Abstract
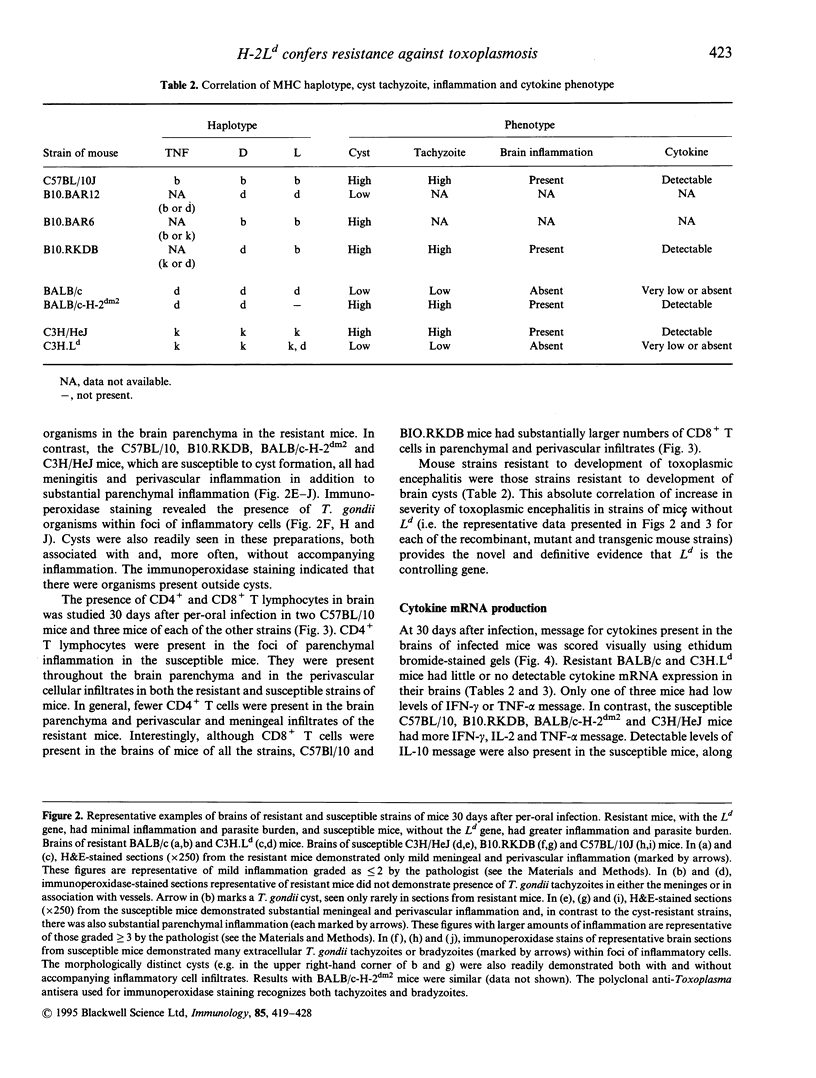
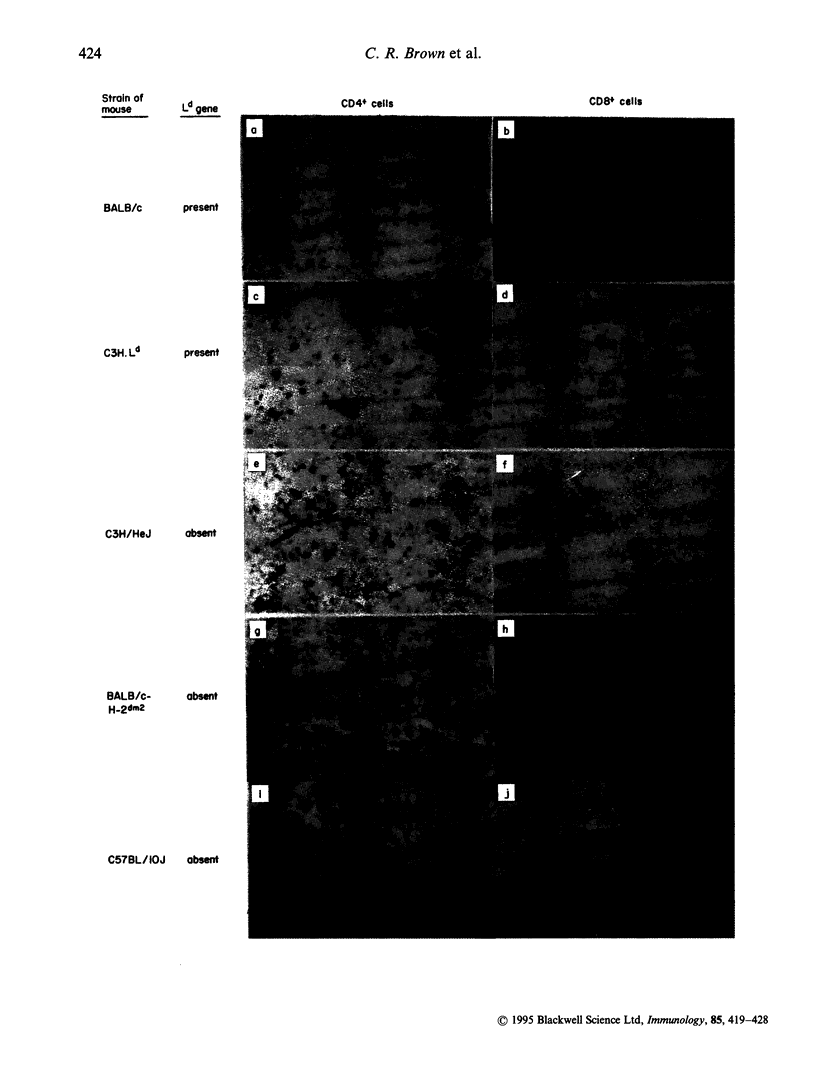
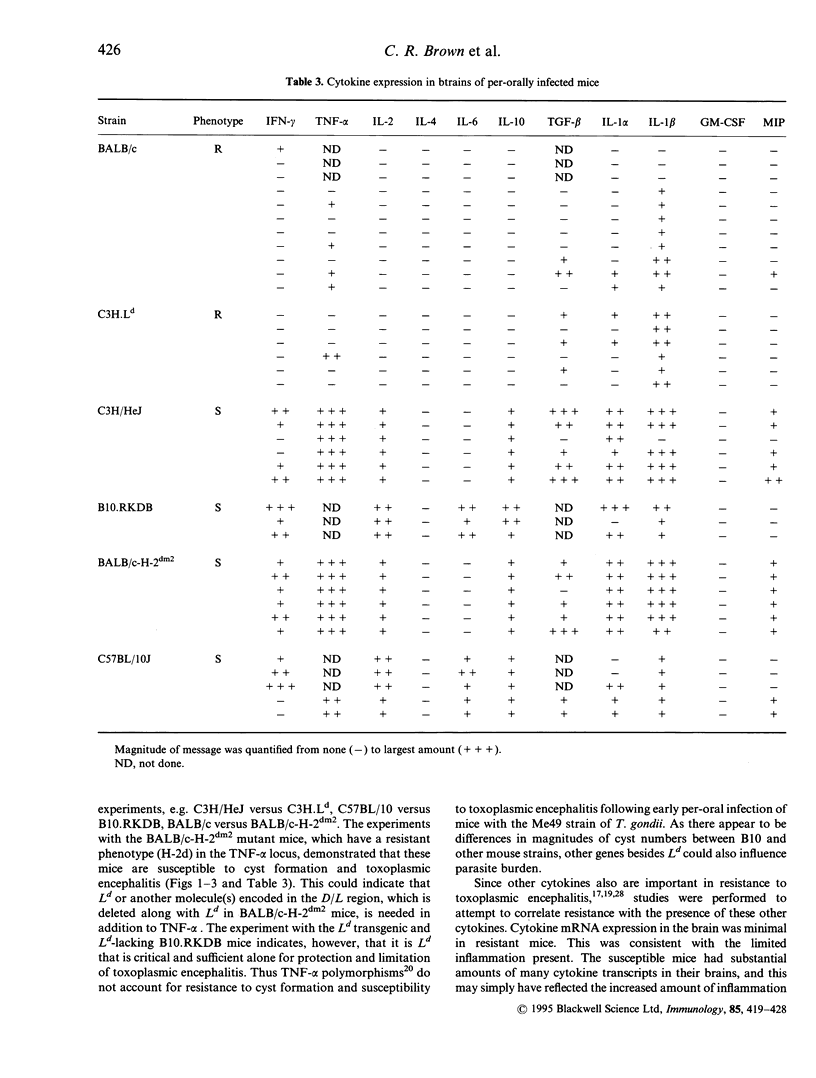
Control of resistance to cyst burden following per-oral infection with Toxoplasma gondii has been mapped previously to a region of mouse chromosome 17 of approximately 140 kb. This region is contiguous with and contains the class I gene, Ld. Resistance to development of toxoplasmic encephalitis has also been reported to be controlled by genes in this region of H-2. TNF-alpha, D and L genes, as well as unidentified genes, are also in this region. The work described here was performed to identify definitively the gene(s) in this 140 kb region that confers resistance to cysts and encephalitis. The study demonstrates that relative resistance to T. gondii organisms and cyst burden in brain, and toxoplasmic encephalitis, 30 days following per-oral T. gondii infection is correlated absolutely with the presence of the Ld gene in inbred, recombinant, mutant and C3H.Ld transgenic mice. Mice with the Ld gene had lower cyst burdens and less encephalitis than those without the Ld gene. Specifically, 30 days after infection mice with the Ld gene had minimal perivascular inflammation and meningeal inflammation and very few Toxoplasma cysts or organisms in immunoperoxidase-stained preparations of their brains. Mice without the Ld gene had a similar pattern of inflammation, but in addition they had collections of inflammatory cells in the brain parenchyma. Free tachyzoites were found within these foci of inflammation and cysts were present in these areas as well as in contiguous areas without inflammatory cells. There were CD4+ and CD8+ T lymphocytes in the areas of inflammation