both IL-4 and IFN-gamma production among circulating, pollen-reactive cells in individuals with seasonal allergic rhinitis. The distinction in the allergen-driven cytokine responses elicited from normal and atopic donors was underscored by examination of the ratios of IFN-gamma:IL-4 synthesis. Non-atopic individuals exhibited intense IFN-gamma dominance of the T-cell response, in marked contrast to that observed among grass pollen-sensitive individuals (median IFN-gamma:IL-4 ratios of 14.0 versus 0.096, P = 0.000002). The observation that essentially all individuals produced IFN-gamma (+/- IL-4) following antigen stimulation in vitro argues that the most relevant consideration in determining susceptibility to immediate hypersensitivity versus clinical tolerance to environmental allergens is not a genetically defined capacity to recognize the antigen (i.e. if allergen-reactive T cells are present in that individual) but the nature of the cytokine response.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Cavaillon J. M., Fitting C., Guinnepain M. T., Rassemont R., David B. Lymphocyte proliferative responses to the purified Dermatophagoides farinae major allergen in untreated and hyposensitized atopic patients. Allergy. 1988 Feb;43(2):146–151. doi: 10.1111/j.1398-9995.1988.tb00409.x. [DOI] [PubMed] [Google Scholar]
- Chan S. C., Kim J. W., Henderson W. R., Jr, Hanifin J. M. Altered prostaglandin E2 regulation of cytokine production in atopic dermatitis. J Immunol. 1993 Sep 15;151(6):3345–3352. [PubMed] [Google Scholar]
- Chrétien I., Van Kimmenade A., Pearce M. K., Banchereau J., Abrams J. S. Development of polyclonal and monoclonal antibodies for immunoassay and neutralization of human interleukin-4. J Immunol Methods. 1989 Feb 8;117(1):67–81. doi: 10.1016/0022-1759(89)90120-8. [DOI] [PubMed] [Google Scholar]
- Del Prete G. F., De Carli M., D'Elios M. M., Maestrelli P., Ricci M., Fabbri L., Romagnani S. Allergen exposure induces the activation of allergen-specific Th2 cells in the airway mucosa of patients with allergic respiratory disorders. Eur J Immunol. 1993 Jul;23(7):1445–1449. doi: 10.1002/eji.1830230707. [DOI] [PubMed] [Google Scholar]
- Del Prete G., Tiri A., Maggi E., De Carli M., Macchia D., Parronchi P., Rossi M. E., Pietrogrande M. C., Ricci M., Romagnani S. Defective in vitro production of gamma-interferon and tumor necrosis factor-alpha by circulating T cells from patients with the hyper-immunoglobulin E syndrome. J Clin Invest. 1989 Dec;84(6):1830–1835. doi: 10.1172/JCI114368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham S. R., Ying S., Varney V. A., Jacobson M. R., Sudderick R. M., Mackay I. S., Kay A. B., Hamid Q. A. Cytokine messenger RNA expression for IL-3, IL-4, IL-5, and granulocyte/macrophage-colony-stimulating factor in the nasal mucosa after local allergen provocation: relationship to tissue eosinophilia. J Immunol. 1992 Apr 15;148(8):2390–2394. [PubMed] [Google Scholar]
- Gajewski T. F., Joyce J., Fitch F. W. Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J Immunol. 1989 Jul 1;143(1):15–22. [PubMed] [Google Scholar]
- Gauchat J. F., Gauchat D., Qiu G., Mandallaz M., Stadler B. M. Detection of cytokine mRNA in polyclonally-, antigen- or allergen-stimulated mononuclear cells. Immunol Rev. 1991 Feb;119:147–161. doi: 10.1111/j.1600-065x.1991.tb00582.x. [DOI] [PubMed] [Google Scholar]
- Grewe M., Gyufko K., Schöpf E., Krutmann J. Lesional expression of interferon-gamma in atopic eczema. Lancet. 1994 Jan 1;343(8888):25–26. doi: 10.1016/s0140-6736(94)90879-6. [DOI] [PubMed] [Google Scholar]
- Jujo K., Renz H., Abe J., Gelfand E. W., Leung D. Y. Decreased interferon gamma and increased interleukin-4 production in atopic dermatitis promotes IgE synthesis. J Allergy Clin Immunol. 1992 Sep;90(3 Pt 1):323–331. doi: 10.1016/s0091-6749(05)80010-7. [DOI] [PubMed] [Google Scholar]