and throughout the brain parenchyma. Mice that were resistant to cysts and encephalitis had little detectable brain cytokine mRNA expression, while mice that were susceptible had elevated levels of mRNA for a wide range of cytokines, consistent with their greater amounts of inflammation. The present work definitively demonstrates that a Ld-restricted response decreases the number of organisms and cysts within the brain and thereby limits toxoplasmic encephalitis and levels of interferon-gamma (IFN-gamma), tumour necrosis factor-alpha (TNF-alpha), interleukin-2 (IL-2), IL-6, IL-10, transforming growth factor-beta (TGF-beta), IL-1 alpha, IL1 beta and macrophage inhibiting protein (MIP) mRNA in the brain 30 days after per-oral infection.
Full text
PDF


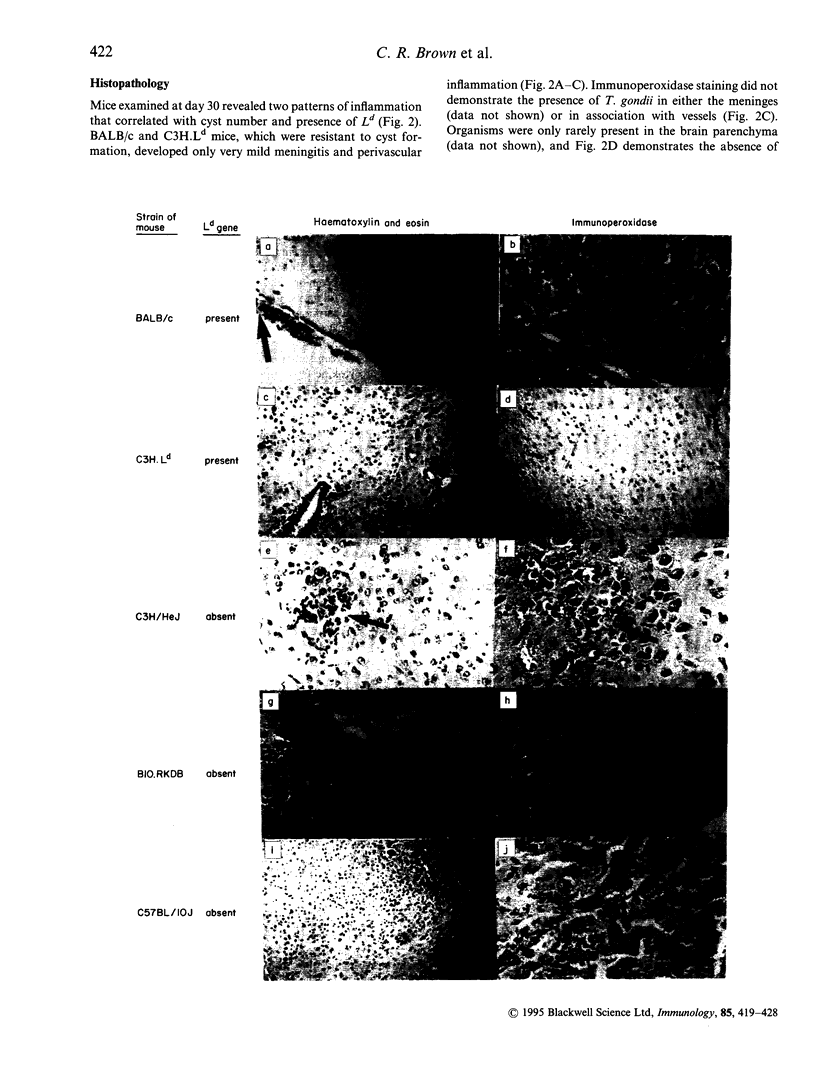



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich C. J., Hammer R. E., Jones-Youngblood S., Koszinowski U., Hood L., Stroynowski I., Forman J. Negative and positive selection of antigen-specific cytotoxic T lymphocytes affected by the alpha 3 domain of MHC I molecules. Nature. 1991 Aug 22;352(6337):718–721. doi: 10.1038/352718a0. [DOI] [PubMed] [Google Scholar]
- Araujo F. G., Williams D. M., Grumet F. C., Remington J. S. Strain-dependent differences in murine susceptibility to toxoplasma. Infect Immun. 1976 May;13(5):1528–1530. doi: 10.1128/iai.13.5.1528-1530.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell J. M., Roberts C. W., Alexander J. Influence of genes within the MHC on mortality and brain cyst development in mice infected with Toxoplasma gondii: kinetics of immune regulation in BALB H-2 congenic mice. Parasite Immunol. 1993 Jun;15(6):317–324. doi: 10.1111/j.1365-3024.1993.tb00616.x. [DOI] [PubMed] [Google Scholar]
- Brown C. R., McLeod R. Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J Immunol. 1990 Nov 15;145(10):3438–3441. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Conley F. K., Jenkins K. A. Immunohistological study of the anatomic relationship of toxoplasma antigens to the inflammatory response in the brains of mice chronically infected with Toxoplasma gondii. Infect Immun. 1981 Mar;31(3):1184–1192. doi: 10.1128/iai.31.3.1184-1192.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran L. W., Zeller J. C., Lundy J. K., Chang-Miller A., Krco C. J., David C. S., Pease L. R. Genetic analysis of the H-2D region using a new intra-D-region recombinant mouse strain. J Immunol. 1987 Oct 15;139(8):2818–2824. [PubMed] [Google Scholar]
- Gazzinelli R. T., Eltoum I., Wynn T. A., Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-alpha and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol. 1993 Oct 1;151(7):3672–3681. [PubMed] [Google Scholar]
- Gazzinelli R. T., Hakim F. T., Hieny S., Shearer G. M., Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991 Jan 1;146(1):286–292. [PubMed] [Google Scholar]
- Hunter C. A., Roberts C. W., Alexander J. Kinetics of cytokine mRNA production in the brains of mice with progressive toxoplasmic encephalitis. Eur J Immunol. 1992 Sep;22(9):2317–2322. doi: 10.1002/eji.1830220921. [DOI] [PubMed] [Google Scholar]
- Jones T. C., Erb P. H-2 complex-linked resistance in murine toxoplasmosis. J Infect Dis. 1985 Apr;151(4):739–740. doi: 10.1093/infdis/151.4.739. [DOI] [PubMed] [Google Scholar]
- Khan I. A., Ely K. H., Kasper L. H. Antigen-specific CD8+ T cell clone protects against acute Toxoplasma gondii infection in mice. J Immunol. 1994 Feb 15;152(4):1856–1860. [PubMed] [Google Scholar]