- Karp C. L., el-Safi S. H., Wynn T. A., Satti M. M., Kordofani A. M., Hashim F. A., Hag-Ali M., Neva F. A., Nutman T. B., Sacks D. L. In vivo cytokine profiles in patients with kala-azar. Marked elevation of both interleukin-10 and interferon-gamma. J Clin Invest. 1993 Apr;91(4):1644–1648. doi: 10.1172/JCI116372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay A. B., Ying S., Varney V., Gaga M., Durham S. R., Moqbel R., Wardlaw A. J., Hamid Q. Messenger RNA expression of the cytokine gene cluster, interleukin 3 (IL-3), IL-4, IL-5, and granulocyte/macrophage colony-stimulating factor, in allergen-induced late-phase cutaneous reactions in atopic subjects. J Exp Med. 1991 Mar 1;173(3):775–778. doi: 10.1084/jem.173.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemeny D. M., Urbanek R., Ewan P., McHugh S., Richards D., Patel S., Lessof M. H. The subclass of IgG antibody in allergic disease: II. The IgG subclass of antibodies produced following natural exposure to dust mite and grass pollen in atopic and non-atopic individuals. Clin Exp Allergy. 1989 Sep;19(5):545–549. doi: 10.1111/j.1365-2222.1989.tb02431.x. [DOI] [PubMed] [Google Scholar]
- Kimura J. Y., Ohta N., Ishii A., Nagano T., Usui M. Functional characterization of lymphocyte response to fractionated house dust mite antigens (Dermatophagoides pteronyssinus) in atopic and non-atopic individuals. Immunology. 1990 Jul;70(3):385–390. [PMC free article] [PubMed] [Google Scholar]
- Lagoo A. S., Lagoo-Deenadayalan S., Lorenz H. M., Byrne J., Barber W. H., Hardy K. J. IL-2, IL-4, and IFN-gamma gene expression versus secretion in superantigen-activated T cells. Distinct requirement for costimulatory signals through adhesion molecules. J Immunol. 1994 Feb 15;152(4):1641–1652. [PubMed] [Google Scholar]
- Li Y., Stefura W. P., Simons F. E., Jay F. T., HayGlass K. T. Limiting dilution analysis of antigen stimulated IL-4 and IFN-gamma production in human mononuclear cell populations. J Immunol Methods. 1994 Oct 14;175(2):169–179. doi: 10.1016/0022-1759(94)90360-3. [DOI] [PubMed] [Google Scholar]
- Maggi E., Parronchi P., Manetti R., Simonelli C., Piccinni M. P., Rugiu F. S., De Carli M., Ricci M., Romagnani S. Reciprocal regulatory effects of IFN-gamma and IL-4 on the in vitro development of human Th1 and Th2 clones. J Immunol. 1992 Apr 1;148(7):2142–2147. [PubMed] [Google Scholar]
- Manetti R., Parronchi P., Giudizi M. G., Piccinni M. P., Maggi E., Trinchieri G., Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993 Apr 1;177(4):1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ownby D. R., Buckley R. H. Lymphocyte responses to ragweed antigens from different sources. J Allergy Clin Immunol. 1979 Jan;63(1):65–66. doi: 10.1016/0091-6749(79)90164-7. [DOI] [PubMed] [Google Scholar]
- Paganelli R., Scala E., Capobianchi M. R., Fanales-Belasio E., D'Offizi G., Fiorilli M., Aiuti F. Selective deficiency of interferon-gamma production in the hyper-IgE syndrome. Relationship to in vitro IgE synthesis. Clin Exp Immunol. 1991 Apr;84(1):28–33. [PMC free article] [PubMed] [Google Scholar]
- Paliard X., de Vries J. E., Spits H. Comparison of lymphokine secretion and responsiveness of human T cell clones isolated in IL-4 and in IL-2. Cell Immunol. 1991 Jul;135(2):383–393. doi: 10.1016/0008-8749(91)90283-h. [DOI] [PubMed] [Google Scholar]
- Paliard X., de Waal Malefijt R., Yssel H., Blanchard D., Chrétien I., Abrams J., de Vries J., Spits H. Simultaneous production of IL-2, IL-4, and IFN-gamma by activated human CD4+ and CD8+ T cell clones. J Immunol. 1988 Aug 1;141(3):849–855. [PubMed] [Google Scholar]
- Parronchi P., Macchia D., Piccinni M. P., Biswas P., Simonelli C., Maggi E., Ricci M., Ansari A. A., Romagnani S. Allergen- and bacterial antigen-specific T-cell clones established from atopic donors show a different profile of cytokine production. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4538–4542. doi: 10.1073/pnas.88.10.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W. E., Seder R. A. Lymphocyte responses and cytokines. Cell. 1994 Jan 28;76(2):241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- Phillips L., Chambers C., Underdown B. J., Zimmerman B. Lymphocyte proliferation to antigen E: demonstration of the restriction of antigen E-specific T cells to ragweed-allergic donors. J Allergy Clin Immunol. 1987 Jun;79(6):933–940. doi: 10.1016/0091-6749(87)90243-0. [DOI] [PubMed] [Google Scholar]