- Luft B. J., Hafner R., Korzun A. H., Leport C., Antoniskis D., Bosler E. M., Bourland D. D., 3rd, Uttamchandani R., Fuhrer J., Jacobson J. Toxoplasmic encephalitis in patients with the acquired immunodeficiency syndrome. Members of the ACTG 077p/ANRS 009 Study Team. N Engl J Med. 1993 Sep 30;329(14):995–1000. doi: 10.1056/NEJM199309303291403. [DOI] [PubMed] [Google Scholar]
- McGuire W., Hill A. V., Allsopp C. E., Greenwood B. M., Kwiatkowski D. Variation in the TNF-alpha promoter region associated with susceptibility to cerebral malaria. Nature. 1994 Oct 6;371(6497):508–510. doi: 10.1038/371508a0. [DOI] [PubMed] [Google Scholar]
- McLeod R., Eisenhauer P., Mack D., Brown C., Filice G., Spitalny G. Immune responses associated with early survival after peroral infection with Toxoplasma gondii. J Immunol. 1989 May 1;142(9):3247–3255. [PubMed] [Google Scholar]
- McLeod R., Estes R. G., Mack D. G., Cohen H. Immune response of mice to ingested Toxoplasma gondii: a model of toxoplasma infection acquired by ingestion. J Infect Dis. 1984 Feb;149(2):234–244. doi: 10.1093/infdis/149.2.234. [DOI] [PubMed] [Google Scholar]
- McLeod R., Skamene E., Brown C. R., Eisenhauer P. B., Mack D. G. Genetic regulation of early survival and cyst number after peroral Toxoplasma gondii infection of A x B/B x A recombinant inbred and B10 congenic mice. J Immunol. 1989 Nov 1;143(9):3031–3034. [PubMed] [Google Scholar]