- Quint D. J., Bolton E. J., McNamee L. A., Solari R., Hissey P. H., Champion B. R., MacKenzie A. R., Zanders E. D. Functional and phenotypic analysis of human T-cell clones which stimulate IgE production in vitro. Immunology. 1989 May;67(1):68–74. [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–257. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- Rousset F., Robert J., Andary M., Bonnin J. P., Souillet G., Chrétien I., Brière F., Pène J., de Vries J. E. Shifts in interleukin-4 and interferon-gamma production by T cells of patients with elevated serum IgE levels and the modulatory effects of these lymphokines on spontaneous IgE synthesis. J Allergy Clin Immunol. 1991 Jan;87(1 Pt 1):58–69. doi: 10.1016/0091-6749(91)90213-8. [DOI] [PubMed] [Google Scholar]
- Secrist H., Chelen C. J., Wen Y., Marshall J. D., Umetsu D. T. Allergen immunotherapy decreases interleukin 4 production in CD4+ T cells from allergic individuals. J Exp Med. 1993 Dec 1;178(6):2123–2130. doi: 10.1084/jem.178.6.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Surcel H. M., Troye-Blomberg M., Paulie S., Andersson G., Moreno C., Pasvol G., Ivanyi J. Th1/Th2 profiles in tuberculosis, based on the proliferation and cytokine response of blood lymphocytes to mycobacterial antigens. Immunology. 1994 Feb;81(2):171–176. [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Hu-Li J., Seder R. A., Fazekas de St Groth B., Paul W. E. Interleukin 4 suppresses interleukin 2 and interferon gamma production by naive T cells stimulated by accessory cell-dependent receptor engagement. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5914–5918. doi: 10.1073/pnas.90.13.5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H., Matthes T., Carballido-Perrig N., Zubler R. H., Kindler V. Differential induction of T cell cytokine mRNA in Epstein-Barr virus-transformed B cell clones: constitutive and inducible expression of interleukin-4 mRNA. Eur J Immunol. 1993 Apr;23(4):899–903. doi: 10.1002/eji.1830230420. [DOI] [PubMed] [Google Scholar]
- Tang M., Kemp A. Production and secretion of interferon-gamma (IFN-gamma) in children with atopic dermatitis. Clin Exp Immunol. 1994 Jan;95(1):66–72. doi: 10.1111/j.1365-2249.1994.tb06016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M., Kemp A., Varigos G. IL-4 and interferon-gamma production in children with atopic disease. Clin Exp Immunol. 1993 Apr;92(1):120–124. doi: 10.1111/j.1365-2249.1993.tb05957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troye-Blomberg M., Riley E. M., Kabilan L., Holmberg M., Perlmann H., Andersson U., Heusser C. H., Perlmann P. Production by activated human T cells of interleukin 4 but not interferon-gamma is associated with elevated levels of serum antibodies to activating malaria antigens. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5484–5488. doi: 10.1073/pnas.87.14.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga E. A., Snoek M., Bos J. D., Jansen H. M., Kapsenberg M. L. Comparison of diversity and function of house dust mite-specific T lymphocyte clones from atopic and non-atopic donors. Eur J Immunol. 1990 Jul;20(7):1519–1526. doi: 10.1002/eji.1830200717. [DOI] [PubMed] [Google Scholar]
- Wierenga E. A., Snoek M., de Groot C., Chrétien I., Bos J. D., Jansen H. M., Kapsenberg M. L. Evidence for compartmentalization of functional subsets of CD2+ T lymphocytes in atopic patients. J Immunol. 1990 Jun 15;144(12):4651–4656. [PubMed] [Google Scholar]
- Wu C. Y., Demeure C., Kiniwa M., Gately M., Delespesse G. IL-12 induces the production of IFN-gamma by neonatal human CD4 T cells. J Immunol. 1993 Aug 15;151(4):1938–1949. [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- Yang X., Gieni R. S., Mosmann T. R., HayGlass K. T. Chemically modified antigen preferentially elicits induction of Th1-like cytokine synthesis patterns in vivo. J Exp Med. 1993 Jul 1;178(1):349–353. doi: 10.1084/jem.178.1.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Hayglass K. T. Allergen-dependent induction of interleukin-4 synthesis in vivo. Immunology. 1993 Jan;78(1):74–79. [PMC free article] [PubMed] [Google Scholar]
- van der Pouw Kraan C. T., Aalberse R. C., Aarden L. A. IgE production in atopic patients is not related to IL-4 production. Clin Exp Immunol. 1994 Aug;97(2):254–259. doi: 10.1111/j.1365-2249.1994.tb06077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