- Parker S. J., Roberts C. W., Alexander J. CD8+ T cells are the major lymphocyte subpopulation involved in the protective immune response to Toxoplasma gondii in mice. Clin Exp Immunol. 1991 May;84(2):207–212. doi: 10.1111/j.1365-2249.1991.tb08150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrinaci S., Hanson J., David C. Hierarchy in the assembly of HLA-B27 and HLA-Cw3 molecules in transgenic mice. Immunogenetics. 1994;39(2):130–137. doi: 10.1007/BF00188616. [DOI] [PubMed] [Google Scholar]
- Rubocki R. J., Hansen T. H., Lee D. R. Molecular studies of murine mutant BALB/c-H-2dm2 define a deletion of several class I genes including the entire H-2Ld gene. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9606–9610. doi: 10.1073/pnas.83.24.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley L. D., Adams L. B., Fukutomi Y., Krahenbuhl J. L. Tumor necrosis factor-alpha triggers antitoxoplasmal activity of IFN-gamma primed macrophages. J Immunol. 1991 Oct 1;147(7):2340–2345. [PubMed] [Google Scholar]
- Suzuki Y., Conley F. K., Remington J. S. Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J Immunol. 1989 Sep 15;143(6):2045–2050. [PubMed] [Google Scholar]
- Suzuki Y., Joh K., Orellana M. A., Conley F. K., Remington J. S. A gene(s) within the H-2D region determines the development of toxoplasmic encephalitis in mice. Immunology. 1991 Dec;74(4):732–739. [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Orellana M. A., Wong S. Y., Conley F. K., Remington J. S. Susceptibility to chronic infection with Toxoplasma gondii does not correlate with susceptibility to acute infection in mice. Infect Immun. 1993 Jun;61(6):2284–2288. doi: 10.1128/iai.61.6.2284-2288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. M., Grumet F. C., Remington J. S. Genetic control of murine resistance to Toxoplasma gondii. Infect Immun. 1978 Feb;19(2):416–420. doi: 10.1128/iai.19.2.416-420.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]